Abstract
Drug-induced liver damage is a frequently encountered clinical table caused by many drugs. Cetirizine is a widely preferred and prescribed antihistaminic agent for allergic disorders due to its non-sedative properties. In view of the literature, we present four cases of hepatotoxicity due to cetirizine use. We conclude that in patients with high levels of liver enzymes of unknown origin, cetirizine as well as other hepatotoxic drugs should be reconsidered.
Keywords: cetirizine, hepatotoxicity
Introduction
Cetirizine is a cholinergic, nonsedating, second-generation histamine-1 receptor-blocking agent widely used in allergic disorders. Its elimination has been reported to be slow in the elderly and in people with liver disease [1]. Severe liver failure and cholestatic and hypersensitivity hepatitis induced by antihistamines such as cyproheptadine, loratadine and terfenadin have been reported previously [2,3]. Increases of alanine aminotransferase (ALT) [2], hepatitis [4–8], and cholestasis [9–11] due to cetirizine use have been also reported. Here we report four hepatotoxicity cases resulting from cetirizine use and review the literature.
Case presentation
Case 1: A 46-year-old male patient was admitted with complaints of fatigue over the past seven days. His medical history revealed that he had been diagnosed with contact dermatitis and that 10 mg/day cetirizine had been started by a dermatology specialist three days previously. He had no history of alcohol use nor any other drug use or chronic disease. His physical examination was normal. Biochemical tests revealed total cholesterol 228 mg/dL (normal range 140–220 mg/dL) and triglycerides 163 mg/dL (normal range 40–160 mg/dL); total bilirubin, total protein, albumin, globulin, lactate dehydrogenase (LDH), amylase and fasting blood glucose levels were within normal limits. Leucocyte, erythrocyte and thrombocyte counts were normal. HBsAg, anti-HCV, anti-HAV IgM, Anti-HEV, CMV IgM, and EBV-VCA were negative; his anti-HB level was positive. IgG, IgM and IgA levels were within normal ranges, while IgE was high (303 IU; normal range 0–100 IU). Antinuclear antibody (ANA), antimitochondrial antibody (AMA) and anti-smooth muscle antibody (ASMA) were negative. Abdominal ultrasound examination of the liver was normal. In the peripheral blood smear, 66% neutrophils, 28% lymphocytes, 4% eosinophils and 2% monocytes were detected. Serum iron level, iron-binding capacity, ferritin, ceruloplasmin and thyroid function tests (TFTs) were within normal limits. Liver biopsy was planned but was not approved by the patient. Cetirizine was discontinued immediately. He was advised not to take any medicine and to avoid any intake harmful to the liver. In the follow-up two weeks later, ALT was 45 U/L (normal range 13–40 U/L), and gamma-glutamyl transferase (GGT) was 264 U/L (normal range 9–50 U/L). In the second follow-up, GGT was 95 U/L (normal range 9–50 U/L), and ALT, aspartate aminotransferase (AST) and other tests were within normal ranges two months later.
Case 2: A 21 year-old female patient was admitted with complaints of generalized jaundice and fatigue. She had been started on cetirizine five days previously for complaints of itching. No other drug or alcohol use was involved. Her physical examination was normal except for jaundice. Total bilirubin was 6.3 mg/dL, and direct bilirubin was 2.4 mg/dL, while total cholesterol, triglycerides, total protein, albumin and whole blood counts were within normal range. Iron parameters and TFT were normal. HBsAg, anti-HBc IgM, anti-HCV, anti-HBs, anti-HAV IgM, CMV IgM, EBV-VCA and Salmonella and Brucella agglutination levels were negative. ANA, AMA and ASMA were negative. Abdominal ultrasonography revealed mild hepatomegaly and hepatosteatosis grade I. Liver biopsy was planned but the patient did not approve. Cetirizine-induced toxic hepatitis was considered, and the drug was stopped. In the follow-up, ALT was 139 U/L, AST 53 U/L, alkaline phosphatase (ALP) 71 U/L (normal range 40–140 U/L) and GGT 20 U/L, while bilirubins returned to normal two weeks later. All biochemical tests returned to their normal ranges a month later.
Case 3: A 66-year-old female patient was admitted with fatigue and jaundice. She had been started on cetirizine for the complaint of itching a week ago. She had no alcohol or any other drug use. Her physical examination revealed jaundice of the skin and sclera and palpable liver under her ribcage at the midclavicular line. Total, direct and indirect bilirubins were 9.36, 6.39 and 2.97 mg/dL, respectively, and eosinophils were increased in the peripheral smear. Iron parameters, TFT and other biochemical values were normal. ANA, AMA and ASMA were negative. HBsAg, anti-HBc, anti-HCV, anti-HBs, anti-HAV IgM, CMV IgM, EBV-VCA and Salmonella and Brucella agglutination tests were negative. Abdominal ultrasonography revealed hepatomegaly, and magnetic resonance cholangiopancreatography (MRCP) was normal. Liver biopsy revealed enlarged cells with macrosteatosis, cholestasis and mixed inflammatory infiltration with increased eosinophils in portal areas and parenchyma (Figure 1). Cetirizine was immediately discontinued. In the follow-up, GGT was 90 U/L, while AST, ALT and other biochemical tests were all normal three weeks later.
Figure 1.
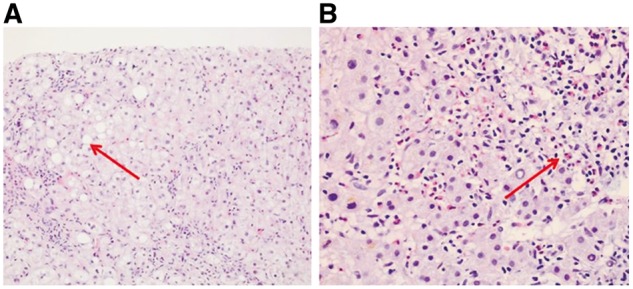
Liver biopsy of Case 3 shows macrosteatosis places (A, hematoxylin-eosin staining, x100) and mixed inflammatory infiltration including eosinophils; (B, hematoxylin-eosin, x200) in portal areas and parenchyma.
Case 4: A 40-year-old female patient, who had been prescribed cetirizine, 10 mg/day, four days previously by a dermatologist because of generalized itching complaint, was admitted to our department with the complaint of fatigue. She did not have a history of any other drug or alcohol use. Her physical examination, iron parameters, TFT and other laboratory test results were normal except for AST, ALT, ALP and GGT. HBsAg, anti-HBs, anti-HBc IgM, anti-HAV IgM, anti-HCV, CMV IgM, EBV-VCA and Salmonella and Brucella agglutination tests were negative. Abdominal ultrasonography was normal. Cetirizine was discontinued. Liver biopsy was not performed due to lack of the patient’s approval. In follow up as month later, her biochemical values had returned to normal.
Demographic features and laboratory test results of these four cases at their admission are presented in Table 1.
Table 1.
Patient characteristics and laboratory test results
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Age (years) | 46 | 21 | 66 | 40 |
| Sex | Male | Female | Female | Female |
| Duration of cetirizine use (days) | 3 | 5 | 7 | 4 |
| IgE level (IU) | 303 | 30 | 17 | 36 |
| Eosinophil count | 420 | 100 | 1000 | 100 |
| AST (NR: 10–40 U/L) | 419 | 188 | 685 | 47 |
| ALT (NR: 13–40 U/L) | 461 | 443 | 667 | 59 |
| ALP (NR: 40–140 U/L) | 153 | 404 | 111 | 843 |
| GGT (NR: 9–50 U/L) | 545 | 44 | 144 | 411 |
| R ratio | 10,57 | 3,84 | 21,1 | 0,24 |
| Hepatic damage type | Hepatocellular | Mixed | Hepatocellular | Cholestatic |
| RUCAM score | 10 | 10 | 11 | 9 |
ALP: alkaline phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; GGT: gamma-glutamyl transferase; NR: normal range; R ratio = (ALT/ upper limit of normal range) / (ALP/ upper limit of normal range); RUCAM: Roussel Uclaf Causality Assessment Method
Discussion
Drug-induced liver damage is a frequently encountered clinical table and is encountered as side effects of many drugs. Asymptomatic patients may be admitted with elevated liver function tests results as well as with hepatitis findings. There are three types of hepatotoxic drug reaction based on biochemical parameters: hepatocellular damage, cholestatis and mixed type. Classification is based on the following formula using ALP and ALT levels: R ratio = (ALT/ upper limit of normal range) / (ALP/ upper limit of normal range). (1) If the R ratio is ≥ 5 or ALT exceeds twice the upper limit, the reaction is classified as hepatocellular toxicity; (2) if the R ratio is ≤ 2 and ALT is over the normal limit, the reaction is designated as cholestatic toxicity; and (3) if the R ratio is between 2 and 5, the reaction is mixed type [12]. Based on this classification, Case 1 and Case 3 were evaluated as hepatocellular, Case 2 was mixed, and Case 4 was the cholestatic type.
Hepatotoxicity can also be categorized as immune and non-immune. Hepatotoxicity involving the immune system is also referred to as allergic reaction or hypersensitivity [13]. The presence of eosinophils in a peripheral blood smear, increased eosinophil count and elevated blood IgE level in Case 1 suggested hypersensitivity.
Since our patients had no history of alcohol use, blood transfusion, tooth extraction, any surgical operation, close contact with hepatitis patients, history of a systemic disease or any other drug use, cetirizine was considered to the likely cause for the increased values of the liver tests (AST, ALT, ALP, GGT and total bilirubin). Any other sources causes that may cause hepatotoxicity such as viral causes, autoimmune causes, gallstones, hemochromatosis and endocrinological causes (hypothyroidism and hyperthyroidism) hepatitis were ruled out through viral serology, ultrasonography, ANA, AMA, ASMA, ferritin, iron, serum iron-binding capacity, free T3, free T4 and TSH examinations. If available, measurement of serum cetirizine level, or challenge test could have supported the strenght of our diagnosis. In diagnosing drug–induced hepatotoxicity, measurement the levels of liver-kidney microsomal antibody (anti-LKM2), and the antibody against cytochrome P450 may be useful [5]. In our cases, re-use of the drug did not encountered. Tests to measure the serum levels of liver-kidney microsomal antibody (anti-LKM2), and the antibody against cytochrome P450 were not available. Liver biopsy was approved and performed in only one patient.
A markedly high GGT level immediately suggested a toxic cause [2,4]. Drug-induced hepatitis has been reported in many studies [14]. High levels of liver enzymes and possible hepatitis development have been shown in various studies [2,3]. Although it has been suggested that elevated liver enzyme levels could sometimes result from cetirizine use [1,2], hepatitis has been reported in only five case reports in the literature [4–8]. In two of the four cases, cetirizine was used for allergic dermatitis, but it was discontinued due to elevated levels of liver enzymes. When the drug was later accidentally re-administered to the same patient , liver enzymes were again reported to be increased [4,5]. It is interesting that in our four cases, all enzyme levels except for GGT were within normal limits at the follow-up two months after cetirizine had been stopped. The literature reveals liver function tests returning to normal limits within three months in terfenadin and cyproheptadine-induced hepatitis cases [15,16]. We conclude that, in patients with high levels of liver enzymes and hepatitis of unknown cause, cetirizine as well as other hepatotoxic drugs should be suspected.
Conflict of interest statement: none declared.
References
- 1. Campoli-Richards D, Buckley M, Fitton A.. Cetirizine. A a review of its pharmacological properties and clinical potential in allergic rhinitis, pollen-induced asthma, and chronic urticaria. Drugs 1990;40:762–81. [DOI] [PubMed] [Google Scholar]
- 2. Arendt C, Bernheim J.. Double-blind comparison of maintenance treatment of chronic idiopathic urticaria by cetirizine and terfenadine. Curr Ther Res 1989;46:724–34. [Google Scholar]
- 3. Larrey D, Palazzo L, Benhamou JP.. Terfenadine and hepatitis. Ann Intern Med 1985;103:634. [DOI] [PubMed] [Google Scholar]
- 4. Bera F, Sipruhis JP, Jonville-Bera AP, et al. Cytolytic hepatic involvement after administration of cetirizine (Zyrtec (in French). Gastroenterol Clin Biol 1993;17:770–1. [PubMed] [Google Scholar]
- 5. Pompili M, Basso M Grieco A. et al. Recurrent acute hepatitis associated with use of cetirizine. Ann Pharmacother 2004;38:1844–7. [DOI] [PubMed] [Google Scholar]
- 6. Sanchez-Lombrana JL, Alvarez RP, Saez LR, et al. Acute hepatitis associated with cetirizine intake. J Clin Gastroenterol 2002;34:493–5. [DOI] [PubMed] [Google Scholar]
- 7. Watanabe M, Kohge N, Kaji T.. Severe hepatitis in a patient taking cetirizine. Ann Intern Med 2001;135:142–3. [DOI] [PubMed] [Google Scholar]
- 8. Jurawan R, Smith A.. Severe hepatitis in a primary sclerosing cholangitis patient receiving recent cetirizine therapy. N Z Med J 2010;123:106–7. [PubMed] [Google Scholar]
- 9. Díaz-Sánchez A, Marín-Jiménez I, Aldeguer M.. Benign recurrent intrahepatic cholestasis simulating cetirizine-induced toxic hepatitis (in Spanish). Gastroenterol Hepatol 2010;33:68–9. [DOI] [PubMed] [Google Scholar]
- 10. Rodríguez-Gómez SJ, Zamora-Martínez T, Bailador-Andrés C, et al. Severe intrahepatic cholestasis associated with cetirizine (in Spanish). Gastroenterol Hepatol 2009;32:383–4. [DOI] [PubMed] [Google Scholar]
- 11. Fong DG, Angulo P, Burgart LJ. et al. Cetirizine-induce cholestasis. J Clin Gastroenterol 2000;31:250–3. [DOI] [PubMed] [Google Scholar]
- 12. Danan G, Benichou C.. Causality assessment of adverse reactions to drugs-I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol 1993;46:1323–30. [DOI] [PubMed] [Google Scholar]
- 13. Leise MD, Poterucha JJ, Talwalkar JA.. Drug-induced liver injury. Mayo Clin Proc 2014;89:95–106 [DOI] [PubMed] [Google Scholar]
- 14. Chitturi S, Teoh NC, Farrell GC.. Hepatic drug metabolism and liver disease caused by drugs In: Feldman M, Friedman LS, Brandt LJ (eds). Gastrointestinal and Liver Disease, volum 2, 10th ed.Philadelphia: Saunders, 2016, 1442–77. [Google Scholar]
- 15. Sahai A, Villeneuve JP.. Terfanadine–induced cholestatic hepatitis. Lancet 1996;348:552–3. [DOI] [PubMed] [Google Scholar]
- 16. Freneaux E, D Larrey, Berson A, et al. Hepatitis caused by cyproheptadine (Periactine). A case and review of the literature (in French). Gastroenterol Clin Biol 1988;12:573–5. [PubMed] [Google Scholar]


