Abstract
Large-scale genome-wide association studies have consistently shown that genetic variation in CACNA1C, a gene that encodes calcium voltage-gated channel subunit alpha1C, increases risk for psychiatric disorders. CACNA1C encodes the Cav1.2 subunit of voltage-gated calcium channels, which themselves have been functionally implicated in a broad spectrum of neuropsychiatric syndromes. Research has concentrated on uncovering the underlying biological mechanisms that could be responsible for this increased risk. This review presents an overview of recent findings regarding Cacna1c variation in animal models, particularly focusing on behavioral phenotypes associated with neurodevelopmental disorders such as cognition, anxiety and depressive phenotypes, and fear conditioning. The impact of reduced gene dosage of Cacna1c on adult hippocampal neurogenesis is also assessed, including new data from a novel Cacna1c+/− rat model.
Keywords: cacna1c, animal models, genetics, psychiatry, neurobiology, phenotypes, neurogenesis
Introduction
Genetic Association
The growth of psychiatric genetics has heralded in a new era of knowledge about psychiatric and neurodevelopmental disorders. Genome-wide association studies (GWAS) have been highly influential in identifying common variation in genes that are over or underrepresented in individuals with a certain disorder. These studies identify single nucleotide polymorphisms (SNPs) that occur throughout the genome that increase risk for neuropsychiatric disorders. One of the first, and now well replicated, GWAS finding in psychiatry was the association of SNP rs1006737 within the calcium voltage-gated channel subunit alpha1c (CACNA1C) gene with bipolar disorder.1 This association was confirmed in a larger data set,2 and subsequent studies showed a further association of this SNP with schizophrenia, major depressive disorder (MDD) and autism (table 1). Further SNPs within CACNA1C have since been associated with these disorders in multiple studies (table 1).
Table 1.
Summary of Published Association Studies of SNPs Within CACNA1C With Psychiatric/Neurodevelopmental Disorders
| SNP | Disorder | Risk Allele | Main References |
|---|---|---|---|
| rs1006737 | BPD | A | Ferreira et al2 |
| Sklar et al1 | |||
| Green et al3 | |||
| Gonzalez et al4 | |||
| Liu et al5 | |||
| Ruderfer et al6 | |||
| Lett et al7 | |||
| SCZ | A | Green et al8 | |
| Nyegaard et al9 | |||
| He et al10 | |||
| Ivorra et al11 | |||
| Guan et al12 | |||
| Zheng et al13 | |||
| Hori et al14 | |||
| Ruderfer et al6 | |||
| Autism | G | Li et al15 | |
| MDD | A | Liu et al5 | |
| Green et al8 | |||
| Wray et al16 | |||
| Casamassima et al17 | |||
| rs4765905 | SCZ | A | Hamshere et al18 |
| Takahashi et al19 | |||
| Autism | G | Li et al15 | |
| rs4765913 | BPD | A | Ripke et al20 |
| Mühleisen et al21 | |||
| SCZ | A | Ripke et al20 | |
| MDD | A | Ripke et al20 | |
| rs1024582 | BPD, SCZ, ADHD, MDD, autism | A | Cross-Disorder Group of the Psychiatric Genomics Consortium22 |
| rs2007044 | SCZ | A | Ripke et al20 |
| Pardiñas et al23 | |||
| rs7297582 | BPD | T | Liu et al5 |
| MDD | T | Liu et al5 | |
| rs12898315 | SCZ | A | Pardiñas et al23 |
| rs10744560 | BPD | T | Stahl et al24 |
Note: BPD, bipolar disorder; SCZ, schizophrenia; MDD, major depressive disorder; ADHD, attention deficit hyperactivity disorder.
The majority of these SNPs are in known linkage disequilibrium with each other, except rs7297582 and rs12898315, potentially due to the fact they are less studied. The SNPs lie within introns, within predicted enhancers which can interact with the CACNA1C transcription start site25 and therefore may determine gene expression.26,27 rs1006737 has been shown to be an expression quantitive trait loci (eQTL) for CACNA1C expression: associated with decreased expression.27
CACNA1C SNPs were found to have shared effects across attention deficit hyperactivity disorder (ADHD), autism, BPD, SCZ, and MDD,22 implying that common variation in CACNA1C may be associated with particular symptom clusters instead of one particular disorder.
In addition to GWAS findings, large exome sequencing studies have shown that rare disruptive mutations within calcium ion channels are enriched in patients with schizophrenia28 and autism.29,30 Furthermore, missense mutations in exon 8, or the alternatively spliced exon 8a, of CACNA1C can cause an autosomal dominant genetic disorder named Timothy syndrome (TS).31 TS is a multisystem channelopathy characterized by cardiac defects, craniofacial abnormalities, autism, and cognitive impairments. There are 2 common types of TS characterized by mutation; TS1 (G406R in exon 8a) and the more severe form TS2 (G406R or G402S in exon 8). Both TS1 and TS2 are characterized by gain-of-function mutations in CACNA1C.32
Cacna1c, Gene Transcription, and Synaptic Plasticity
CACNA1C encodes for the alpha1c subunit of the Cav1.2 L-type voltage-gated calcium channel (LTCC). This subunit forms the pore through which calcium influxes into a cell and initiates downstream signaling cascades.33 LTCCs have a prominent role in controlling gene expression through coupling membrane depolarization with cAMP response element-binding protein (CREB) phosphorylation via local Ca2+/calmodulin-dependent protein kinase II (CaMKII) signaling.34 CREB can bind to a critical Ca2+ response element within brain-derived neurotrophic factor (BDNF) to trigger its transcription.35,36 This pathway, and particularly CREB and BDNF, are thought to be essential for learning and memory processes. Synaptic plasticity, which is thought to underlie learning and memory, can be modulated by LTCCs37,38; LTCC antagonists reduce induction of long-term potentiation (LTP) in the CA1 of the rat hippocampus.39 Cav1.2 knockdown models have shown reduced CREB transcription and hippocampal LTP,40,41 implicating the important of these channels in gene expression and plasticity.
This review aims to give a brief overview of the current phenotypes relevant to psychiatric and neurodevelopmental disorders studied so far in animal models of Cacna1c/Cav1.2 dysfunction, including new findings on impacts on neurogenesis in a rat model of reduced gene dosage of Cacna1c.
Models
Genetic Cacna1c/Cav1.2 rodent models have mostly concentrated on reduced gene dosage. Some studies utilize a constitutive heterozygote model (Cacna1c+/−) to study gene dosage effects as the homozygote model is embryonically lethal. However, other studies have utilized region-specific complete knockouts of Cacna1c (Cacna1c−/−) driven by specific promotors to disentangle the neuronal contribution of this gene compared with the cardiac properties. Bader and colleagues42 developed a genetic mouse model based on TS2. While both homozygote and heterozygote knockout of exon 8a were lethal, a heterozygote model that included an inverted neomycin cassette was viable (TS2_neo).42 An overview of the genetic Cacna1c/Cav1.2 mouse models and their associated phenotypes are presented in table 2.
Table 2.
Overview of the Currently Studied Mouse Models of Cacna1c Dysfunction and Their Associated Psychiatric and Mood Phenotypes
| Study | Model | Phenotype |
|---|---|---|
| Bader et al42 | TS2_neo+/− | ↓ novelty induced locomotion |
| ↓ sociability | ||
| ↑ cued and contextual fear memory | ||
| ↓ extinction of fear memory | ||
| ↑ preservation in Y maze | ||
| Kabitzke et al43 | TS2_neo+/− | ↓ social-induced locomotion |
| ↓ sociability | ||
| Dao et al44 | Cacna1c +/− | ↓ exploratory activity in females |
| ↓ locomotion in females | ||
| ↓ depressive phenotype | ||
| ↑ anxiety in females | ||
| Bader et al42 | Cacna1c +/− | ↓ basal and novelty induced locomotion |
| ↑ anxiety | ||
| Bavley et al45 | Cacna1c +/− | ↓ depressive phenotype |
| Moosmang et al40 | Cacna1c −/− (forebrain only) | ↓ spatial discrimination |
| McKinney et al46 | Cacna1c −/− (forebrain excitatory neurons only) | No effect on contextual fear memory |
| White et al47 | Cacna1c −/− (forebrain only) | ↓ long-term spatial memory |
| Langwieser et al48 | Cacna1c −/− (CNS only) | No effect on cued fear memory |
| Lee et al49 | Cacna1c −/− (prefrontal cortex only) | ↑ anxiety |
| Temme et al50 | Cacna1c −/− (neurons only) | ↓ context discrimination |
| ↓ spatial memory (complex task) | ||
| ↓ neurogenesis | ||
| Lee et al51 | Cacna1c −/− (forebrain only) | ↓ neurogenesis |
| Kabir et al52 | Cacna1c −/− (prefrontal cortex only) | ↓ depressive phenotype |
| Dedic et al53 | Cacna1c +/− (excitatory neurons only) | ↓ sociability |
| ↓ depressive phenotypes | ||
| ↑ susceptibility to chronic social defeat stress | ||
| ↑ anxiety |
Motor Function
Neurodevelopmental disorders, particularly autism, can present with neurological disturbance of the motor system resulting in abnormal gait54,55 and dysfunctions in movement planning and execution.56 Bader et al42 reported that TS2_neo mice had similar motor abilities and reflexes in their home cage, however had decreased locomotion when placed in a novel environment. Consistently, another study reported that while TS2_neo mice had no deformities in gait, they had reduced locomotion in social tests such as reciprocal social interaction, urine open field test and increased freezing in the Smartcube platform challenge.43Cacna1c+/− mice were reported to be markedly hypoactive in both a home cage and novel environment,42 however studies on Cacna1c+/− mice using a rotarod paradigm40,44,48 did not report any differences in motor ability or co-ordination. A prefrontal cortex specific elimination of Cacna1c also did not result in any different basal locomotor behavior.49 Dao et al44 also reported no genotype differences in motor activity in the home cage however did report a slight hypoactivity in females in the open field test, as well as reduced exploratory activity in the holeboard test.
The role of Cav1.2 in motor activity thus requires further clarification, models suggest that dysfunction in Cacna1c may lead to elements of hypoactivity. It is important to consider that this reduced locomotion could in part reflect an indication of anxiety in contrast to a motor deficit per se.
Sociability
Social interactions, and the perceptions of them, are often altered in psychiatric patients. TS2_neo mice show no sociability defects in the 3 chamber test43 and maintained social memory,42 however present decreased activity in social interactions. They also initiate less social events, but maintain them longer.42,43 The Cacna1c+/− knock out mouse did not show any differences in social beahvior,42 however a Cacna1c+/− excitatory neuron knockout showed decreased sociality.53 This suggests that some subtle elements of social interactions may be affected in Cav1.2 dysfunction, but no global social deficits are present.
Fear Conditioning
Aversive associative learning processes such as fear conditioning can be used to investigate learning, memory, and cognitive processes in animal models. They can give us an understanding on the neural circuity, ie, affected in a wide range of psychiatric disorders. Interestingly, it has been shown that Cav1.2 levels are increased in the amygdala following fear conditioning.57 In genetic models, deletion of Cav1.2 in the anterior cingulate cortex results in decreased observational fear learning, where unconditioned mice develop freezing behavior by observing conditioned mice receiving foot shocks.58 TS2-neo mice can acquire cued fear conditioning correctly, however demonstrate increased freezing in context and cue recalls, as well as reduced extinction.42 The authors suggest that this is due to an enhanced perseverance of both tone and context memory. However, other models of Cacna1c knockdown do not show alterations of fear memory. Animals with neuron specific knockout of Cacan1c−/− show no impairments in acquisition, consolidation or recall of auditory,48 or contextual50 fear conditioning paradigms. However, Temme et al50 did show significant context discrimination deficits in their neuronal knockout model. The Cacna1c−/− (forebrain excitatory neurons only) model also maintained successful consolidation and extinction of conditioned fear.46 This disparity between the Cacna1c knockdown models and TS models is interesting and may suggest that there are some compensatory adaptations.48 Future studies on Cacna1c+/− models would be beneficial to further investigate Cav1.2’s influence over fear memory.
Anxiety and Depressive Phenotypes
Cacna1c +/− mice have shown decreased depressive-related phenotypes as assessed by the tail suspension test45 at 5–7 days following a chronic stress. Cacna1c heterozygosity has also been associated with protection against depressive-like phenotypes in the forced swim, sucrose preference, and tail suspension tests.44,52,53 However, Cacna1c+/− deletion during development increases susceptibility to chronic social defeat stress.53 In addition, a gene × environment human study revealed that SNPs in CACNA1C interact with trauma to predict depressive symptoms,53 suggesting that depressive phenotypes may be subject to environment factors interacting with CACNA1C.
Dao et al44 reported increased anxiety-related phenotypes in female Cacna1c+/− mice only.44 Increased anxiety-like phenotypes in males has been reported in Cacna1c+/− mice in an annex test,42 dark-light box,53 and in the open field,49 however, these findings are not consistent across all models.40,45 The TS2-neo model has not been associated with alterations in anxiety.42,43
The association between Cav1.2 and anxiety is still not fully understood. However, the current literature seems to suggest that Cacan1c heterozygosity may result in increased anxiety and this effect may be stronger in females.
Cognition
Elements of cognitive dysfunction, such as working memory, are common in psychiatric disorders and may represent core features of these conditions.59 The SNP rs1006737 was associated with increased prefrontal activity during executive cognition in healthy humans60 and impaired logical memory performance14 in those with schizophrenia. SNP rs2007044 was also associated with poor working memory in schizophrenia patients, potentially through decreased prefrontal cortex connectivity to other cortical regions.61
No significant differences were seen between TS2-neo mice and wild-types in the procedural T-maze,43 however, increased preservative behavior was observed in the Y maze.42 Elements of spatial memory have been shown to be affected in Cacna1c−/− conditional forebrain knockout mice.40,47,50 In the Morris water maze, knockout mice could learn the spatial task correctly,46,47 but they display spatial memory impairments when tested 30 days later.47 In a neuronal specific Cacna1c knockdown, mice could successful learn a simple Morris water maze but had profound deficits in the acquisition of spatial learning within a more complex maze when visual cues around the room were limited.50 Impairments in a water maze spatial discrimination task have also been reported.40
These findings have implications for understanding how genetic variants can have an impact on underlying cognitive impairments in psychiatric disorders, however more research is needed to clarify the primary domains affected. It will also be important to test animal models on tasks with a high degree of translational potential, such as rodent analogues of human touchscreen tasks, to facilitate future integrative research and drug development.
Neurogenesis
Cav1.2 may be required for more complex cognitive behaviors such as limited cued Morris water mazes where allocentric spatial representations are required. Data have shown that adult hippocampal neurogenesis is required for formation of complex forms of spatial representations but not simple,62 mirroring results seen in cognitive tasks following Cav1.2 knockdown. This suggests a possible deficit in adult hippocampus may be responsible for elements of behavior dysfunction in these models.
Psychiatric disorders, and in particular mood disorders, have been linked to alterations in adult neurogenesis.63 In rodents and humans, neurogenic niches have been found in the ventricular-subventricular zone in the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus (DG) in the hippocampus.64 These neurogenic cells of the hippocampus have been commonly associated with psychiatric and affective disorders63 although this is still controversial in the current literature. Adult hippocampal neurogenesis is a complex multistep process, ie, necessary for the generation of new neurons from neural stem cells (NSCs). A range of psychotropic medications have been associated with increasing neurogenesis in rodent models (including SSRIs, selective SNRIs, and tricyclic antidepressants).65 There is also increasingly evidence that hippocampal neurogenesis contributes to some forms of hippocampus-dependent learning and memory.66 There are many factors regulating this process: environmental cues, growth factors such as BDNF, glucocorticoids, and neurotransmitters.63 As the literature suggests that stress may interact with CACNA1C to cause depressive symptoms, and CACNA1C is known to mediate BDNF production, it may be hypothesized that Cav1.2 has a role in neurogenesis, through interacting with stress or BDNF.
LTCCs have been shown to regulate the conversion of adult hippocampal neural precursors to immature neurons in a bidirectional manner.67 This agrees with findings in genetic models; Cacna1c−/− deletions in the forebrain and neurons both show decreases in immature neurons (table 3). In the forebrain-Cav1.2 knockout, this was attributed to increased cell death of young neurons, correlated with decreased BDNF levels.51 However, in a pan-neuronal Cacna1c deletion marked decreases in cell proliferation were seen which is likely to be the cause of decreased numbers of immature neurons.50 Völkening et al68 deleted Cacna1c on type 1 cells and reported decreased proliferation and immature neuron production (table 3). These mice also showed deficits in a pattern separation paradigm—a type of learning thought to require intact hippocampal neurogenesis.68
Table 3.
Summary of the Findings Involving Hippocampal Neurogenesis in Rodent Models of Cacna1c/Cav1.2 Dysfunction
| Model | Proliferation Rate | Immature Neurons | Cell Survival | Dentate Gyrus Size | |
|---|---|---|---|---|---|
| Lee et al51 | Forebrain-Cav1.2 CkO (−/−) | n.d. | ↓ | ↓ | n.d. |
| Temme et al50 | Neuron-specific deletion of Cav1.2 CkO (−/−) | ↓ | ↓ | n.d. | |
| Völkening et al68 | TgGLAST-CreERT2 /Cacna1cfl/fl /RCE:loxP mice (Mouse model with Cacna1c-deficient Type-1 cells) | ↓ | ↓ | ||
| Moon et al, this study | Cacna1c+/− rat model | ↓ | n.d. | n.d. |
Note: n.d., no significant difference seen.
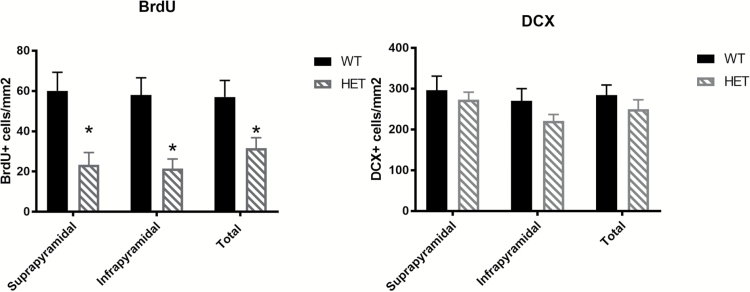
We have used a novel Cacna1c heterozygote (Cacna1c+/−) rat model to investigate if these findings could be replicated in another rodent species.69 We show a marked decrease in cells incorporating 5-bromo-2-deoxyuridine (BrdU)—a nucleotide analogue that marks dividing cells—suggesting that proliferation is significantly decreased in the SGZ in this model, confirming a key role for Cacna1c in neurogenesis across species. However, we do not see any difference in the number of immature neurons, contrasting with the findings in the mouse models (table 3, figures 1 and 2). This may be due to compensatory mechanisms such as decreased apoptosis resulting in increased cell survival. Further studies assessing long-term survival, over following the proliferation, survival, differentiation, and integration of newly born neurons following BrdU incorporation, would give valuable insight into the functional consequences of Cacna1c knockdown on psychology and behavior related to this process.
Fig. 1.
Cacna1c +/− rats show decreased BrdU, a marker of cell proliferation, in both suprapyramidal and infrapyramidal blades of the dentate gyrus (F = 11.9133, P = .0043, one-way ANOVA). There are no differences in doublecortin positive cells between Cacna1c+/− rats and wild-type littermates. Bars represent normalized mean per mm2 ±SEM, n = 8/genotype, all males.
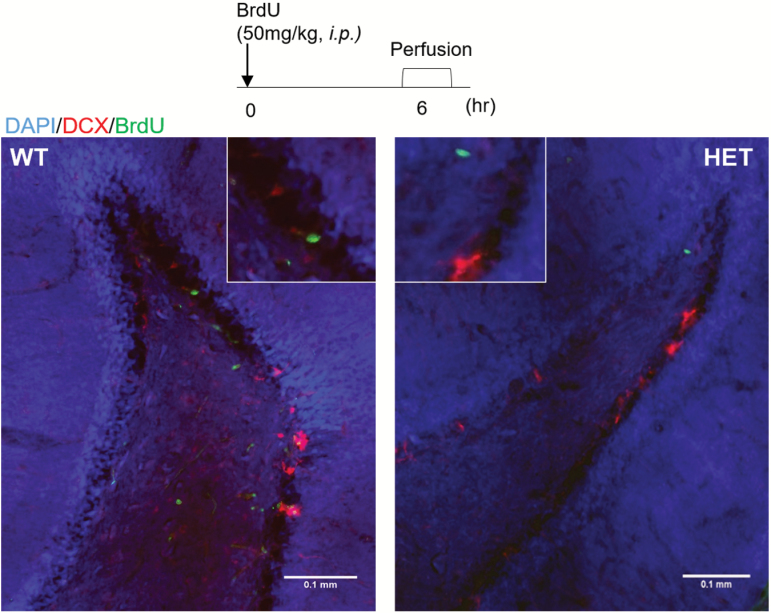
Fig. 2.
Representative immunofluorescent image of BrdU+ cells (green) and DCX+ cells (red) in the dentate gyrus of the hippocampus. For color, please see the figure online.
Conclusions
Associations of psychiatric disorders with the CACNA1C locus has been one of the most robust findings from genetic studies in mental health. This has led to the investigation of a number of animal models of genetic variation in Cacna1c to study potential risk pathways. These models have yielded some clues as to functional impacts—including potential alterations in motor behaviors, social interactions as well as increased anxiety, and preservative behavior. Interestingly, there may also be a subtle anti-depressive effect of a reduced gene dosage of Cacna1c, although interactions with stress may alter this phenotype.
Cav1.2 also appears to play an essential role in elements of hippocampal neuron production, suggesting that the alterations seen in neurogenesis in rodent models have also play a part in other phenotypes seen. This is of interest as disruptions in SGZ neurogenesis have been associated with both psychiatric disorders and treatment response. However, more work is needed to determine if this, in fact, a causative effect.
There are, of course, limitations to the work so far. The majority of the Cacna1c+/− models have focused on reduced gene dosage, whereas some of the genetic literature suggests that both loss and gain-of-function phenotype may be relevant to disease. Additionally, it is important to note that there is an imprecise relationship between rodent behavioral tests and human psychiatric disorders. Further studies using translational tasks and assessments in both animal models and human subjects with specific genetic variants in CACNA1C will be needed to build up the knowledge required for potential therapeutic targeting of LTCCs and associated pathways in psychiatric disorders.
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Laboratory Animals
All procedures were carried out in accordance with local ethics guidelines, the UK Home Office Animals Act 1986 and the European Communities Council Directive of 24 November 1986 (86/609/EEC). For further details on methods, please see supplementary material.
Funding
This work was supported by the Medical Research Council (PhD scholarship awarded to A.M.) and a Wellcome Trust Strategic Award (503147).
Acknowledgment
The authors report no conflict of interest.
References
- 1. Sklar P, Smoller JW, Fan J, et al. Whole-genome association study of bipolar disorder. Mol Psychiatry. 2008;13:558–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferreira MA, O’Donovan MC, Meng YA, et al. ; Wellcome Trust Case Control Consortium. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008;40:1056–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Green EK, Hamshere M, Forty L, et al. ; WTCCC. Replication of bipolar disorder susceptibility alleles and identification of two novel genome-wide significant associations in a new bipolar disorder case–control sample. Mol Psychiatry. 2013;18:1302–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gonzalez S, Xu C, Ramirez M, et al. Suggestive evidence for association between L-type voltage-gated calcium channel (CACNA1C) gene haplotypes and bipolar disorder in Latinos: a family-based association study. Bipolar Disord. 2013;15:206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu Y, Blackwood DH, Caesar S, et al. ; Wellcome Trust Case Control Consortium. Meta-analysis of genome-wide association data of bipolar disorder and major depressive disorder. Mol Psychiatry. 2011;16:2–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ruderfer DM, Fanous AH, Ripke S, et al. ; Schizophrenia Working Group of the Psychiatric Genomics Consortium; Bipolar Disorder Working Group of the Psychiatric Genomics Consortium; Cross-Disorder Working Group of the Psychiatric Genomics Consortium. Polygenic dissection of diagnosis and clinical dimensions of bipolar disorder and schizophrenia. Mol Psychiatry. 2014;19:1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lett TA, Zai CC, Tiwari AK, et al. ANK3, CACNA1C and ZNF804A gene variants in bipolar disorders and psychosis subphenotype. World J Biol Psychiatry. 2011;12:392–397. [DOI] [PubMed] [Google Scholar]
- 8. Green EK, Grozeva D, Jones I, et al. ; Wellcome Trust Case Control Consortium. The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Mol Psychiatry. 2010;15:1016–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nyegaard M, Demontis D, Foldager L, et al. CACNA1C (rs1006737) is associated with schizophrenia. Mol Psychiatry. 2010;15:119–121. [DOI] [PubMed] [Google Scholar]
- 10. He K, An Z, Wang Q, et al. CACNA1C, schizophrenia and major depressive disorder in the Han Chinese population. Br J Psychiatry. 2014;204:36–39. [DOI] [PubMed] [Google Scholar]
- 11. Ivorra JL, Rivero O, Costas J, et al. Replication of previous genome-wide association studies of psychiatric diseases in a large schizophrenia case–control sample from Spain. Schizophr Res. 2014;159:107–113. [DOI] [PubMed] [Google Scholar]
- 12. Guan F, Zhang B, Yan T, et al. MIR137 gene and target gene CACNA1C of miR-137 contribute to schizophrenia susceptibility in Han Chinese. Schizophr Res. 2014;152:97–104. [DOI] [PubMed] [Google Scholar]
- 13. Zheng F, Zhang Y, Xie W, et al. Further evidence for genetic association of CACNA1C and schizophrenia: new risk loci in a Han Chinese population and a meta-analysis. Schizophr Res. 2014;152:105–110. [DOI] [PubMed] [Google Scholar]
- 14. Hori H, Yamamoto N, Fujii T, et al. Effects of the CACNA1C risk allele on neurocognition in patients with schizophrenia and healthy individuals. Sci Rep. 2012;2:634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li J, Zhao L, You Y, et al. Schizophrenia related variants in CACNA1C also confer risk of autism. PLoS One. 2015;10:e0133247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wray NR, Pergadia ML, Blackwood DH, et al. Genome-wide association study of major depressive disorder: new results, meta-analysis, and lessons learned. Mol Psychiatry. 2012;17:36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Casamassima F, Huang J, Fava M, et al. Phenotypic effects of a bipolar liability gene among individuals with major depressive disorder. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:303–309. [DOI] [PubMed] [Google Scholar]
- 18. Hamshere ML, Walters JT, Smith R, et al. ; Schizophrenia Psychiatric Genome-wide Association Study Consortium; Wellcome Trust Case Control Consortium+; Wellcome Trust Case Control Consortium 2. Genome-wide significant associations in schizophrenia to ITIH3/4, CACNA1C and SDCCAG8, and extensive replication of associations reported by the schizophrenia PGC. Mol Psychiatry. 2013;18:708–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takahashi S, Glatt SJ, Uchiyama M, Faraone SV, Tsuang MT. Meta-analysis of data from the psychiatric genomics consortium and additional samples supports association of CACNA1C with risk for schizophrenia. Schizophr Res. 2015;168:429–433. [DOI] [PubMed] [Google Scholar]
- 20. Ripke S, Neale BM, Corvin A, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mühleisen TW, Leber M, Schulze TG, et al. Genome-wide association study reveals two new risk loci for bipolar disorder. Nat Commun. 2014;5:3339. [DOI] [PubMed] [Google Scholar]
- 22. Psychiatric Genetics Cross Disorder Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pardiñas AF, Holmans P, Pocklington AJ, et al. ; GERAD1 Consortium; CRESTAR Consortium; GERAD1 Consortium; CRESTAR Consortium; GERAD1 Consortium; CRESTAR Consortium. Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat Genet. 2018;50:381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stahl E, Breen G, Forstner A, et al. Genomewide association study identifies 30 loci associated with bipolar disorder. bioRxiv. 2018:173062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roussos P, Mitchell AC, Voloudakis G, et al. A role for noncoding variation in schizophrenia. Cell Rep. 2014;9:1417–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gershon ES, Grennan K, Busnello J, et al. A rare mutation of CACNA1C in a patient with bipolar disorder, and decreased gene expression associated with a bipolar-associated common SNP of CACNA1C in brain. Mol Psychiatry. 2014;19:890–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eckart N, Song Q, Yang R, et al. Functional characterization of schizophrenia-associated variation in CACNA1C. PLoS One. 2016;11:e0157086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Purcell SM, Moran JL, Fromer M, et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Rubeis S, He X, Goldberg AP, et al. ; DDD Study; Homozygosity Mapping Collaborative for Autism; UK10K Consortium. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515:209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jiang YH, Yuen RK, Jin X, et al. Detection of clinically relevant genetic variants in autism spectrum disorder by whole-genome sequencing. Am J Hum Genet. 2013;93:249–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dick IE, Joshi-Mukherjee R, Yang W, Yue DT. Arrhythmogenesis in timothy syndrome is associated with defects in Ca(2+)-dependent inactivation. Nat Commun. 2016;7:10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Splawski I, Timothy KW, Sharpe LM, et al. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. [DOI] [PubMed] [Google Scholar]
- 33. Ortner NJ, Striessnig J. L-type calcium channels as drug targets in CNS disorders. Channels (Austin). 2016;10:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wheeler DG, Groth RD, Ma H, et al. Ca(V)1 and Ca(V)2 channels engage distinct modes of Ca(2+) signaling to control CREB-dependent gene expression. Cell. 2012;149:1112–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tao X, West AE, Chen WG, Corfas G, Greenberg ME. A calcium-responsive transcription factor, CaRF, that regulates neuronal activity-dependent expression of BDNF. Neuron. 2002;33:383–395. [DOI] [PubMed] [Google Scholar]
- 36. Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. [DOI] [PubMed] [Google Scholar]
- 37. Weisskopf MG, Bauer EP, LeDoux JE. L-type voltage-gated calcium channels mediate NMDA-independent associative long-term potentiation at thalamic input synapses to the amygdala. J Neurosci. 1999;19:10512–10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Degoulet M, Stelly CE, Ahn KC, Morikawa H. L-type Ca²⁺ channel blockade with antihypertensive medication disrupts VTA synaptic plasticity and drug-associated contextual memory. Mol Psychiatry. 2016;21:394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Freir DB, Herron CE. Inhibition of L-type voltage dependent calcium channels causes impairment of long-term potentiation in the hippocampal CA1 region in vivo. Brain Res. 2003;967:27–36. [DOI] [PubMed] [Google Scholar]
- 40. Moosmang S, Haider N, Klugbauer N, et al. Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J Neurosci. 2005;25:9883–9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Striessnig J, Koschak A, Sinnegger-Brauns MJ, et al. Role of voltage-gated L-type Ca2+ channel isoforms for brain function. Biochem Soc Trans. 2006;34:903–909. [DOI] [PubMed] [Google Scholar]
- 42. Bader PL, Faizi M, Kim LH, et al. Mouse model of timothy syndrome recapitulates triad of autistic traits. Proc Natl Acad Sci U S A. 2011;108:15432–15437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kabitzke PA, Brunner D, He D, et al. Comprehensive analysis of two Shank3 and the Cacna1c mouse models of autism spectrum disorder. Genes Brain Behav. 2018;17:4–22. [DOI] [PubMed] [Google Scholar]
- 44. Dao DT, Mahon PB, Cai X, et al. ; Bipolar Genome Study (BiGS) Consortium. Mood disorder susceptibility gene CACNA1C modifies mood-related behaviors in mice and interacts with sex to influence behavior in mice and diagnosis in humans. Biol Psychiatry. 2010;68:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bavley CC, Fischer DK, Rizzo BK, Rajadhyaksha AM. Cav1.2 channels mediate persistent chronic stress-induced behavioral deficits that are associated with prefrontal cortex activation of the p25/Cdk5-glucocorticoid receptor pathway. Neurobiol Stress. 2017;7:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McKinney BC, Sze W, White JA, Murphy GG. L-type voltage-gated calcium channels in conditioned fear: a genetic and pharmacological analysis. Learn Mem. 2008;15: 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. White JA, McKinney BC, John MC, Powers PA, Kamp TJ, Murphy GG. Conditional forebrain deletion of the L-type calcium channel Ca V 1.2 disrupts remote spatial memories in mice. Learn Mem. 2008;15:1–5. [DOI] [PubMed] [Google Scholar]
- 48. Langwieser N, Christel CJ, Kleppisch T, Hofmann F, Wotjak CT, Moosmang S. Homeostatic switch in hebbian plasticity and fear learning after sustained loss of Cav1.2 calcium channels. J Neurosci. 2010;30:8367–8375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee AS, Gonzales KL, Lee A, et al. Selective genetic deletion of cacna1c in the mouse prefrontal cortex. Mol Psychiatry. 2012;17:1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Temme SJ, Bell RZ, Fisher GL, Murphy GG. Deletion of the mouse homolog of CACNA1C disrupts discrete forms of hippocampal-dependent memory and neurogenesis within the dentate gyrus. eNeuro. 2016;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lee AS, De Jesús-Cortés H, Kabir ZD, et al. The neuropsychiatric disease-associated gene cacna1c mediates survival of young hippocampal neurons. eNeuro. 2016;3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kabir ZD, Lee AS, Burgdorf CE, et al. Cacna1c in the prefrontal cortex regulates depression-related behaviors via REDD1. Neuropsychopharmacology. 2017;42:2032–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dedic N, Pöhlmann ML, Richter JS, et al. Cross-disorder risk gene CACNA1C differentially modulates susceptibility to psychiatric disorders during development and adulthood. Mol Psychiatry. 2018;23:533–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hallett M, Lebiedowska MK, Thomas SL, Stanhope SJ, Denckla MB, Rumsey J. Locomotion of autistic adults. Arch Neurol. 1993;50:1304–1308. [DOI] [PubMed] [Google Scholar]
- 55. Kindregan D, Gallagher L, Gormley J. Gait deviations in children with autism spectrum disorders: a review. Autism Res Treat. 2015;2015:741480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mari M, Castiello U, Marks D, Marraffa C, Prior M. The reach-to-grasp movement in children with autism spectrum disorder. Philos Trans R Soc Lond B Biol Sci. 2003;358:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shinnick-Gallagher P, McKernan MG, Xie J, Zinebi F. L-type voltage-gated calcium channels are involved in the in vivo and in vitro expression of fear conditioning. Ann N Y Acad Sci. 2003;985:135–149. [DOI] [PubMed] [Google Scholar]
- 58. Jeon D, Kim S, Chetana M, et al. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat Neurosci. 2010;13:482–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Millan MJ, Agid Y, Brüne M, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11:141–168. [DOI] [PubMed] [Google Scholar]
- 60. Bigos KL, Mattay VS, Callicott JH, et al. Genetic variation in CACNA1C affects brain circuitries related to mental illness. Arch Gen Psychiatry. 2010;67:939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cosgrove D, Mothersill O, Kendall K, et al. ; Wellcome Trust Case Control Consortium. Cognitive characterization of schizophrenia risk variants involved in synaptic transmission: evidence of CACNA1C’s role in working memory. Neuropsychopharmacology. 2017;42:2612–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dupret D, Revest JM, Koehl M, et al. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008;3:e1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Apple DM, Fonseca RS, Kokovay E. The role of adult neurogenesis in psychiatric and cognitive disorders. Brain Res. 2017;1655:270–276. [DOI] [PubMed] [Google Scholar]
- 64. Urbán N, Guillemot F. Neurogenesis in the embryonic and adult brain: same regulators, different roles. Front Cell Neurosci. 2014;8:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Malberg JE. Implications of adult hippocampal neurogenesis in antidepressant action. J Psychiatry Neurosci. 2004;29:196–205. [PMC free article] [PubMed] [Google Scholar]
- 66. Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory?Nat Rev Neurosci. 2010;11:339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. [DOI] [PubMed] [Google Scholar]
- 68. Völkening B, Schönig K, Kronenberg G, Bartsch D, Weber T. Deletion of psychiatric risk gene Cacna1c impairs hippocampal neurogenesis in cell-autonomous fashion. Glia. 2017;65:817–827. [DOI] [PubMed] [Google Scholar]
- 69. Horizon. Cacna1c knockout rat TGRA6930 2018. https://www.horizondiscovery.com/cacna1c-knockout-rat-tgra6930. Accessed February 5, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.