Abstract
Bartonella sp. are a common cause of culture-negative infective endocarditis. Glomerulonephritis is a well-documented consequence of the immune activation associated with infective endocarditis. However, Cryoglobulinemia has not previously been reported in association with Bartonella infective endocarditis. Below we report a case of a 48-year-old male with Bartonella henselae infective endocarditis complicated by cryoglobulinemia and multifocal proliferative glomerulonephritis, highlighting a possible link between Bartonella sp. infection and type III cryoglobulinemia.
Keywords: Cryoglobulinemia, Bartonella, infective endocarditis, glomerulonephritis
CASE REPORT
A 48-year-old male with a history of aortic insufficiency, bioprosthetic aortic valve replacement, and complete atrioventricular block with a permanent pacemaker presented to the emergency room with progressive shortness of breath on exertion, night sweats, and an erythematous macular rash on his lower extremities. He lived in rural Pennsylvania with dogs and cats as pets and had recently been released from a 1-year period of incarceration, during which there had been multiple lice outbreaks. Physical examination was significant for a diastolic murmur. Laboratory studies revealed pancytopenia, paraproteinemia, and acute kidney injury; a urine analysis showed evidence of hematuria and proteinuria. Serum C3 and C4 levels were low, and testing for HIV, hepatitis B and C (HBV, HCV), antinuclear antibodies (ANAs), rheumatoid factor (RF), and antineutrophil antibodies (ANCAs) was negative. Serum cryoglobulin screen was positive. Abdominal imaging showed no evidence of hydronephrosis or nephrolithiasis. Transesophageal echocardiogram demonstrated abnormal thickening of all the prosthetic leaflets and a mobile echo density attached to the right ventricular lead. Three sets of blood cultures off antibiotics were negative. Serologies for Coxiella burnetii, Bartonella henselae, and quintana as work-up for culture-negative endocarditis were sent. His serologies were positive for Bartonella quintana IgG (1:1024) and mildly positive IgM, and he was started on treatment with doxycycline and rifampin. Due to worsening renal function, he underwent a renal biopsy, which revealed cryoglobulinemia-associated multifocal proliferative glomerulonephritis (MPGN) with cellular crescents (Figures 1 and 2). Characterization of the cyroglobulins revealed a type III pattern. He received steroids and underwent plasmapheresis and was commenced on intermittent hemodialysis (iHD). Later serum DNA polymerase chain reaction (PCR), performed at Quest Diagnostics, was positive for Bartonella henselae. He underwent aortic valve replacement and pacemaker removal. 16 S ribosomal sequencing of the valve tissue, performed at the University of Washington, was positive for B. henselae. At 3-month follow-up, the patient was doing well and had stopped iHD.
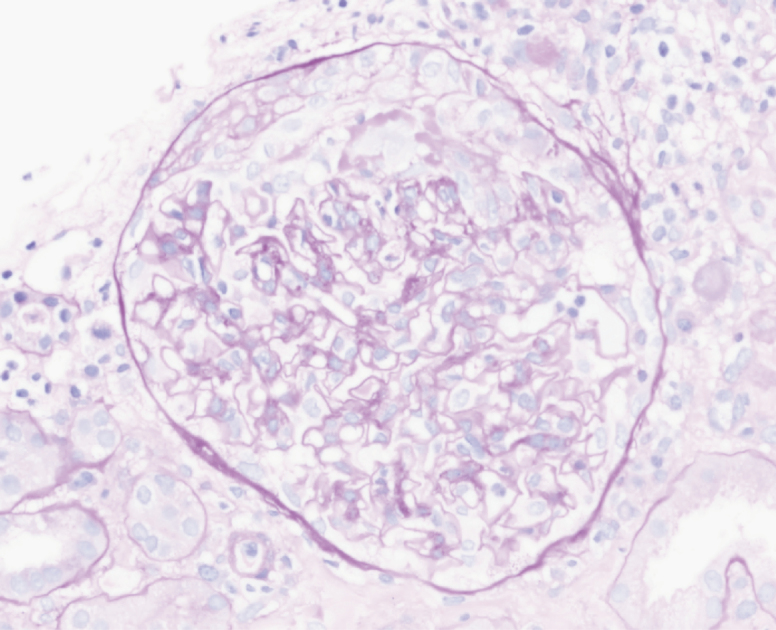
Figure 1.
Renal biopsy with Periodic acid-Schiff staining demonstrating crescents.
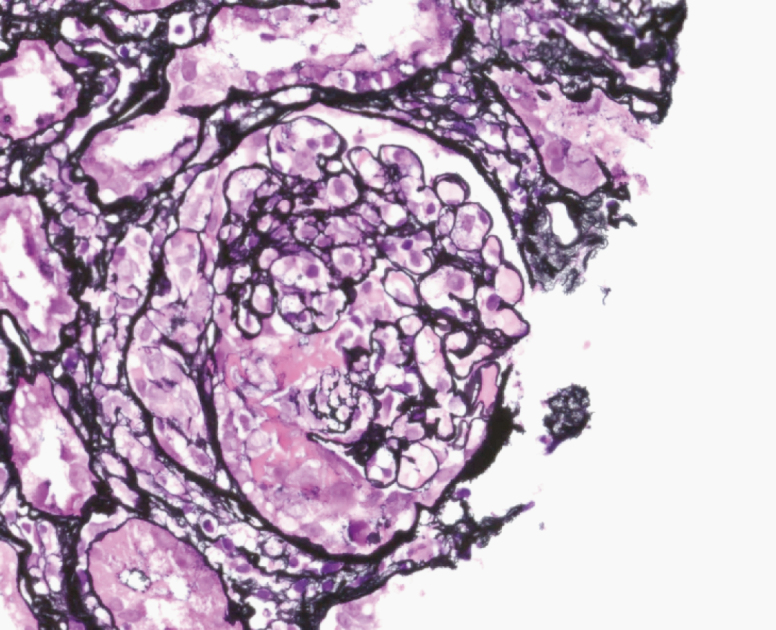
Figure 2.
Renal biopsy with Jones staining demonstrating crescents and glomerular basement membrane breaks and duplication.
DISCUSSION
Since initially being reported as a cause of endocarditis in 1993 [1, 2], Bartonella sp. have become increasingly appreciated as an etiology of culture-negative infective endocarditis (IE). Of the Bartonella sp., B. quintana is most commonly implicated in IE, followed by B. henselae [3]. Factors associated with Bartonella sp. infection include low socioeconomic status, homelessness, alcoholism, body louse infestation, contact with cats, and valvular disease [4]. Renal disease in the setting of IE is common and can be due to prerenal azotemia, septic emboli, or immunological phenomena leading to glomerulonephritis. In 1 case series, 40% of Bartonella patients had some degree of renal impairment; however, renal impairment due to glomerular disease is a rare occurrence. To date, there have been 11 reported cases of Bartonella IE with glomerulonephritis. Eight of these cases were ANCA induced [5]. However, Cryoglobulinemia with associated MPGN has not previously been reported in association with Bartonella IE. Mixed-type cryoglobulinemia (type II and II) results from chronic inflammatory states and has been linked to infectious pathogens, including chronic HCV and HBV infections and occasionally IE [6, 7]. Historical studies that previously found a high prevalence of mixed cyroglulinemaemia with IE did not include patients with Bartonella sp. due to lack of recognition of Bartonella as an implicating pathogen at the time [8].
Despite advances in our recognition of Baronella sp. as a major cause of culture-negative IE, diagnosis remains challenging and requires a high index of suspicion. Due to its fastidious nature, the yield of standard culturing methods is low, and hence they are seldom relied on [9]. Serologies by enzyme-linked immunosorbent assay (ELISA) and indirect fluorescence antibody testing (IFA) have become reference methods. Bartonella titers >1:800 have been shown to have a high positve predictive value (95.5%) for IE; however, there are many limitations to serological testing [10]. Bartonella IgM is frequently negative (or low) in acute infection [10]. Also, there is frequent cross-reactivity across Bartonella species and with C. burnetii and Chlamydia spp., as in our case [10]. Serology can frequently be positive in both species. Advances in diagnostic DNA PCR amplification and 16S rRNA sequencing have made these diagnostic methods preferred given their high sensitivity and specificity, especially when applied to cardiac valve tissue, even in the setting of antibiotic therapy [11]. Robust prospective data to guide therapy in Bartonella IE are lacking. Current guidelines based upon expert panel opinion recommend 2 weeks of aminoglycoside therapy plus 6 weeks or more of doxycycline [12].
The above case of a 48-year-old male with valvular diseases and zoonotic exposure who later developed Bartonella infective endocarditis complicated by MPGN highlights a novel association between Bartonella infectious endocarditis and type III cryoglobulinemia-induced glomerulonephritis. In future cases of endocarditis with glomerular disease where no contributing infectious etiology has been elucidated, Bartonella sp. infection should be considered, especially in the setting of classical risk factors.
Acknowledgments
Potential conflicts of interest. None of the authors have any potential conflicts of interest or funding sources to report. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Daly JS, Worthington MG, Brenner DJ, et al. . Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J Clin Microbiol 1993; 31:872–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Spach DH, Callis KP, Paauw DS, et al. . Endocarditis caused by Rochalimaea quintana in a patient infected with human immunodeficiency virus. J Clin Microbiol 1993; 31:692–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fournier PE, Thuny F, Richet H, et al. . Comprehensive diagnostic strategy for blood culture-negative endocarditis: a prospective study of 819 new cases. Clin Infect Dis 2010; 51:131–40. [DOI] [PubMed] [Google Scholar]
- 4. Raoult D, Fournier PE, Drancourt M, et al. . Diagnosis of 22 new cases of Bartonella endocarditis. Ann Intern Med 1996; 125:646–52. [DOI] [PubMed] [Google Scholar]
- 5. Raybould JE, Raybould AL, Morales MK, et al. . Bartonella endocarditis and pauci-immune glomerulonephritis: a case report and review of the literature. Infect Dis Clin Pract 2016; 24:254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kodo K, Hida M, Omori S, et al. . Vasculitis associated with septicemia: case report and review of the literature. Pediatr Nephrol 2001; 16:1089–92. [DOI] [PubMed] [Google Scholar]
- 7. Belizna CC, Hamidou MA, Levesque H, et al. . Infection and vasculitis. Rheumatology 2009; 48:475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DREYFUSS F, LIBRACH G. Cold precipitable serum globulins (cold fractions, cryoglovulins) in subacute bacterial endocarditis. J Lab Clin Med 1952; 40:489–97. [PubMed] [Google Scholar]
- 9. Gandhi TN Slater LN, Welch DF, Koehler JE. Bartonella, including cat-scratch disease. In: Bennett JE, Dolin R, Blaser MJ, ed. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Disease. 8th ed Philadelphia, PA: Elsevier Press; 2015:2649–63. [Google Scholar]
- 10. Fournier PE, Mainardi JL, Raoult D. Value of microimmunofluorescence for diagnosis and follow-up of Bartonella endocarditis. Clin Diagn Lab Immunol 2002; 9:795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fournier PE, Lelievre H, Eykyn SJ, et al. . Epidemiologic and clinical characteristics of Bartonella quintana and Bartonella henselae endocarditis: a study of 48 patients. Medicine 2001; 80:245–51. [DOI] [PubMed] [Google Scholar]
- 12. Rolain JM, Brouqui P, Koehler JE, et al. . Recommendations for treatment of human infections caused by Bartonella species. Antimicrob Agents Chemother 2004; 48:921–33. [DOI] [PMC free article] [PubMed] [Google Scholar]