Abstract
Background and aims
Patients in the intensive care unit (ICU) with acute pancreatitis (AP) are at risk for extra-pancreatic complications given their severe illness and prolonged length of stay. We sought to determine the rate of extra-pancreatic complications and its effect on length of stay (LOS) and mortality in ICU patients with AP.
Methods
We performed a retrospective cohort study of ICU patients admitted to a tertiary-care center with a diagnosis of AP. A total of 287 ICU patients had a discharge diagnosis of AP, of which 163 met inclusion criteria. We calculated incidence rates of extra-pancreatic complications and performed a univariate and multi-variable analysis to determine predictors of LOS and mortality.
Results
There were a total of 158 extra-pancreatic complications (0.97 extra-pancreatic complications per patient). Ninety-five patients had at least one extra-pancreatic complication, whereas 68 patients had no extra-pancreatic complications. Patients with extra-pancreatic complications had a significantly longer LOS (14.7 vs 8.8 days, p < 0.01) when controlling for local pancreatic complications. Patients with non-infectious extra-pancreatic complications had a higher rate of mortality (24.0% vs 16.2%, p = 0.04). Patients requiring dialysis was an independent predictor for LOS and mortality (incidence risk ratio [IRR] 1.73, 95% confidence interval [CI]: 1.263–2.378 and IRR 1.50, 95% CI 1.623–6.843, p < 0.01) on multi-variable analysis. Coronary events were also a predictor for mortality (p = 0.05). Other extra-pancreatic complications were not significant.
Conclusions
Extra-pancreatic complications occur frequently in ICU patients with AP and impact LOS. Patients with non-infectious extra-pancreatic complications have a higher mortality rate. After controlling for local pancreatic complications, patients requiring dialysis remained an independent predictor for LOS and mortality.
Keywords: Severe acute pancreatitis, extra-pancreatic complications, intensive care unit, length of stay, in-hospital mortality, infections
INTRODUCTION
Acute pancreatitis (AP) is an acute inflammatory condition presenting with severe, persistent epigastric pain with nausea and vomiting [1]. It is the leading discharge diagnosis of all gastrointestinal disorders [2]. The majority of patients with AP have a mild, self-limiting course without organ failure or pancreatic complications (i.e. necrosis or fluid collections). However, approximately 15–25% of patients have severe AP requiring close monitoring in the intensive care unit (ICU), given its relatively high morbidity and mortality [3, 4]. The mortality from severe AP is approximately 30%, but may be as high as 47% in patients with persistent multi-organ system dysfunction and/or infected necrosis [5–8]. Additionally, the presence of extra-pancreatic complications contributes to morbidity and mortality [9, 10].
Broadly, extra-pancreatic complications can be divided into infectious events and non-infectious events. Infectious extra-pancreatic complications have been previously reported in patients hospitalized with AP but not specifically in patients admitted to ICUs [9, 10]. Moreover, non-infectious extra-pancreatic complications have not been explored in this population.
A systematic review reported the incidence of infectious extra-pancreatic complications as 32% [9]. Further, in 2008, one population-based study explored the impact of hospital-acquired infections on outcomes for all patients diagnosed with AP. The authors found a significantly longer length of stay (LOS) and high mortality in patients with hospital-acquired infections [10]. Several studies have documented the impact of non-infectious complications on LOS, hospital costs and mortality in hospitalized patients of other similarly morbid conditions [11–15].
Patients admitted to the ICU with AP are particularly at risk for extra-pancreatic complications given their severe illness and extended LOS. However, little is known about the impact of infectious and non-infectious extra-pancreatic complications in patients with AP admitted to the ICU. Hence, we conducted a review of all patients admitted to the ICU for AP that aimed to determine the rate of extra-pancreatic complications and their effect on LOS and mortality. Additionally, we sought to determine independent predictors for LOS and mortality.
PATIENTS AND METHODS
Study population and study design
We performed a retrospective review of patients with a discharge diagnosis of AP (ICD-9 code: 577.1) who were admitted to the ICU at Beth Israel Deaconess Medical Center in Boston, Massachusetts, between 1 January 2008 and 1 October 2015. AP was diagnosed in patients who presented with at least two of the three following findings: (i) classic abdominal pain, (ii) lipase greater than three times the upper limit of normal and (iii) imaging findings suggestive of AP [16]. Diagnosis of AP in intubated or non-verbal patients was made using the latter two criteria. Patients who did not meet these criteria were excluded. Additional exclusion criteria included patients with a history of chronic pancreatitis, previous pancreatic surgery, concurrent pancreatic, biliary, duodenal or ampulla carcinoma, patients with a prior history of severe or necrotizing pancreatitis and patients admitted to an outside hospital for greater than 2 days. Lastly, patients who developed AP while admitted to the hospital for another reason were excluded.
Patients were admitted to the ICU if they met criteria for severe AP (i.e. end-organ failure and/or local pancreatic complications) or if they required vasopressor support, respiratory support by invasive and non-invasive mechanical ventilation, insulin drips for diabetic ketoacidosis, frequent medication administration (i.e. for alcohol withdrawal), airway monitoring for altered consciousness or close monitoring as deemed necessary by the admitting clinician. While admitted to the ICU, patients received standard institutional-based care, including stress-ulcer prophylaxis, deep-venous thrombosis prophylaxis and, when needed, urinary catheter and venous catheter care. Local pancreatic complications were defined as sterile intra-abdominal collections (i.e. necrosis or fluid collections) or infectious collections (i.e. abscesses).
Data collection and extra-pancreatic complications
Institutional-board review approval was obtained prior to initiating the study in compliance with institutional ethical standards. Demographic, laboratory, microbiologic, radiographic (i.e. number of imaging tests), procedural (i.e. dialysis, intubation and endoscopy) and LOS data were collected. Additionally, in-hospital death and presence of local pancreatic complication were recorded. A bedside index for severity in acute pancreatitis (BISAP) score and Charlson comorbidity index (CCI) was calculated for patients from admission data [17, 18]. The BISAP score was calculated using the blood urea nitrogen, mental status, presence of systemic inflammatory response syndrome, age greater than 60 and presence of pleural effusions on admission. The CCI was calculated retrospectively by reviewing the patient’s medical history as noted on the admission note. Presence of infectious and non-infectious extra-pancreatic complications was noted. Definitions of extra-pancreatic complications are summarized in Table 1.
Table 1.
Definitions of extra-pancreatic complications
| Event | Definition |
|---|---|
| Ventilator-associated pneumonia | Positive culture data from a respiratory sample collected from an endotracheal tube |
| Hospital-acquired pneumonia | Positive culture data from an expectorated or induced respiratory sample collected in a patient hospitalized for greater than 48 h |
| Aspiration pneumonia | Positive culture data from a respiratory sample after a documented aspiration event in a patient hospitalized for greater than 48 h |
| Catheter-associated urinary-tract infection | Positive urinary culture data in patient with an indwelling urinary catheter or in patient with a recent history of an indwelling urinary catheter (i.e. within 48 h) |
| Central or peripheral line-associated blood-stream infection | Positive blood culture data in patient with a peripheral or central blood (arterial or venous) catheter or a recent history of a peripheral or central blood catheter (i.e. within 48 h) in greater than one culture bottle |
| Non-line-associated blood-stream infection | Positive blood culture data in greater than one culture bottle in a patient hospitalized for greater than 48 h |
| Clostridium difficile-associated diarrhea | Toxin assay or PCR-positive stool assay for Clostridium difficile in a patient hospitalized for at least 48 h |
| Cardiac arrest | Loss of pulse either resulting in death or requiring cardiopulmonary resuscitation in patient hospitalized for at least 48 h |
| Fall with injury | A fall resulting in bodily harm (i.e. fracture) in patient hospitalized for at least 48 h |
| Gastrointestinal bleed | Gastrointestinal bleeding in any part of the gastrointestinal tract during ICU stay, with endoscopic evidence or based on clinical note in discharge summary |
| Acute coronary syndrome | An ST-segment elevation myocardial infarction or non-ST-segment elevation myocardial infarction documented by cardiology consultation note |
| Initiation of hemodialysis | Patient with a new requirement for renal replacement therapy while admitted |
| Delirium | Acute confusional state marked by gross inattentiveness and waxing and waning changes in mental status in the absence of an underlying primary central nervous event |
Main outcomes and statistical analysis
Our primary outcome was in-hospital mortality and our secondary outcomes were total and ICU LOS. Qualitative data are expressed as absolute numbers and percentages, and quantitative data are expressed as median and range. We compared total hospital and ICU LOS, and mortality between the groups for statistical significance using t-test of proportions. We then repeated analysis to control for local pancreatic complications. We hypothesized that patients with in-hospital extra-pancreatic complications would have a higher mortality rate, which, based on one prior study, was 17%. To detect a difference in 17%, assuming an error of 5%, we calculated our sample size to be 168. Univariate analysis of patients without local pancreatic complications for LOS and mortality was completed using a t-test of proportions. Furthermore, multi-variable analysis of the significant findings from univariate analysis was done using a negative binomial model for LOS and using logistical regression for mortality. Lastly, we completed a Fisher T-test to determine whether there was a significant association between BISAP scores, CCI scores and the number of extra-pancreatic complications.
RESULTS
Study population and patient characteristics
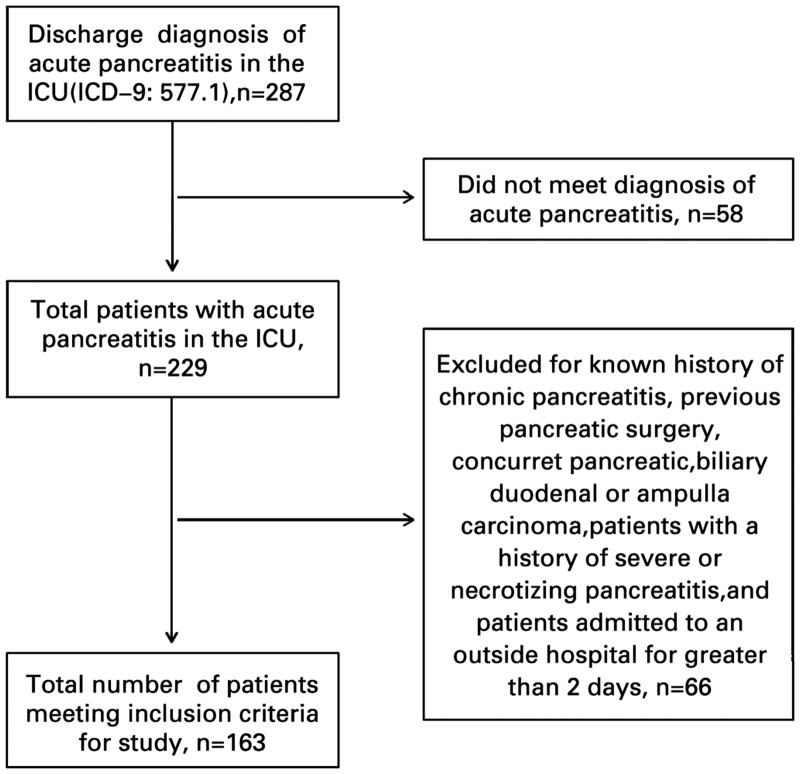
Two hundred eighty-seven ICU patients were identified with a discharge diagnosis of AP during the study period, of which 163 met inclusion criteria for this study (Figure 1). The majority of patients were male (98/163, 60.1%) and Caucasian (116/163, 71.7%) with a mean age of 58 years. The most common etiology was gallstone-related disease (57/163, 35.0%). Patient characteristics are outlined in Table 2.
Figure 1.
Patient-eligibility flowchart.
Table 2.
Patient characteristics
| Patient characteristic | No extra-pancreatic | At least one extra-pancreatic | p-value |
|---|---|---|---|
| complications (n = 68) | complication (n = 95) | ||
| Mean age (SD) | 58.0 (16.3) | 57.9 (18.5) | 0.98 |
| Male gender, n (%) | 38 (55.8) | 60 (63.1) | 0.35 |
| Prior history of acute pancreatitis, n (%) | 19 (27.9) | 22 (23.2) | 0.49 |
| History of congestive heart failure, n (%) | 7 (10.3) | 11 (11.6) | 0.80 |
| History of myocardial infarction, n (%) | 7 (10.3) | 10 (10.5) | 0.96 |
| History of chronic kidney disease, n (%) | 6 (8.8) | 10 (10.5) | 0.72 |
| Diabetes with end-organ damage, n (%) | 3 (4.4) | 5 (5.3) | 0.92 |
| Mild liver disease, n (%) | 7 (10.3) | 8 (8.4) | 0.29 |
| Moderate to severe liver disease, n (%) | 1 (1.5) | 4 (4.2) | 0.32 |
| Mean CCI (SD) | 1.28 (1.40) | 1.41 (1.69) | 0.60 |
| Mean age-adjusted CCI (SD) | 2.78 (2.38) | 2.96 (2.65) | 0.66 |
| Mean BISAP (SD) | 1.97 (1.17) | 2.10 (1.21) | 0.51 |
| Presence of local pancreatic complication (fluid collection or necrosis), n (%) | 22 (32.4) | 38 (40.0) | 0.32 |
| Mean ICU length of stay (SD) | 4.1 (5.7) | 9.8 (15.2) | <0.01 |
| Mean total length of stay (SD) | 12.0 (9.4) | 18.0 (14.7) | <0.01 |
| Mean admission blood urea nitrogen (mg/dL) | 23.7 | 29.9 | 0.08 |
| Mean admission white blood cell count (1000/µL) | 15.2 | 13.6 | 0.12 |
| Mean admission lipase (IU/L) | 2108 | 1583 | 0.33 |
| Mean admission hematocrit (%) | 38.5 | 37.4 | 0.40 |
| Mean admission lactate (mmol/L) | 2.3 | 2.7 | 0.36 |
| Mean admission albumin (g/dL) | 3.6 | 3.3 | 0.06 |
| Medical ICU admission, n (%) | 65 (95.6) | 62 (65.3) | 0.48 |
| Mortality rate, n (%) | 5 (7.4) | 14 (14.7) | 0.15 |
| Mean number of images | 6.06 | 13.36 | <0.01 |
SD, standard deviation; CCI, Charlson comorbidity index; BISAP, bedside index of severity in acute pancreatitis; ICU, intensive care unit.
Extra-pancreatic complications
One hundred fifty-eight extra-pancreatic complications (0.97 events/patient) occurred during the study period. Ninety-five patients (58.3%, 95/163) had at least one extra-pancreatic complication, whereas 68 patients (41.7%, 68/163) had no any extra-pancreatic complications. There were an equal number of infectious and non-infectious complications (79 events each). The most common infectious extra-pancreatic complications in this cohort were catheter-associated urinary-tract infection and hospital-acquired pneumonia (both 17/79, 21.5%). Together, respiratory infections (hospital-acquired pneumonia, ventilator-associated pneumonia and aspiration pneumonia) composed the largest component of the infectious extra-pancreatic complications (34/79, 43.0%). Delirium was the most common non-infectious extra-pancreatic complication in the ICU (32/79, 40.5%). Figure 2 illustrates the distribution of extra-pancreatic complications.
Figure 2.
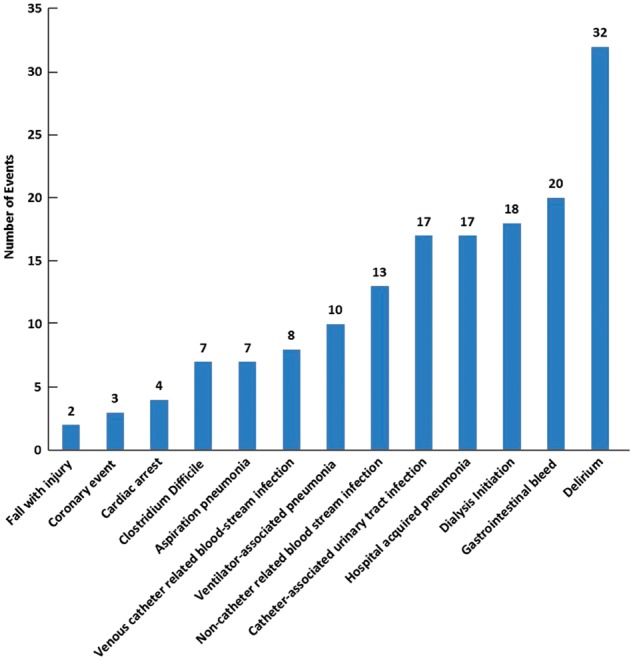
Histogram of extra-pancreatic complications.
Patients with extra-pancreatic complications had significantly longer ICU and total LOS than those without extra-pancreatic complications (9.8 vs 4.1 days, p < 0.01 and 18.0 vs 12.0 days, p < 0.01, respectively). The overall mortality rate was 11.7% (19/163). There was no significant difference in mortality between those with extra-pancreatic complications and those without extra-pancreatic complications (14.8% vs 7.4%, p = 0.15). Moreover, there was significantly more imaging performed (p < 0.01) in patients with extra-pancreatic complications. There were no statistically significant differences in age, gender, CCI, BISAP, etiology of AP or other admission laboratory values between the two groups (Table 2).
Patients with local pancreatic complications
There were 60 patients with local pancreatic complications in the study period (36.8%, 60/163). Patients with local pancreatic complications had longer total LOS than those without local pancreatic complications (21.3 vs 12.1 days, p < 0.01) as well as longer ICU LOS (11.1 vs 5.2 days, p < 0.01). There were no differences in mortality (p = 0.13) or number of events (p = 0.89); however, there were more imaging studies in the patients with pancreatic complications (p < 0.01; Table 3).
Table 3.
Patients with local pancreatic complications
| Pancreatic complications | No pancreatic complications | p-value | |
|---|---|---|---|
| (n = 60) | (n = 103) | ||
| Mean total length of stay, days (SD) | 21.3 (14.8) | 12.1 (10.7) | <0.01 |
| Mean ICU length of stay, days (SD) | 11.1 (15.5) | 5.2 (9.8) | <0.01 |
| Mortality, n (%) | 10 (16.7) | 9 (8.7) | 0.13 |
| Mean number of events | 0.97 | 0.96 | 0.89 |
| Mean number of images | 15 | 8 | <0.01 |
SD, standard deviation; ICU, intensive care unit.
After excluding patients with local pancreatic complications, 57 of the 103 patients had a total of 99 extra-pancreatic complications (1.73 events/patient), of which 56 were infectious extra-pancreatic complications and 43 were non-infectious extra-pancreatic complications. Patients with extra-pancreatic complications had longer total and ICU LOS (14.7 vs 8.8 days, p < 0.01 and 7.1 vs 2.9 days, p = 0.03). The mortality rate in this group was 8.7% (9/103) and there was no difference in mortality rates between those with extra-pancreatic complications and without extra-pancreatic complications (12.3% vs 4.3%, p = 0.16). Lastly, there were significantly more imaging studies in patients with extra-pancreatic complications than without (10.9 vs 4.80 images/patient, p = 0.03).
Univariate analysis
Patients with infectious extra-pancreatic complications had longer total LOS (14.6 vs 10.6 days, p = 0.05). Specifically, patients with hospital-acquired pneumonia had a significantly longer total LOS (23.1 vs 14.6 days, p = 0.01). Further, patients with non-venous catheter-related blood-stream infections had significantly longer ICU LOS (11.3 vs 4.5 days, p = 0.04). No other infectious extra-pancreatic complications had significantly longer ICU or total LOS. Moreover, mortality was not affected by any infectious extra-pancreatic complication (Table 4).
Table 4.
P-values from univariate analysis of patients with and without extra-pancreatic complications for total and intensive care unit length of stay and mortality
| Event | Total length of stay | ICU length of stay | Mortality |
|---|---|---|---|
| Hospital-acquired pneumonia | 0.01 | 0.07 | 0.96 |
| Aspiration pneumonia | 0.26 | 0.40 | 0.53 |
| Ventilator acquired pneumonia | 0.81 | 0.80 | 0.40 |
| Catheter-associated urinary-tract infection | 0.24 | 0.95 | 0.82 |
| Line-associated blood-stream infection | 0.34 | 0.28 | 0.48 |
| Non-line-associated blood-stream infection | 0.16 | 0.04 | 0.88 |
| All blood-stream infections | 0.42 | 0.21 | 0.82 |
| Clostridium difficile-associated diarrhea | 0.59 | 0.30 | 0.53 |
| Delirium | <0.01 | 0.01 | 0.23 |
| Fall with injury | 0.94 | 0.91 | 0.66 |
| Coronary event | 0.78 | 0.88 | 0.04 |
| Gastrointestinal bleeding | 0.04 | 0.04 | 0.88 |
| Cardiac arrest | 0.49 | 0.36 | 0.53 |
| Dialysis initiation | <0.01 | <0.01 | 0.04 |
| Any infectious complication | 0.05 | 0.30 | 0.77 |
| Any non-infectious complication | <0.01 | 0.01 | 0.04 |
Patients with non-infectious extra-pancreatic complications had significantly longer total and ICU LOS (16.7 vs 9.5 days, p < 0.01 and 8.4 vs 3.4 days, p = 0.01, respectively) and had significantly more in-hospital deaths than those without non-infectious extra-pancreatic complications (24.0% vs 16.2%, p = 0.04). Specifically, total and ICU LOS was longer in patients with delirium (19.0 vs 15.2 days, p < 0.01 and 10.1 vs 4.1 days, p = 0.01, respectively), gastrointestinal bleeding (17.7 vs 11.5 days, p = 0.04 and 11.2 vs 4.6 days, p = 0.04, respectively) and patients requiring dialysis (24.1 vs 11.1 days, p < 0.01 and 16.6 vs 4.2 days, p < 0.01, respectively). Lastly, mortality rates were significantly higher in patients with coronary events (33.3% vs 8.0%, p = 0.04) and in patients requiring dialysis (25.0% vs 9.4%, p = 0.04).
Multi-variable analysis
On multi-variable analysis, patients’ requiring dialysis was an independent predictor for total and ICU LOS (IRR 1.73, 95% CI: 1.263–2.378, p < 0.01 and IRR 3.20, 95% CI: 1.878–5.463, p < 0.01), whereas other variables were not (all p > 0.05, Table 5). Similarly, patients’ requiring dialysis was an independent predictor for in-hospital mortality (IRR 1.50, 95% CI 1.623–6.843, p < 0.01), along with coronary events (IRR 14.61, 95% CI: 1.008–24.534, p = 0.05).
Table 5.
Multi-variable analysis for predictors of length of stay and mortality
| Event | Incidence risk ratio | p-value | 95% confidence interval |
|---|---|---|---|
| Total length of stay | |||
| Hospital-acquired pneumonia | 1.36 | 0.10 | 0.944–1.951 |
| Dialysis | 1.73 | <0.01 | 1.263–2.378 |
| Gastrointestinal bleeding | 1.32 | 0.09 | 0.957–1.815 |
| Delirium | 1.26 | 0.09 | 0.962–1.651 |
| ICU length of stay | |||
| Hospital-acquired pneumonia | 1.50 | 0.18 | 0.825–2.713 |
| Non-line blood-stream infection | 1.41 | 0.21 | 0.768–3.139 |
| Dialysis | 3.20 | <0.01 | 1.878–5.463 |
| Gastrointestinal bleeding | 1.56 | 0.11 | 0.898–2.705 |
| Delirium | 1.53 | 0.06 | 0.979–2.392 |
| Mortality | |||
| Coronary event | 14.61 | 0.05 | 1.008–24.534 |
| Dialysis | 1.50 | <0.01 | 1.623–6.843 |
Association between severity scores and extra-pancreatic complications
There was a significant association between having extra-pancreatic complications events and higher BISAP score using an a priori cut-off score of 3 (p = 0.02). On post-hoc analysis, we adjusted the cut-off to 2 and 4 and there was a significant association at 4 (p = 0.05), although the association was no longer significant when the cut-off was reduced to 2 (p > 0.05).
A significant association did not exist for either the CCI or the age-adjusted CCI when an a priori cut-off set at 3 (p = 0.44). On post-hoc analysis, the cut-off was adjusted to 2, 4, 5 and 6, and there remained no significant associations (p > 0.05).
DISCUSSION
Only a minority of patients with AP require ICU level of care; however, mortality may be as high as 30%. While it is known that the severity of AP predicts mortality, the impact of in-hospital extra-pancreatic complications, however, is not well studied. In this study, we sought to determine the rate of extra-pancreatic complications in patients admitted to the ICU with AP and to identify predictors for LOS and mortality.
Our study demonstrates the significant impact that extra-pancreatic complications have on outcomes in ICU patients with AP. We report an extra-pancreatic complications event rate of nearly one event per patient and, when controlling for patients with local pancreatic complications, an event rate of greater than one event per patient. Moreover, these events prolong both ICU and total LOS, and increase in-hospital mortality especially in patients with non-infectious extra-pancreatic complications. Above all, even after controlling for patients with local pancreatic complications, patients requiring dialysis remained the strongest predictor of total and ICU LOS as well as in-hospital mortality.
To date, infectious extra-pancreatic complications have been reported in patients hospitalized with AP, but not specifically in patients admitted to the ICU. In a systematic review, Brown et al. compiled 1700 patients admitted for AP from 19 studies and reported an incidence rate of 32%, with the most common infections being respiratory infections, blood-stream infection and urinary-tract infections [9]. Similarly, our study found that respiratory infections, blood-stream infection and urinary-tract infections were the most common infectious extra-pancreatic complications in patients admitted with AP to the ICU. We, however, reported a higher incidence (48.5%) of infectious extra-pancreatic complications which likely reflects our sicker ICU-only cohort. Further, Wu et al. conducted a population-based assessment to determine the rate of hospital-acquired infections using a large national database. The authors showed that patients with infectious extra-pancreatic complications had longer LOS [10], which was corroborated in our study.
Of the non-infectious extra-pancreatic complications, gastrointestinal bleeding has been previously reported in patients hospitalized for AP with an incidence ranging from 3.7 to 17.8% and with a significantly higher mortality rate [14, 19–21]. Our study found an incidence of 12.2% but no significant difference in mortality. Our institution regularly practices stress-ulcer prophylaxis whereas previous studies did not mention use of prophylaxis. While it is unclear what effect this practice had on the incidence of gastrointestinal bleeding in our study, at least one study has demonstrated the efficiency of the use of a proton pump inhibitor on the prevention of stress ulcers in patients with AP [22]. Our study also found that patients who suffered from gastrointestinal bleeding had longer total and ICU LOS, which is in keeping the largest population-based study [21].
Our study is the first to report other non-infectious complications in ICU patients with AP. One such complication is delirium, which is an acute confusional state with waxing and waning changes in mental status precipitated by an underlying acute condition [12]. The incidence of delirium in hospitalized patients has been estimated at 30%. ICU-related conditions including sepsis, diabetic keto-acidosis and heart-failure delirium range between 16 and 89% [12–13, 23–25]. These studies have also demonstrated longer hospitalization and higher mortality rates [23]. In our study, the incidence of delirium was 19.6% (32/163) and our patients had significantly longer LOS, in keeping with these studies that explored similarly morbid conditions.
Hospitalized patients are also at risk for falling [26]. Specifically, patients with delirium, patients with ischemic strokes, those in geriatric units and those with cancer are at higher risk for falls, with some studies quoting a 10% risk of fall [11, 26, 27]. In contrast, there was a relatively low rate of falls (1.2%, 2 falls in 158 complications) in our patient population in spite of receiving narcotics and other sedatives, perhaps due to the relatively lower rate of delirium (19.6%) and younger patient population.
Acute coronary syndrome was a rare event in our cohort (1.8%, 3/163), which is in keeping with several published case reports [28–31]. The incidence is lower than in similarly morbid conditions such as sepsis, where the incidence has been reported as 4.1% [32, 33]. The mortality rate in our study for patients with acute coronary syndrome was significantly higher and was an independent predictor for mortality in our cohort, similarly to patients with sepsis [30]. Along with acute coronary syndrome, cardiac arrest is another important complication that rarely occurred in our study (2.5%, 4/163) as reported in other studies [34, 35]. The overall incidence of in-hospital cardiac arrest has been quoted as 1.6 arrests per 1000 admissions, which is lower than in our cohort of ICU patients and is explained by our sicker population. Further studies are needed to explore the relationship between cardiac complications and AP.
Transient or persistent acute renal failure is a marker for severity in patients diagnosed with AP [1, 3–5, 36]. Patients whose renal function does not improve and those who develop severe hypervolemia, uremia or electrolyte disturbances may require urgent therapy via hemodialysis. The incidence of ICU patients with AP requiring urgent hemodialysis is not well reported. Studies reported a rate of 3% in all patients admitted for AP as well as in other critically ill patients [37, 38]. In our cohort, the incidence rate was 8.7%, likely due to a sicker ICU population. Importantly, our study demonstrates that renal failure is an independent predictor for LOS and mortality, even after controlling for the presence of local pancreatic complications. These findings further emphasize the critical impact that renal failure has on outcomes in patients with AP.
Several studies have demonstrated that comorbid conditions impact outcomes in patients with AP, including organ failure and mortality [38–40]. BISAP scores of greater than 3 have a mortality rate ranging between 5 and 22%, whereas scores less than 2 have lower mortality [17, 40]. Furthermore, a BISAP score of 3 had 99.1% specificity for severe AP in one study [41]. A CCI score of greater than 3 also had a higher rate of mortality [42]. However, little is known about the correlation between BISAP and CCI and rates of extra-pancreatic complications. Using this information, we set a cut-off of 3 to determine whether there is an association between BISAP and extra-pancreatic complications as well as CCI and extra-pancreatic complications. In our study, BISAP score had a significant association in predicting extra-pancreatic complications, whereas comorbidity scores did not.
Naturally, in our study, patients with local pancreatic complications had longer hospital and ICU LOS and thus confound LOS measurements in patients with extra-pancreatic complications. However, after excluding these patients, LOS remained significantly longer in patients with extra-pancreatic complications, suggesting that these complications independently prolong hospitalization.
Because of the retrospective nature of our study and its inherit limitations, more studies are needed to understand the impact that these extra-pancreatic complications have on mortality and LOS. Further, as CCI scores were calculated retrospectively, there may have been significant bias in its calculation. Thus, it is unclear what impact comorbidity has on extra-pancreatic complications. In our study, we report that there was not a statistically significant difference in our primary outcome, highlighting that our study was underpowered. Thus, larger studies are necessary to further investigate what impact in-hospital complications have on mortality in patients with AP. While admission to the ICU had clear criteria, discharge criteria from the ICU were not clearly delineated and were left to the clinician, which confounds our LOS analysis. Moreover, in an effort to reduce in-hospital complications, there may have been institutional practice changes to improve care. It is unclear what effect, if any, these had on our study, as we did not account for them. Lastly, we did not collect data on whether patients with pancreatic fluid collections required endoscopic, surgical or percutaneous drainage, which could confound our results. Studies that further clarify the impact of these fluid collections on in-hospital complications are needed.
In summary, both infectious and non-infectious extra-pancreatic complications occur frequently in patients with AP who are admitted to the ICU and impact LOS when present. After controlling for local pancreatic complications, patients with renal failure requiring dialysis remained the strongest predictor for LOS and mortality.
Conflict of interest statement: none declared.
References
- 1. Swaroop VS, Chari ST, Clain JE.. Severe acute pancreatitis. JAMA 2004;291:2865–8. [DOI] [PubMed] [Google Scholar]
- 2. Peery AF, Dellon ES, Lund J. et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012;143:1179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Robert JH, Frossard JL, Mermillod B. et al. Early prediction of acute pancreatitis: prospective study comparing computed tomography scans, Ranson, Glascow, Acute Physiology and Chronic Health Evaluation II scores, and various serum markers. World J Surg 2002;26:612–9. [DOI] [PubMed] [Google Scholar]
- 4. Fagenholz PJ, Castillo CF-D, Harris NS. et al. Increasing United States hospital admissions for acute pancreatitis, 1988-2003. Ann Epidemiol 2007;17:491.e1–7. [DOI] [PubMed] [Google Scholar]
- 5. Forsmark CE, Vege SS, Wilcox CM.. Acute pancreatitis. N Engl J Med 2016;375:1972–81. [DOI] [PubMed] [Google Scholar]
- 6. Gloor B, Müller CA, Worni M. et al. Late mortality in patients with severe acute pancreatitis. Br J Surg 2001;88:975–9. [DOI] [PubMed] [Google Scholar]
- 7. Cavallini G, Frulloni L, Bassi C. et al. Prospective multicentre survey on acute pancreatitis in Italy (ProInf-AISP): results on 1005 patients. Dig Liver Dis 2004;36:205–11. [DOI] [PubMed] [Google Scholar]
- 8. Agarwal S, George J, Padhan RK. et al. Reduction in mortality in severe acute pancreatitis: a time trend analysis over 16 years. Pancreatology 2016;16:194–9. [DOI] [PubMed] [Google Scholar]
- 9. Brown LA, Hore TA, Phillips ARJ. et al. A systematic review of the extra-pancreatic infectious complications in acute pancreatitis. Pancreatology 2014;14:436–43. [DOI] [PubMed] [Google Scholar]
- 10. Wu BU, Johannes RS, Kurtz S. et al. The impact of hospital-acquired infection on outcome in acute pancreatitis. Gastroenterology 2008;135:816–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gettens S, Fulbrook P.. Fear of falling: association between the Modified Falls Efficacy Scale, in-hospital falls and hospital length of stay. J Eval Clin Pract 2015;21:43–50. [DOI] [PubMed] [Google Scholar]
- 12. Han JH, Eden S, Shintani A. et al. Delirium in older emergency department patients is an independent predictor of hospital length of stay. Acad Emerg Med 2011;18:451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hsieh SJ, Madahar P, Hope AA. et al. Clinical deterioration in older adults with delirium during early hospitalisation: a prospective cohort study. BMJ Open 2015;5:e007496.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shen HN, Lu CL, Li CY.. The effect of gastrointestinal bleeding on outcomes of patients with acute pancreatitis: a national population-based study. Pancreatology 2012;12:331–6. [DOI] [PubMed] [Google Scholar]
- 15. Pendlebury S, Lovett N, Smith S. et al. Observational, longitudinal study of delirium in consecutive unselected acute medical admissions: age-specific rates and associated factors, mortality and re-admission. BMJ Open 2015;5:e007808.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Banks PA, Bollen TL, Dervenis C. et al. Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102–11. [DOI] [PubMed] [Google Scholar]
- 17. Wu BU, Johannes RS, Sun X. et al. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut 2008;57:1698–703. [DOI] [PubMed] [Google Scholar]
- 18. Charlson ME, Pompei P, Ales KL. et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 19. Zhan XB, Guo XR, Yang J. et al. Prevalence and risk factors for clinically significant upper gastrointestinal bleeding in patients with severe acute pancreatitis. J Dig Dis 2015;16:37–42. [DOI] [PubMed] [Google Scholar]
- 20. Sharma PK, Madan K, Garg PK.. Hemorrhage in acute pancreatitis: should gastrointestinal bleeding be considered. An organ failure? Pancreas 2008;36:141–5. [DOI] [PubMed] [Google Scholar]
- 21. Rana SS, Sharma V, Bhasin DK. et al. Gastrointestinal bleeding in acute pancreatitis: etiology, clinical features, risk factors and outcome. Trop Gastroenterol 2015;36:31–5. [DOI] [PubMed] [Google Scholar]
- 22. No authors listed. Prevention of acute stress ulcer and gastrointestinal bleeding in patients with acute necrotizing pancreatitis. Anesteziol Reanimatol 2011;6:74–8. [PubMed] [Google Scholar]
- 23. Inouye SK, Rushing JT, Foreman MD. et al. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med 1998;13:234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bergeron N, Dubois M-J, Dumont M. et al. Intensive care delirium screening checklist: evaluation of a new screening tool. Intensive Care Med 2001;27:859–64. [DOI] [PubMed] [Google Scholar]
- 25. Ely WE, Girard TD, Shintani AK. et al. Apolipoprotein E4 polymorphism as a genetic predisposition to delirium in critically ill patients. Crit Care Med 2007;35:112–7. [DOI] [PubMed] [Google Scholar]
- 26. De Carle AJ, Kohn R.. Risk factors for falling in a psychogeriatric unit. Int J Geriatr Psychiatry 2001;16:762–7. [DOI] [PubMed] [Google Scholar]
- 27. Hendrich A, Nyhuis A, Kippenbrock T. et al. Hospital falls: development of a predictive model for clinical practice. Appl Nurs Res 1995;8:129–39. [DOI] [PubMed] [Google Scholar]
- 28. Vasantha Kumar A, Mohan Reddy G, Anirudh Kumar A.. Acute pancreatitis complicated by acute myocardial infarction: a rare association. Indian Heart J 2013;65:474–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Phadke MS, Punjabi P, Sharma S. et al. Acute pancreatitis complicated by ST-elevation myocardial infarction. J Emerg Med 2013;44:932–5. [DOI] [PubMed] [Google Scholar]
- 30. Hsu P-C, Lin T-H, Su H-M. et al. Acute necrotizing pancreatitis complicated with ST elevation acute myocardial infarction: a case report and literature review. Kaohsiung J Med Sci 2010;26:200–5. [DOI] [PubMed] [Google Scholar]
- 31. Asfalou I, Miftah F, Kendoussi M. et al. Myocardial infarction as complication of acute pancreatitis. Ann Fr Anesth Reanim 2011;30:77–9. [DOI] [PubMed] [Google Scholar]
- 32. Smilowitz NR, Gupta N, Guo Y. et al. Comparison of outcomes of patients with sepsis with versus without acute myocardial infarction and comparison of invasive versus noninvasive management of the patients with infarction. Am J Cardiol 2016;117:1065–71. [DOI] [PubMed] [Google Scholar]
- 33. Dalager-Pedersen M, Sogaard M, Schonheyder HC. et al. Risk for myocardial infarction and stroke after community-acquired bacteremia: a 20-year population-based cohort study. Circulation 2014;129:1387–96. [DOI] [PubMed] [Google Scholar]
- 34. Li B, Ke L, Shen X. et al. Successful cardiopulmonary cerebral resuscitation in patient with severe acute pancreatitis. Am J Emerg Med 2015;33:1108.e5–7. [DOI] [PubMed] [Google Scholar]
- 35. Imperadore F, Accardi R, Spagnolli W. et al. Heart arrest caused by ventricular fibrillation in acute biliary pancreatitis: a case report and etiopathogenetic hypothesis. Ital Heart J Suppl 2000;1:419–22. [PubMed] [Google Scholar]
- 36. Frost L, Pedersen RS, Ostgaard SE. et al. Prognosis in acute pancreatitis complicated by acute renal failure requiring dialysis. Scand J Urol Nephrol 1990;24:257–60. [PubMed] [Google Scholar]
- 37. Wald R, McArthur E, Adhikari NKJ. et al. Changing incidence and outcomes following dialysis-requiring acute kidney injury among critically ill adults: a population-based cohort study. Am J Kidney Dis 2015;65:870–7. [DOI] [PubMed] [Google Scholar]
- 38. Murata A, Ohtani M, Muramatsu K. et al. Influence of comorbidity on outcomes of older patients with acute pancreatitis based on a national administrative database. Hepatobiliary Pancreat Dis Int 2015;14:422–8. [DOI] [PubMed] [Google Scholar]
- 39. Weitz G, Woitalla J, Wellhöner P. et al. Comorbidity in acute pancreatitis relates to organ failure but not to local complications. Z Gastroenterol 2016;54:226–30. [DOI] [PubMed] [Google Scholar]
- 40. Yadav J, Yadav SK, Kumar S. et al. Predicting morbidity and mortality in acute pancreatitis in an Indian population: a comparative study of the BISAP score, Ranson’s score and CT severity index. Gastroenterol Rep 2016;4:216–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee PJW, Bhatt A, Lopez R. et al. Thirty-day readmission predicts 1-year mortality in acute pancreatitis. Pancreas 2016;45:561–4. [DOI] [PubMed] [Google Scholar]
- 42. McNabb-Baltar J, Ravi P, Isabwe GA. et al. A population-based assessment of the burden of acute pancreatitis in the United States. Pancreas 2014;43:687–91. [DOI] [PubMed] [Google Scholar]