Abstract
We have previously shown that the wheat (Triticum aestivum) TaADF gene expression level is correlated with the plants capacity to tolerate freezing. Sequence analysis revealed that this gene encodes a protein homologous to members of the actin-depolymerizing factor (ADF)/cofilin family. We report here on the characterization of the recombinant TaADF protein. Assays for ADF activity showed that TaADF is capable of sequestering actin, preventing nucleotide exchange, and inducing actin depolymerization. In vitro phosphorylation studies showed that TaADF is a substrate for a wheat 52-kD kinase. The activity of this kinase is modulated by low temperature during the acclimation period. Western-blot analyses revealed that TaADF is expressed only in cold-acclimated Gramineae species and that the accumulation level is much higher in the freezing-tolerant wheat cultivars compared with the less tolerant ones. This accumulation was found to be regulated by a factor(s) encoded by a gene(s) located on chromosome 5A, the chromosome most often found to be associated with cold hardiness. The induction of an active ADF during cold acclimation and the correlation with an increased freezing tolerance suggest that the protein may be required for the cytoskeletal rearrangements that may occur upon low temperature exposure. These remodelings might be important for the enhancement of freezing tolerance.
Acquisition of freezing tolerance (FT) in plants is associated with numerous physiological and genetic alterations. These changes are triggered by a period of low temperature (LT) exposure and are necessary to protect critical cell structures and vital physiological processes during freezing. The alterations include increased levels of sugars, soluble proteins, proline, and organic acids, the appearance of new enzyme isoforms, and modifications in the lipid membrane composition (Hughes and Dunn, 1996; Thomashow, 1999). They are regulated by a complex, multigenic system that is programmed at the gene expression level. To elucidate the molecular basis underlying this system, identification of cold-regulated genes and study of their function and regulation are required.
Several LT-regulated cDNAs and their products have been isolated and characterized in many species (Thomashow, 1999). However, their exact functions are still unknown. In wheat (Triticum aestivum), the cold-regulated TaADF cDNA (previously called Wcor719) shows a high homology to plant, animal, and yeast actin-binding proteins (Danyluk et al., 1996). The actin depolymerizing factors (ADF) are part of the ADF/cofilin group, a family of small proteins (15–22 kD) that includes cofilin, destrin, depactin, and actophorin (Staiger et al., 1997; Lappaleinen et al., 1998). The members of this family can be described as stimulus-responsive modulators of the cell actin cytoskeleton dynamics. They show actin-monomer binding, actin-filament binding/severing, and nucleotide/monomer dissociation-inhibiting activities in vitro (Lappalainen et al., 1997; McGough and Chiu, 1999). ADF/cofilin dissociation-inhibiting activity creates a shift in the equilibrium between ADP-actin monomers and ATP-actin monomers toward the high-energy form, which has been suggested to promote rapid actin polymerization and cytoskeletal reorganization (Aderem, 1992). Overexpression of cofilin was studied in slime mold (Aizawa et al., 1996) and in the bacterium Listeria monocytogenes (Carlier et al., 1997), two motile unicellular organisms. The slime mold cells showed a development of thick actin cables, dramatic membrane ruffling, and increased motility. A 2-fold increase in cell movement was observed in L. monocytogenes. In addition to cell movement, ADF/cofilin-like proteins perform essential functions in the yeast Saccharomyces cerevisiae (Moon et al., 1993), in the nematode Caenorhabditis elegans (McKim et al., 1994), and in fruit fly (Gunsalus et al., 1995). Using Arabidopsis ADF1, Carlier and colleagues (1997) have suggested that one of the main functions of ADF is to increase the turnover rate of actin filaments. This would change the kinetic parameters of actin assembly and disassembly in an end-directed fashion, controlling the dynamics and the length of actin filaments in vivo. McGough and Chiu (1999) recently showed that ADF/cofilin weakens and disrupts lateral actin-actin contacts.
Several cellular processes are associated with the reorganization of the actin cytoskeleton in plants. These include cell division and differentiation, stomatal movement, gravitropic tip growth, light-induced plastid migration, wound repair, response to pathogen attack, pollen development, nuclear migration, cytoplasmic streaming, secretion, cell wall biosynthesis, and transmembrane signaling (Aon et al., 1999). Actin filaments are tightly linked to the plasma membrane and believed to be involved in signal transduction events in plants (Aon et al., 1999). Disruption or reorganization of the cytoskeleton could thus impair or modify the activity of signaling molecules associated with cytoskeletal elements.
Plant ADF/cofilin family members share approximately 30% amino acid identity with the vertebrate family members (Danyluk et al., 1996; Lopez et al., 1996), and the primary structure and actin binding/depolymerizing activities are generally conserved among species (Aizawa et al., 1995; Jiang et al., 1997; Lappaleinen et al., 1998). Based on the above information and on the identification of a wheat cold-regulated ADF homolog, we designed the present experiments to determine the function and regulation of TaADF. Our data demonstrate for the first time to our knowledge that LT induces the accumulation of an ADF protein in Gramineae species. This suggests that important changes in the actin cytoskeletal architecture may occur during LT acclimation, and that these modifications may be related to cell survival under freezing conditions.
RESULTS
Protein Production and Purification
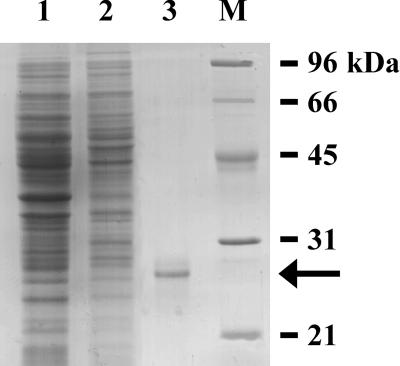
The TaADF cDNA was cloned previously and the expression was characterized at the mRNA level (Danyluk et al., 1996). To characterize the TaADF protein, the cDNA was expressed in Escherichia coli. After isopropylthio-β-galactoside (IPTG) induction, the recombinant His-tagged TaADF protein was purified by affinity chromatography to approximately 90% purity (Fig. 1, lane 3). The molecular mass of the recombinant TaADF calculated by SDS-PAGE (27 kD) is higher than the predicted mass of approximately 19 kD (15.8 kD ADF plus 3.3 kD fusion peptide). The discrepancy between the apparent and calculated mass has often been observed for stress-induced proteins (Sarhan et al., 1997). This protein was used directly in the in vitro ADF activity and phosphorylation assays. For antibody production, the protein was further purified to almost 100% purity by electroelution of the 27-kD band from a preparative SDS-PAGE gel.
Figure 1.
Expression and purification of the recombinant TaADF protein. Total proteins were extracted from non-transformed (Lane 1) and transformed (Lane 2) E. coli cells after IPTG-induction. Lane 3, Eluate of metal-binding chromatography. Proteins were separated by SDS-PAGE and stained with Coomassie Brilliant Blue. The arrow indicates the position of the 27-kD band corresponding to the TaADF recombinant protein. The protein identity was confirmed by total amino acid composition analysis. Lane M, Molecular mass markers.
TaADF Is an ADF
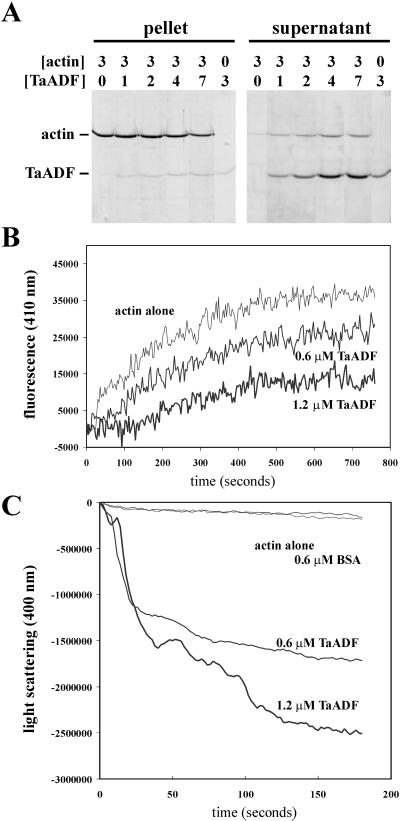
Sequence analysis of TaADF revealed a high level of similarity between the predicted encoded product and proteins of the ADF/cofilin family present in several eukaryote species. To determine whether TaADF does indeed have an effect on actin dynamics, a number of in vitro biochemical assays were performed using the recombinant protein and yeast actin. First, cosedimentation assays (Fig. 2A) showed that TaADF is able to interact with actin at pH 7.4, as revealed by the higher proportion of actin present in the supernatant fraction when TaADF is included to varying concentrations. Similar results were obtained when the assays were done at pH 8.0. No actin was released in the supernatant when the assays were performed at pH 6.8. These results reveal that the activity of the wheat ADF shows the same pH sensitivity as other members of the ADF/cofilin family. Second, the ability of TaADF to interact with actin-ATP monomers was assessed by its ability to inhibit nucleotide exchange by the actin monomer (Hawkins et al., 1993). The results show that TaADF interferes with nucleotide exchange in a concentration-dependent manner (Fig. 2B). Third, the actin-depolymerizing activity of TaADF on polymerized purified yeast actin was followed by the decrease of light scattering at 400 nm. As shown in Figure 2C, TaADF behaves as a concentration-dependent ADF. Together these results provide compelling evidence that the wheat TaADF protein is an active ADF.
Figure 2.
TaADF is an active ADF. Purified actin and TaADF were used at the indicated concentrations (micromolars) in the different assays. A, Cosedimentation assays. F-actin and TaADF were mixed and incubated, and then polymerized actin was pelleted. The pellet and supernatant fractions were analyzed by SDS-PAGE and Coomassie Brilliant Blue staining. B, Actin nucleotide exchange assays. The interaction of TaADF with G-actin ATP was determined by the inhibition of actin nucleotide exchange using etheno-ATP (Molecular Probes, Eugene, OR) and fluorescence detection. C, Actin depolymerization assays. F-actin depolymerization by TaADF was followed by the decrease of light scattering at 400 nm. Bovine serum albumin (BSA) was used as a negative control.
TaADF Is Phosphorylated by a Wheat Protein Kinase
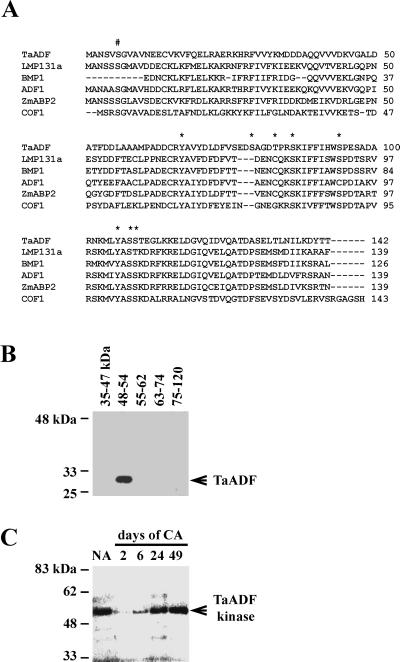
From the deduced TaADF protein sequence analysis and data from characterization of other ADF/cofilins we predicted putative targets for protein kinases (http://genome.cbs.dtu.dk/services/NetPhos). An alignment of TaADF and other ADF/cofilins and the possible phosphorylation sites are shown in Figure 3A. In vitro phosphorylation assays were performed to determine if TaADF is a phosphorylation target in wheat. The results in Figure 3B demonstrate that, at least in vitro, TaADF serves as a substrate for wheat protein kinases with molecular mass in the range of 48 to 54 kD. The kinase(s) acting on TaADF exhibits an absolute requirement for Mn2+, a property that has been noted in plant receptor-like protein kinases.
Figure 3.
TaADF acts as a substrate for a 48- to 54-kD wheat protein kinase. A, Alignment of the plant ADF/cofilins and their known/putative phosphorylation sites. LMP131a, lily ADF (Z14110); BMP1, Brassica ADF (Z14109); ADF1, Arabidopsis ADF (U48938); ZmABP2, maize actin-binding protein (X97725); and COF1, yeast cofilin (D13230). #, Conserved phosphorylation site in several eukaryotic ADFs; Asterisk, predicted phosphorylation sites in TaADF. B, SDS-PAGE was used to fractionate proteins from NA wheat (cv Norstar). After in-gel renaturation, slices of the gel corresponding to the molecular mass interval indicated above each lane were ground with purified recombinant TaADF and incubated with [γ-32P]ATP. The mixture was then loaded on a new SDS-PAGE gel and the TaADF band was revealed by autoradiography. C, In-gel phosphorylation assay of control and cold-acclimated wheat extracts. Protein extracts from wheat (cv Norstar) were separated on an SDS-PAGE gel containing 0.1 mg/mL of the recombinant TaADF. After in-gel renaturation of the proteins, the gel was incubated in kinase buffer with [γ-32P]ATP, and the band was revealed by autoradiography. 2 through 49, Duration of cold acclimation (in days).
In-gel kinase activity assays (Fig. 3C) confirmed that TaADF is phosphorylated mostly by a 52-kD kinase. This kinase is present and active in nonacclimated (NA) plants, even though TaADF is undetectable (see Fig. 4A). The phosphorylation activity drops to a barely detectable level at the beginning of the acclimation period and recovers gradually after that. After 49 d, the kinase activity was comparable with that of control plants. Inhibition of the activity of this kinase seems to be consequential to the initiation of the cold acclimation (CA) process. Substitution of TaADF by casein or myelin-binding protein (MBP) as phosphorylation substrate revealed a 52-kD kinase of identical kinetics for casein (but not MBP), as well as additional kinases .The fact that this 52-kD kinase is present in NA plants (when TaADF is not expressed) and that it can phosphorylate another substrate (casein) suggests that it is likely to be a multifunctional kinase. Identification of the 52-kD TaADF kinase should allow us to determine if the phosphorylation status of TaADF affects its depolymerization activity.
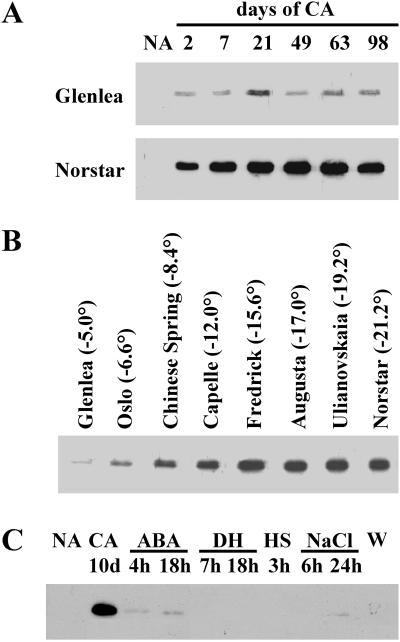
Figure 4.
TaADF expression is up-regulated by LT exposure. Soluble proteins were extracted from wheat plants and analyzed by western blotting using the TaADF antiserum. A, Accumulation kinetics of TaADF during CA in spring (cv Glenlea) and winter (cv Norstar) wheat. 2 through 98, Duration of CA (in days). B, Relative abundance of TaADF in wheat cultivars with different levels of FT, after 49 d of cold acclimation. The LT50 (temperature at which 50% of the plants are killed) is given for each cultivar (in °C). C, Effect of different stresses on the accumulation of TaADF in the cultivar Norstar. ABA, Treated with 10−4 M abscisic acid for 4 and 18 h; DH, dehydrated for 7 and 18 h; HS, heat-shocked for 3 h at 40°C; NaCl, salt stressed for 6 and 24 h with 0.5 m NaCl; W, 18 h after wounding stress.
TaADF Expression Correlates with CA
To determine the implication of TaADF in CA, western-blot analyses were performed to evaluate the protein level at different stages of acclimation. As shown in Figure 4A, TaADF is undetectable in NA control plants (24°C; NA), but accumulates to a high level when the plants are exposed to LT (4°C; 2–98 d). Norstar, a hardy cultivar (LT50, −21°C), exhibits a constant accumulation of the protein up to 49 to 63 d of CA, whereas Glenlea, a less tolerant one (LT50; −5°C), shows a lower level and shorter (21 d) accumulation period. After 21 d, the TaADF accumulation in Norstar is about 10-fold higher than that in Glenlea. This pattern of expression during CA is similar to that observed for other cold-induced wheat proteins (Sarhan et al., 1997; Danyluk et al., 1998). Proteins from wheat cultivars showing different levels of FT were also analyzed. The results in Figure 4B show that after 49 d of CA, the accumulation level of TaADF is much higher in the freezing tolerant cultivars compared with the less tolerant ones.
To determine whether the TaADF accumulation is specifically regulated by LT, plants were subjected to different treatments that elicit typical stress responses. The results in Figure 4C indicate that abscisic acid and NaCl treatments induce a very low level of protein accumulation compared to LT exposure. Water stress, wounding, and heat shock did not induce its accumulation. The protein expression data are consistent with the mRNA expression data reported previously (Danyluk et al., 1996) and suggest that the TaADF accumulation is LT-specific. This high specificity of induction has not been seen with the other cold-induced proteins isolated in our laboratory.
The TaADF protein is present to a similar level in the leaf, crown, and root tissues. Subcellular fractionation experiments revealed that it is found only in the soluble cytosolic fraction, not associated with any cellular structures (data not shown). Analysis of proteins from plant and animal tissues such as tobacco, cucumber, strawberry, Arabidopsis, alfalfa, human and mouse peripheral blood, calf leukocytes, and various tissues from trout did not reveal the presence of the ADF orthologues. On the other hand, a protein of identical Mr was detected in rye and barley. These results suggest that the antibody raised against TaADF is specific to the Gramineae ADFs.
Genes on Chromosome 5A Regulate TaADF Expression
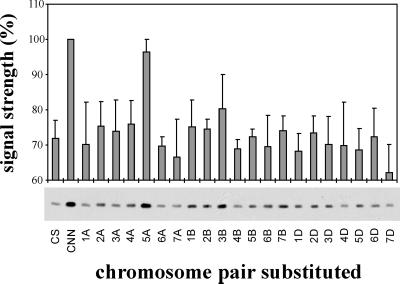
The accumulation of the TaADF protein was studied in a chromosome substitution series in which, for each line, one pair of chromosomes from the hardy winter Cheyenne cultivar is substituted for the homologous pair in the less hardy Chinese Spring cultivar (Fig. 5). After 21 d of acclimation the protein level was much higher in the hardy cultivar Cheyenne than in the less hardy Chinese Spring. Substitution of chromosome 5A induced the accumulation of TaADF in the Chinese Spring background to a level comparable with the one observed in Cheyenne. These results suggest that the expression of TaADF during CA is, at least in part, regulated by factor(s) encoded by gene(s) found on chromosome 5A. Two other cold-induced genes from wheat (wcor410 and wcs120) were also shown to be regulated by the same chromosome (Sarhan et al., 1997; Danyluk et al., 1998).
Figure 5.
Factor(s) encoded by genes located on chromosome 5A regulate(s) the expression of TaADF. Plants of the Chinese Spring (CS)/Cheyenne (CNN) chromosome substitution series were CA for 21 d, then proteins were extracted and analyzed by western blotting using the TaADF antibody. The TaADF abundance was determined by densitometric reading of the western blots. The signal intensity has been corrected for the protein load, as estimated by densitometric reading of the Coomassie Brilliant Blue-stained gels. Expression level in CNN was set to 100%. Values represent the means ± se from three independent experiments.
DISCUSSION
During the course of our studies on CA in wheat, we have isolated the TaADF cDNA, which was predicted to encode an ADF. Increasing evidence has suggested that proteins of the ADF family could be involved in the actin cytoskeleton rearrangement and signal transduction events occurring when plants are subjected to stress conditions (Aon et al., 1999). The present study was thus aimed at the characterization and expression analysis of the encoded protein.
In vitro assays using the recombinant protein revealed that TaADF behaves like an active ADF. It is well known that the activity of ADF/cofilins is influenced by several factors, including pH, phosphorylation, and phosphoinositide binding. The TaADF activity showed a pH dependency similar to the Arabidopsis ADF1 (Carlier et al., 1997), the porcine cofilin (Yonezawa et al., 1985), and the human ADF (Hawkins et al., 1993). These data suggest that the intracellular activities of ADF may be, at least in part, regulated by small changes in pH. Yoshida (1994) has shown that LT induces cytoplasm acidification in cold-sensitive mung bean cells harvested in the early growth period, but not in cold-tolerant cells harvested in the later phase of growth. The stability of pH in cold-tolerant cells may provide a mechanism to maintain ADF activity in hardy wheat cultivars.
It is becoming increasingly evident that signal transduction of environmental and developmental stimuli in plants involves signaling pathways similar to those found in yeast and animal systems. Available evidence suggest that calcium, phosphorylation cascades, and phosphoinositides metabolism could be involved in the perception of LT and initiation of the acclimation process in plants (Drøbak, 1993; Jonak et al., 1996; Smolenska-Sym and Kacperska, 1996; Monroy et al., 1997; Vazquez-Tello et al., 1998; Thomashow, 1999). The general kinase activity was shown to increase upon LT exposure of wheat and alfalfa (Monroy et al., 1997; Vazquez-Tello et al., 1998). Plant MAP kinase-like activities have been identified and shown to respond to a number of stimuli, including cold and drought (Jonak et al., 1996).
Post-translational modification of ADF by phosphorylation is a major factor affecting its activity. Vertebrates ADF are phosphorylated on the consensus Ser-3 adjacent to Gly-4 (Ser-6 and Gly-7 in plant ADFs; Lopez et al., 1996). Rapid dephosphorylation of ADF and cofilin has been observed in various stimulated cells (Davidson and Haslam, 1994; Saito et al., 1994; Samstag et al., 1994; Kanamori et al., 1995). These stress-induced dephosphorylations coincided with changes in cytoskeleton organization and assembly. Morgan and colleagues (1993) showed that phosphorylation inhibits the G-actin binding and F-actin depolymerizing activities of ADF. It appears that specific kinases are needed since the ADF was not phosphorylated by ubiquitous protein kinases (calmodulin kinase II, protein kinase C, protein kinase AMP dependent, and myosin light chain-activated kinase). In vitro phosphorylation by size-selected wheat protein kinases indicates that, like other ADFs, actin-modulating activity of TaADF could be regulated by such a modification. The major kinase detected was a renaturable 52-kD protein, and its activity was found to be regulated by LT. The decrease in activity observed in the early period of CA could result from catabolic degradation of the kinase or its post-translational modification. However, it is unlikely that initiation of the acclimation period would lead to such a rapid catabolism of the kinase since there is no available evidence suggesting an increased activity of protein degradation pathways at LT. On the other hand, the rapid change in kinase activity could result from a post-translational modification, such as phosphorylation, that would occur upon exposure of the plant to LT.
The TaADF protein accumulates during the acclimation period to a higher level and for a longer period of time in hardy cultivars compared with sensitive ones. Fowler and colleagues (1996) have reported a gradual loss of LT tolerance after 49 d of acclimation, which is approximately the time when vernalization saturation is complete for hardy cultivars. These periods coincide with the maximum cold tolerance achieved by the cultivars. Worth mentioning is the fact that the kinetics of TaADF protein expression is different from the mRNA expression pattern (Danyluk et al., 1996). During the acclimation period, TaADF mRNA level is maximal after 1 or 2 d and slowly decreases afterward, whereas protein accumulation increases and peaks at 49 d in the hardy cultivars. These results suggest that the protein is highly stable and/or that the mRNA translation efficiency at LT is increased. It is interesting that the purified Arabidopsis ADF1 was found to be stable for at least 4 weeks when kept at 4°C (Carlier et al., 1997). It is likely that the depolymerizing activity of the highly abundant TaADF protein in the later phase of the acclimation period might need to be down-regulated by the TaADF 52-kD kinase.
As reported for WCS120 and WCOR410 (two cold-induced protein families from wheat), TaADF expression is in part regulated by factor(s) encoded by genes located on chromosome 5A (Limin et al., 1997; Danyluk et al., 1998), where major vernalization and cold hardiness quantitative trait loci have been mapped. Substitution of the Cheyenne chromosome 5A in Chinese Spring brought the TaADF accumulation close to the level of the hardy Cheyenne cultivar. This effect on protein expression is the strongest observed among the cold-induced proteins tested in our laboratory. This suggests that TaADF expression could be more dependent on the factors encoded by genes on chromosome 5A. These factors may link cold perception and gene induction or may promote the accumulation of cold-regulated gene products. They could also be responsible for the differential expression of TaADF among the different cultivars. Detailed genetic analysis and molecular isolation of the regulators on chromosome 5A, the “master switch,” will help us understand their biological significance in the development of FT (Sarhan and Danyluk, 1998). Knowledge gained from these studies will help determine whether FT in cereals can be improved by modulating the expression level of the regulators in the less tolerant and sensitive Gramineae species.
The induction of an ADF protein by LT suggests that actin reorganization may occur during the acclimation process. In support of this it was shown that the expression of elongation factor-1, a potent actin cytoskeleton rearrangement factor (Kielbassa et al., 1995), is up-regulated by LT in barley (Dunn et al., 1993) and potato (Rorart et al., 1997). Wheat elongation factor-1 has just been isolated in our laboratory and its characterization is under way. It has been hypothesized that acclimating plant cells need a dynamic, localized, and coordinated actin turnover (Carlier et al., 1997; Aon et al., 1999). This reorganization could thus have a repercussion on most of the cytoskeletal-associated processes. For example, actin filament dynamics could be implicated in the cell volume changes observed during CA. Expansion-induced cell lysis is a form of cell and membrane injury that occurs during freeze/thaw cycles (Thomashow, 1999). For the cells to survive, their plasma membranes and ultrastructures must be able to withstand the efflux and influx of water. Few published studies have reported on the relationship between the cytoskeleton and cellular adjustment during CA. Kerr and Carter (1990) showed that LT causes microtubule depolymerization in winter rye root tips and that the level of depolymerization was related to the degree of FT, suggesting that microtubule depolymerization is important in FT.
Although several questions are still unanswered, the accumulation of an ADF protein during LT offers new perspectives that should help understand the overall mechanism of CA and FT of plants. Immunocytological experiments will help determine if the actin cytoskeleton undergoes major restructuration during LT exposure and will help clarify the possible association between TaADF and actin. In addition, the characterization of the cold-regulated 52-kD TaADF kinase is needed to determine its function during CA. Yeast and animal ADFs interact with actin and this activity is regulated by phosphorylation and binding to the potent second messenger PIP2 (Yonezawa et al., 1990; Morgan et al., 1993). It remains to be determined if this is also the case with TaADF. Given the major roles played by phosphorylation and phosphoinositides in signaling events, it is possible that the TaADF may be involved in LT signaling in plants. Detailed experiments are required to confirm this hypothesis.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Winter and spring wheat (Triticum aestivum; hexaploid AABBDD genome) seeds were germinated in water-saturated vermiculite for 7 d at 25°/20°C (day/night) with a 15-h photoperiod under an irradiance of 250 μmol m−2 s−1 and a relative humidity of 75%. At the end of this period plants were maintained under the same conditions (controls, NA) or exposed to CA conditions and other stresses as described previously (Danyluk et al., 1996) and in the figure legends. For the substitution experiments, 21 wheat lines were used (hexaploid wheat is composed of three genomes, each of which comprise seven chromosome pairs), in which one pair of chromosomes from winter wheat Cheyenne was substituted for the homologous pair in the Chinese Spring background (Limin et al., 1997).
Protein Purification and Antibody Production
The pTaADF cDNA clone was isolated previously from a Lambda Zap II library constructed from CA winter wheat (cv Norstar; Danyluk et al., 1996). The TaADF protein was produced in Escherichia coli as an N-terminal His-tagged fusion using the pTrc-His vector (Invitrogen, Carlsbad, CA). The recombinant proteins were purified on His-Bind resin (Novagen, Madison, WI) after a 6-h induction with 1 mm IPTG according to standard procedures (Invitrogen). For antibody production, the major 27-kD protein band was further purified by preparative SDS-PAGE and electroelution.
Plant Protein Extraction and Western Blotting
Total soluble proteins from aerial tissues were extracted in 3 volumes of Tris HCl buffer, 100 mm, pH 9.6; phenylmethylsulfonyl fluoride, 1 mm) and the extracts were cleared by centrifugation at 10,000g for 15 min. Protein concentration was determined and western analyses were performed as described (Vazquez-Tello et al., 1998), using a 1:10,000 dilution of the TaADF antiserum and a 1:25,000 dilution of the horseradish peroxidase-coupled secondary antibody (Jackson Immunoresearch Laboratories, West Grove, PA). Reactive proteins were detected using the enhanced chemiluminescence detection kit (Amersham, Buckinghamshire, UK).
ADF Activity Assays
Yeast actin was purified by elution from a DNAseI column, as described (Goode et al., 1999) with the following modification: instead of the NH4Cl precipitation, the formamide eluate was dialyzed overnight against three changes of G-buffer (5 mm Tris-HCl, pH 7.4, 0.2 mm ATP, 0.2 mm dithiothreitol, and 0.2 mm CaCl2) and then concentrated in Centriprep 10 devices (Amicon, Beverly, MA).
For cosedimentation assays, actin was diluted to 9 μm in G-buffer and both the actin and TaADF were precleared in a TLA-100 rotor (Beckman Instruments, Fullerton, CA) at 90,000 rpm for 20 min. Initiation mix (2 m KCl, 40 mm MgCl2, and 5 mm ATP) was added to the actin supernatant and the actin was allowed to polymerize for 1 h at room temperature. TaADF was then added to varying concentrations in reactions containing 3 μm actin. After 30 min at room temperature, the reactions were spun as above and equivalent proportions of supernatant and pellet samples were run on 15% (w/v) SDS-PAGE gels. The nucleotide exchange and F-actin depolymerization assays were performed essentially as described (Lappalainen et al., 1997).
Phosphorylation Assays
Total proteins (30 μg) from control plants were fractionated on a 9% (w/v) SDS-PAGE gel and renatured in situ as described (Usami et al., 1995). Pre-stained markers (Bio-Rad) were used to cut gel slices corresponding to molecular mass intervals. Each slice was ground in 100 μL kinase assay buffer {20 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid], pH 7.2, 10 mm MgCl2, 2 mm MnCl2, and 20 μm ATP}. The kinase reaction was initiated by the addition of 10 μCi [γ-32P]ATP and 3 μg purified TaADF, and allowed to proceed for 2 h at 25°C. An aliquot (10 μL) of each reaction was analyzed by SDS-PAGE and visualized by autoradiography. For in-gel kinase assay, total proteins (20 μg) from control and CA plants were fractionated on a SDS-PAGE gel containing 0.1 mg/mL of TaADF. Renaturable protein kinase activity (Usami et al., 1995) was assayed by incubating the gel for 1 h in kinase buffer containing [γ-32P]ATP, and phosphorylation of TaADF was detected by autoradiography.
ACKNOWLEDGMENTS
We would like to thank Dr. Brian Fowler (University of Saskatchewan, Saskatoon) for providing some of the plant material. We also thank Dr. David G. Drubin (University of California, Berkeley) and Dr. Rajinder S.S. Dhindsa (McGill University, Montreal) for use of their laboratory facilities.
Footnotes
This work was supported by research grants from the Natural Sciences and Engineering Research Council of Canada and Fonds pour la Formation de Chercheurs et l'Aide à la Recherche (to F.S.).
LITERATURE CITED
- Aderem A. Signal transduction and the actin cytoskeleton: the role of MARCKS and profilin. Trends Biochem Sci. 1992;17:438–443. doi: 10.1016/0968-0004(92)90016-3. [DOI] [PubMed] [Google Scholar]
- Aizawa H, Sutoh K, Tsubuki S, Kawashima S, Ishii A, Yahara I. Identification, characterization, and intracellular distribution of cofilin in Dictyostelium discoideum. J Biol Chem. 1995;278:10923–10932. doi: 10.1074/jbc.270.18.10923. [DOI] [PubMed] [Google Scholar]
- Aizawa H, Sutoh K, Yahara I. Overexpression of cofilin stimulates bundling of actin filaments, membrane ruffling, and cell movement in Dictyostelium. J Cell Biol. 1996;132:335–344. doi: 10.1083/jcb.132.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aon MA, Cortassa S, Gomez Casati DF, Iglesias AA. Effects of stress on cellular infrastructure and metabolic organization in plant cells. Int Rev Cytol. 1999;194:239–273. doi: 10.1016/s0074-7696(08)62398-0. [DOI] [PubMed] [Google Scholar]
- Carlier MF, Laurent V, Santolini J, Melki R, Didry D, Xia GX, Hong Y, Chua NH, Pantaloni D. Actin depolymerizing factor (ADF/Cofilin) enhances the rate of filament turnover: implication in actin-based motility. J Cell Biol. 1997;136:1307–1323. doi: 10.1083/jcb.136.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danyluk J, Carpentier E, Sarhan F. Identification and characterization of a low temperature regulated gene encoding an actin-binding protein from wheat. FEBS Lett. 1996;389:324–327. doi: 10.1016/0014-5793(96)00599-6. [DOI] [PubMed] [Google Scholar]
- Danyluk J, Perron A, Houde M, Limin A, Fowler B, Benhamou N, Sarhan F. Accumulation of an acidic dehydrin in the vicinity of the plasma membrane during CA of wheat. Plant Cell. 1998;10:623–638. doi: 10.1105/tpc.10.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson MM, Haslam RJ. Dephosphorylation of cofilin in stimulated platelets: role for a GTP-binding protein and Ca2+ Biochem J. 1994;301:41–47. doi: 10.1042/bj3010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drøbak BK. Plant phosphoinositides and intracellular signaling. Plant Physiol. 1993;102:705–709. doi: 10.1104/pp.102.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn MA, Morris A, Jack PL, Hughes MA. A low temperature-responsive translation elongation factor 1α from barley (Hordeum vulgare L.) Plant Mol Biol. 1993;23:221–225. doi: 10.1007/BF00021434. [DOI] [PubMed] [Google Scholar]
- Fowler BD, Chauvin LP, Limin AE, Sarhan F. The regulatory role of vernalization in the expression of low-temperature induced genes in wheat and rye. Theor Appl Genet. 1996;93:554–559. doi: 10.1007/BF00417947. [DOI] [PubMed] [Google Scholar]
- Goode BL, Wong JJ, Butty AC, Peter M, McCormack AL, Yates JR, Drubin DG, Barnes G. Coronin promotes the rapid assembly and cross-linking of actin filaments and may link the actin and microtubule cytoskeletons in yeast. J Cell Biol. 1999;144:83–98. doi: 10.1083/jcb.144.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus KC, Bonaccorsi S, Williams E, Verni F, Gatti M, Golberg ML. Mutations in twinstar, a Drosophila gene encoding a cofilin/ADF homologue, result in defects in centrosome migration and cytokinesis. J Cell Biol. 1995;131:1243–1259. doi: 10.1083/jcb.131.5.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins M, Pope B, Maciver SK, Weeds AG. Human actin depolymerizing factor mediates a pH-sensitive destruction of actin filaments. Biochemistry. 1993;32:9985–9993. doi: 10.1021/bi00089a014. [DOI] [PubMed] [Google Scholar]
- Hughes MA, Dunn MA. The molecular biology of plant acclimation to low temperature. J Exp Bot. 1996;47:291–305. [Google Scholar]
- Jiang CJ, Weeds AG, Khan S, Hussey PJ. F-actin and G-actin binding are uncoupled by mutation of conserved tyrosine residues in maize actin depolymerizing factor (ZmADF) Proc Natl Acad Sci USA. 1997;94:9973–9978. doi: 10.1073/pnas.94.18.9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonak C, Kiegerl S, Ligterink W, Barker PJ, Huskisson NS, Hirt H. Stress signaling in plants: a mitogen-activated protein kinase pathway is activated by cold and drought. Proc Natl Acad Sci USA. 1996;96:11274–11279. doi: 10.1073/pnas.93.20.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori T, Hayakawa T, Suzuki M, Titani K. Identification of two 17-kDa rat parotid gland phosphoproteins, subjects for dephosphorylation upon beta-adrenergic stimulation, as destrin- and cofilin-like proteins. J Biol Chem. 1995;270:8061–8067. doi: 10.1074/jbc.270.14.8061. [DOI] [PubMed] [Google Scholar]
- Kerr GP, Carter JV. Relationship between freezing tolerance of root-tip cells and cold stability of microtubules in rye (Secale cereale L. cv Puma) Plant Physiol. 1990;93:77–82. doi: 10.1104/pp.93.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielbassa K, Müller HJ, Meyer HE, Marks F, Gfchwedt M. Protein kinase Cd-specific phosphorylation of the elongation factor eEF-1α and an eEF-1α peptide at threonine 431. J Biol Chem. 1995;270:6156–6162. doi: 10.1074/jbc.270.11.6156. [DOI] [PubMed] [Google Scholar]
- Lappalainen P, Fedorov EV, Fedorov AA, Almo SC, Drubin DG. Essential functions and actin-binding surfaces of yeast cofilin revealed by systematic mutagenesis. EMBO J. 1997;16:5520–5530. doi: 10.1093/emboj/16.18.5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappaleinen P, Kessels MM, Cope MJTV, Drubin D. The ADF homology (ADF-H) domain: a highly exploited actin-binding module. Mol Biol Cell. 1998;9:1951–1959. doi: 10.1091/mbc.9.8.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limin AE, Danyluk J, Chauvin LP, Fowler DB, Sarhan F. Chromosome mapping of low-temperature induced Wcs120 family genes and regulation of cold-tolerance expression in wheat. Mol Gen Genet. 1997;253:720–727. doi: 10.1007/s004380050376. [DOI] [PubMed] [Google Scholar]
- Lopez I, Anthony RG, Maciver SK, Jiang CJ, Khan S, Weeds A, Hussey PJ. Pollen specific expression of maize genes encoding actin depolymerizing factor-like proteins. Proc Natl Acad Sci USA. 1996;93:7415–7420. doi: 10.1073/pnas.93.14.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGough A, Chiu W. ADF/cofilin weakens lateral contacts in the actin filament. J Mol Biol. 1999;291:513–519. doi: 10.1006/jmbi.1999.2968. [DOI] [PubMed] [Google Scholar]
- McKim KM, Matheson C, Marra MA, Wakarchuk MF, Baillie DL. The Caenorhabditis elegans unc-60 gene encodes proteins homologous to a family of actin binding proteins. Mol Gen Genet. 1994;242:346–357. doi: 10.1007/BF00280425. [DOI] [PubMed] [Google Scholar]
- Monroy AF, Labbé E, Dhindsa RS. Low temperature perception in plants: effect of cold on protein phosphorylation in cell-free extracts. FEBS Lett. 1997;410:206–209. doi: 10.1016/s0014-5793(97)00589-9. [DOI] [PubMed] [Google Scholar]
- Moon AL, Janmey PA, Louie KA, Drubin DG. Cofilin is an essential component of the yeast cortical cytoskeleton. J Cell Biol. 1993;120:421–435. doi: 10.1083/jcb.120.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan TE, Lockerbie RO, Minamide LS, Browning MD, Bamburg JR. Isolation and characterization of a regulated form of actin depolymerizing factor. J Cell Biol. 1993;122:623–633. doi: 10.1083/jcb.122.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorart T, Irzykowski W, Grygorowicz WJ. Isolation and expression of novel cold-induced genes in potato (Solanum sogorandinum) Plant Sci. 1997;124:69–78. [Google Scholar]
- Saito T, Lamy F, Roger PP, Lecoq R, Dumont JE. Characterization and identification as cofilin and destrin of two thyrotropin- and phorbol ester- regulated phosphoproteins in thyroid cells. Exp Cell Res. 1994;212:49–61. doi: 10.1006/excr.1994.1117. [DOI] [PubMed] [Google Scholar]
- Samstag Y, Eckerskorn C, Wesselborg S, Henning S, Wallich R, Meuer SC. Costimulatory signal for human T-cell activation induces nuclear translocation of Pp19/cofilin. Proc Natl Acad Sci USA. 1994;91:4494–4498. doi: 10.1073/pnas.91.10.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarhan F, Danyluk J. Engineering cold-tolerant crops: throwing the master switch. Trends Plant Sci. 1998;3:289–290. [Google Scholar]
- Sarhan F, Ouellet F, Vazquez-Tello A. The wcs120 gene family in wheat: a useful model to study the molecular genetics of cold acclimation in cereals. Physiol Plant. 1997;101:439–445. [Google Scholar]
- Smolenska-Sym G, Kacperska A. Inositol 1,4,5-triphosphate formation in leaves of winter oilseed rape plants in response to freezing, tissue water potential and abscisic acid. Physiol Plant. 1996;96:692–698. [Google Scholar]
- Staiger CJ, Gibbon BC, Kovar DR, Zonia LE. Profilin and actin-depolymerizing factor: modulators of actin organization in plants. Trends Plant Sci. 1997;2:275–281. [Google Scholar]
- Thomashow MF. Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:571–599. doi: 10.1146/annurev.arplant.50.1.571. [DOI] [PubMed] [Google Scholar]
- Usami S, Banno H, Ito Y, Hishihama R, Machida Y. Cutting activates a 46-kilodalton protein kinase in plant. Proc Natl Acad Sci USA. 1995;92:8660–8664. doi: 10.1073/pnas.92.19.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Tello A, Ouellet F, Sarhan F. Low-temperature-stimulated phosphorylation regulates the binding of nuclear factors to the promoter of wcs120, a wheat cold-specific gene. Mol Gen Genet. 1998;257:157–166. doi: 10.1007/s004380050635. [DOI] [PubMed] [Google Scholar]
- Yonezawa N, Nishida E, Iida K, Yahara I, Sakai H. Inhibition of the interactions of cofilin, destrin, and deoxyribonuclease I with actin by phosphoinositides. J Biol Chem. 1990;265:8382–8386. [PubMed] [Google Scholar]
- Yonezawa N, Nishida E, Sakai H. pH control of actin polymerization by cofilin. J Biol Chem. 1985;260:14410–14412. [PubMed] [Google Scholar]
- Yoshida S. Low temperature-induced cytoplasmic acidosis in cultured mung bean (Vigna radiata L. Wilczek) cells. Plant Physiol. 1994;104:1131–1138. doi: 10.1104/pp.104.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]