Abstract
Background
Our recent study showed the efficacy and safety of vedolizumab in the treatment of chronic antibiotic-refractory pouchitis. However, there are no published studies on its efficacy and safety in Crohn’s disease (CD) of the pouch. The aim of this study was to assess the efficacy and safety of vedolizumab in those patients.
Methods
This case series included all eligible patients with CD of the pouch from our prospectively maintained, IRB-approved Pouchitis Registry from 2015 to 2017. Disease activity in pouch patients can be monitored using the modified Pouchitis Disease Activity Index (mPDAI). mPDAI is the 18-point pouchitis disease activity index consisting of three principal component scores: symptom (range, 0–6 points), endoscopy, (range 0–6 points), and histology (range, 2–6 points). Pre- and post- treatment (minimum 6 months) pouchoscopy and clinical visits were used to calculate mPDAI.
Results
A total of 12 patients were included in this study, who had restorative proctocolectomy with ileal pouch anal anastomosis for medically refractory ulcerative colitis (UC). The mean age at the time of pre-colectomy diagnosis of UC was 25.0 ± 11.5 years. The mean current age was 41.0 ± 12.1 years, nine (75.0%) were female, three (25.0%) had smoked and eight (66.7%) had used anti-tumor necrosis factor agents prior to vedolizumab use. The mean duration of vedolizumab use was 1.0 ± 6.4 years. There was a significant reduction in mPDAI symptom subscores after vedolizumab therapy (3.50 ± 1.93 vs 5.08 ± 0.79, P = 0.015). The pre- and post-treatment mean endoscopy subscores were 1.25 ± 1.36 and 0.91 ± 1.50 in the afferent limb (P = 0.583); 2.58 ± 1.68 and 2.27 ± 2.05 (P = 0.701) in the pouch body; and 2.67 ± 1.93 and 2.09 ± 2.12 (P = 0.511) in the cuff, respectively. None of the patients experienced side effects throughout the vedolizumab therapy.
Conclusion
The findings of our study suggests that vedolizumab appears to be effective and safe in reducing the symptoms in patients with CD of the pouch.
Keywords: Anti-tumor necrosis factor agent, Crohn’s disease, ileal pouch, restorative proctocolectomy, vedolizumab
Introduction
Restorative proctocolectomy with ileal pouch anal anastomosis (IPAA) is the treatment of choice for medically refractory ulcerative colitis (UC), colitis-associated dysplasia and familial adenomatous polyposis (FAP) [1]. Some patients who undergo IPAA for UC may develop early-onset Crohn’s disease (CD) of the pouch or de novo CD of the pouch (months to years after IPAA) [2]. A collaborative endoscopy evaluation, histology, radiographic imaging and examination under anesthesia are often required for an accurate diagnosis, disease classification, management and prognosis [3]. Documented frequencies of CD of the pouch in patients who underwent IPAA for UC ranged from 2.7% to 13% [4]. Recurrent CD in the ileal pouch is a common cause for pouch failure, requiring pouch excision or permanent diversion [5–8].
Crohn's disease of the pouch has three phenotypes: inflammatory, fibrostenotic and fistulizing [4]. Each phenotype is associated with different risk factors and clinical presentations. The etiology and pathogenesis of CD of the pouch are not clear. Patients should be strongly encouraged to avoid smoking cigarettes and non-steroidal anti-inflammatory drug (NSAID) use. Medical treatment plays an important role in inflammatory and fistulizing CD of the pouch. Reported pharmaceutical agents for CD of the pouch include budesonide, infliximab and adalimumab [9,10].
Vedolizumab is a gut-specific monoclonal antibody, which acts on α4β7 isomer of integrin and blocks gut lymphocyte trafficking [11], which was approved by the Food and Drug Administration of US for the induction and maintenance of remission in moderate to severe CD and UC. Clinically we found that vedolizumab appeared to be safe and effective in the treatment of chronic antibiotic-refractory pouchitis. However, the efficacy and safety of vedolizumab in CD of the pouch have not been systemically evaluated. The aim of the study was to evaluate the efficacy and safety of vedolizumab in CD of the pouch by measuring the modified Pouchitis Disease Activity Index (mPDAI) symptom and endoscopy subscores pre and post treatment.
Patients and methods
This case series study included all eligible patients with CD of the pouch from our prospectively maintained, Institutional Review Board (IRB)-approved Pouchitis Registry from June 2015 until June 2017. Demographics, comorbidities and pouch complications were extracted from a prospectively maintained pouch database. Office visit notes, follow-up notes, admission records, operation reports and other medical records were carefully reviewed.
Inclusion and exclusion criteria
Inclusion criteria were patients with (i) an age older than 18 years, (ii) pelvic ileal pouch, (iii) diagnosis of CD of the pouch and (iv) being treated or with a history of treatment with vedolizumab. They were excluded if they had underlying FAP or CD with a diverting ileostomy.
Clinical variables
A retrospective chart review was performed by one investigator (F.K.) to extract relevant data and demographic information, including age, gender, body mass index (BMI), smoking history, family history of inflammatory bowel disease (IBD), chronic medical issues, history of colon cancer and clinical symptoms including presence or absence of extra-intestinal manifestations. Pouch-related variables were also collected including indication of pouch surgery, duration of disease, type of pouch, history of pouchitis and past or current use of immunomodulators (azathioprine [AZA], 6-mercaptopurine [6-MP], methotrexate [MTX], anti-tumor necrosis factor [TNF] or anti-integrin agents). CD of the pouch was diagnosed by a combined assessment of endoscopic, histologic and radiographic features [12].
In addition, we recorded information regarding vedolizumab-related adverse events, exacerbation of extra-intestinal manifestations and pouch failure (defined by need for pouch revision or excision and permanent ileostomy). The diagnosis of CD of the pouch was made on the basis of a triad of compatible symptoms and endoscopic and histologic findings. Pre- and post-treatment (minimum 6 months) pouchoscopy and clinical visits were used to calculate mPDAI symptom (range 0–6) and endoscopy (range 0–6) subscores.
Outcome measurement
The primary outcomes were to assess the improvement or reduction in the mPDAI symptom and endoscopy subscores after 6 months of vedolizumab therapy for CD of the pouch. The mPDAI symptom index is the most commonly used diagnostic instrument. mPDAI is the 18-point pouchitis disease activity index consisting of three principal component scores: symptom (range, 0–6 points), endoscopy, (range 0–6 points), and histology (range, 2–6 points). Symptoms include stool frequency, rectal bleeding, fecal urgency/abdominal cramps, and fever. Endoscopic criteria include presence of edema, granularity, friability, loss of vascular pattern, mucus exudates and ulceration. Histology evaluation consists of polymorphic nuclear leukocyte infiltration and degree of ulceration. Pre- and post- treatment (minimum 6 months) pouchoscopy and clinical visits were used to calculate mPDAI. Endoscopy subscores are evaluated in the afferent limb, pouch body and cuff. The secondary outcome was adverse events related to the use of vedolizumab.
Statistical analysis
Data are presented as mean ± standard deviation, median (25th, 75th percentiles) or frequency (percentage). A Sign test was used to assess whether the difference between post- and pre-vedolizumab mPDAI scores was significantly different than 0. Univariable analysis was conducted to assess factors associated with changes in mPDAI. All analyses were performed using SAS (version 9.4, The SAS Institute, Cary, NC) and a P < 0.05 was considered statistically significant.
Results
A total of 12 patients who received vedolizumab for CD of the pouch were included in the analysis. Six (50.0%) had inflammatory phenotype of CD of the pouch and six (50.0%) had fibrostenotic phenotype of CD of the pouch. They were followed for treatment response and adverse events at 6 months post therapy.
Demographic and clinical variables
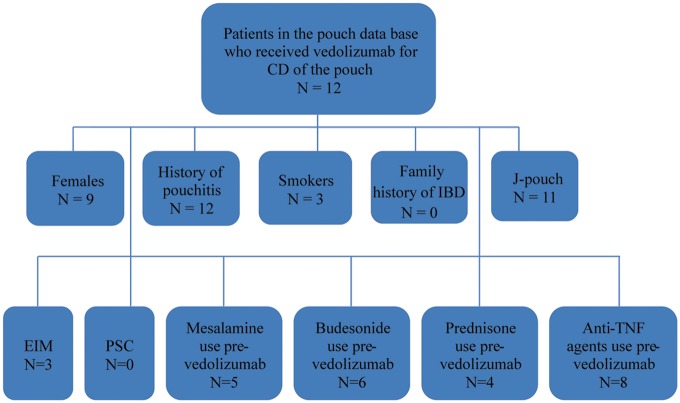
The mean age at the time of diagnosis of UC was 25.0 ± 11.5 years. The mean age of the studied population was 41.0 ±12.1 years. Out of 12 patients, 9 (75.0%) were female, 9 (75.0%) were Caucasian and 3 (25.0%) had a history of smoking. None of the patients had concurrent primary sclerosing cholangitis. None reported a family history of IBD or colon cancer. Three (25.0%) reported other extra-intestinal manifestations. A patient-selection flow diagram is shown in Figure 1.
Figure 1.
Flow diagram for patient selection. CD, Crohn’s disease; EIM, extra-intestinal manifestations; PSC, primary sclerosing cholangitis.
All studied patients had undergone restorative proctocolectomy with ileal pouch anal anastomosis with restorative IPAA due to medical refractory UC. Eleven out of 12 patients (91.7%) had J pouches. One (8.3%) patient underwent one-stage pouch surgery, seven (58.3%) had two-stage pouch surgery and four (33.4%) had three-stage pouch surgery. The median duration from the pouch construction to data sensor was 12.0 ± 9.7 years. The average duration of vedolizumab use was 12.2 ± 6.4 months. Patients were followed for treatment response and adverse events at 6 months post therapy. A summary of demographic and clinical characteristics of patients included in the study is shown in Table 1.
Table 1.
Demographic and clinical characteristics in patients with Crohn’s disease of the pouch
| Factor | Total (N =12) |
|---|---|
| Age at ulcerative diagnosis, years | 25.0 ± 11.5 |
| Mean age of patients, years | 41.0 ± 12.1 |
| Recent body mass index, kg/m2 | 23.7 ± 4.7 |
| Body mass index before vedolizumab use, kg/m2 | 23.2 ± 4.2 |
| Mean age of pouch duration, years | 12.0 ± 9.7 |
| Smoking, n (%) | 3.0 (25.0) |
| Excessive alcohol use, n (%) | 0 |
| Family history of colon cancer, n (%) | 0 |
| Family history of IBD, n (%) | 0 |
| Duration of vedolizumab use, months | 12.2 ± 6.4 |
| Phenotype of disease, n (%) | |
| Fibrostenotic | 6.0 (50.0) |
| Inflammatory | 6.0 (50.0) |
| Pouch type, n (%) | |
| J | 11.0 (91.7) |
| S | 1.0 (8.3) |
| Stage of pouch surgery, n (%) | |
| 1 | 1.0 (8.3) |
| 2 | 7.0 (58.3) |
| 3 | 4.0 (33.4) |
| Presence of extra-intestinal manifestations, n (%) | 3.0 (25.0) |
| Primary sclerosing cholangitis, n (%) | 0 |
| Mesalamine use pre vedolizumab, n (%) | 5.0 (41.7) |
| Concurrent vedolizumab and mesalamine use, n (%) | 3.0 (25.0) |
| Oral budesonide use pre vedolizumab, n (%) | 6.0 (50.0) |
| Concurrent vedolizumab and oral budesonide use, n (%) | 4.0 (33.3) |
| Prednisone use pre vedolizumab, n (%) | 4.0 (33.3) |
| Concurrent vedolizumab and prednisone use, n (%) | 2.0 (16.7) |
| Anti-TNF agent use pre vedolizumab, n (%) | 8.0 (66.7) |
IBD, inflammatory bowel disease; TNF, anti-tumor necrosis factor.
Concurrent medical therapy
Five patients (41.7%) used mesalamine, six (50.0%) took budesonide and four (33.3%) took prednisone prior to using vedolizumab. Concurrent medical therapy was also noted. Three patients (25.0%) took mesalamine, four (33.3%) used budesonide and two (16.7%) took prednisone while being treated with vedolizumab. Eight (66.7%) had used anti-TNF agents prior to vedolizumab use.
Efficacy and safety of vedolizumab
Patients on vedolizumab therapy were noted to have statistically significantly lower mPDAI symptom subscores post treatment using univariate analysis. Numerical improvement in endoscopy subscores was also noted with vedolizumab use. Eight (66.7%) patients demonstrated significant reduction in mPDAI symptom subscores before and after vedolizumab therapy (5.08 ± 0.79 vs 3.50 ± 1.93, P = 0.015). Pre- and post-vedolizumab therapy mPDAI symptom subscores for 12 patients included in this study are depicted in Figure 2.
Figure 2.
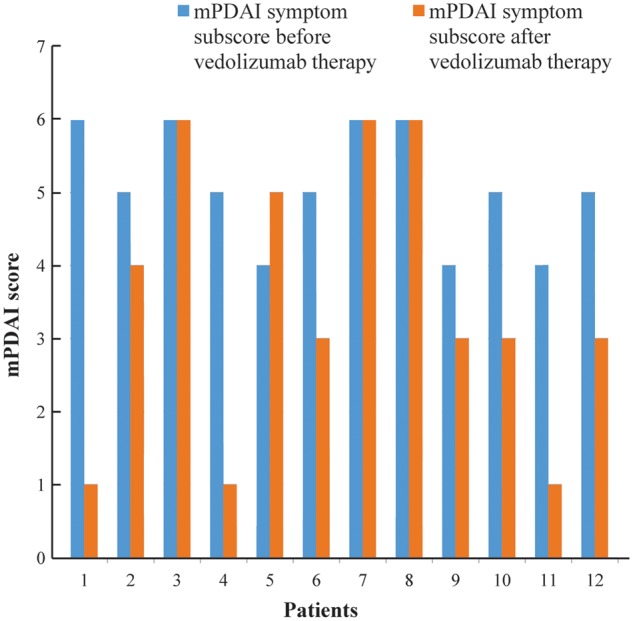
Modified Pouchitis Disease Activity Index (mPDAI) symptom subscores for 12 patients before and after vedolizumab therapy.
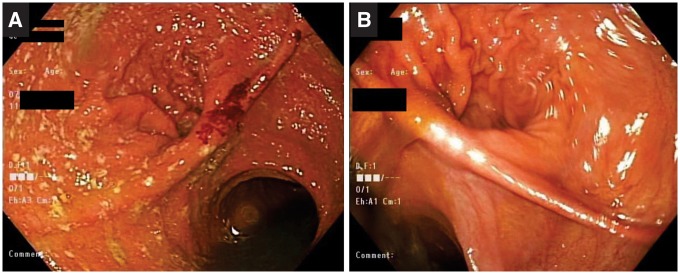
Ten (83.3%) patients showed significant improvement in mPDAI endoscopy subscores in the afferent limb, pouch body and cuff. The pre- and post-treatment mean endoscopy subscores were 1.25 ± 1.36 and 0.91 ± 1.50 in the afferent limb (P = 0.583); 2.58 ± 1.68 and 2.27 ± 2.05 (P = 0.701) in the pouch body; and 2.67 ± 1.93 and 2.09 ± 2.12 (P = 0.511) in the cuff, respectively (Table 2). None of the patients reported any side effects throughout the therapy. Pouchoscopy pictures before and after vedolizumab therapy are shown in Figure 3.
Table 2.
Comparison of modified Pouchitis Disease Activity Index (mPDAI) symptom and endoscopy subscores pre and post treatment with vedolizumab
| Pre treatment | Post treatment | P-value | |
|---|---|---|---|
| mPDAI symptom subscore | 5.08 ± 0.79 | 3.50 ± 1.93 | 0.015 |
| mPDAI endoscopy subscore in the afferent limb | 1.25 ± 1.36 | 0.91 ± 1.50 | 0.583 |
| mPDAI endoscopy subscore in the pouch body | 2.58 ± 1.68 | 2.27 ± 2.05 | 0.701 |
| mPDAI endoscopy subscore in the cuff | 2.67 ± 1.93 | 2.09 ± 2.12 | 0.511 |
Figure 3.
Pouchoscopy before (A) and after (B) vedolizumab therapy.
Discussion
Our study showed that vedolizumab appeared to be effective in the treatment of the majority of patients with CD of the pouch by significantly reduced mPDAI symptom subscores and numerically reduced endoscopy subscores in the afferent limb, pouch body and cuff. The agent was also well tolerated. Some of those patients had failed therapy with corticosteroids or anti-TNF biologics.
IPAA is a well-established and possibly curative surgical procedure for patients with UC. Some patients are subsequently found to develop CD in the ileal pouch. CD of the pouch is being increasingly recognized in IPAA patients. The exact etiology and pathogenesis of CD of the pouch remain to be determined. However, genetic and environmental factors may contribute. The mucosal inflammation of CD of the pouch can be assessed by mPDAI symptom and endoscopy subscores in the afferent limb, pouch body and cuff [13].
There are three phenotypes of CD of the pouch: inflammatory, fibrostenotic and fistulizing [4]. Clinical manifestations of CD of the pouch vary upon phenotypes, disease location and degree of inflammation. Radiographic findings include nodules, thickened folds, ulcers, cobblestoning, sacculations, strictures, sinus tracks, fistulas or other abnormalities involving the pouch body or distal ileum [14].
Reported risk factors for CD of the pouch include the presence of family history of CD, smoking, longer duration of pouch, pre-operative diagnosis of indeterminate colitis and seropositivity for anti-Saccharomyces cerevisiae-IgA antibody [15–18]. Another study showed that Ashkenazi Jewish ethnicity was associated with a higher risk for pouch complications, including CD of the pouch, suggesting a role of genetic predisposition [19].
The management of CD of the pouch has been challenging. Due to relapsing nature of the disease and various complications such as strictures, fistulas and abscesses, patients usually require aggressive medical management with various combination of antibiotics, corticosteroids, immunomodulators and anti-TNF [20]. Infliximab and adalimumab have been investigated for the treatment of CD of the pouch. A short-term follow-up study was conducted on 26 patients with an IPAA who received infliximab infusion. Findings showed that 16 out of 26 patients (62%) had a complete response, 6 (23%) had a partial response and 4 (15%) had no response [9]. Our group studied the efficacy and safety of adalimumab in treating CD of the pouch. Seven patients (41.2%) had a complete symptom response and six (35.3%) had a partial response at 4 weeks post therapy. Significant improvement was also noted with the PDAI endoscopy subscores at week 4 [10]. Despite aggressive medical therapy, approximately 10–48% of patients develop refractory CD in the ileal pouch requiring excision of the pouch with a permanent end ileostomy [5–8].
Vedolizumab has been used off-label for the treatment of chronic antibiotic-refractory pouchitis and has been shown to be beneficial with symptomatic and endoscopic improvement [21,22]. In a small case series of four patients, we showed improvement in symptoms and endoscopic appearance of pouch after 3 months of therapy with vedolizumab in patients who initially failed all other therapies including anti-TNF agents [23]. Our recent study on the efficacy and safety of vedolizumab in chronic antibiotic-refractory pouchitis demonstrated significant improvement in both endoscopic and total mPDAI scores after 3 months of therapy (submitted for publication).
To the best of our knowledge, this is the first study to illustrate the use of vedolizumab to treat CD of the pouch. This study also demonstrated that vedolizumab is safe to use in UC. There are no published data to date on its efficacy and safety in CD of the pouch. Vedolizumab treatment in CD of the pouch has not been previously investigated, largely due to a relatively small number of patients scattered in private and academic practices. Taking advantage of established Pouchitis Clinic, we were able to evaluate 12 patients and performed statistical analyses. The efficacy of vedolizumab was supported by mPDAI symptom subscore as well as endoscopy subscores.
Our study is unique, as it is the first in the literature to provide information on vedolizumab therapy in UC patients who have undergone IPAA and developed CD of the pouch. Vedolizumab therapy may be recommended as the next treatment option in patients with CD of the pouch who showed no improvement with steroids and other biological therapies such as anti-TNFs. Its use will be advantageous in the long term by reducing the risk for pouch failure.
There are limitations to our study. First, there is the possibility of referral bias, as all patients in the study were seen in the setting of a subspecialty Pouchitis Clinic in which patients with a spectrum of pouch disorders were diagnosed and managed. Second, we included patients who used vedolizumab from June 2015 until June 2017. The sample size was small and the study was not controlled, as CD of the pouch is not common in the general population and vedolizumab has not become its standard treatment to date. The findings of the current study will help to set the stage for future randomized–controlled trials.
In conclusion, our study demonstrated that vedolizumab appears to be effective in the treatment of CD of the pouch by significantly reducing mPDAI symptom subscores and numerically reducing endoscopy subscores in the afferent limb, pouch body and cuff. The agent appears to be well tolerated.
Conflict of interest statement: none declared.
Acknowledgements
F.K.—data gathering and entry; F.K., A.S. and X.-H.G.—manuscript preparation; J.P. and B.S.—concept, general supervision and manuscript revisions.
Funding
Dr. Bo Shen has received research grant from Takeda Pharmaceutical Company.
References
- 1. Fazio VW, Ziv Y, Church JM. et al. Ileal pouch-anal anastomosis: complications and function in 1005 patients. Ann Surg 1995;222:120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu H, Shen B.. Crohn’s disease of the pouch: diagnosis and management. Expert Rev Gastroenterol Hepatol 2009;3:155–65. [DOI] [PubMed] [Google Scholar]
- 3. Gu J, Remzi FH, Lian L. et al. Practice pattern of ideal pouch surveillance in academic medical centers in the United States. Gastroenterol Rep 2016;4:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shen B, Remzi FH, Lavery IC. et al. A proposed classification of ileal pouch disorders and associated complications after restorative proctocolectomy. Clin Gastroenterol Hepatol 2008;6:145–58. [DOI] [PubMed] [Google Scholar]
- 5. Deutsch AA, McLeod RS, Cullen J. et al. Results of the pelvic-pouch procedure in patients with Crohn’s disease. Dis Colon Rectum 1991;34:475–7. [DOI] [PubMed] [Google Scholar]
- 6. Grobler SP, Hosie KB, Affie E. et al. Outcome of restorative proctocolectomy when the diagnosis is suggestive of Crohn’s disease. Gut 1993;34:1384–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Keighley MR. The final diagnosis in pouch patients for presumed ulcerative colitis may change to Crohn’s disease: patients should be warned of the consequences. Acta Chir Iugosl 2000;47:27–31. [PubMed] [Google Scholar]
- 8. Mylonakis E, Allan RN, Keighley MR.. How does pouch construction for a final diagnosis of Crohn’s disease compare with ileoproctostomy for established Crohn’s proctocolitis? Dis Colon Rectum 2001;44:1137–43. [DOI] [PubMed] [Google Scholar]
- 9. Colombel JF, Ricart E, Loftus EV. et al. Management of Crohn’s disease of the ileoanal pouch with infliximab. Am J Gastroenterol 2003;98:2239–44. [DOI] [PubMed] [Google Scholar]
- 10. Shen B, Remzi FH, Lavery IC. et al. Administration of adalimumab in the treatment of Crohn’s disease of the ileal pouch. Aliment Pharmacol Ther 2009;29:519–26. [DOI] [PubMed] [Google Scholar]
- 11. Feagan BG, Rutgeerts P, Sands BE. et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 12. Du P, Sun C, Ashburn J. et al. Risk factors for Crohn’s disease of the neo-small intestine in ulcerative colitis patients with total proctocolectomy and primary or secondary ileostomies. J Crohns Colitis 2015;9:170–6. [DOI] [PubMed] [Google Scholar]
- 13. Shen B, Achkar JP, Connor JT. et al. Modified pouchitis disease activity index: a simplified approach to the diagnosis of pouchitis. Dis Colon Rectum 2003;46:748–53. [DOI] [PubMed] [Google Scholar]
- 14. Wagner-Bartak NA, Levine MS, Rubesin SE. et al. Crohn’s disease in the ileal pouch after total colectomy for ulcerative colitis: findings on pouch enemas in six patients. Am J Roentgenol 2005;184:1843–7. [DOI] [PubMed] [Google Scholar]
- 15. Melmed GY, Fleshner PR, Bardakcioglu O. et al. Family history and serology predict Crohn’s disease after ileal pouch-anal anastomosis for ulcerative colitis. Dis Colon Rectum 2008;51:100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shen B, Remzi FH, Hammel JP. et al. Family history of crohn’s disease is associated with an increased risk for Crohn’s disease of the pouch. Inflamm Bowel Dis 2009;15:163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shen B, Fazio VW, Remzi FH. et al. Risk factors for diseases of ileal pouch-anal anastomosis after restorative proctocolectomy for ulcerative colitis. Clin Gastroenterol Hepatol 2006;4:81–9. [DOI] [PubMed] [Google Scholar]
- 18. Delaney CP, Remzi FH, Gramlich T. et al. Equivalent function, quality of life and pouch survival rates after ileal pouch-anal anastomosis for indeterminate and ulcerative colitis. Ann Surg 2002;236:43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tyler AD, Milgrom R, Xu W. et al. Antimicrobial antibodies are associated with a crohn’s disease-like phenotype after ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol 2012;10:507–12. [DOI] [PubMed] [Google Scholar]
- 20. Achkar JP, Shen B.. Medical management of postoperative complications of inflammatory bowel disease: pouchitis and Crohn’s disease recurrence. Curr Gastroenterol Rep 2001;3:484–90. [DOI] [PubMed] [Google Scholar]
- 21. Fazio VW, Kiran RP, Remzi FH. et al. Ileal pouch anal anastomosis: analysis of outcome and quality of life in 3707 patients. Ann Surg 2013;257:679–85. [DOI] [PubMed] [Google Scholar]
- 22. Shen B, Lashner BA.. Can we immunogenotypically and immunophenotypically profile patients who are at risk for pouchitis? Am J Gastroenterol 2004;99:442–4. [DOI] [PubMed] [Google Scholar]
- 23. Shen B, Achkar JP, Lashner BA. et al. Endoscopic and histologic evaluation together with symptom assessment are required to diagnose pouchitis. Gastroenterolology 2001;121:261–7. [DOI] [PubMed] [Google Scholar]