Abstract
Erectile dysfunction (ED) is defined as the inability to attain or keep an erection of the penis, and this has become a prevalent male sexual disorder. Rodents are employed by many studies to research the physiology/pathology of erectile function. Erectile function in rodents can be evaluated by measuring the intracavernosal pressure (ICP). In practice, ICP can be monitored following electrical stimulation of the cavernous nerves (CNs). The arterial pressure of the carotid artery (the mean arterial pressure) is used as the reference for ICP. Using ICP recording protocols, many key parameters of erectile function can be measured from the ICP response curve. The ICP measurement provides more information than the apomorphine-induced penile erection test, and is cheaper than telemetric monitoring of the corpus spongiosum penis, making this method the most popular one to evaluate erectile function. However, compared to the easily-performed APO-induced erectile function test, successful ICP recordings require attention to detail, practice, and adherence to the operation method. In this work, an introduction to ICP recording in rats is provided to complement the procedure efficiently.
Keywords: Biology, Issue 136, Cavernous Nerve, Erectile Dysfunction, Intracavernosal Pressure, Mean Arterial Pressure, Penis, Rodent
Introduction
ED is defined as the inability to attain or keep a penile erection, and has become a common male sexual disorder1. Experimental animals are used and provide reproducible models to investigate erectile function2. For a long time, several larger animal models have been employed for investigating erectile function3,4,5. Although rodents are relatively small compared to other animals, they are also used for the study of male erectile dysfunction due to exhibiting several advantages6. First, the morphological and functional sexual characteristics of humans are recapitulated in rodents. Second, compared to larger animals used in ED studies, rodents are more economical to purchase, house, and maintain. Third, genetically modified rodent models provide advantages in reproducible and subsequent behavioral as well as neurophysiological studies. Therefore, rodents have quickly become the primary animals used in the study of male erectile dysfunction.
Benefiting from a pure genetic background and consistent culture conditions, rodent models have provided consistently reproducible data5,6,7,8. Among the numerous available studies related to many aspects of erectile functions, the apomorphine (APO)-induced erectile response test and the electric-stimulation-induced ICP response test are the most widely used methods that reliably reflect erectile function9,10,11,12. The APO-induced erectile function test, developed by Heaton et al.13, is a bio-assay that utilizes the phenomenon that administration of apomorphine to rats elicits erections and yawns. As an easy, noninvasive, and stable bio-assay to evaluate erectile function, the APO-induced erectile function test is widely used in many studies. However, this assay does not adequately reflect the quality of erections or the dynamic changes in blood flow associated with an erectile response14. ICP measurements were initially developed by Quinlan et al.15. In this method, a catheter is placed into the carotid artery to measure systemic blood pressure, and another catheter is inserted into the crus corpus cavernosum to record the ICP. Before or during the ICP recording, a vasoactive agent and/or electrical field stimulation of the major pelvic ganglion (MPG) or CN were often given to the rats14. This assay has been a reliable tool for evaluating the therapies and medicines for ED, and will likely be used as a vital evaluation method in the future6.
Compared to the easily-performed APO-induced erectile function test, successful ICP recordings require attention to detail, practice, and adherence to the operation method. Therefore, here, we provide a detailed description of how to perform ICP recording.
Protocol
Three-month-old and 18-month-old Sprague-Dawley rats were used in the present study. All animals were handled in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals. Procedures involving animal subjects were approved by the local Institutional Animal Care and Ethics Committee, with an effort to minimize animal suffering. The protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at Nanjing Tech University (Nanjing, China).
The rats were divided into two groups according to their age and preliminary performance in the APO-induced erectile function test prior to ICP recording: the young normal group (YN group) and aged erectile dysfunction group (AE group)10.
1. Preparation Before Surgery
Manually make a pair of bipolar electrodes for the ICP recording (Figure 1). Slightly bend the ends of electrodes, and adjust the distance between two electrodes to 1-2 mm wide, as shown in Figure 1A.
Connect the electrodes to the stimulator using two crocodile clamps (Figure 1A,B).
Assemble the catheter system: First, connect a hypodermic 23G needle to a 3-way stopcock with tubing, then connect the stopcock to the pressure transducer. Next, attach a 10-mL syringe to the third end of stopcock to provide heparin saline.
Carefully check for leakage after filling the whole system with heparin saline (200 U/mL). Then turn the 3-way stopcock to close the syringe channel or the pressure transducer channel (Figure 1C).
Lift the needle 20 cm up the level of the wooden pad. Then calibrate the pressure recording system to 20 cm H2O. After that, move the height of the needle to verify the accuracy of the recording system. Repeat the calibration until the accuracy was confirmed.
Transfer the rats from the animal facility to the surgery room, and allow them to become accustomed to the surgery room for at least 30 min.
The autoclaved instruments are sprayed with 70% ethanol just before surgery

2. Surgery Procedure
Anesthetize the rat with an intraperitoneal injection of sodium pentobarbital at a dose of 45 mg/kg body weight, and wait for 5-10 min. Pinch the toes to confirm a proper anesthetization.
Shave the fur of the abdomen and neck with an electric shaver, and place the rat on its back on a heating pad.
Wipe the surgery area with 10% povidone-iodine solution soaked cotton balls followed by 70% ethanol soaked cotton balls. Also, apply ophthalmic ointment to prevent the eyes from drying out.
- Catheterize the left carotid artery.
- Grasp the skin of the neck with forceps and make a horizontal incision in the middle of the neck. Incise the muscles, carefully expose the left carotid artery, and isolate a 5-mm section of the vessel.
- Carefully separate the carotid artery from the vagus nerve using forceps, draw a silk suture under the carotid artery, and put a loose tie on the caudal end of the vessel, then make another tight knot on the cranial end of the vessel.
- Caudally clamp the vessel with a bulldog clamp above the suture to stop the blood flow.
- Carefully make an incision on the vessel with microsurgical scissors, and insert the arterial catheter towards the heart with the assistance of the micro-dissecting hook and forceps.
- Fasten the loose caudal ligature around the catheter to secure it. Remove the bulldog clamp to recover the blood flow.
- Isolate the CN and Place the Electrode
- Lift the skin and muscle of the abdomen with a pair of forceps. With the dissecting scissors, cut through the lower abdomen to the penis to make a midline incision.
- Gently push the intestine with swab into the upper part of the abdominal cavity.
- Grasp the bladder with a pair of forceps and pull out the bladder from the abdominal cavity. Expose the ventral lobes of the prostate, which is located on the ventral portion of the urethra.
- Pull out the ventral lobes of the prostate, seminal vesicle, and vas deferens to expose the dorsal lobe of the prostate. Find the point of adhesion of the vas deferens and prostate.
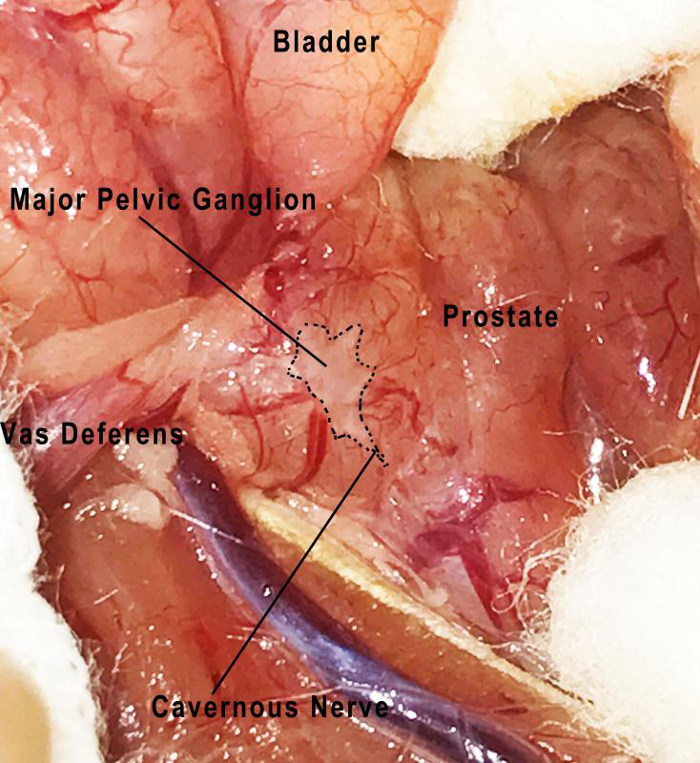
- Separate the space between the prostate and vas deferens. Carefully expose the fibrous capsule, which is located posterior to the junction point of the prostate and vas deferens. Then find the major pelvic ganglion (Figure 2). NOTE: The major pelvic ganglion and cavernous nerves can be seen at the surface of the prostate.
- Carefully dry CN area with a sterile swab. Carefully isolate and hook the right cavernous nerve with the bipolar electrodes.
- Catheterize the Left Crura
- Cut a small incision in the skin of the penis with dissecting scissors, and then carefully denude the skin of the penis shaft.
- Dissect the striated penile musculature. Find the upper branch of pubis bone.
- Expose the bulbospongiosus muscle, which covers the spongious bulb.
- Divide the bulbospongiosus muscle from the ischiocavernosus muscle by using curved forceps.
- Carefully isolate the ischiocavernosus muscle with curved forceps, and then cut the ischiocavernosus muscle to expose the white tunica albuginea of the crus corpus cavernosum.
- Following the anatomical direction of the crus corpus cavernosum, carefully insert the needle into the crus corpus cavernosum through the white tunica albuginea. NOTE: This is a crucial step for successful catheterization. A small amount of heparinized saline can be injected, and a slight penile tumescence should be observed, if the needle has been inserted correctly.
- Carefully release the needle and avoid any sliding of the needle or disruption of the connecting tube. Inspect for any leakage.
3. Stimulate the CN
Open the software program for pressure signal recording and start the pressure signal recording.
Set the parameters of the stimulation: 15 Hertz, pulse width of 5 milliseconds, 5 volts, and a 60 s duration. Stimulate CN at a frequency of 15 Hz with a pulse width of 5 ms. NOTE: A sharp rise of the ICP can be observed while applying the electrical stimulation.
Allow a 30-minute rest interval between stimulations. The maximum of consecutive stimulation in each animal is three times.
4. End the Procedure
After recording, administer euthanasia by injecting an overdose of pentobarbital sodium at a dose of 150 mg/kg body weight. Confirm the death of rats by checking their arterial pressure. Remove the rats, and clean the surgery tools.
5. Data Analysis
Save and export the data from the software. The response is commonly expressed as the ratio of ICP to systemic mean arterial pressure (MAP). The ratio of peak ICP/MAP was calculated to evaluate the erectile function.
Pool data from at least five rats, and analyze with statistical software. Differences are considered statistically significant when p < 0.05, using Student's t-test.
Representative Results
Numerous studies have shown that erectile dysfunction in aged males is becoming a common problem. However, medical treatment is limited in the management of aging-related ED16. In rodent models of aging-related ED, many therapies are tested on the erectile function of aged rats. As we have introduced above, the ICP recording test could be used to distinguish the ED animals from the total population of experimental animals, which is also valuable to quantify the effect of potential treatment or drugs on erectile function.
As illustrated in Figure 3A, the typical ICP response curve of the ED group (Aged ED rats, AE, 18-month-old) was much lower than the control group's curve (Young normal rats, YN, 3-month-old). Usually, the highest ICP was chosen for statistical analysis. After calculating the ratio of ICP/MAP, the pooled data from 5 rats showed that the ICP/MAP ratio in AE group also decreased significantly compared with that in control groups (Figure 3B). Besides these two key parameters, the peak ICP, the plateau ICP, the detumescence time, the duration of response, and the area under the curve all significantly decreased in aged ED rats (Table 1). These ICP recording data provide a quantitative measurement method to reflect erectile function.
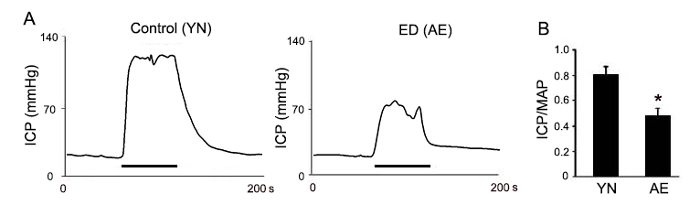
Figure 1: Manually made catheter, bipolar electrodes, and the stimulator and signal recording system for ICP recording. Both the catheter and electrodes are connected to the stimulator and signal recording system equipment. The catheter ends are inserted into the left artery or crus corpus cavernosum to record the pressure. The electrodes are placed beneath the cavernous nerves (CNs). (A) The ends of the electrodes are slightly bent.(B) The stimulator and signal recording system equipment. (C) Manually assembled catheter. Please click here to view a larger version of this figure.
Figure 2: The anatomic position of the major pelvic ganglion and cavernous nerves. As shown in the figure, the major pelvic ganglion is located at the lateral border of the prostate. The cavernous nerve in a rat is a distinct nerve which extends from the major pelvic ganglion. Please click here to view a larger version of this figure.
Figure 3: Representative ICP recording of differently aged rats. (A) Representation of ICP changes during cavernous nerve stimulation. Control group (the young normal rats, YN, 3-month-old rats); ED group (the aged ED rats, AE, 18-month-old). The bar under ICP response curve represents the timing of the electrical stimulation.(B) The erectile function index (intracavernous pressure/mean arterial pressure) of different experiment group is displayed. Data from at least five rats are presented as mean ±standard deviation; differences are considered statistically significant when *p < 0.05, using Student's t-test. Please click here to view a larger version of this figure.
| Young normal (YN) | Aged Erectile dysfunction (AE) | |
| (Mean±SD) | (Mean±SD) | |
| basal ICP | 22.3±3.7 (mmHg) | 21.9±5.2 (mmHg) |
| peak ICP | 172.8±7.6 (mmHg) | 105.4±4.9 (mmHg) * |
| plateau ICP | 165.4±2.5 (mmHg) | 86.5±4.1 (mmHg) ** |
| latency to erection | 10.3±1.6 (s) | 15.1±2.3 (s) |
| detumescence time | 46.7±2.6 (s) | 11.8±3.3 (s) * |
| duration of response | 107.2±3.7 (s) | 71.7±4.2 (s) * |
| area under the curve | 17436.9±736.4 | 6426±428.3 ** |
| number of erections | 3±0.0 | 3±0.0 |
Table 1: Parameters of erectile function in young and aged erectile dysfunction rats. The basal intracavernous pressure (ICP), peak ICP, plateau ICP, latency to erection, detumescence time, duration of response, area under the ICP time response curve, and the number of erections observed in 30 min are calculated and expressed as mean ±standard deviation (n=5). Differences are considered statistically significant when *p < 0.05 or **p < 0.01, using Student's t-test.
| Troubleshooting Symptoms | Possible Causes and Suggestions |
| No pressure of ICP or MAP | Equipment problem: Check the status of equipment |
| Leakage problem: Check the tube, make sure the connection is intact, then check the stopcock, and whether it is in the appropriate position | |
| Low pressure increased after electrical stimulation | Inadequate or no stimulation: Check the connection of the electrodes from the stimulator to CN, try repositioning the electrodes |
| Damage to the CN: try stimulating contralateral CN | |
| Leakage: Check the insertion site of ICP catheter, as leakage from insertion site will decrease the ICP | |
| Bleeding | Check the bleeding site; if the artery is perforated, end the experiment. If the breeding happens in the ICP insertion site, re-puncture is very tricky, perhaps impossible |
Table 2: Troubleshooting for the ICP recording procedure. Three common symptoms in ICP surgery, possible causes, and suggestions.
Discussion
As a direct measure of erectile function, ICP is a reliable method14. It allows for the acquisition of data on basal ICP, peak ICP, plateau ICP, time to erection and detumescence time, duration of response, etc. Besides these direct measured parameters, there are some other index parameters: (1) "T80", the time to reach 80% of peak ICP; (2) "D20", the time to decrease to 20% of peak ICP; (3) "ΔT80", the rate of increase in pressure (per second) at T80; and (4) "ΔD20", the rate of decrease in pressure (per second) at D20. These parameters allow for the quantification of each stage and the quality of the ICP response, which can reflect the impact of disease and examine the efficacy of the drugs14,17. This ICP recording method is widely used in ED animal models. In this protocol, we compared the ICP response in young and aged ED rats. If administration of drugs via intracavernous catheter is needed, it could be done by placement of another intracavernous catheter into the contralateral crus corpus cavernosum17. The total volume of injection should be less than 0.1 mL. Systematic drug administration can be performed via intraperitoneal or subcutaneous injection. If intravenous administration is needed, the right jugular vein could be considered.
Although ICP surgery is slightly technically challenging and requires an in-depth understanding of lower abdominal and pelvic organs anatomy, with time and effort one can master the protocol. Since the catheters and electrodes are manually made by the experimenter, the pressure transducer needs to be checked and calibrated every time before starting the experiment. The electrodes should be thin and the distance between the two electrodes should be small enough to stimulate the nerve efficiently. It is important to avoid stretching or other damage to the nerve while placing the electrodes. It is critical to precisely insert the needle into the left crura, without any sliding or dropping off. Three common symptoms are listed in Table 2, which could be considered when some symptoms occur.
This ICP recording method is also transferrable to mice. Nevertheless, the bodies of mice are smaller than rats, making ICP recording in mice harder. If ICP recordings were conducted in mice, smaller sized hypodermic needles could be used.
ICP recording also has its limitations and other methods may be considered. Usually, the ICP recording surgery is performed on anesthetized animals, and the animals are sacrificed after short-term ICP measurement, which is not suitable for longitudinal monitoring of ICP. A significant improvement of direct ICP recording method is performing ICP recordings on conscious, freely moving rats, which is well-described in the protocols written by Hedlund et al.17 This method provides an opportunity to record the intracavernosal pressure for longer periods of time. Recently, advancements have been made to include a telemetric approach applied in conscious, freely moving animals18,19,20. Although the telemetric approach has many advantages over traditional ICP recording, it requires expensive equipment to convert and receive the converted pressure signals. For overcoming the catheterization method, Adachi et al. describe a way to examine erectile responses by continuous measuring of the diameter of penises in rats21. In this method, a pair of piezoelectric crystals is placed to the shaft of the corpus cavernosum penis. Two parameters are defined to evaluate the erectile response: (1) "D-max": the maximal developed penile diameter during measurement; and (2) "T50%": the time from the maximum response to 50% recovery of the maximum response in anesthetized rats. With this protocol, the changes of the diameter of the penis also faithfully reflect the quality of erectile response; furthermore, the tissue is not injured during the entire measurement. However, like the telemetric approach, this sonomicrometry method also requires specific and relatively expensive equipment to convert and receive the diameter changes of the penis.
In summary, ICP recording is an accurate, relatively convenient, and inexpensive method. We believe this introduction can help extend the use of ICP method, and further advance knowledge of the pathology and physiology of erectile dysfunction.
Disclosures
The authors have nothing to disclose
Acknowledgments
This work was supported by the Fundamental Research Funds for the Central Universities (020814380018, 020814380077), The China Scholarship Council (CSC, No. 201606195024), Natural Science Foundation of Jiangsu Province (BK20160138), and Key Project supported by Science and Technology Development Foundation, Nanjing Medical University (2014NJMUZD053).
References
- NIH Consensus Conference. Impotence. NIH Consensus Development Panel on Impotence. JAMA. 1993;270(1):83–90. [PubMed] [Google Scholar]
- Chung E, De Young L, Brock GB. Investigative models in erectile dysfunction: a state-of-the-art review of current animal models. J Sex Med. 2011;8(12):3291–3305. doi: 10.1111/j.1743-6109.2011.02505.x. [DOI] [PubMed] [Google Scholar]
- Lue TF, Takamura T, Schmidt RA. Hemodynamics of erection in the monkey. J Urol. 1983;130(6):1237–1241. doi: 10.1016/s0022-5347(17)51768-1. [DOI] [PubMed] [Google Scholar]
- Carati CJ, Creed KE, Keogh EJ. Autonomic control of penile erection in the dog. J Physiol. 1987;384:525–538. doi: 10.1113/jphysiol.1987.sp016468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steers WD, Mallory B, de Groat WC. Electrophysiological study of neural activity in penile nerve of the rat. Am J Physiol. 1988;254(6 Pt 2):R989–R1000. doi: 10.1152/ajpregu.1988.254.6.R989. [DOI] [PubMed] [Google Scholar]
- Kapoor MS, Khan SA, Gupta SK. Animal models of erectile dysfunction. J Pharmacol Toxicol Methods. 2015;76:43–54. doi: 10.1016/j.vascn.2015.07.013. [DOI] [PubMed] [Google Scholar]
- Burnett AL, Lowenstein CJ, Bredt DS. Nitric oxide: A physiologic mediator of penile erection. Science. 1992;257(5068):401–403. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- Oh TY, Kang KK, Ahn BO. Erectogenic effect of the selective phosphodiesterase type 5 inhibitor, DA-8159. Arch Pharm Res. 2000;23(5):471–476. doi: 10.1007/BF02976575. [DOI] [PubMed] [Google Scholar]
- Ouyang B, Sun X, Han D. Human urine-derived stem cells alone or genetically-modified with FGF2 Improve type 2 diabetic erectile dysfunction in a rat model. PLoS One. 2014;9(3):e92825. doi: 10.1371/journal.pone.0092825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F, Xu J, Zhang Q. Identification and characterization of the MicroRNA profile in aging rats with erectile dysfunction. J Sex Med. 2014;11(7):1646–1656. doi: 10.1111/jsm.12500. [DOI] [PubMed] [Google Scholar]
- Cho MC, Park K, Kim SW. Restoration of erectile function by suppression of corporal apoptosis, fibrosis and corporal veno-occlusive dysfunction with rho-kinase inhibitors in a rat model of cavernous nerve injury. J Urol. 2015;193(5):1716–1723. doi: 10.1016/j.juro.2014.10.099. [DOI] [PubMed] [Google Scholar]
- Hannan JL, Matsui H, Sopko NA. Caspase-3 dependent nitrergic neuronal apoptosis following cavernous nerve injury is mediated via RhoA and ROCK activation in major pelvic ganglion. Sci Rep. 2016;6:29416. doi: 10.1038/srep29416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton JP, Varrin SJ, Morales A. The characterization of a bio-assay of erectile function in a rat model. J Urol. 1991;145(5):1099–1102. doi: 10.1016/s0022-5347(17)38543-9. [DOI] [PubMed] [Google Scholar]
- Mehta N, Sikka S, Rajasekaran M. Rat as an animal model for male erectile function evaluation in sexual medicine research. J Sex Med. 2008;5(6):1278–1283. doi: 10.1111/j.1743-6109.2008.00854.x. [DOI] [PubMed] [Google Scholar]
- Quinlan DM, Nelson RJ, Partin AW. The rat as a model for the study of penile erection. J Urol. 1989;141(3):656–661. doi: 10.1016/s0022-5347(17)40926-8. [DOI] [PubMed] [Google Scholar]
- Albersen M, Orabi H, Lue TF. Evaluation and treatment of erectile dysfunction in the aging male: A mini-review. Gerontology. 2012;58(1):3–14. doi: 10.1159/000329598. [DOI] [PubMed] [Google Scholar]
- Hedlund P, Matsumoto K, Andersson K-E. Animal Models of Erectile Dysfunction. Curr Prot Pharmacol. 2005;29:5.41.1–5.41.22. doi: 10.1002/0471141755.ph0541s29. [DOI] [PubMed] [Google Scholar]
- Giuliano F, Bernabe J, Rampin O. Telemetric monitoring of intracavernous pressure in freely moving rats during copulation. J Urol. 1994;152(4):1271–1274. doi: 10.1016/s0022-5347(17)32566-1. [DOI] [PubMed] [Google Scholar]
- Bernabe J, Rampin O, Giuliano F. Intracavernous pressure changes during reflexive penile erections in the rat. Physiol Behav. 1995;57(5):837–841. doi: 10.1016/0031-9384(94)00309-s. [DOI] [PubMed] [Google Scholar]
- Bernabe J, Rampin O, Sachs BD. Intracavernous pressure during erection in rats: an integrative approach based on telemetric recording. Am J Physiol. 1999;276(2 Pt 2):R441–R449. doi: 10.1152/ajpregu.1999.276.2.R441. [DOI] [PubMed] [Google Scholar]
- Adachi H, Kodama K, Ishihara H. Evaluation of erectile response by continuous measurement of penile diameter in rats. J Pharmacol Toxicol Methods. 1999;41(4):147–152. doi: 10.1016/s1056-8719(99)00034-9. [DOI] [PubMed] [Google Scholar]


