Abstract
The fusion of 3D anatomical models derived from high-fidelity pre-interventional computed tomography angiography (CTA), and x-ray (XR) fluoroscopy to facilitate anatomical guidance is of huge interest for complex cardiac interventions like TAVI procedures with cerebral protection. Co-registration of CTA and XR has been introduced either based on additional intraoperative non-/contrast-enhanced cone-beam computed tomography (CBCT) or two separate aortograms. With the related increase of radiation exposure and/or contrast agent (CA) dose, a potential additional risk for the patient is introduced. Here, we propose a modified co-registration approach making use of arteriograms of the iliofemoral arteries, routinely performed during the femoral puncture and sheath introduction. On-the-fly refinement of the co-registration during the on-going procedure enables accurate co-registration without any additional angiograms, thus reducing CA, XR dose and procedure time, while simultaneously improving operator confidence and procedure safety.
Keywords: Medicine, Issue 136, image-based intervention guidance, image fusion, x-ray guidance, CT angiography, double-filter cerebral protection system, transcatheter aortic valve implantation
Introduction
Image fusion (IF) is the process of superimposing datasets acquired at the different time- and viewpoints on different modalities into a single-frame of reference1. XR is the most frequently used imaging modality for intervention guidance. Even though, providing high temporal and spatial resolution, XR has low dimensionality (2D projections) and lacks anatomical details. 3D organ shape models derived from e.g. high-quality pre-interventional CTA data superimposed onto the live fluoroscopy image can augment XR by relevant anatomic soft-tissue structures. Prerequisite step for IF is the co-registration of the different imaging modalities.
Typically, co-registration of preoperative 3D image datasets with XR fluoroscopy involves one of the following techniques2: a) image-based 3D-3D registration of the preoperative 3D dataset with an intraoperative non-/contrast-enhanced CBCT dataset3,4,5,6, or b) direct image-based 2D-3D registration, where two angiographic images with a minimum of 30° angular spacing7,8 are used for co-registration.
With the recent introduction of fusion packages on commercial XR systems, IF can be made more readily available for a wide range of applications. Using those systems, we have previously shown that it is technically feasible and safe to overlay an aortic root model by means of direct image-based 2D-3D registration for supporting transfemoral transcatheter aortic valve implantation (TAVI)8. Without compromising the overall CA or XR dose, IF proved itself highly valuable during TAVI procedure by adding 3D anatomical details to the conventional XR fluoroscopy image, especially during deployment of the cerebral protection device. However, the additional acquisition of the aortograms used for the co-registration required additional CA and XR dose. Therefore, an optimized workflow providing accurate IF without the need of any additional aortograms was highly desirable.
Here, we present an approach to improved co-registration of pre-interventional CTA with real-time XR without requiring any additional CA or C-arm CT scans for IF. The femoral access TAVI is performed as described elsewhere9,10,11. Briefly, both femoral arteries are accessed: one for the guidance of the contralateral puncture, followed by placement of a pigtail catheter through a 6F sheath to allow arteriography during placement of the valve prosthesis; the second for placement of the valve delivery system and subsequent balloon valvuloplasty and device placement. Angiographic confirmation of appropriate puncture is performed in our institution as a standard of care for localization of the puncture height (above the femoral bifurcation) and estimation of the position of the covered stent in case of access-related complications12. For capturing embolic debris, a double-filter cerebral protection system is introduced after insertion of the TAVI delivery sheath prior to the passage of the aortic arch with any further device.
We use arteriograms performed routinely during the puncture of the femoral arteries to establish the initial co-registration. On-the-fly refinement of the co-registration is subsequently performed during the on-going procedure using the position of the pigtail catheter within the aortic root, the double-filter cerebral embolic protection system in the supraaortal vessels and the aortograms performed before the implantation of the valve prosthesis, thus ensuring accurate model overlay at any time point during the intervention.
Protocol
The study protocol is in compliance with the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's ethics committee. Written informed consent was obtained from all individual participants included in the study (CSI-Ulm, clinicaltrials.gov NCT02162069).
1. CT Examination
Perform cardiac CT angiography with retrospective ECG-gating using 80 mL of iodinated contrast media injected at a flow rate of 4.0 mL/s followed by a flushing bolus of 70 mL 0.9% saline solution.
Acquire data cranio-caudally extending from the femoral arteries to the subclavian arteries with the following acquisition parameters: the matrix of 512 × 512 pixels, slice thickness of 1 mm, slice spacing of 0.7 mm.
Reconstruct images at 30% of the R-R interval using iterative reconstruction algorithm.
2. Image Segmentation and Model Generation
Import CT data from an external device or an internal archiving system into image fusion software by drag-and-dropping the dataset or subsets of data for the selected patient into the Patients view within the application.
Start automatic segmentation of the selected CT volume by double-clicking on the image series. Note: Segmentation of the heart chambers (left and right ventricle, left and right atrium, myocardium) and great vessels (abdominal aorta, vena cava, and coronary sinus) is started automatically and will be displayed in the Segmentation work step.
Go through the image slices and verify if the edges of the aorta and left ventricle are correctly detected and if necessary edit the automatic segmentation in the Tissue window using the Edit button.
To perform manual segmentation of additional structures, use the Add button in the Tissue window.
Use existing editing tools to fill the structure (Inject Dye) and drag the edges of the structure (Drag Edge) in 2D views or remove structure parts with the Free-form Cut in the 3D view.
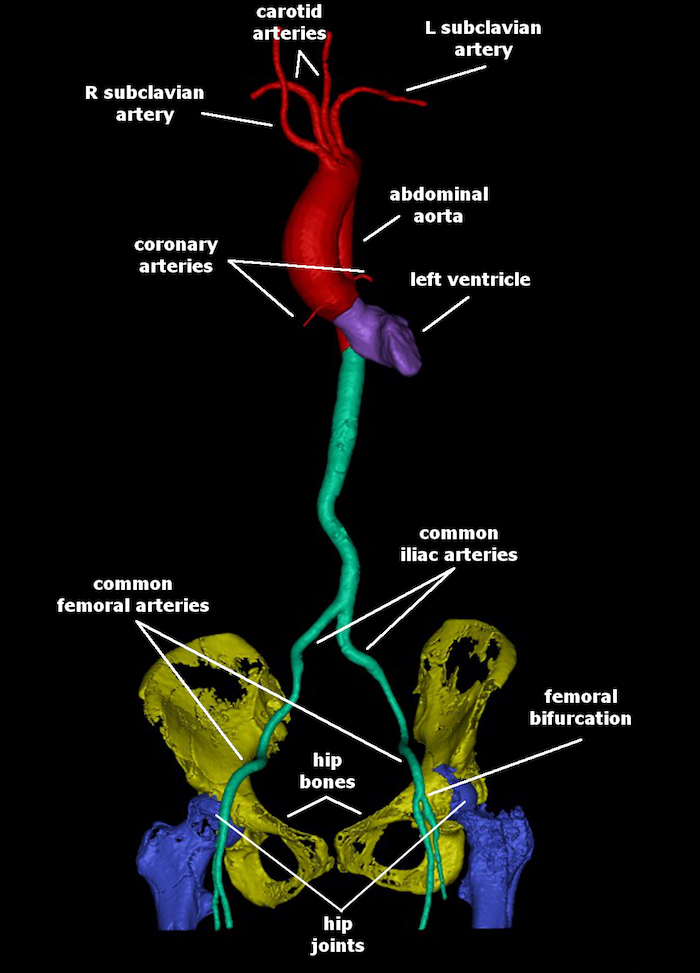
Perform manual segmentation of the main trunks of the left and right coronary arteries, main artery branches (brachiocephalic artery with right carotid and right subclavian arteries, left common carotid artery and left subclavian artery), left and right iliofemoral arteries, hip bones, and hip joints as described in steps 2.4-2.5 (Figure 1). Note: Depending on the quality of the CT volume this may take 20 to 30 min.
3. Image Co-registration and Fusion
In the Patients view merge the selected patient with the current patient in the XR system by choosing the respective action in the right-click-context-menu. Note: Now the Registration and Live work steps are active and can be used.
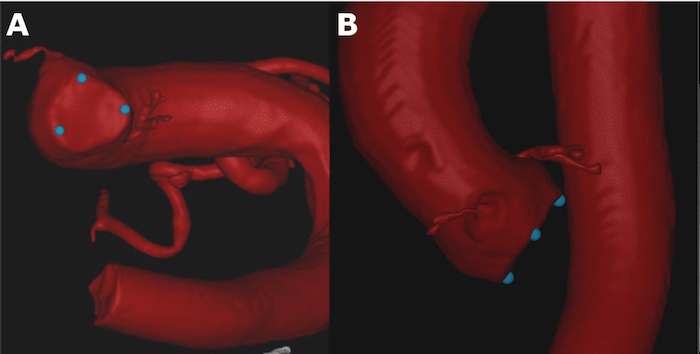
In the Live work step, click Add new tag point in the Tag points window to place three reference markers at aortic valve cusps for facilitating the choice of the optimal projection of the annular plane during the intervention (Figure 2).
Go to the Registration work step to acquire the XR runs and to perform the co-registration of the segmented models with XR. Note: XR runs acquired with at least 30° angular distance are required for reliable registration.
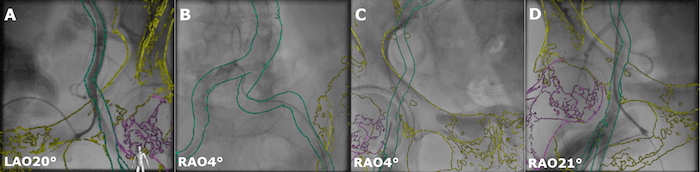
Copy the angiographic projection of the appropriate puncture in ~LAO 20 - 30° orientation (or ~RAO 20 - 30° depending on the initial puncture side) into the Reference view 1 by clicking the button Copy to Reference View 1.
Copy following x-ray projections acquired in ~AP orientation during visualization of the transition from the contralateral A. iliaca communis to the A. femoralis communis into the Reference view 2 by clicking the button Copy to Reference View 2.
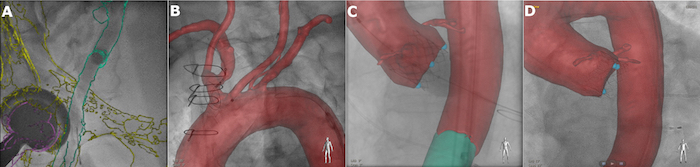
Use the interaction tools Registration Pan, Registration Rotate (for in-plane rotation), Registration Roll (for 3D rotation) to manually align the model of the iliofemoral arteries with the acquired XR projections (Figure 3A-B).
Use hip bones and hip joints visible in the XR images as additional landmarks during alignment of the overlay in the iliofemoral region. Note: Now the model is linked to the XR system geometry, and the overlay is automatically being adapted to the current XR projection orientation, magnification, and patient table position.
Guide the puncture of the common femoral artery on the sheath side (Figure 3C) by using the coarse initial co-registration performed in step 3.6.
Record an angiographic projection of the device sheath femoral artery in ~RAO 20 - 30° (or respectively ~LAO 20 - 30°) by clicking the button Copy to Reference View and finalize the image co-registration in the iliofemoral region (Figure 3D). Note: Since the patient is positioned differently during CT scan and the intervention, registration based on the iliofemoral structures provides only limited accuracy in other regions. Thus, manual refinement of the co-registration in the thoracic region is required.
To use these data for further overlay re-alignment, copy any additional acquired projections to the “Reference View” during the transition from the iliofemoral to the thoracic region as well as any catheterization of the brachiocephalic trunk via the right radial artery.
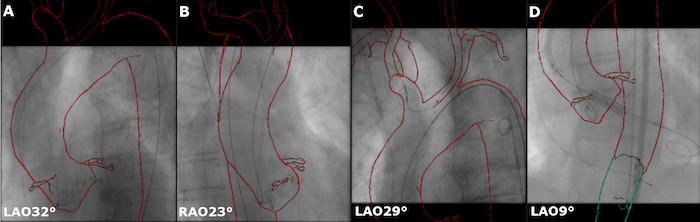
After placing the pigtail catheter in the aortic arch, acquire two fluoroscopic projections without CA in LAO 30 - 40° and RAO 20 - 30° orientation and copy them in the Reference view 1 and Reference view 2.
Use the interaction tools Registration Pan, Registration Rotate (for in-plane rotation), Registration Roll (for 3D rotation) to manually adjust the registration in the thoracic region (Figure 4A-B).
Guide the placement of the protection device without administration of additional CA purely based on the anatomic overlay (Figure 4C).
For further refinement, manually correct the overlay position as described in step 3.12 on each acquired XR projection during the entire course of the intervention, ensuring accurate overlay at any time point. Note: Use aortograms acquired according to the routine procedure for verifying the correct position of the delivery system as landmarks for the overlay adjustment (Figure 4D).
Representative Results
We introduced a novel co-registration approach for image fusion during TAVI, which allows overlaying the patient-specific anatomic model onto the live XR images during the entire TAVI procedure without the need for any additional aortograms.
Several interventional steps will benefit from the IF: (1) guidance of the puncture of the common femoral artery above the femoral bifurcation on the sheath side (Figure 5A); (2) accurate placement of the cerebral embolic protection device even in very tortuous anatomies based solely on the anatomic overlay (Figure 5B); (3) visualization of the model in arbitrary C-arm angulation without any XR exposure and CA administration (Figure 2), as well as identification of the optimal view before the valve deployment (Figure 5C); (4) alignment of the valve prosthesis even in very complex anatomies (Figure 5D).
In the related clinical study13, the proposed IF approach could prove significant reduction of the CA volume [80 (50 - 95) vs. 100 (80 - 110) mL, p = 0.010], overall procedure time [48 (41 - 58) vs. 61 (53 - 67) min, p = 0.002] and procedure XR dose [51 (42 - 55) vs. 64 (49 - 81) Gy cm2, p = 0.032] in comparison with a matched control group.
Figure 1 : 3D models of the CTA-based segmented aortic arch with coronary, carotid and subclavian arteries (red), iliac and femoral arteries (green), left ventricle (purple), hip bones (yellow), and hip joints (blue). Please click here to view a larger version of this figure.
Figure 2: Manual setting of the reference markers (blue dots) at the aortic valve cusps for facilitating the choice of the optimal projection of the annular plane. A. Oblique view. B. Orthogonal view. Please click here to view a larger version of this figure.
Figure 3: Registration between the 3D model and XR system in the iliofemoral region.A. Arteriogram of the punctured non-device sheath femoral artery in LAO 20°. B. Arteriogram of the iliac arteries in RAO 4° orientation. C. Puncture of the vessel selected for the delivery sheath in RAO 4°. D. Confirmation of the wire position inside the vessel selected for the delivery sheath in RAO 21°. Please click here to view a larger version of this figure.
Figure 4: Refinement of the co-registration in the thoracic region. The overlay is re-aligned based on two reference projections of the abdominal aorta after insertion of the pigtail catheter A. acquired in LAO 32° and B. RAO 23°. Further refinements may be done after the placement of C. the cerebral embolic protection device and D. based on the angiographic projections of the aortic annulus. Please click here to view a larger version of this figure.
Figure 5: Examples of successful image fusion.A. Femoral puncture. B. Placement of the cerebral embolic protection device. C. Valve deployment (C-arm is aligned perpendicular to the annular plane as it can be seen via three markers set at the aortic annulus aligned along a straight line). D. Valve prosthesis after deployment in the aortic annulus. Please click here to view a larger version of this figure.
Discussion
The main focus of this study was to investigate the feasibility of IF without modifying the clinically established TAVI workflow. Whereas the gold standard for the co-registration of the pre-interventional CTA data and XR fluoroscopy uses dedicated aortograms8, we propose using multiple approximate registrations with on-the-fly refinements to provide accurate 3D model overlay during the entire course of the intervention.
The continuous manual registration refinement requires additional trained staff to assist the interventional team from the control room. The required refinements are difficult to perform at the tableside since the related user interactions impact the clinically established interventional workflow. However, even though the co-registration based on the acquisition of two dedicated aortograms is potentially easier to realize at the tableside, the 3D overlay would not be available during femoral access, and any motion of the patient would spoil the accuracy of the registration.
Even the use of static roadmaps, not considering the cardiac and respiratory motion, appeared promising for femoral puncture, placement of the embolic protection device and initial alignment of the valve prosthesis. However, the usage of cardiac phase synchronized anatomic models in combination with respiratory motion compensation may further increase the usefulness of the proposed approach by accounting for dynamic changes in the aortic annulus position, especially during the valve deployment.
3D model overlay represents another incremental advancement to improve the navigation of complex interventions leading to an improved procedural safety and efficacy14. Co-registration of the pre-interventional CT data to live fluoroscopy by means of contrast-enhanced CBCT may raise concerns regarding high XR dose and additional CA, despite its reported success6,15. Using native CBCT instead is limited by the poor visibility of the target structures and causes difficulties in registration and highly trained operators are necessary, making the workflow less straightforward6,16. Even though using two additional aortograms for the co-registration allowed for accurate co-registration, no reduction of neither CA nor XR dose could be achieved8. The proposed co-registration approach yields significant reduction of the fluoroscopy dose, contrast volume and overall procedure time while maintaining the usual workflow during the implantation. Furthermore, it already supports femoral artery puncture by superimposing respective models during the initial phase of the intervention.
The current limitation of the technique results from the static nature of the anatomical models and non-deformable rigid registration, potentially causing overlay inaccuracy. In particular, different patient positioning between pre-interventional imaging and the intervention as well as thin, tortuous structures which easily deform during device manipulation may cause a mismatch between the anatomical models and the live situation.
The proposed protocol as such is applicable to a huge variety of transcatheter interventions. With the increasing complexity of these procedures, the demand for improved guidance will steadily rise. Procedures on the verge for the clinical routine like the percutaneous treatment of the tricuspid valve as well as procedures already clinically established like atrial or ventricular ablation will likely benefit from the suggested approach. Even though the procedure is explained in terms of one specific system, the approach should be easily transferable to other systems as long as a similar software solution is available.
Disclosures
On behalf of all authors, the corresponding authors state that there are no relationships that could be construed as a conflict of interest.
Acknowledgments
The authors would like to thank the Ulm University Center for Translational Imaging MoMAN for its support.
References
- Sánchez Y, et al. Navigational Guidance and Ablation Planning Tools for Interventional Radiology. Current Problems in Diagnostic Radiology. 2017;32(2):225–233. doi: 10.1067/j.cpradiol.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Schwein A, et al. Feasibility of three-dimensional magnetic resonance angiography-fluoroscopy image fusion technique in guiding complex endovascular aortic procedures in patients with renal insufficiency. Journal of Vascular Surgery. 2017;65(5):1440–1452. doi: 10.1016/j.jvs.2016.10.083. [DOI] [PubMed] [Google Scholar]
- Ierardi AM, et al. Fusion of CT Angiography or MR Angiography with Unenhanced CBCT and Fluoroscopy Guidance in Endovascular Treatments of Aorto-Iliac Steno-Occlusion: Technical Note on a Preliminary Experience. CardioVascular and Interventional Radiology. 2015;39(1):111–116. doi: 10.1007/s00270-015-1158-4. [DOI] [PubMed] [Google Scholar]
- Sailer AM, et al. CTA with Fluoroscopy Image Fusion Guidance in Endovascular Complex Aortic Aneurysm Repair. European Journal of Vascular and Endovascular Surgery. 2014;47(4):349–356. doi: 10.1016/j.ejvs.2013.12.022. [DOI] [PubMed] [Google Scholar]
- McNally MM, et al. Three Dimensional Fusion CT Decreases Radiation Exposure, Procedure Time and Contrast Use during Fenestrated Endovascular Aortic Repair. Journal of Vascular Surgery. 2015;61(2):309–316. doi: 10.1016/j.jvs.2014.07.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaswamy A, Tuzcu EM, Kapadia SR. Integration of MDCT and fluoroscopy using C-arm computed tomography to guide structural cardiac interventions in the cardiac catheterization laboratory. Catheterization and Cardiovascular Interventions. 2015;85(1):139–147. doi: 10.1002/ccd.25392. [DOI] [PubMed] [Google Scholar]
- Movassaghi B, Rasche V, Grass M, Viergever MA, Niessen WJ. A quantitative analysis of 3-D coronary modeling from two or more projection images. IEEE Transactions on Medical Imaging. 2004;23(12):1517–1531. doi: 10.1109/TMI.2004.837340. [DOI] [PubMed] [Google Scholar]
- Vernikouskaya I, et al. Image-guidance for transcatheter aortic valve implantation (TAVI) and cerebral embolic protection. International Journal of Cardiology. 2017;249:90–95. doi: 10.1016/j.ijcard.2017.09.158. [DOI] [PubMed] [Google Scholar]
- Ramlawi B, Anaya-Ayala JE, Reardon MJ. Transcatheter Aortic Valve Replacement (TAVR): Access Planning and Strategies. Methodist DeBakey Cardiovascular Journal. 2012;8(2):22–25. doi: 10.14797/mdcj-8-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöhrle J, et al. Transfemoral Aortic Valve Implantation with the New Edwards Sapien 3 Valve for Treatment of Severe Aortic Stenosis-Impact of Valve Size in a Single Center Experience. PLOS ONE. 2016;11(3):e0151247. doi: 10.1371/journal.pone.0151247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger J, Gonska B, Otto M, Rottbauer W, Wöhrle J. Cerebral Embolic Protection During Transcatheter Aortic Valve Replacement Significantly Reduces Death and Stroke Compared With Unprotected Procedures. JACC: Cardiovascular Interventions. 2017;10(22):2297–2303. doi: 10.1016/j.jcin.2017.06.037. [DOI] [PubMed] [Google Scholar]
- Seeger J, Gonska B, Rodewald C, Rottbauer W, Wöhrle J. Impact of suture mediated femoral access site closure with the Prostar XL compared to the ProGlide system on outcome in transfemoral aortic valve implantation. International Journal of Cardiology. 2016;223:564–567. doi: 10.1016/j.ijcard.2016.08.193. [DOI] [PubMed] [Google Scholar]
- Vernikouskaya I, et al. Patient-specific registration of 3D CT angiography (CTA) with X-ray fluoroscopy for image fusion during transcatheter aortic valve implantation (TAVI) increases performance of the procedure. Clinical Research in Cardiology. 2018. In Press. [DOI] [PubMed]
- Eng MH, Kim MS. Fluoroscopy and CT fusion overlay-greater than the sum of their parts. Catheterization and Cardiovascular Interventions. 2015;85(1):148–149. doi: 10.1002/ccd.25741. [DOI] [PubMed] [Google Scholar]
- John M, et al. System to guide transcatheter aortic valve implantations based on interventional C-arm CT imaging. Medical Image Computing and Computer-Assisted Intervention. 2010;13(Pt 1):375–382. doi: 10.1007/978-3-642-15705-9_46. [DOI] [PubMed] [Google Scholar]
- Lu Y, Sun Y, Liao R, Ong SH. A pre-operative CT and non-contrast-enhanced C-arm CT registration framework for trans-catheter aortic valve implantation. Computerized Medical Imaging and Graphics. 2014;38(8):683–695. doi: 10.1016/j.compmedimag.2014.06.021. [DOI] [PubMed] [Google Scholar]


