Abstract
Background
Mass distributions of oral azithromycin have long been used to eliminate trachoma, and they are now being proposed to reduce childhood mortality. The observed benefit appears to be augmented with each additional treatment, suggesting a possible community-level effect. Here, we assess whether 2 biannual mass treatments of preschool children affect the community’s gut microbiome at 6 months after the last distribution.
Methods
In this cluster-randomized controlled trial, children aged 1–60 months in the Dossa region of Niger were randomized at the village level to receive a single dose of azithromycin or placebo every 6 months. Fecal samples were collected 6 months after the second treatment for metagenomic deep sequencing. The prespecified primary outcome was the Euclidean PERMANOVA of the gut microbiome, or effectively the distance between the genus-level centroid at the community level, with the secondary outcome being the Simpson’s α diversity.
Results
In the azithromycin arm, the gut microbial structures were significantly different than in the placebo arm (Euclidean PERMANOVA, P < .001). Further, the diversity of the gut microbiome in the azithromycin arm was significantly lower than in the placebo arm (inverse Simpson’s index, P = .005).
Conclusions
Two mass azithromycin administrations, 6 months apart, in preschool children led to long-term alterations of the gut microbiome structure and community diversity. Here, long-term microbial alterations in the community did not imply disease but were associated with an improvement in childhood mortality.
Clinical Trials Registration
Keywords: antibiotics, azithromycin, children, gut microbiome, randomized controlled trial
Azithromycin is a broad-spectrum bacteriostatic macrolide with a long half-life and excellent tissue penetration [1]. The drug has been used extensively for the treatment of respiratory, enteric, and genitourinary infections [1]. In addition, the World Health Organization has recommended mass oral azithromycin treatment as part of an effort to eliminate blinding trachoma [2–4], and >700 million doses of azithromycin have been distributed for this purpose. Other uses are now being proposed, including to improve childhood mortality.
Macrolides Oraux pour Réduire les Décès avec un Oeil sur la Résistance (MORDOR; clinical trial registration NCT02048007) was a large double-masked, cluster-randomized controlled trial that took place between 2015 and 2017 in 3 countries: Niger, Malawi, and Tanzania. This trial found a significant 18% reduction in childhood mortality with mass azithromycin administrations in Niger [5].The mechanism of mortality reduction is not clear, although the functional state of the gut microbiome has been linked in numerous ways to a child’s health and disease [6]. Its microbial composition, diversity, and intestinal pathogen abundances can be significantly altered for days to weeks after systemic antibiotic administration [7–10]. Longer-term persistence of the individual treatment and its functional effects, however, have been difficult to demonstrate. Here, we tested whether the gut microbiome in preschool children was affected 6 months after the previous antibiotic distribution in the relatively antibiotic-naïve, high–childhood mortality setting of Niger.
METHODS
Study Setting and Design
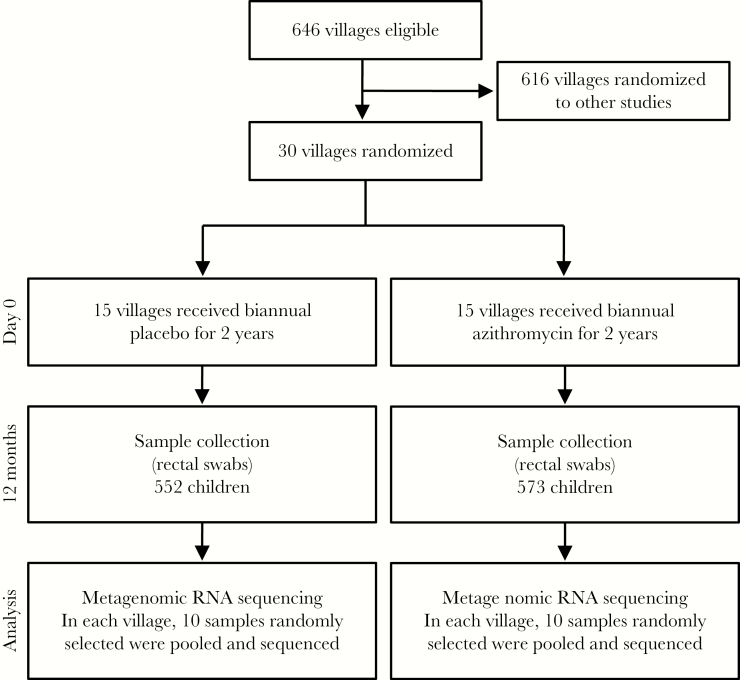
We obtained ethical approval for the study from the University of California San Francisco (UCSF) Committee for Human Research and the Ethical Committee of the Niger Ministry of Health (Institutional Review Board No. 10-01036). The study was undertaken in accordance with the Declaration of Helsinki. This study was a double-masked, cluster randomized controlled clinical trial with a prespecified primary outcome conducted in Niger. In Niger, 594 villages were randomized to receive 1 oral administration of azithromycin (Pfizer, New York, NY; height-based dosing to roughly 20 mg/kg) or placebo every 6 months for 2 years (Figure 1). Thirty communities were randomly selected for close monitoring, including the collection of rectal swabs from children. Baseline demographics of these 30 communities are displayed in Table 1.
Figure 1.
Trial profile. Children aged 1–60 months, from 30 villages, were randomly assigned to either the placebo or azithromycin-treated groups. Rectal samples from 10 children in each village were pooled and subjected to metagenomic RNA sequencing and analysis.
Table 1.
Baseline Characteristics
| Children Aged 0–60 mo | Placebo, Mean (95% CI) | Azithromycin, Mean (95% CI) | P Valuea |
|---|---|---|---|
| Age, mo | 33.9 (32.9–34.9) | 31.5 (30.6–32.4) | .30 |
| Female, % | 54.0 (51.2–56.7) | 48.0 (45.8–50.2) | .36 |
Abbreviation: CI, confidence interval; mo, months.
aWilcoxon rank-sum test for continuous variables and Fisher exact test for categorical variables.
The randomization sequence was generated by T.C.P. and implemented by T.C.P., K.J.R., and Pfizer personnel (necessary for packaging). Allocation concealment was attained by assigning communities simultaneously. Trained study personnel directly observed both antibiotic and placebo being taken by study subjects. We obtained oral consent from guardians of children before treatment distribution. No incentives were offered for participation of this study. The prespecified primary outcome was the Euclidean PERMANOVA P value for differences in the gut microbiome between treatment groups at the 12-month time point. We anticipated that the inclusion of 30 communities and a pooled sample from 10 children per community would provide 80% power to detect a mean 0.75 SD difference in within-group L2-norm (Euclidean) distance from between-group distance. Similarly, 30 communities would provide 80% power to detect a 0.75-SD decrease in alpha diversity using an inverse Simpson’s index.
Sample Processing and Sequencing
Rectal samples were collected in the field at 12 months (6 months after the second treatment). For each study participant, examiners changed into clean gloves, placed the swab 1–3 cm into the anus and rotated 360 degrees, repeating until stool was visible on the swab. Swabs were immediately placed into a Stool Nucleic Acid Collection and Transport Tube containing Norgen Stool Preservative (Norgen, Ontario, Canada). Samples were immediately placed on ice in the field and transported to the study center for storage at –20°C while in Niger and shipped to UCSF for long-term storage at –80°C. Samples were de-identified. Researchers performing the assays were masked. Ten samples from each village were randomly chosen for pooling and subsequent library preparation and sequencing (Figure 1). RNA was extracted from 300 fecal samples using the Norgen Stool RNA Isolation Kit (Norgen, Ontario, Canada), per the manufacturer’s instructions. The RNA concentration of each sample was quantified using the Qubit RNA HS Assay Kit (ThermoFisher Scientific, Waltham, MA) and normalized for pooling. The RNA from the 300 samples was pooled into 30 samples (10 samples per village). Five microliters of the pooled RNA was used to prepare libraries using the New England Biolabs’ (NEB’s) NEBNext RNA First Strand Synthesis Module (E7525) and NEBNext Ultra Directional RNA Second Strand Synthesis Module (E7550) to generate double-stranded cDNA. The cDNA was converted to Illumina libraries using the NEBNext Ultra II DNA Library Prep Kit (E7645) according to the manufacturer’s recommendation and then amplified with 14 polymerase chain reaction cycles. Samples were sequenced on the Illumina HiSeq 4000 using 125-nucleotide (nt) paired-end sequencing.
Metagenomic Sequencing Analyses and Statistical Methods
An initial human-sequence removal step was accomplished by alignment of all paired-end reads to the human reference genome 38 (hg38) and the Pantroglodytes genome (panTro4, 2011, UCSC), using the Spliced Transcripts Alignment to a Reference (STAR) aligner (v. 2.5.1b) [11]. Unaligned reads were quality-filtered using PriceSeqFilter with the “-rnf 90” and “-rqf 85 0.98” settings [12]. Reads that passed quality control were then subjected to duplicate removal. The remaining reads that were at least 95% identical were compressed by cd-hit-dup (v. 4.6.1) [13]. Paired reads were then assessed for complexity by compression with the Lempel-Ziv-Welch algorithm [14]. Read pairs with a compression space savings score lower than 0.45 were removed. Another round of human reads removal was performed using the very-sensitive-local mode of Bowtie2 (v. 2.2.4) with the same hg38 and panTro4 reference genomes described above [15]. The remaining nonhost read pairs were then passed onto Centrifuge (v. 1.0.3) to align to the entire NCBI nonredundant collection, downloaded on February 28, 2016 [16]. This reference database contains completed genomes, draft genomes, and partial genomes. Overall, it comprises ~75 000 genera, including many eukaryotes.
The primary outcome of this study was conducted on the pooled samples. Specifically, we used PERMANOVA to compare within-group and between-group Euclidean distances among samples [17]; significance was evaluated by a permutation test that compares true distances with the distribution when assigned to groups by chance. The primary outcome was prespecified to capture any differences in the structure of the gut microbial communities between treatment groups. The secondary outcome was the alpha diversity (inverse Simpson’s) at the genus level of the microbiome of children within villages at 12 months. As a diversity measure, Simpson’s index is weighted toward the abundant taxonomic groups in a population [18]. Therefore, as a sensitivity analysis, we also computed Shannon’s alpha diversity, a measure that is relatively more influenced by rare taxonomic groups in a population. Alpha and gamma diversities (Simpson’s and Shannon’s) were each assessed using equations described by Jost [18, 19]. We computed the difference in means of diversities by arm, that is, The permutation based P values for testing were computed as the proportion of permutations with Δ larger than the observed Δ value for n = 10 000. Analyses were implemented in the R software environment (http://cran.r-project.org/), version 3.1.3, package ‘vegetarian.’
RESULTS
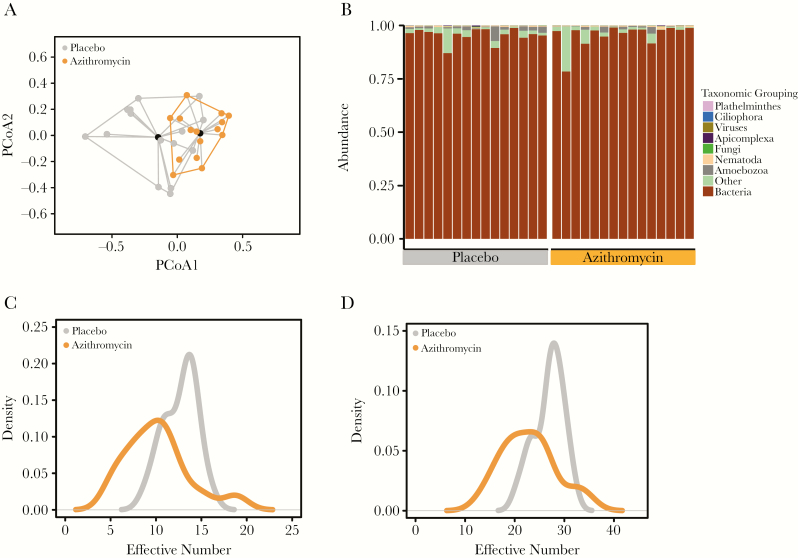
Treatment with azithromycin resulted in a significant change in the bacterial gut microbiome composition. Specifically, the stool bacterial communities treated with azithromycin were significantly closer to each other (smaller Euclidean distance within groups) than they were to those in placebo communities (P < .001) (Figure 2A, Table 2) at 12 months. This result remained unchanged when the Euclidean distance was used for all taxa including nonbacterial (P < .001). This is consistent with expectations, as the predominant fraction of the gut flora is bacterial (Figure 2B) and the main effect of azithromycin is antibacterial [1, 20, 21]. Indeed, PERMANOVA analysis limited to other taxonomic groups did not reveal statistically significant differences between treatment arms (Table 2).
Figure 2.
Two biannual treatments of azithromycin cause long-term alterations of the gut microbial communities of children. A, Principal coordinate analyses of the Euclidean distances for the placebo (gray) and the azithromycin-treated (orange) villages. Beta-dispersions were similar (P = .20) between treatment groups. B, Stacked bar graph of the abundance of the different taxonomic groups. Distributions of the inverse Simpson’s diversity index (C) and Shannon’s diversity index (D) for the placebo and azithromycin-treated villages. Abbreviation: PCoA, principal coordinate.
Table 2.
Difference in Gut Microbiome Between Treatment Groups
| Taxonomic Group | Euclidean Distance, P Value | Manhattan Distance, P Value |
| All | <.001 | <.001 |
| Bacteria | <.001 | <.001 |
| Fungi | .85 | .94 |
| Viruses | .93 | .83 |
| Nematoda | .26 | .08 |
| Plathelminth | .97 | .97 |
| Ciliophora | .16 | .14 |
| Apicomplexa | .58 | .60 |
| Amoebozoa | .17 | .20 |
Significant P values are bolded.
A prespecified secondary analysis of this study was the community-level (alpha) bacterial diversity (inverse Simpson’s). Here, we found that the inverse Simpson’s diversity index was decreased in the azithromycin arm (9.1; 95% CI, 7.8–10.8) compared with the placebo arm (12.4; 95% CI, 11.5–13.4, P = .005) (Figure 2C). Similarly, Shannon’s diversity was reduced after azithromycin treatment (22.2; 95% CI, 19.9–25.1) compared with placebo (26.7; 95% CI, 25.2–28.3; P = .01) (Figure 2D, Table 3). To evaluate the richness in each treatment group, we compared the gamma diversity, which showed that the diversity was significantly higher in the placebo arm compared with the azithromycin arm (Table 3). These results demonstrate that 2 biannual administrations of single-dose azithromycin to all preschool children are sufficient to induce long-lasting (at least 6 months’ duration) alterations in the gut microbiome at the community level.
Table 3. .
Bacterial Diversity by Treatment Groups at 12 Months
| Diversity | Placebo Effective Number (95% CI) | Azithromycin Effective Number (95% CI) | PValuea | |
|---|---|---|---|---|
| Alpha | Simpson (inverse) | 12.4 (11.5–13.4) | 9.1 (7.8–10.8) | .005 |
| Shannon (exponential: eH’) | 26.7 (25.2–28.3) | 22.2 (19.9–25.1) | .01 | |
| Gamma | Simpson (inverse) | 15.5 (14.1–16.3) | 10.5 (8.9–12.7) | .003 |
| Shannon (exponential: eH’) | 32.2 (30.0–33.2) | 26.9 (23.4–30.2) | .03 | |
DISCUSSION
Generally, a reduction in the gut microbiome diversity at the individual level has been associated with a pathologic state, ranging from Clostridium difficile colitis to the development of obesity and diabetes [22–24]. This study illustrates that such an association does not hold at the community level. Here, mass azithromycin distribution was associated with a community reduction in gut microbiome diversity, and in the companion mortality study of communities drawn from the same overall pool, the same treatment was associated with a significant improvement in childhood mortality [5]. The exact mechanisms by which an alteration in the gut microbiome at the community level may lead to a reduction in childhood deaths in Niger are unclear. Respiratory infections, diarrhea, and malaria are among the major causes of childhood mortality in sub-Saharan African countries [25–27]. Given that there is mounting evidence to support the role of the gut microbiome in modulating the immune system [28, 29], it is possible that changes in the gut microbiome community in these children made them less susceptible to infections. At 12 months, however, we could not demonstrate that the protective effect of azithromycin was due to the reduction of intestinal pathogens. It remains to be seen if there will be a reduction in malaria or respiratory infections in the MORDOR trial. Most likely, multiple mechanisms are contributing to the reduction in childhood mortality.
Several limitations apply to the interpretation of this study. Relatively antibiotic-naïve communities, like those in this study, have become exceedingly rare in the antibiotic era. Further, with the exception of mass drug distribution, coordination of antibiotic treatment to every preschool child in a community simply does not happen in the developing or developed countries. Also, because the rectal samples were pooled for sequencing, alterations in the gut microbiome can only be applied to changes at the community level. Given these limitations, we cannot generalize our findings to regions with greater background antibiotic use or lower childhood mortality.
In summary, two mass biannual azithromycin administrations in preschool children led to long-term alterations of the gut microbiome structure and community diversity. This same treatment resulted in a reduction in pediatric deaths. Determining the exact molecular mechanism(s) of azithromycin’s effect on mortality may help clarify possible large-scale implementation.
Acknowledgments
We thank Derek Bogdanoff in the UCSF Center for Advanced Technology for his expertise and assistance operating the Illumina sequencer.
Financial support. This study was supported by the Bill and Melinda Gates Foundation (grant No. 1032340 to T.M.L.), the National Eye Institute of the National Institutes of Health (award No. K08EY026986 to T.D.), the Research to Prevent Blindness Career Development Award (to T.D.), and an unrestricted grant from Research to Prevent Blindness.
Potential conflicts of interest. All authors declare no competing financial interests. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. McMullan BJ, Mostaghim M. Prescribing azithromycin. Aust Prescr 2015; 38:87–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amza A, Kadri B, Nassirou B, et al. A cluster-randomized trial to assess the efficacy of targeting trachoma treatment to children. Clin Infect Dis 2017; 64:743–50. [DOI] [PubMed] [Google Scholar]
- 3. Bhosai SJ, Bailey RL, Gaynor BD, Lietman TM. Trachoma: an update on prevention, diagnosis, and treatment. Curr Opin Ophthalmol 2012; 23:288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Porco TC, Gebre T, Ayele B, et al. Effect of mass distribution of azithromycin for trachoma control on overall mortality in Ethiopian children: a randomized trial. JAMA 2009; 302:962–8. [DOI] [PubMed] [Google Scholar]
- 5. Keenan JD, Bailey RL, West SK, et al. ; MORDOR Study Group Azithromycin to reduce childhood mortality in Sub-Saharan Africa. N Engl J Med 2018; 378:1583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Knight R, Callewaert C, Marotz C, et al. The microbiome and human biology. Annu Rev Genomics Hum Genet 2017; 18:65–86. [DOI] [PubMed] [Google Scholar]
- 7. Yassour M, Vatanen T, Siljander H, et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med 2016; 8:343ra81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doan T, Acharya NR, Pinsky BA, et al. Metagenomic DNA sequencing for the diagnosis of intraocular infections. Ophthalmology 2017; 124:1247–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grassly NC, Praharaj I, Babji S, et al. The effect of azithromycin on the immunogenicity of oral poliovirus vaccine: a double-blind randomised placebo-controlled trial in seronegative Indian infants. Lancet Infect Dis 2016; 16:905–14. [DOI] [PubMed] [Google Scholar]
- 10. Parker EPK, Praharaj I, John J, et al. Changes in the intestinal microbiota following the administration of azithromycin in a randomised placebo-controlled trial among infants in South India. Sci Rep 2017; 7:9168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 2013; 29:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Doan T, Acharya NR, Pinsky BA, et al. Metagenomic DNA sequencing for the diagnosis of intraocular infections. Ophthalmology 2017; 124:1247–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fu L, Niu B, Zhu Z, et al. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics 2012; 28:3150–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ziv J, Lempel A. A universal algorithm for sequential data compression. IEEE Trans Inf Theory 1977; 23:337–43. [Google Scholar]
- 15. Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods 2012; 9:357–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim D, Song L, Breitwieser FP, Salzberg SL. Centrifuge: rapid and sensitive classification of metagenomic sequences. Genome Res 2016; 26:1721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anderson MJ, Walsh DC. ERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: what null hypothesis are you testing?Ecol Monogr 2013; 83:557–74. [Google Scholar]
- 18. Jost L. Entropy and diversity. Oikos 2006; 113:363–75. [Google Scholar]
- 19. Jost L. Partitioning diversity into independent alpha and beta components. Ecology 2007; 88:2427–39. [DOI] [PubMed] [Google Scholar]
- 20. Kitzis MD, Goldstein FW, Miégi M, Acar JF. In-vitro activity of azithromycin against various Gram-negative bacilli and anaerobic bacteria. J Antimicrob Chemother 1990; 25(Suppl A):15–8. [DOI] [PubMed] [Google Scholar]
- 21. Lukehart SA, Fohn MJ, Baker-Zander SA. Efficacy of azithromycin for therapy of active syphilis in the rabbit model. J Antimicrob Chemother 1990; 25(Suppl A):91–9. [DOI] [PubMed] [Google Scholar]
- 22. Mosca A, Leclerc M, Hugot JP. Gut microbiota diversity and human diseases: should we reintroduce key predators in our ecosystem?Front Microbiol 2016; 7:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Young VB, Schmidt TM. Antibiotic-associated diarrhea accompanied by large-scale alterations in the composition of the fecal microbiota. J Clin Microbiol 2004; 42:1203–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mai V, Braden CR, Heckendorf J, et al. Monitoring of stool microbiota in subjects with diarrhea indicates distortions in composition. J Clin Microbiol 2006; 44:4550–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ogbo FA, Agho K, Ogeleka P, et al. ; Global Child Health Research Interest Group Infant feeding practices and diarrhoea in sub-Saharan African countries with high diarrhoea mortality. PLoS One 2017; 12:e0171792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. UNICEF/WHO. Diarrhoea: Why Children Are Still Dying and What Can Be Done. New York: UNICEF/WHO; 2010. [Google Scholar]
- 27. See CW, O’Brien KS, Keenan JD, et al. The effect of mass azithromycin distribution on childhood mortality: beliefs and estimates of efficacy. Am J Trop Med Hyg 2015; 93:1106–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature 2016; 535:65–74. [DOI] [PubMed] [Google Scholar]
- 29. Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature 2016; 535:75–84. [DOI] [PubMed] [Google Scholar]