Abstract
Airway secretions contain a large number of immune-related cells, e.g., neutrophils, macrophages, and lymphocytes, which can be used as a major resource to evaluate a variety of pulmonary diseases, both for research and clinical purposes. However, due to the heterogeneous and viscous nature of patient mucus, there is currently no reliable dissociation method that does not damage the host immune cells in the patient airway secretion. In this research, we introduce a sample preparation method that uses inertial microfluidics for the patient's immune assessment. Regardless of the heterogeneous fluidic properties of the clinical samples, the proposed method recovers more than 95% of neutrophils from airway secretion samples that are diluted 1,000-fold with milliliters of clean saline. By recirculating the concentrated output stream to the initial sample reservoir, a high concentration, recovery, and purity of the immune cells are provided; recirculation is considered a trade-off to the single-run syringe-based operation of inertial microfluidics. The closed-loop operation of spiral microfluidics provides leukocytes without physical or chemical disturbance, as demonstrated by the phorbol 12-myristate 13-acetate (PMA)-induced elastase release of sorted neutrophils.
Keywords: Immunology and Infection, Issue 136, Inertial microfluidics, airway secretion, label-free cell sorting, heterogeneous biofluid, neutrophil enrichment, patient sample preparation
Introduction
Since cells are encapsulated in a large amount of mucus in airway secretions, the functional assessment of leukocytes by an in vitro assay has been hindered. Dithiothreitol (DTT) is the most common lysis buffer to dissociate and homogenize the sputum for cytological analysis and detection of mediators while providing tolerable viability of isolated cells1,2. However, DTT can interfere with surface-bound antigens of airway neutrophils, resulting in the disruption of neutrophil function such as elastase and myeloperoxidase (MPO) release2,3.Therefore, few studies of human airway neutrophil function have been conducted with peripheral blood neutrophils, which may not reveal the physiological characteristics of pulmonary4. Meanwhile, inertial microfluidics has made advances in isolating cells from various patient biomatrices5,6.The equilibrium between inertial lift forces and Dean drag aligns the particle/cell according to their size, which allows label-free particle separation7. Our group previously introduced a sample preparation method for circulating tumor cells8,9, pathogens in blood8, cells from a suspension culture10,11,12, and polymorphonuclear leukocytes (PMNs) from blood13,14.
Here, we introduce a protocol to prepare immune cells from a patient's airway secretions using closed-loop inertial microfluidics for a downstream in vitro assay, such as the neutrophil elastase (NE) assay. This method provides both high concentration and recovery, especially when there is a significant overlap in the lateral direction of the cell/particle from which the cell/particle-of-interest is to be removed, which is commonly observed in clinical samples. By recirculating the Inner wall (IW)-focused large particles or cells back to the input sample tube, the particle or cell-of-interest concentrates in the original reservoir, while background fluids with small mucin aggregates pass through the waste reservoir. Despite the heterogeneous fluidic properties of clinical samples, the proposed method recovers consistently above 95% of neutrophils from airway secretion samples that are diluted 1,000-fold with a clean saline solution (~1 mL). By contrast, the lysis method presents a wide range of PMNs recovery rates depending on the sample condition. The proposed protocol captures leukocytes in a label-free manner with no physical or chemical disruption, which provides the possibility to harvest delicate cells from clinically challenging biometrics with minimally invasive procedures.
Protocol
The sample collection was approved by the University of Pittsburgh Institutional Review Board (IRB# PRO16060443, PRO10110387). All experiments are performed under a biosafety cabinet with the proper personal protective equipment.
1. Device Fabrication and Soft Lithography
NOTE: Standard soft lithography techniques15,16 were used to create the polydimethylsiloxane (PDMS) microchannel.
Mix the PDMS precursor in a 10:1 ratio of base and curing agent.
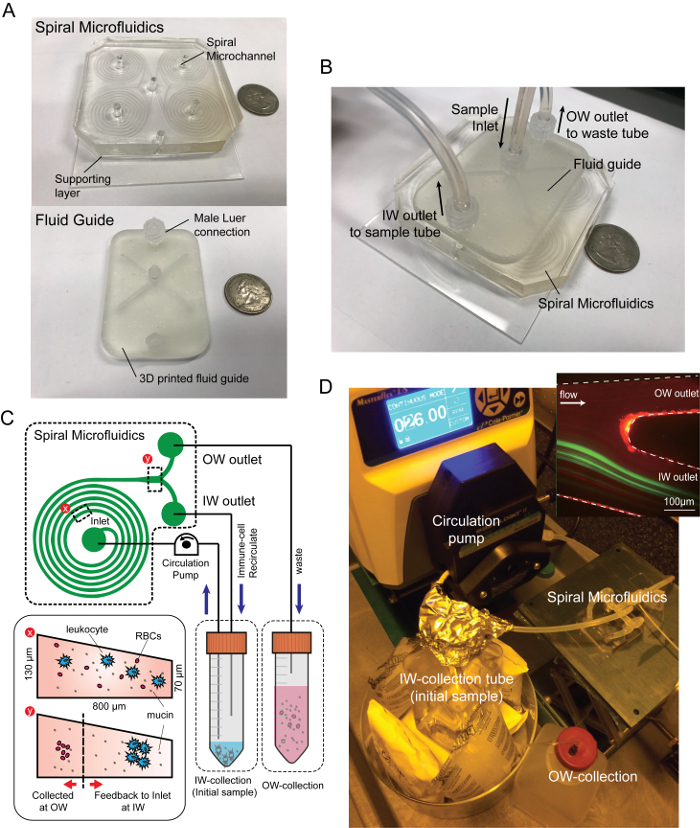
Pour 30 g of the PDMS precursor mixture on the micro-machined aluminum mold (see Figure 1 for dimensions) and 20 g of the PDMS precursor mixture on the 100 mm Petri dish. NOTE: The Petri dish is used to make the thick film of PDMS (~3 mm) for the supporting layer. The supporting layer of PDMS provides uniform physical surface properties throughout the microfluidics. Alternatively, a thin PDMS film on the glass slide can be used. The master mold with specific channel dimensions was designed and fabricated by micro-milling on the aluminum sheet. The spiral channel used in this study was an 8-loop spiral microchannel with one inlet and two outlets, with the radius increasing from 8 mm to 24 mm for efficient sized-based separation.
Place the mold and the Petri dish into a vacuum desiccator to degas until no bubbles are visible on the surface, typically 5 - 10 min. Use a house-vacuum for the desiccator.
Release the vacuum and place the mold and the Petri dish in a 90 °C oven for 1 h. NOTE: A hotplate or other heating tools can be used instead of an oven.
Remove the mold and the Petri dish from the oven and allow it to cool at room temperature for 10 min.
Carefully cut out the outline and punch the fluid access holes on the device using a 2 mm puncher. NOTE: The size of the punch can vary depending on the size of the tubing or fluid guide.
Adhere a tape to the channel side of the device and thick film of PDMS, and peel carefully with forceps to remove dust.
Treat both the channel side of the device and plain PDMS with air plasma and bond with the prepared supporting layer of plain PDMS (Figure 1A).
Place the chip on the 50 mm x 70 mm glass slide and place it in a 70 °C oven for at least 30 min to enhance the bonding strength.
2. Tracheal Secretion Collection from Mechanically Ventilated Patients
NOTE: Tracheal secretions can be obtained from mechanically ventilated patients during normal routine airway care by using a protocol modified from conventional methods to accommodate the standard, adult ventilated patient. Samples were de-identified and sent immediately for processing.
If tracheal aspiration is indicated, use a catheter to extract airway secretions.
Advance the catheter carefully half-way into the endotracheal tube and instill 5 mL of 0.9% sterile normal saline from a pre-filled 10 mL syringe.
When the catheter is advanced fully, or resistance is met, aspirate the secretions and collect into a sterile sputum collection container.
Collect 10 mL of tracheal secretions in a sterile sputum container and place it on ice. Send samples immediately for processing.
3. Tracheal Secretion Sample Preparation
NOTE: All experiments must be performed under a biosafety cabinet with the proper personal protective equipment.
Disperse 1 mL of airway secretion samples in 9 mL of phosphate-buffered saline (PBS) using a plastic 10 mL syringe (for a 10x dilution). Use a blunt pipette to homogenize the mucus sample.
Strain the diluent with a 40 µm nylon cell strainer to remove large chunks or blood clots, which can block the microfluidics access hole or the channel. Place the sample on the ice after processing.
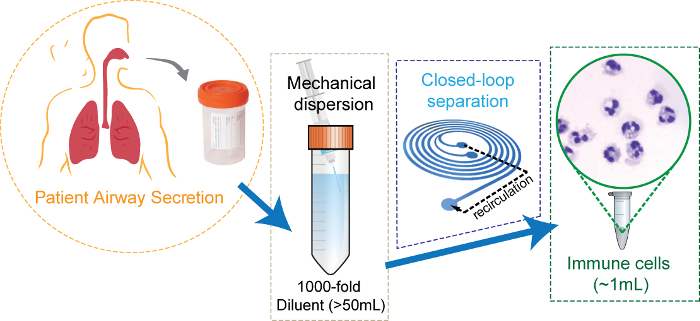
Disperse the diluent of each sample using a 100x volume of PBS buffer, resulting in a 1,000x diluted suspension. Place the sample on the ice during the entire dissociation operation.
4. Experimental Setup
NOTE: All experiments must be performed under a biosafety cabinet with the proper personal protective equipment.
Assemble the PDMS chip with the fluid guide to apply uniform flow to each of the four spiral microchannels (Figure 1B). NOTE: Four channels were used to increase the throughput by parallelization, as well as optimization of the volume and cell density of the resulting suspension. The fluid guide is designed and made with a stereolithography type 3D printer with clear resin. The inlet and the outlet port of the fluid guide use female Luer connectors for ease of connection and stable sealing during the operation.
Connect a 1/16-inch male Luer connector to the inlet, the inner wall (IW) outlet, and the outer wall (OW) outlet port of the fluid guide and connect a silicone tubing to the sample suspension.
Insert blunt tips to each end of the inlet and outlets to reach the bottom of the sample reservoir.
Connect the peristaltic pump with the inlet tubing.
Place the end of the inlet tubing and IW outlet tubing in the sample reservoir and place the end of the OW outlet tubing in the waste reservoir (Figure 1C, D).
Place the 50 mL tube of filtered PBS without calcium and magnesium at the sample reservoir.
Start pumping at a low flow rate (~1 mL/min) to prime the device. NOTE: When bubbles are captured in the channel, push the top of the channel with the tweezer to destabilize and eliminate air bubbles. When the device is fully filled with buffer solution, change the PBS tube to prepare the airway secretion sample tube.
When the sample is placed in the sample reservoir, switch on the peristaltic pump and set the flow rate at 4 mL/min (Figure 1C, D).
When the sample volume reaches the designated volume (~1 mL), stop the operation and disconnect the silicone tubing. NOTE: The proposed method is more efficient at reducing a large volume of diluent (>50 mL) into a micro-centrifuge tube volume (~1 mL) (Figure 2).
5. Flow Cytometry Analysis
NOTE: To compare the dissociation methods (microfluidics and lysis), flow cytometry with immunofluorescence was used.
Add fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD66b monoclonal antibody and allophycocyanin (APC)-conjugated mouse anti-human CD45 monoclonal antibody to the initial suspensions (step 3.3) and resulting suspensions (step 4.9) (200 µL of each antibody) in the ratio of 1:25 v/v.
Incubate the samples at 4 °C in the dark for 30 min.
Analyze both samples on a flow cytometer to quantify the PMNs. NOTE: The population that is FITC-APC double positive, CD66b positive and CD45 positive was considered to be neutrophils. Recovery was calculated by the ratio of the total cell numbers of the input and the resulting suspension.
6. NE Release Analysis
NOTE: To compare the functionality of the isolated neutrophil by the microfluidics and lysis method, an NE assay kit was used.
Add PMA (provided in the assay kit) to each resulting suspension (obtained at step 4.9) to a final concentration of 50 nM.
Incubate the samples at 37 °C for 2 h.
Centrifuge the suspensions at 300 x g for 10 min.
Transfer 10 µL of each supernatant to a 96-well plate (provided in the assay kit). NOTE: A 96-well microplate with a flat bottom and black polystyrene can be used as well.
Add 90 µL of diluted assay buffer (provided in the assay kit) to each well.
Add 10 µL of the elastase substrate (Z-Ala-Ala-Ala-Ala 2Rh110, provided in the assay kit).
Incubate for 1.5 h at 37 °C.
Read the 96-well plate using a fluorometer at an excitation wavelength of 485 nm and an emission wavelength of 525 nm. NOTE: The level of NE was divided by the PMN count from the flow cytometry analysis.
Representative Results
We achieved transparent immune-cell suspensions with both DTT mucolysis and microfluidics dissociation (Figure 3A). Microfluidics dissociation collected 4.40 x 105 PMNs on average (2.1 x 104 to 5.60 x 105 PMNs, n = 6) from airway secretion samples diluted 1,000-fold (50 mL total volume) in 1 mL of clean suspension. Compared to the initial diluent, 94.0% PMNs (CD66b+/CD45+) were recovered in a small volume, consistently over 6 clinical samples. However, the DTT mucolysis method showed a 53.5% recovery on average with significant sample-to-sample variations (30.8 - 96.0%) (Figure 3B).
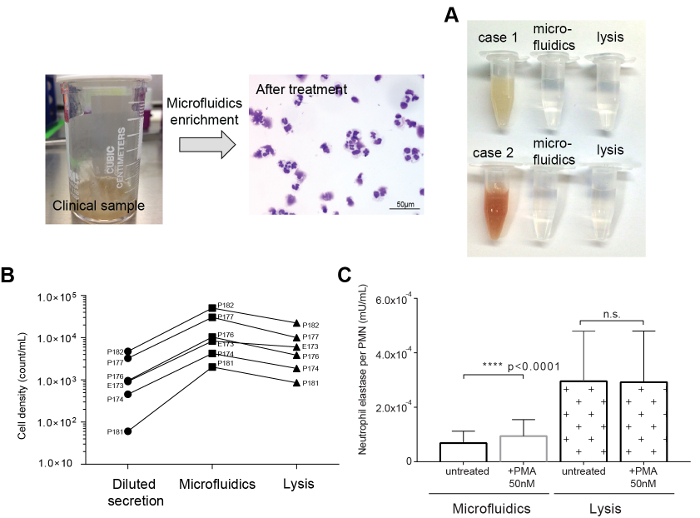
Next, the release of elastase was examined with a commercial NE assay kit as a proof-of-concept. Without external stimulation, the sample prepared by the mucolytic method showed a higher NE level than the sample from the microfluidics method. PMA was added to the resulting suspensions from both the microfluidics and mucolytic dissociation methods to trigger the NE release. We tested each method with airway secretion samples from 6 different patients. Immune cells separated with microfluidics presented intact functionality, showing an increased amount of NE release by PMA stimulation. On the other hand, cells prepared by the mucolytic dissociation method showed an inconsistent increase of NE release (Figure 3C).
Figure 1: Spiral microfluidic device and experimental settings. Photograph images of (A) spiral microfluidics and (B) the device assembled with the fluidic adaptor. (C) Schematic diagram of closed-loop separation using spiral inertial microfluidics. By recirculating the particle/cell concentrated stream (IW outlet) back into the original sample reservoir, immune-related cells were concentrated in a small volume while the background fluid containing mucin aggregates and minor RBCs were continuously removed through the waste tube connected to the OW outlet. (D) Experimental setup and the fluorescent image of a 10 µm particle, the trajectory (green) and the outlet of the spiral channel (upper right). Please click here to view a larger version of this figure.
Figure 2: Schematic diagram of the airway secretion sample preparation using closed-loop inertial microfluidics. Clinically induced airway secretion dispersed mechanically in 1,000x volume of buffer solution. The diluent is concentrated by microfluidics, resulting in ~1 mL of cell suspension with the clear background. Adapted with permission from Ryu et al., Copyright (2017) American Chemical Society17. Please click here to view a larger version of this figure.
Figure 3: Representative comparisons of the DTT mucolytic method to the microfluidic dissociation method. (A) Tracheal secretions sample (left) and microscopic image after the microfluidic dissociation. (B) Photographic image of the original and resulting suspensions. (C) Comparison of PMN recovery rates between the mucolytic and microfluidics methods. (D) Comparison of NE release of separated neutrophils by the microfluidics and DTT mucolytic methods before and after PMA stimulation. NE release was significantly triggered by PMA on the sample from microfluidic separation (p <0.0001). Please click here to view a larger version of this figure.
Discussion
In inertial microfluidics, particle and cells localize at a certain lateral position in a micro-channel based on their size5,18,19,20. Due to the combined effect of the Dean drag force and the inertial lift force in the curved microchannel, large particles or neutrophils (>10 µm) are located inside the channel and small particles, mucus aggregates, and debris smaller than 6 µm are positioned outside the channel at certain flow velocity conditions10,11,13,14.
However, there is a trade-off between the concentration and recovery of the separation when using microfluidics, especially when the separation size range of the particle or the cell of interest overlaps with other cells/particles. To achieve a high concentration of sorted particles and cells, the separation range needs to be narrow and requires a fine-tuning with microscopic observation for each sample run; this limits the usage of inertial microfluidics on heterogeneous biometrics, such as airway secretions.
Here, we introduce a clinical airway secretions sample preparation method for in vitro assays using closed-loop inertial microfluidics (Figure 1B, C). The closed-loop operation by recirculation of the particle/cell through a focusing outlet achieved both high concentration factors and high recovery rates. The OW outlet flows into the waste tube, and the IW outlet is continuously fed to the original sample tube. Cells larger than 10 µm in diameter remain in the initial sample tube, thus, increasing the cell density of the sample, while the background fluid is eliminated to the waste tube. The high dilution rate allows clearance of the background fluid, including debris and mucus. Also, regardless of the condition of patient sample, the proposed method can uniformly dissociate and isolate immune cells.
DTT is a widely used mucin lysis buffer that cleaves disulfide bonds in proteoglycan aggregation to separate cell content from the mucilage1,21,22. However, DTT reportedly interferes with the white blood cell surface antigens, which can affect leukocyte function2. In our study, the cellular contents recovered by DTT dissociation were inconsistent across samples, because of non-uniform mucolytic efficiency and clogging of the filter. This becomes more critical for the preparation of initial samples with small volumes and relatively thick airway secretion samples. On the other hand, the method using microfluidics enabled the consistent recovery of cell contents in heterogeneous patient samples (Figure 3C). As shown in Figure 3B, we could consistently isolate the cell contents of the airway secretion in a clean buffer regardless of the original sample state, i.e., bloody, tenacious, or watery. Compared to the conventional method, the proposed method has less debris in the recovered sample, which minimizes possible interference of debris in downstream biochemical analyses.
NE release was examined to evaluate the functional integrity of captured neutrophils by both enrichment methods (Figure 3D). Neutrophils remove foreign organic molecules through NE release23,24,25. PMA is commonly used to activate neutrophils in vitro26. Cells isolated by the microfluidics and the mucolytic (DTT) methods were stimulated with 50 nM of PMA. We tested each method with human airway secretion samples (labeled as P173, P174, P176, P177, P181, and P182). Samples concentrated by microfluidics showed an increase in NE by PMA stimulation for all 6 samples. In contrast, only 3 of the DTT treated samples could induce increased NE activity after PMA stimulation. In addition, the neutrophils isolated by the DTT mucolytic method showed much higher baseline activity than the neutrophils isolated by microfluidics. These results showed possible chemical disturbance of the NE machinery, i.e., disruption of the surface bound antigen2,3, which suggests that the microfluidics method is an improved method in terms of preserving the neutrophil function than DTT homogenization. The proposed dissociation method also operates in an automated and fully contained manner, minimizing the exposure of patient samples with potential hazards of the external environment.
However, the proposed method requires a high flow rate and, therefore, a stable fluidic connection. Also, due to the inherent fluctuation of the peristaltic circulation pump, the IW outlet dimension must be greater than the OW outlet dimension, otherwise throughput may be compromised. We expect that the proposed method will provide higher throughput if there is a circulation pump that can provide a more stable flow rate.
By providing a dissociation protocol to capture neutrophils from clinical airway secretions, the method presented in this study is expected to expand the scope of clinical research on respiratory diseases, such as pneumonia, asthma, cystic fibrosis, and chronic obstructive pulmonary disease.
Disclosures
The authors have filed a patent application on the technology described here.
Acknowledgments
This work was supported by NIH/NIAID (R21AI119042) as well as NIH U24 Sample Sparing assay program (U24-AI118656).
References
- Hamid Q, et al. Methods of sputum processing for cell counts, immunocytochemistry and in situ hybridisation. European Respiratory Journal. 2002;20(Supplement 37):19S–23S. doi: 10.1183/09031936.02.00001902. [DOI] [PubMed] [Google Scholar]
- van Overveld FJ, et al. Effects of homogenization of induced sputum by dithiothreitol on polymorphonuclear cells. J Physiol Pharmacol. 2005;56(Suppl 4):143–154. [PubMed] [Google Scholar]
- Qiu D, Tan WC. Dithiothreitol has a dose-response effect on cell surface antigen expression. J Allergy Clin Immunol. 1999;103(5 Pt 1):873–876. doi: 10.1016/s0091-6749(99)70432-x. [DOI] [PubMed] [Google Scholar]
- Usher LR, et al. Induction of Neutrophil Apoptosis by the Pseudomonas aeruginosa Exotoxin Pyocyanin: A Potential Mechanism of Persistent Infection. The Journal of Immunology. 2002;168(4):1861–1868. doi: 10.4049/jimmunol.168.4.1861. [DOI] [PubMed] [Google Scholar]
- Di Carlo D. Inertial microfluidics. Lab Chip. 2009;9(21):3038–3046. doi: 10.1039/b912547g. [DOI] [PubMed] [Google Scholar]
- Martel JM, Toner M. Inertial focusing dynamics in spiral microchannels. Phys Fluids. 2012;24(3):32001. doi: 10.1063/1.3681228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, et al. Fundamentals and applications of inertial microfluidics: a review. Lab Chip. 2016;16(1):10–34. doi: 10.1039/c5lc01159k. [DOI] [PubMed] [Google Scholar]
- Hou HW, Bhattacharyya RP, Hung DT, Han J. Direct detection and drug-resistance profiling of bacteremias using inertial microfluidics. Lab Chip. 2015;15(10):2297–2307. doi: 10.1039/c5lc00311c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warkiani ME, et al. Ultra-fast, label-free isolation of circulating tumor cells from blood using spiral microfluidics. Nat Protoc. 2016;11(1):134–148. doi: 10.1038/nprot.2016.003. [DOI] [PubMed] [Google Scholar]
- Warkiani ME, Tay AK, Guan G, Han J. Membrane-less microfiltration using inertial microfluidics. Sci Rep. 2015;5:11018. doi: 10.1038/srep11018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warkiani ME, Wu L, Tay AK, Han J. Large-Volume Microfluidic Cell Sorting for Biomedical Applications. Annu Rev Biomed Eng. 2015;17:1–34. doi: 10.1146/annurev-bioeng-071114-040818. [DOI] [PubMed] [Google Scholar]
- Kwon T, et al. Microfluidic Cell Retention Device for Perfusion of Mammalian Suspension Culture. Sci Rep. 2017;7(1):6703. doi: 10.1038/s41598-017-06949-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Guan G, Hou HW, Bhagat AA, Han J. Separation of leukocytes from blood using spiral channel with trapezoid cross-section. Anal Chem. 2012;84(21):9324–9331. doi: 10.1021/ac302085y. [DOI] [PubMed] [Google Scholar]
- Guan G, et al. Spiral microchannel with rectangular and trapezoidal cross-sections for size based particle separation. Sci Rep. 2013;3:1475. doi: 10.1038/srep01475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotz K, Cheng X, Toner M. PDMS Device Fabrication and Surface Modification. J Vis Exp. 2007. p. e319. [DOI] [PMC free article] [PubMed]
- Duffy DC, McDonald JC, Schueller OJA, Whitesides GM. Rapid Prototyping of Microfluidic Systems in Poly(dimethylsiloxane) Analytical Chemistry. 1998;70(23):4974–4984. doi: 10.1021/ac980656z. [DOI] [PubMed] [Google Scholar]
- Ryu H, et al. Patient-Derived Airway Secretion Dissociation Technique To Isolate and Concentrate Immune Cells Using Closed-Loop Inertial Microfluidics. Anal Chem. 2017;89(10):5549–5556. doi: 10.1021/acs.analchem.7b00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach AJ, Di Carlo D. Continuous scalable blood filtration device using inertial microfluidics. Biotechnol Bioeng. 2010;107(2):302–311. doi: 10.1002/bit.22833. [DOI] [PubMed] [Google Scholar]
- Di Carlo D, Irimia D, Tompkins RG, Toner M. Continuous inertial focusing, ordering, and separation of particles in microchannels. Proc Natl Acad Sci U S A. 2007;104(48):18892–18897. doi: 10.1073/pnas.0704958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang N, et al. Fundamentals of elasto-inertial particle focusing in curved microfluidic channels. Lab Chip. 2016;16(14):2626–2635. doi: 10.1039/c6lc00376a. [DOI] [PubMed] [Google Scholar]
- Lotvall J, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011;127(2):355–360. doi: 10.1016/j.jaci.2010.11.037. [DOI] [PubMed] [Google Scholar]
- Houston N, et al. Sputum neutrophils in cystic fibrosis patients display a reduced respiratory burst. J Cyst Fibros. 2013;12(4):352–362. doi: 10.1016/j.jcf.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Janoff A, Scherer J. Mediators of inflammation in leukocyte lysosomes. IX. Elastinolytic activity in granules of human polymorphonuclear leukocytes. J Exp Med. 1968;128(5):1137–1155. doi: 10.1084/jem.128.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata K, Hagio T, Matsuoka S. The role of neutrophil elastase in acute lung injury. Eur J Pharmacol. 2002;451(1):1–10. doi: 10.1016/s0014-2999(02)02182-9. [DOI] [PubMed] [Google Scholar]
- Rubin BK. Plastic Bronchitis. Clin Chest Med. 2016;37(3):405–408. doi: 10.1016/j.ccm.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Kokot K, Teschner M, Schaefer RM, Heidland A. Stimulation and inhibition of elastase release from human neutrophils dependent on the calcium messenger system. Miner Electrolyte Metab. 1987;13(2):133–140. [PubMed] [Google Scholar]


