Abstract
Catatonia is an independent syndrome that co-occurs with several mental and medical conditions. We performed a systematic literature review in PubMed/Scopus until February 2017 and meta-analyzed studies reporting catatonia prevalence. Across 74 studies (cross-sectional = 32, longitudinal = 26, retrospective = 16) providing data collected from 1935 to 2017 across all continents, mean catatonia prevalence was 9.0% (k = 80, n = 110764; 95% CI = 6.9–11.7, I2 = 98%, publication bias P < .01), decreasing to 7.8% (k = 19, n = 7612, 95% CI = 7–8.7, I2 = 38.9%) in a subgroup with low heterogeneity. Catatonia prevalence was 23.9% (k = 8, n = 1168, 95% CI = 10–46.9, I2 = 96%) in patients undergoing ECT/having elevated creatinine phosphokinase. Excluding ECT samples, the catatonia prevalence was 8.1% (k = 72, n = 109606, 95% CI = 6.1–10.5, I2 = 98%, publication bias P < .01), with sensitivity analyses demonstrating that country of study origin (P < .001), treatment setting (P = .003), main underlying condition (P < .001), and sample size (P < .001)moderated catatonia prevalence, being highest in Uganda (48.5%, k = 1) and lowest in Mexico (1.9%, 95% CI = 0.4–8.8, I2 = 67%, k = 2), highest in nonpsychiatric out- or inpatient services (15.8%, 95% CI = 8.1–28.4, I2 = 97%, k = 15)and lowest in psychiatric outpatients services (3.2%, 95% CI = 1.7–6.1, I2 = 50%, k = 3), highest in presence of medical or neurological illness with no comorbid psychiatric condition (20.6%, 95% CI = 11.5–34.2, I2 = 95%, k = 10)and lowest in mixed psychiatric samples (5.7%, 95% CI = 4.2–7.7, I2 =98%, k = 43), highest in studies with sample sizes <100 (20.7%, 95% CI = 12.8–31.6, I2 = 90%, k = 17) and lowest in studies with sample sizes >1000 (2.3%, 95% CI = 1.3–3.9, I2 = 99%, k = 16). Meta-regression showed that smaller sample size (P < .01) and less major depressive disorder (P = .02) moderated higher catatonia prevalence. Year of data collection did not significantly moderate the results. Results from this first meta-analysis of catatonia frequencies across time and disorders suggest that catatonia is an epidemiologically and clinically relevant condition that occurs throughout several mental and medical conditions, whose prevalence has not decreased over time and does not seem to depend on different rating scales/criteria. However, results were highly heterogeneous, calling for a cautious interpretation.
Keywords: catatonia, meta-analysis, DSM5, severe mental illness, prevalence
Introduction
Catatonia is a complex psychopathological and clinical condition.1 The difficulties in the clinical diagnosis, conceptualization, and management of catatonia have been described as “the catatonic dilemma.”2,3 Clinical manifestations of catatonia are extremely heterogeneous and at least 40 separate signs of catatonia have been described,4 making recognition challenging with frequent misdiagnoses.5
The nosological conceptualization of catatonia ranges from the original concept catatonia as an independent syndrome as per Kahlbaum and Heckers, to the inclusion of catatonia into Kraepelin’s nomenclature of dementia praecox (although he acknowledged Kahlbaum’s theory, but coming to different nosology though), which influenced various diagnostic classifications to include a catatonia subtype of schizophrenia.6 Despite Bleuler’s assimilation of catatonia as a feature of schizophrenia, and despite DSM-III and -IV including the subtype of catatonic schizophrenia, which each ignored Taylor and Abrams’s reports describing a high frequency of catatonia in bipolar disorder (BD) rather than schizophrenia, the literature as well as DSM-5 gradually, but only partially,6,7 acknowledged Kahlbaum’s and Hecker’s original categorization of catatonia as an independent syndrome.8,9
In recent years, catatonia has accrued renewed interest in clinical research, as shown by an exponentially growing number of published studies (from a total of 1660 hits with “catatonia” search in PubMed from database inception until 2000, 546 from 2000 to 2010, and 738 just between 2010 and May 2017). Some authors10–15 proposed refined criteria to identify catatonia as a self-standing syndrome with its core clinical features that can be addressed by effective therapies, such as benzodiazepines (BDZs) and electroconvulsive treatment (ECT).1 However, international classification systems of psychiatric diseases have only partially accepted these proposals. In 2013, the Diagnostic and Statistical Manual of Psychiatric Disorders (DSM5)16 classified catatonia not as separate syndrome but as a “specifier,” which may occur among virtually all psychiatric disorders (mainly neurodevelopmental, psychotic, and mood disorders) as well as medical conditions and which may be associated to drug treatments (mainly antipsychotics). However, a change toward catatonia as an independent syndrome has been considered, but not made yet.9
Due to the fact that theoretical frameworks, nosological concepts, and rating scales/criteria used to diagnose catatonia have changed across time, and since pharmacological treatments for a wide range of nonpsychotic conditions shifted from mainly first-generation antipsychotics (FGAs) to second-generation antipsychotics (SGAs) that are associated with less extrapyramidal symptoms (with EPS being a risk factor for catatonia),17,18 it may be expected and has been described that the epidemiology of catatonia may have decreased over time.19,20
However, data on time trends are scarce and isolated and the prevalence of catatonia likely varies based on several factors, including the specific population and sampling frame, underlying diagnoses, definition of catatonia, country/continent of study origin, and time of the study, which may also be related to differences in specific medication and dosing patterns of antipsychotics.
Recent studies have reported that the prevalence of catatonia rates is around 10% among acute psychiatric inpatients,10,21 with apparently different prevalence ranges according to the underlying or comorbid condition, ie, 4%–67% for schizophrenia, 14%–71% for mood disorders, and 4%–46% for medical conditions.
As an additional complication, however, is that catatonia definitions vary according to the number, duration, definition, and severity of signs and symptoms, with different criteria having been used in different studies. Moreover, there are several different rating scales or catatonia criteria definitions that have been proposed to detect and measure catatonic symptomatology. A recent narrative review of different catatonia scales which have been used/developed in the last 3 decades22 identified the following: Modified Rogers Scale (MRS) in 1991,23,24 Rogers Catatonia Scale revised (RCS) in 1996,25 Bush–Francis Catatonia Rating Scale (BFCRS) in 1996,4 Northoff Catatonia Rating Scale (NCRS) in 1999,26 and the Catatonia Rating Scales in 2000.27 In addition, Carrol also proposed the Kanner Scale in 2000.28 Furthermore, frequencies of catatonia measured with these different rating scales may differ substantially (range: 3.4% to 10.3%).29 Despite the heterogeneous nature and theoretical background of the above-mentioned rating scales, which could raise the concern about the validity of the measured clinical syndrome, they have all shown high sensitivity/specificity.
Moreover, some studies have reported changes in rates of catatonic schizophrenia during different time periods at single sites,19,30 suggesting that at least catatonic schizophrenia may have become less common during the course of the 20th century. However, this supposedly decreased incidence may be influenced by several factors, including the selection of the type and dose regimen of FGAs or SGAs, to rating scales/criteria used to diagnose catatonia, or likelihood of misdiagnoses (secondary to neuroleptic malignant syndrome or rapid BDZ/anti-parkinsonian agent discontinuation), which has not been systematically and quantitatively assessed.13,31
Since the epidemiology of catatonia remains poorly investigated and has not been systematically evaluated, we conducted a systematic review and meta-analysis plus moderator analysis of the prevalence of catatonia and of potential moderators.
Methods
Search Strategy and Study Selection
This systematic review adhered to the MOOSE guidelines32 and PRISMA statement.33
Four authors divided into two pairs (GP, AG, BR, LM) independently searched PubMed, and Scopus from database inception until February 11, 2017, using the following search terms: (“catatonia” [MeSH Terms] OR “catatonia” [All Fields]) OR (“catatonia” [MeSH Terms] OR “catatonia” [All Fields] OR “catatonic” [All Fields]). We also checked the reference list of included articles and of relevant reviews. Studies were deemed eligible if they reported the prevalence of catatonia, or data allowing to compute it, in a psychiatric or medical clinical sample, with data gathered after 1935.
Data Extraction
Four authors divided into two pairs (GP, AG, BR, LM) independently extracted data, using a predetermined extraction form, including: catatonia prevalence (or variables needed to compute it), author, year of publication, year of data collection, country/continent of data collection, study design, setting, demographic characteristics, underlying main condition, employed catatonia rating scale used to diagnose catatonia, and percentage of subjects diagnosed with schizophrenia, major depressive disorder (MDD), BD, or other primary diagnoses, and prescription of FGAs or SGAs.
Quality Assessment
Two authors (BR, MS) independently assessed the quality of included studies with the Newcastle-Ottawa Scale (NOS), with a score of ≤5 (out of 9) indicating high risk of bias.34
Meta-analysis
Due to the anticipated heterogeneity, we utilized a random effects meta-analysis and calculated pooled prevalence and 95% confidence intervals (CIs) with comprehensive meta-analysis (CMA, version 3). Heterogeneity was assessed with the Cochrane Q and I2 statistics for each analysis.35 We conducted meta-regression analyses with CMA for outcomes with high heterogeneity (I2 > 50% and/or P < .05) and reported by ≥4 studies to investigate potential moderators of the observed catatonia prevalence. We conducted sensitivity analyses according to country, continent, rating scale/criteria used to define catatonia, treatment setting (nonpsychiatric out- or inpatient units, psychiatric inpatient units or psychiatric outpatients services), period of data collection, main underlying/co-morbid clinical condition (specific psychiatric diagnosis, or absence of any psychiatric condition, ie, medical/neurological condition) and quality of the study (post hoc, using the NOS score >5 as the threshold for high quality studies). We also investigated the following moderators: sample size, year of data collection, mean age, percentage of males, percent of subjects with schizophrenia, with mood disorders, with MDD, with BD, taking antipsychotics (FGAs and/or SGAs).
Publication bias was assessed via visual inspection of funnel plots and with the Begg–Mazumdar Kendall’s tau36 and Egger bias test.37 In case that publication bias was suspected, we calculated the trim and fill adjusted analysis38 to remove the most extreme small studies from the positive side of the funnel plot, and recalculated the effect size at each iteration until the funnel plot was symmetric around the (new/adjusted) effect size.
Whenever studies reported more than one prevalence result, providing frequencies for different catatonia criteria/rating scales, we averaged the frequencies to yield one single prevalence result for these studies and avoid double counting of subjects in the main analyses.
Given the fact that studies restricted to patients undergoing ECT or those with elevated levels of creatinine phosphokinase (CPK) inherently contain a selection bias toward higher catatonia prevalence in these enriched samples, we reported outcomes separately in these studies and the remaining ones not restricting their samples in this way. Thereafter, we conducted a comprehensive series of subgroup analyses or sensitivity analyses, adding also treatment setting (nonpsychiatric in- or outpatient services and psychiatric in- and outpatient units or services) and subgroups of studies with ascending numbers of included patients (as we found that sample size had a significant effect on the catatonia prevalence (ie, n ≥ 100, n ≥ 200, n ≥ 300, n ≥ 500, n ≥ 750, n ≥ 1000) in order to find possible explanations of the high observed heterogeneity. Finally, we trimmed down the same number of studies on both sides of the catatonia point prevalence of the studies not focusing on ECT/elevated CPK samples in an ascending fashion until we had a final study sample without significant heterogeneity, reporting this post hoc exploratory result in comparison to the overall prevalence to provide a benchmark of a nonheterogeneous catatonia prevalence estimate not influenced by outliers with overly high or low results, assessing the range and robustness of our primary findings.
Results
Search Results
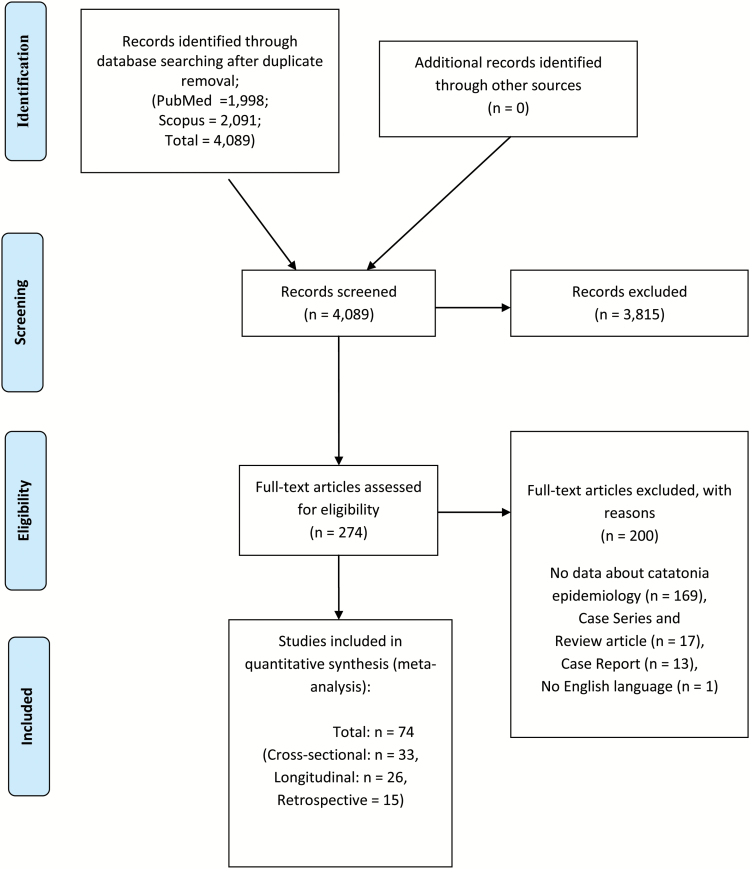
Out of initial 4089 hits in PubMed and Scopus after duplicate removal, 3813 studies were excluded after title/abstract reading. Full text articles of 276 studies were assessed, with further exclusion of 201 studies due to several reasons specified in figure 1. Finally, we included 73 studies that provided data about the prevalence of catatonia.20,21,26,29,39–107
Fig. 1.
PRISMA flowchart.
Characteristics of Included Studies
All included studies’ main features are reported in table 1.
Table 1.
Study, Sample and Catatonia Definition Characteristics of Included Studies (Organized by Median Year of Data Collection)
| Study | NOS scale Quality | Country | Region | Median Year of Data Collection | Sample Size | Study | Age | Population | Psychiatric Diagnosis | Medical, Neurologic, or Intellectual Disability Comorbidity | Catatonia Definition |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baran et al39 | 3 | Hungary | Europe | 1935 | 23 | LG | AD | Psychiatric inpatients, ECT | Mixed | N | ICD-10 |
| Flekkoy40 | 4 | Norway | Europe | 1956 | 72 | CS | AD | Psychiatric inpatients | SCZ | N | Study-defined criteria |
| *Guggenheim and Babigian41 | 6 | USA | North America | 1963 | 39475 | CS | AD | Psychiatric inpatients | Mixed, SCZ | N | Study-defined criteria |
| Kimura et al42 | 4 | Japan | Asia | 1967 | 173 | LG | C/A | Psychiatric outpatients | SCZ | N | Study-defined criteria |
| Kleinhaus et al43 | 8 | Israel | Asia | 1970 | 568 | LG | AD | Psychiatric inpatients | SCZ | N | ICD-10 |
| Petho et al44 | 8 | Hungary | Europe | 1971 | 276 | LG | AD | Psychiatric inpatients | Mixed | N | Leonhard’s criteria |
| Bland45 | 4 | USA | North America | 1972 | 1556 | CS | AD | Psychiatric inpatients | SCZ | N | ICD-8 |
| Scharfetter46 | 6 | Switzerland | Europe | 1973 | 140 | LG | AD | Psychiatric inpatients | SCZ | N | ICD |
| Serban47 | 6 | USA | North America | 1975 | 641 | CS | AD | Psychiatric inpatients | SCZ | N | DSM-II |
| Tsoi48 | 4 | Singapore | Asia | 1975 | 423 | CS | AD | Psychiatric inpatients | SCZ | N | ICD-9 + Bleuler |
| Strian and Klicpera49 | 6 | Germany | Europe | 1976 | 225 | LG | AD | Psychiatric inpatients | SCZ | N | ICD |
| Ihezue and Kumaraswamy50 | 7 | Nigeria | Africa | 1978 | 204 | LG | AD | Psychiatric inpatients | SCZ | N | Study-defined criteria |
| Beckmann et al51 | 6 | Germany | Europe | 1992 | 749 | CS | AD | Psychiatric inpatients and outpatients | SCZ | N | Leonhard’s criteria |
| Northoff et al52 | 6 | Germany | Europe | 1992 | 1143 | CS | AD | Psychiatric inpatients | Mixed | Y | Rosebush + Lohr and Wiesniwski |
| Beratis et al53 | 5 | Greece | Europe | 1993 | 374 | R | AD | Psychiatric inpatients | SCZ | N | DSM-III |
| Wing and Shah54 | 6 | UK | Europe | 1994 | 506 | CS | AD | Psychiatric inpatients and outpatients | Autism | N | Study-defined criteria |
| Northoff et al26 | 9 | Germany | Europe | 1994 | 1259 | CS | AD | Psychiatric inpatients | Mixed | N | Rosebush + Lohr and Wiesniwski |
| Peralta et al55 | 8 | Spain | Europe | 1994 | 272 | CS | AD | Psychiatric inpatients | Mixed | N | DSM-III + AMDP |
| Lee56 | 6 | Australia | Oceania | 1994 | 802 | R | AD | Psychiatric inpatients | Mixed | Y | Rosebush + Lohr and Wisenewski + CRS + CSI |
| *Stein et al57 | 5 | Israel | Asia | 1994 | 81 | R | AD, C/A | Psychiatric inpatients, ECT | Mixed | N | DSM-III, IV |
| Lykouras et al58 | 7 | Greece | Europe | 1995 | 120 | CS | AD | Psychiatric inpatients | Mixed | Y | DSM-IV |
| *Stompe et al59 | 7 | Austria | Europe | 1996 | 254 | CS | AD | Psychiatric inpatients | SCZ | N | DSM-IV, ICD-10, Leonhard’s criteria, Bleuler’s definition |
| Bush et al4 | 8 | USA | North America | 1996 | 215 | R | AD | Psychiatric inpatients | Mixed | N | BFCSI |
| Bräunig et al60 | 6 | Germany | Europe | 1997 | 61 | CS | AD | Psychiatric inpatients | BD | N | Braunig criteria |
| Northoff et al61 | 8 | Germany | Europe | 1997 | 385 | CS | AD | Psychiatric inpatients | Mixed | N | BFCRS, Lohr Criteria, Rosebush, Northoff Catatonia Scale |
| Lee62 | 5 | Australia | Oceania | 1997 | 1392 | LG | AD | Psychiatric inpatients | Mixed | Y | Rosebush + Lohr and Wisenewsky |
| Raffin et al63 | 6 | France | Europe | 1997 | 5532 | LG | C/A | Psychiatric inpatients | Mixed | Y | BFCRS |
| Conca et al64 | 7 | Austria | Europe | 1998 | 354 | LG | AD | Psychiatric inpatients | Mixed | N | ICD-10 + AMDP |
| Northoff et al65 | 5 | Germany | Europe | 1998 | 500 | CS | AD | Psychiatric inpatients | Mixed | N | BFCRS, Lohr Criteria, Rosebush, Northoff Catatonia Scale |
| Cohen et al66 | 6 | France | Europe | 1998 | 4976 | LG | C/A | Psychiatric inpatients | Mixed | Y | BFCRS |
| Krüger et al67 | 7 | Germany | Europe | 1999 | 76 | CS | AD | Psychiatric inpatients | SCZ | N | CRS |
| Koch et al68 | 5 | USA | North America | 1999 | 16 | R | AD | Medical and psychiatric inpatients, with elevated CPK | Mixed | Y | DSM-IV, BFCRS |
| Bark et al69 | 9 | Germany | Europe | 2000 | 276 | CS | AD | Psychiatric inpatients | SCZ | N | BFCRS, Lohr Criteria, Rosebush, Northoff Catatonia Scale |
| Stöber70 | 7 | Germany | Europe | 2000 | 749 | CS | AD | Psychiatric inpatients and outpatients | SCZ | N | Leonhard’s criteria |
| Tuerlings et al71 | 1 | Netherlands | Europe | 2000 | 285 | R | AD | Psychiatric inpatients, ECT | Mixed | Y | DSM-IV |
| Peralta and Cuesta72 | 6 | Spain | Europe | 2001 | 187 | CS | AD | Psychiatric inpatients | SCZ | N | Modified Rogers Scale |
| Consoli et al73 | 6 | France | Europe | 2001 | 5532 | LG | C/A | Psychiatric inpatients | Mixed | N | BFCRS |
| Gazdag et al74 | 6 | Hungary | Europe | 2001 | 43 | R | AD | Psychiatric inpatients, ECT | SCZ | N | Study-defined criteria |
| Krüger et al75 | 5 | Germany | Europe | 2002 | 99 | CS | AD | Psychiatric inpatients | BD | N | CRS |
| Ungvari et al76 | 8 | China | Asia | 2003 | 225 | CS | AD | Psychiatric inpatients | SCZ | N | BFCRS |
| Suzuki et al77 | 6 | Japan | Asia | 2004 | 51 | LG | AD | Psychiatric inpatients | SCZ | N | DSM IV |
| Benarous et al78 | 9 | France | Europe | 2004 | 6463 | LG | C/A | Psychiatric inpatients | Mixed | Y | PCRS (from BFCRS) |
| Narayanaswamy et al79 | 6 | India | Asia | 2005 | 7474 | LG | AD | Psychiatric inpatients | Mixed | N | DSM-IV |
| Grover et al80 | 5 | India | Asia | 2005 | 25 | R | C/A | Psychiatric inpatients, receiving ECT | Mixed | Y | BFCRS |
| Cavanna et al81 | 5 | UK | Europe | 2005 | 55 | CS | AD | Medical outpatients | None | Y | BFCRS, BFCSI |
| *Chalasani et al82 | 6 | UK, India | North America, Asia | 2006 | 208 | CS | AD | Psychiatric inpatients | Mixed | Y | DSM-IV, CSI, Morrison |
| Dutt et al83 | 5 | India | Asia | 2006 | 1056 | LG | AD | Psychiatric inpatients | Mixed | Y | BFCRS |
| Ghaziuddin et al84 | 2 | USA | North America | 2006 | 101 | R | C/A | Psychiatric inpatients | Mixed | Y | Study-defined criteria |
| Sayegh and Reid85 | 5 | UK | Europe | 2007 | 453 | LG | AD | Psychiatric inpatients | Mixed | Y | Modified Rogers scale |
| Cottencin et al86 | 5 | France | Europe | 2007 | 656 | LG | AD | Medical inpatients, consultation | Mixed | Y | Carrol |
| Mustafa et al87 | 5 | Kuwait | Asia | 2008 | 729 | R | AD | Psychiatric inpatients | Mixed | N | ICD-10 |
| Kruse et al88 | 4 | USA | North America | 2008 | 32 | R | AD | Medical and psychiatric inpatients | Mixed | Y | Study-defined criteria |
| Yoshimura et al89 | 5 | Japan | Asia | 2009 | 450 | R | AD | Psychiatric inpatients | SCZ | N | DSM-IV, BFCRS |
| Peralta et al90 | 4 | Spain | Europe | 2009 | 200 | LG | AD | Psychiatric inpatients | SCZ | N | DSM-IV |
| Peralta et al91 | 6 | Spain | Europe | 2009 | 200 | LG | AD | Psychiatric inpatients | SCZ | N | Modified Rogers scale |
| Benzoni et al92 | 6 | Italy | Europe | 2009 | 264 | LG | AD | Psychiatric inpatients, ECT | Mixed | Y | DSM-IV |
| Zahid and Ohaeri93 | 4 | Kuwait | Asia | 2009 | 130 | CS | AD | Psychiatric outpatients | SCZ | N | ICD-10 |
| Guinchat et al94 | 4 | France | Europe | 2010 | 58 | R | C/A | Psychiatric inpatients | Autism | Y | Study-defined criteria |
| Menard et al95 | 6 | France | Europe | 2010 | 15 | LG | C/A | Psychiatric inpatients | Mixed | N | BFCRS |
| Grover et al21 | 4 | India | Asia | 2011 | 201 | LG | AD | Psychiatric inpatients | Mixed | N | BFCSI |
| Medda et al96 | 6 | Italy | Europe | 2011 | 447 | CS | AD | Psychiatric inpatients, ECT | BD | N | DSM-V |
| Takahashi et al97 | 5 | Japan, Korea, Taiwan | Asia | 2011 | 324 | LG | AD | Psychiatric outpatients | SCZ | N | DSM-IV |
| *Grover et al98 | 5 | India | Asia | 2012 | 205 | CS | AD | Medical inpatients, consultation | None | Y | DSM5, BFCSI, BFCRS, Fink and Taylor |
| *Jaimes-Albornoz et al99 | 4 | Spain | Europe | 2012 | 348 | CS | AD, E | Medical inpatients, consultation | None | Y | DSM-IV, BFCSI, Fink and Taylor’s criteria |
| Nahar et al100 | 6 | India | Asia | 2013 | 200 | R | AD | Psychiatric inpatients, postpartum psychosis | Postpartum psychosis | N | BFCRS |
| *Sarkar et al29 | 5 | France | Europe | 2013 | 87 | CS | AD | Psychiatric inpatients | Mixed | N | DSM-5, ICD-10, BFCRS, BFCSI, CRS. |
| Ishida et al101 | 6 | Japan | Asia | 2013 | 911 | R | AD | Psychiatric inpatients, involuntarily admitted | Mixed | Y | BFCSI |
| Kakooza et al102 | 6 | Uganda | Africa | 2013 | 33 | CS | C/A | Medical inpatients | None | Y | BFCRS |
| Valencia et al103 | 5 | Mexico | South America | 2014 | 168 | CS | AD | Psychiatric inpatients | SCZ | N | DSM-IV |
| Espinola-Nadurille et al104 | 9 | Mexico | South America | 2014 | 2044 | LG | AD | Medical and psychiatric inpatients | Mixed | Y | DSM-5, BFCRS, BFCSI |
| Rajkumar105 | 6 | India | Asia | 2016 | 66 | CS | AD | Psychiatric inpatients | BD | N | Study-defined criteria. |
| *Kaelle et al106 | 5 | Australia | Oceania | 2014 | 108 | CS | E | Medical inpatients, consultation | Mixed | Y | BFCSI, BFCRS |
| *Usman et al107 | 5 | Nigeria | Africa | 1984, 2004 | 13968 | LG | AD | Psychiatric inpatients | Mixed | N | DSM-IV, BFCSI |
| *Van der Heijden et al20 | 5 | Netherlands | Europe | 1985, 1995, 1998, 2002 | 2805 | R | AD | Psychiatric inpatients | SCZ | N | DSM-III |
| Total: 74 studies | Mean 5.7 (1.5) | 40 Europe, 19 Asia, 8 North America, 3 Africa, 2 Oceania, 2 South America | From 1935 to 2016 | 110,774 | 32 CS, 26 LG, 16R | 63 adults, 11 children/ adolescents, 2 elderly | 55 psychiatric inpatients, 4 psychiatric consultation, 7 ECT treated patients,1 elevated CPK, 4 outpatients, 3 medical inpatients | 37 mixed, 26 SCZ, 4 BD, 2 autism, 4 none, 1 postpartum psychosis | 48 no, 26 yes | 25 Bush-Francis scales, 24 DSM, 11 ICD, 11 no validated tool, 7 Lohr, 7 Rosebush, 4 CRS, 4 Leonhard’s criteria, 3 Northoff, 3 Modified Rogers Scale, 2 CSI, 2 Fink and Taylor, 2 Bleuler, 1 Carrol | |
Note: AD, adults, BD, bipolar disorder; BFCRS: Bush-Francis catatonia rating scale; BFCSI: Bush-Francis catatonia screening instrument; C/A, children/adolescents; CPK, creatinine phosphokinase; CRS, catatonia rating scale; CS, cross-sectional; DSM, Diagnostic and statistical manual; E, elderly; ECT, electroconvulsive therapy; ICD, International classification of diseases; LG, longitudinal; PCRS, pediatric catatonia rating scale; R, retrospective; SCZ, schizophrenia. Asterisk indicates more than one sample in the study, or catatonia defined according to more than one definition.
We included 73 studies with a total population of 110559 subjects from 99 individual samples. Studies were conducted across all continents, with median year of data collection ranging from 1935 to 2017. The majority of studies, 55, included psychiatric inpatients, 7 described patients undergoing ECT, 4 studies provided data from psychiatric consultation services, 4 involved outpatients, while 3 studies included medical inpatients. The populations included subjects with several psychiatric conditions in 36 studies, while the sample was limited to patients diagnosed with schizophrenia in 26 studies, BD in 4 studies, autism in 2 studies, and postpartum psychosis in a single study. A comorbid medical or neurological condition was present in 26 studies. Catatonia was defined according to Bush-Francis scales (k = 25), DSM-any version (k = 24), ICD-any version (k = 11), Lohr’s criteria (k = 7), Rosebush’s criteria (k = 7), Catatonia Rating Scales (k = 4), Leonhard’s criteria (k = 4), Northoff’s criteria (k = 3), Modified Rogers Scale (k = 3), Catatonia Scale Instrument (k = 2), Fink and Taylor’s criteria (k = 2), Bleuler’s criteria (k = 2), or Carrol’s criteria (k = 1), while no validated tool was used in 11 studies. Europe was the most represented continent (k = 40), followed by Asia (k = 19), North America (k = 7), Africa (k = 3), Oceania (k = 2), and South America (k = 2). The design of the study was cross-sectional (k = 32), longitudinal prospective (k = 26), or retrospective (k = 15). The mean Newcastle-Ottawa scale quality score across all studies was 5.6 ± 1.5 (out of a range from 1–9), indicating medium risk of bias.
Meta-analysis of Catatonia Prevalence, Publication Bias, Heterogeneity in the Whole Sample
Results of meta-analysis are reported in detail in table 2.
Table 2.
Meta-analysis of Prevalence of Catatonia in Psychiatric and Medical Patients
| Subgroup/Moderator | Number of Samples | Number of Participants | Prevalence (%) | 95% Confidence Interval (CI) | I 2 (%) | |
|---|---|---|---|---|---|---|
| Lower 95% CI | Upper 95% CI | |||||
| Catatonia (main analysis) | 80 | 110774 | 9.0 | 6.9 | 11.7 | 98 |
| Catatonia (low heterogeneity subgroup) | 19 | 7612 | 7.8 | 7.0 | 8.7 | 39 |
| Catatonia (ECT/elevated CPK subgroup) | 8 | 1168 | 23.9 | 10.0 | 46.9 | 96 |
| Catatonia (no ECT/elevated CPK subgroup) | 72 | 109606 | 8.1 | 6.1 | 10.5 | 98 |
| Sensitivity analyses excluding patients undergoing ECT (based on 92 of 100 samples, from 72 of 80 studies) | ||||||
| Continent (between continent P value = .30) | ||||||
| North America | 8 | 50130 | 11.1 | 5.0 | 22.7 | 99 |
| Asia | 22 | 14113 | 10.0 | 6.3 | 15.4 | 98 |
| Europe | 52 | 37303 | 8.3 | 6.0 | 11.2 | 97 |
| Oceania | 4 | 2410 | 7.1 | 5.8 | 8.8 | 32 |
| Africa | 4 | 28173 | 5.9 | 0.1 | 31.7 | 99 |
| South America | 2 | 2212 | 1.9 | 0.4 | 8.8 | 67 |
| Countrya (between country P value P < .001) | ||||||
| Uganda | 1 | 33 | 48.5 | NA | NA | NA |
| China | 1 | 225 | 32.0 | NA | NA | NA |
| Switzerland | 1 | 140 | 27.1 | NA | NA | NA |
| UK | 6 | 1326 | 19.9 | 8.8 | 38.9 | 96 |
| Hungary | 1 | 276 | 19.6 | 15.3 | 24.7 | NA |
| Singapore | 1 | 423 | 16.8 | NA | NA | NA |
| Austria | 5 | 1370 | 12.1 | 8.1 | 17.6 | 86 |
| India | 12 | 10129 | 11.2 | 5.2 | 22.6 | 98 |
| USA | 8 | 50130 | 11.1 | 5.0 | 22.7 | 99 |
| Spain | 9 | 1631 | 10.7 | 8.2 | 13.8 | 69 |
| Germany | 11 | 5522 | 8.2 | 4.5 | 14.5 | 97 |
| Israel | 1 | 568 | 7.6 | 5.7 | 10.1 | NA |
| Japan | 5 | 1909 | 7.6 | 4.5 | 12.6 | 87 |
| Australia | 4 | 2410 | 7.1 | 5.8 | 8.8 | 32 |
| Netherlands | 4 | 2805 | 6.9 | 3.0 | 15.0 | 96 |
| Norway | 1 | 72 | 5.6 | NA | NA | NA |
| Kuwait | 2 | 859 | 4.9 | 3.2 | 7.6 | 22 |
| Greece | 2 | 494 | 3.9 | 2.5 | 6. | NA |
| France | 12 | 23667 | 3.6 | 2.1 | 6.2 | 95 |
| Nigeria | 3 | 28140 | 2.5 | 0.3 | 17.7 | 99 |
| Mexico | 2 | 2212 | 1.9 | 0.4 | 8.8 | 67 |
| Rating scale/criteria used to diagnose catatonia (between group P value = .13) | ||||||
| Other | 23 | 9430 | 11.9 | 8.7 | 16.1 | 96 |
| ICD | 10 | 4466 | 10.6 | 7.6 | 14.5 | 92 |
| Study-defined | 10 | 48781 | 10.2 | 5.0 | 19.6 | 99 |
| BFCSI | 8 | 1968 | 9,7 | 7.0 | 13.3 | 77 |
| BFCRS | 16 | 25648 | 7.4 | 3.2 | 16.1 | 99 |
| DSM | 25 | 44048 | 5.4 | 3.3 | 8.6 | 98 |
| Period of study conduct (between group P value = .13) | ||||||
| Before 1970 | 5 | 48382 | 5.4 | 2.0 | 13.6 | 99 |
| 1970–1980 | 7 | 3465 | 13.6 | 9.2 | 19.6 | 93 |
| 1980–1990 | 2 | 14880 | 2.4 | 0.2 | 21.7 | 99 |
| 1990–2000 | 26 | 22537 | 6.9 | 4.6 | 10.3 | 98 |
| 2001–2010 | 29 | 39087 | 10.4 | 6.3 | 16.7 | 98 |
| After 2010 | 23 | 5990 | 9.0 | 5.9 | 13.3 | 95 |
| Age group (between group P value = .38) | ||||||
| Adults (>18 years old) | 78 | 106006 | 9.0 | 7.0 | 11.4 | 98 |
| Elderly (>65 years old) | 5 | 552 | 8.5 | 6.4 | 11. | 0 |
| Children/adolescents | 9 | 22883 | 4.9 | 2.1 | 11.0 | 98 |
| Risk of bias (between group P value = .85) | ||||||
| NOS ≤ 5 | 45 | 42202 | 8.6 | 5.9 | 12.3 | 98 |
| NOS > 5 | 47 | 92139 | 8.2 | 6.0 | 11.2 | 99 |
| Main conditiona (between group P value < .001) | ||||||
| Medical or neurological illness | 10 | 1480 | 20.6 | 11.5 | 34.2 | 95 |
| Bipolar disorder | 3 | 226 | 20.1 | 9.6 | 37.3 | 94 |
| Postpartum psychosis | 1 | 200 | 20.0 | NA | NA | NA |
| Autism | 2 | 564 | 11.1 | 3.0 | 33.5 | 93 |
| Schizophrenia | 33 | 20276 | 9.8 | 8.0 | 12.0 | 95 |
| Mixed | 43 | 111595 | 5.7 | 4.2 | 7.7 | 98 |
| Treatment settinga (between group P value = .003) | ||||||
| Nonpsychiatric out- or inpatient units | 15 | 4412 | 15.8 | 8.1 | 28.4 | 97 |
| Psychiatric inpatients units | 74 | 129302 | 7.7 | 6.0 | 9.9 | 99 |
| Psychiatric outpatients services | 3 | 627 | 3.2 | 1.7 | 6.1 | 50 |
| Sample sizea (between group P value < .001) | ||||||
| <100 | 17 | 1069 | 20.7 | 12.8 | 31.6 | 90 |
| ≥100 | 19 | 2324 | 10.9 | 8.2 | 14.3 | 80 |
| ≥200 | 21 | 4766 | 12.9 | 9.7 | 17 | 93 |
| ≥300 | 4 | 1437 | 4.4 | 1.3 | 13.7 | 95 |
| ≥400 | 3 | 1326 | 11.1 | 7.0 | 17.3 | 88 |
| ≥500 | 12 | 8424 | 6.1 | 4.2 | 9.0 | 96 |
| ≥1000 | 16 | 114995 | 2.3 | 1.3 | 3.9 | 99 |
Note: Bold values indicates maini analyses.
aCountry, treatment setting, main underlying condition, and sample size influenced results.
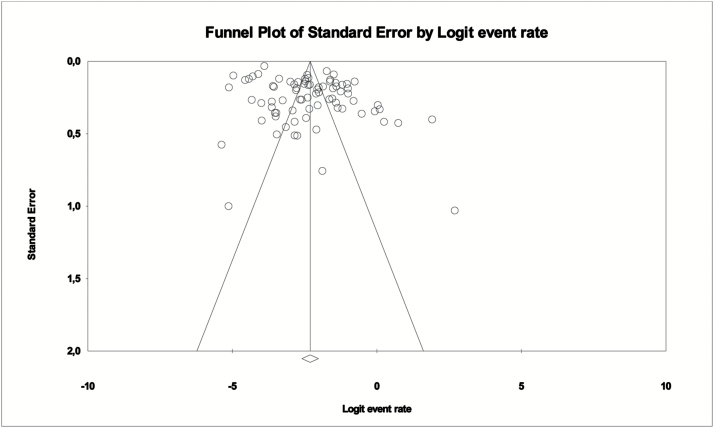
The overall pooled catatonia prevalence across 80 samples from 74 studies (some studies reported results from samples in different countries or time periods) and 110774 individuals was 9.0% (95% CI = 6.9–11.7, I2 = 98%). Heterogeneity was high (I2 = 98%, P < .0001). Publication bias was present according to Egger’s test (5.18, P < .01) and visual funnel plot inspection (figure 2), and results increased to 12.6% (95% CI = 9.1–17.4) after employing the trim and fill procedure and trimming 12 studies.
Fig. 2.
Funnel plot of meta-analysis of catatonia prevalence in whole included clinical populations (80 samples from 74 studies).
Meta-analysis of Catatonia Prevalence, Publication Bias, Heterogeneity, and Categorical Sensitivity Analysis Without Studies Selecting Patients Undergoing ECT or With Elevated CPK Levels
After exclusion of patients undergoing ECT/having elevated CPK, across 72 samples and 109606 subjects the pooled catatonia prevalence was 8.1% (95% CI = 6.1–10.5). Heterogeneity was high (I2 = 98%). Publication bias was evident according to Egger’s test (intercept 4.8, P < .01) and visual funnel plot inspection (supplementary figure 1), and the adjusted point estimate increased after trim and fill analysis (11 studies trimmed) to a catatonia prevalence of 11.3% (95% CI = 8.2–15.3).
In subgroup and sensitivity analyses of studies without ECT/elevated CPK samples the following significant moderators of catatonia prevalence were identified: country (P < .001), ranging from Mexico (1.9%, 95% CI = 0.4–8.8, I2 = 67%, k = 2) to Uganda (48.5%, 95% CI = NA, I2 = NA, k = 1); main underlying condition (P < .001), ranging from mixed psychiatric samples (5.7%, 95% CI = 4.2–7.7, I2 = 98%, k = 43) to medical or neurological illness with no comorbid psychiatric condition (20.6%, 95% CI = 11.5–34.2, I2 = 95%, k = 10); treatment setting (P = .003), ranging from psychiatric outpatient services (3.2%, 95% CI = 1.7–6.1, I2 = 50%, k = 3) to nonpsychiatric/medical out- or inpatient services (15.8%, 95% CI = 8.1–28.4, I2 = 97%, k = 15); sample size (P < .001), ranging from studies with sample sizes >1000 (2.3%, 95% CI = 1.3–3.9, I2 = 99%, k = 16) to sample sizes <100 (20.7%, 95% CI = 12.8–31.6, I2 = 90%, k = 17).
In contrast, the following variables did not significantly moderate catatonia prevalence: continent (P = .30), ranging from South America (1.9%, 95% CI = 0.4–8.8, I2 = 67%, k = 2) to North America (11.1%, 95% CI = 5.0–22.7, I2 = 99%, k = 8); rating scale/criteria (P = .13), ranging from DSM criteria (5.4%, 95% CI = 3.3–8.6, I2 = 98%, k = 25) to studies using other than ICD, DSM, BFCSI, BFCRS, or study defined diagnostic criteria (11.9%, 95% CI = 8.7–16.1, I2 = 96%, k = 23); time of data collection (P = .13), ranging from 1980 to 1990 (2.4%, 95% CI = 0.2–21.7, I2 = 99%, k = 2) to 1970–1980 (13.6%, 95% CI = 9.2–19.6, I2 = 93%, k = 7); age group (P = .38), ranging from children/adolescents (4.9%, 95% CI = 2.1–11, I2 = 98%, k = 9) to adults (9.0%, 95% CI = 7.0–11.4, I2 = 98%, k = 78).
Meta-regression Analysis of Potential Continuous Variable Moderators, in Samples Without Patients Undergoing ECT/Elevated CPK
The only significant continuous moderator variables of greater catatonia prevalence in the entire sample were smaller sample size (P < .001), and lower percentage of patients with MDD (P = .02) (table 3) Moreover, in the subgroup of studies including patients with conditions other than schizophrenia (k = 65), smaller sample size moderated higher prevalence (P < .001).
Table 3.
Mixed Effect Meta-regression of Moderators of Catatonia Prevalence across All Diagnoses, in Schizophrenia, and in Patients With Other Disorders than Schizophrenia (Excluding 8 Studies Restricted to Patients Undergoing Electroconvulsive Treatment or Having Elevated Creatine Phosphokinase)
| Moderator Variable | Number of Comparisons | β | 95% CI | P value | ||
|---|---|---|---|---|---|---|
| All diagnoses | Sample size | 92 | −0.000 | −0.000 | −0.000 | .00009 |
| Year of data collection | 92 | 0.000 | −0.009 | 0.027 | .36 | |
| Mean age | 92 | 0.006 | −0.011 | 0.024 | .48 | |
| % male | 92 | 0.007 | −0.009 | 0.023 | .38 | |
| % schizophrenia | 92 | −0.006 | −0.013 | 0.000 | .06 | |
| % mood disorders | 92 | −0.005 | −0.016 | 0.005 | .36 | |
| % SGA | 15 | −0.004 | −0.022 | 0.013 | .66 | |
| % FGA | 12 | 0.006 | −0.001 | 0.020 | .59 | |
| %MDD | 74 | −0.023 | −0.045 | −0.002 | .02 | |
| %BD | 74 | 0.000 | −0.011 | 0.017 | .65 | |
| Schizophrenia only | Sample size | 33 | −0.000 | −0.000 | 0.000 | .87 |
| Year of data collection | 33 | −0.000 | −0.022 | 0.021 | .93 | |
| Mean age | 33 | 0.000 | −0.045 | 0.045 | .99 | |
| % male | 33 | −0.004 | −0.034 | 0.026 | .79 | |
| %FGA | 5 | −0.003 | −0.014 | 0.008 | .58 | |
| %SGA | 10 | −0.017 | −0.038 | 0.002 | .09 | |
| Other disorders than schizophrenia | Sample size | 59 | −0.000 | −0.000 | −0.000 | .00023 |
| Year of data collection | 59 | 0.031 | −0.001 | 0.064 | .06 | |
| Mean age | 59 | 0.008 | −0.013 | 0.029 | .46 | |
| % male | 59 | 0.009 | −0.010 | 0.029 | .35 | |
| % mood disorders | 59 | −0.006 | −0.021 | 0.009 | .43 | |
| %FGA | 8 | 0.010 | −0.007 | 0.028 | .25 | |
| %SGA | 6 | 0.012 | −0.001 | 0.025 | .07 | |
Note: BD, bipolar disorder; FGA, first-generation antipsychotic; MDD, major depressive disorder; SGA, second-generation antipsychotic; SMI, severe mental illness. Bold P-values: P < .05. Bold values indicates significant moderators.
Meta-analysis of Catatonia Prevalence, Publication Bias, Heterogeneity in Studies Selecting Patients Undergoing ECT or with Elevated CPK Levels
The overall pooled catatonia prevalence across 8 samples and 1168 subjects was 23.9% (95% CI = 10–46.9, I2 = 96%). Heterogeneity was high (I2 = 96%, P < .0001). Publication bias was not evident according to Egger’s test (intercept 7.2, P = .18) or visual funnel plot inspection (supplementary figure 2).
Meta-analysis of Catatonia Prevalence in a Restricted Sample of Studies to Yield Low Heterogeneity
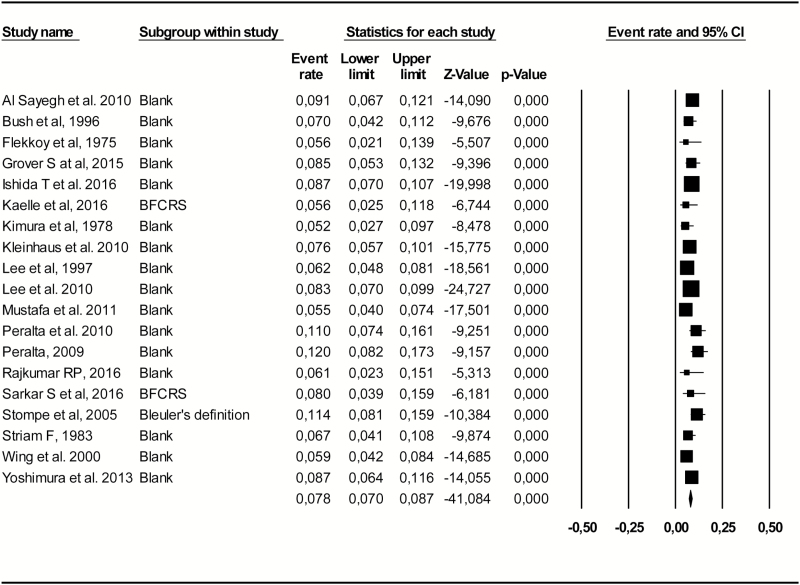
After visual inspection of funnel plot of non-ECT subgroup meta-analysis, we progressively excluded outlying studies with highest and lowest values until the pooled catatonia prevalence estimate had low heterogeneity with an I2 < 50%. Across 19 studies and 7612 subjects, the nonheterogeneous pooled catatonia prevalence was 7.8% (95% CI = 7–8.7, I2 = 39%) (figure 3).
Fig. 3.
Forest plot of catatonia prevalence in a subgroup of 19 studies with reduced heterogeneity (I2 < 50%).
Discussion
This comprehensive meta-analysis, including 74 studies and 107304 individuals from 99 independent samples across all continents, showed that the overall pooled, mean prevalence of catatonia was 9.2% among subjects diagnosed with a variety of psychiatric or medical conditions. Meta-regression results indicated that a lower sample size and a lower proportion of patients diagnosed with MDD significantly moderated higher catatonia prevalence in the entire sample. Finally, despite substantial heterogeneity, catatonia prevalence was not significantly affected by year of study conduct throughout a long-time span ranging from 1935 to 2017, as well as a number of varying patient, system, diagnostic, illness, and treatment variables.
Our results confirm that the catatonia syndrome is not rare among both people with severe mental illnesses and medical conditions. In fact, our findings suggest that catatonia may even be more frequent in patients with a medical condition, progressively decreasing through BD, to autism, schizophrenia, and mixed psychiatric illness, with MDD moderating lower catatonia prevalence. Prior studies suggested that comorbid medical conditions in patients with psychiatric disorders,73 delirium108 and in medically ill samples, especially the presence of encephalitis and seizure disorder, represented risk factors for catatonia.109 Our finding that the catatonia prevalence was higher in BD, even more than in schizophrenia or MDD, is consistent with the prior literature,21,82 with experts underlining the difference between schizophrenia and catatonia as 2 entities,8,110 and the trans-diagnostic nature across mental and psychiatric conditions.111 Moreover, although MDD has long been considered one of the conditions related to catatonia the most,21,108,112 our meta-regression findings suggested MDD as a moderator of lower prevalence of catatonia. However, this result should be interpreted with caution and in light of the absence of “pure” MDD samples precluding isolated sensitivity analysis for this diagnostic subgroup. Furthermore, one potential explanation for the relatively lower catatonia prevalence in samples with more MDD patients may be that with the advent of SGAs for the adjunctive treatment of MDD, doses used for this indication have become much lower than previously when used for psychotic depression or when using FGAs, and the catatonia prevalence in MDD samples may be lowered via a reduced risk for extrapyramidal side effects with low-dose SGA treatment, compared with higher SGA doses when used to treat BD or schizophrenia.
Several limitations of the present meta-analysis need to be considered. These include the limitations of the meta-analyzed sample, such as high heterogeneity of studies, populations, and treatments, as well as assessment and diagnostic strategies for catatonia. Nevertheless, the catatonia prevalence did not seem to be significantly affected by these factors in our meta-regression or sensitivity analyses. Additional limitations that need to be considered when interpreting the results include the presence of publication bias, yet results remained similar after statistical correction for this effect. Furthermore, there was a lack of more specific longitudinal medication-related information, which would have been needed to better disentangle the relationship between antipsychotic and/or other psychotropic medication treatment and catatonia. Moreover, since it may be sometimes difficult to differentiate catatonia from severe EPS or malignant neuroleptic syndrome,113 we cannot exclude some diagnostic imprecision. However, since all of these biases relate to all studies to a large degree and seem relatively independent of when and where the data were collected, the lack of a time trend difference in the frequency of catatonia seems to be a robust finding, withstanding the heterogeneity and potential biases inherent in the meta-analyzed database. Nevertheless, the fact that smaller sample sizes were related to higher catatonia prevalence indicates the potential of a selection bias in smaller sized studies that focused on more enriched samples for the risk and occurrence of catatonia. Thus, the prevalence estimates may be lower in population based samples, even when including patient subgroups with the diagnoses that have been associated with higher frequencies of catatonia. To address this shortcoming, large studies of representative samples are needed in order to further inform the prevalence range and moderator variables of catatonia. Additionally, under-represented countries with extreme prevalence values may have skewed our results. Moreover, no “pure” sample including only patients with MDD were available, precluding any sensitivity analysis for this pure diagnostic subgroup, and relatively few samples including BD patients, rendering the results in patients with affective disorders potentially less robust. Furthermore, the meta-analyzed data rely on published data, and unpublished results could alter the results. Hence, authors should include information on catatonia prevalence when describing their samples.6 Moreover, we included populations affected by potentially severe comorbid conditions, such as those with underlying medical disorders, or psychiatric inpatients, or neurological conditions, such as Tourette’s syndrome, or nodding syndrome, with frequent comorbid catatonic symptoms.81,88,102 Moreover, severity of catatonia was rarely reported. Thus, our catatonia prevalence estimate in clinical population may not be representative of patients affected by milder conditions. Nevertheless, we attempted to investigate this possibility by comparing catatonia prevalence estimates in psychiatric outpatient samples (ie, a potential proxy marker of milder illness) with those in inpatient and nonpsychiatric samples, finding that psychiatric outpatients had lower rates of catatonia, of 3.2%, ie, almost one third of the pooled overall catatonia prevalence, but only 3 small studies (n = 667) were available capturing such populations. Finally, we only used cross-sectional data in our analyses, thus, any causal relationship between catatonia prevalence and investigated moderators remains to be investigated in prospective studies.
Despite these limitations, this is the first comprehensive meta-analysis of the frequency and correlates of catatonia across diverse psychiatric and medical conditions that indicates that catatonia is not a disappearing clinical condition, as had been suggested before.19,20 Overall, our data seem to confirm Kahlbaum’s introduction of the concept of catatonia, around 150 years after he conceptualized it as a syndrome occurring in different psychiatric and medical conditions.8 Since catatonia has been associated with greater illness severity in BD60 and MDD,25 may recur,114 has been associated with an increased risk of suicide,43 and may be life-threatening,115 it deserves ongoing clinical attention. Moreover, when treated appropriately and not co-occurring with or misdiagnosed as schizophrenia,111 eg, when managed with BDZs and/or ECT, catatonia generally has a good outcome.116 In particular, instead of continuing antipsychotics treatment, quick relief from BDZs can actually confirm a diagnosis of catatonia and should encourage an escalation to higher doses of BDZs with a positive response in up to 80% of patients, while nonresponders should prompt referral to undergo ECT to maximize response rates.110 In order not to miss cases with catatonia, sufficiently sensitive and specific rating scales exist.16–23 In fact, at least according to our sensitivity analyses, the catatonia prevalences were not significantly different across rating scales used to diagnose catatonia. Thus, clinicians might feel free chose the scale/criteria they are more used to or that they find most practical. The clinical attention and regular screening for catatonia in patients with suggestive symptoms and signs is particularly relevant, as several authors have suggested that catatonia may remain under-recognized due to lack of awareness or symptomatic overlap with core motor/behavioral symptoms of autism, stuporous, negativistic, or melancholic depression, or negative and disorganized symptoms of schizophrenia.20,117,118
Taken together, our results suggest that beyond specific study settings, time of data collection, rating scales/criteria used to diagnose catatonia or continent, encompassing data from a wide and comprehensive range of settings and clinical conditions, catatonia is a clinically relevant condition that has remained relatively frequent as a feature of medical and psychiatric disorders, whose prevalence (different to some opinions) does not seem to have decreased over a long period of time. Risk factors appear to include underlying medical or neurological conditions. Additional, prospective cohort studies or detailed database and register studies are needed to better understand the current prevalence and risk factors for catatonia. Moreover, additional studies are needed to describe the outcome in specific patient subgroups in response to immediate or delayed identification of catatonia as well as to different interventions.
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Supplementary Material
Acknowledgments
Dr Solmi, Dr stubbs, Dr Fornaro, Dr Monaco, Dr Veronese, Dr Carvalho, Dr Pigato, Dr Roiter, Dr Guaglianone, and Dr Martini have no conflict of interest. Dr Correll has been a consultant and/or advisor to or has received honoraria from: Alkermes, Allergan, Bristol-Myers Squibb, Gerson Lehrman Group, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, Medavante, Medscape, Neurocrine, Otsuka, Pfizer, Sunovion, Takeda, and Teva. He has provided expert testimony for Bristol-Myers Squibb, Janssen, and Otsuka. He served on a Data Safety Monitoring Board for Lundbeck and Pfizer. Dr Correll received grant support from Takeda.
The Behavioral Neurogenetics Center, The Edmond and Lily Safra Children’s Hospital, Sheba Medical Center, Tel Hashomer 52621, Israel; tel: 972-3-530-2663; fax: 972-77-349-8317, e-mail: gothelf@post.tau.ac.il
References
- 1. Fornaro M. Catatonia: a narrative review. Cent Nerv Syst Agents Med Chem. 2011;11:73–79. [DOI] [PubMed] [Google Scholar]
- 2. Brenner I, Rheuban WJ. The catatonic dilemma. Am J Psychiatry. 1978;135:1242–1243. [DOI] [PubMed] [Google Scholar]
- 3. Penland HR, Weder N, Tampi RR. The catatonic dilemma expanded. Ann Gen Psychiatry. 2006;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bush G, Fink M, Petrides G, Dowling F, Francis A. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93:129–136. [DOI] [PubMed] [Google Scholar]
- 5. Fink M, Taylor MA. The many varieties of catatonia. Eur Arch Psychiatry Clin Neurosci. 2001;251(suppl 1):I8–I13. [DOI] [PubMed] [Google Scholar]
- 6. Francis A, Fink M, Appiani F et al. Catatonia in diagnostic and statistical manual of mental disorders, Fifth edition. J ECT. 2010;26:246–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heckers S, Tandon R, Bustillo J. Catatonia in the DSM—shall we move or not?Schizophr Bull. 2010;36:205–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fink M, Shorter E, Taylor MA. Catatonia is not schizophrenia: Kraepelin’s error and the need to recognize catatonia as an independent syndrome in medical nomenclature. Schizophr Bull. 2010;36:314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tandon R, Heckers S, Bustillo J et al. Catatonia in DSM-5. Schizophr Res. 2013;150:26–30. [DOI] [PubMed] [Google Scholar]
- 10. Fink MTM. Catatonia. A Clinician’s Guide to Diagnosis and Treatment. UK: Cambridge University Press. 2003. [Google Scholar]
- 11. Fink M, Taylor MA. The catatonia syndrome: forgotten but not gone. Arch Gen Psychiatry. 2009;66:1173–1177. [DOI] [PubMed] [Google Scholar]
- 12. Fink M. Catatonia: a syndrome appears, disappears, and is rediscovered. Can J Psychiatry. 2009;54:437–445. [DOI] [PubMed] [Google Scholar]
- 13. Caroff SN MS, Campbell EC, Sullivan KA.. Catatonia: From Psychopathology to Neurobiology. USA: American Psychiatric Publishing. 2004. [Google Scholar]
- 14. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6:391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ungvari GS, Goggins W, Leung SK, Lee E, Gerevich J. Schizophrenia with prominent catatonic features (‘catatonic schizophrenia’) III. Latent class analysis of the catatonic syndrome. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:81–85. [DOI] [PubMed] [Google Scholar]
- 16. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 17. Caroff SN, Hurford I, Lybrand J, Campbell EC. Movement disorders induced by antipsychotic drugs: implications of the CATIE schizophrenia trial. Neurol Clin. 2011;29:127–148, viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Solmi M, Murru A, Pacchiarotti I et al. Safety, tolerability, and risks associated with first- and second-generation antipsychotics: a state-of-the-art clinical review. Ther Clin Risk Manag. 2017;13:757–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stompe T, Ortwein-Swoboda G, Ritter K, Schanda H, Friedmann A. Are we witnessing the disappearance of catatonic schizophrenia?Compr Psychiatry. 2002;43:167–174. [DOI] [PubMed] [Google Scholar]
- 20. van der Heijden FM, Tuinier S, Arts NJ, Hoogendoorn ML, Kahn RS, Verhoeven WM. Catatonia: disappeared or under-diagnosed?Psychopathology. 2005;38:3–8. [DOI] [PubMed] [Google Scholar]
- 21. Grover S, Chakrabarti S, Ghormode D, Agarwal M, Sharma A, Avasthi A. Catatonia in inpatients with psychiatric disorders: a comparison of schizophrenia and mood disorders. Psychiatry Res. 2015;229:919–925. [DOI] [PubMed] [Google Scholar]
- 22. Sienaert P, Rooseleer J, De Fruyt J. Measuring catatonia: a systematic review of rating scales. J Affect Disord. 2011;135:1–9. [DOI] [PubMed] [Google Scholar]
- 23. McKenna PJ, Lund CE, Mortimer AM, Biggins CA. Motor, volitional and behavioural disorders in schizophrenia. 2: The ‘conflict of paradigms’ hypothesis. Br J Psychiatry. 1991;158:328–336. [DOI] [PubMed] [Google Scholar]
- 24. Lund CE, Mortimer AM, Rogers D, McKenna PJ. Motor, volitional and behavioural disorders in schizophrenia. 1: Assessment using the Modified Rogers Scale. Br J Psychiatry. 1991;158:323–327, 333. [DOI] [PubMed] [Google Scholar]
- 25. Starkstein SE, Petracca G, Tesón A et al. Catatonia in depression: prevalence, clinical correlates, and validation of a scale. J Neurol Neurosurg Psychiatry. 1996;60:326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Northoff G, Koch A, Wenke J et al. Catatonia as a psychomotor syndrome: a rating scale and extrapyramidal motor symptoms. Mov Disord. 1999;14:404–416. [DOI] [PubMed] [Google Scholar]
- 27. Bräunig P, Krüger S, Shugar G, Höffler J, Börner I. The catatonia rating scale I—development, reliability, and use. Compr Psychiatry. 2000;41:147–158. [DOI] [PubMed] [Google Scholar]
- 28. Carroll BT, Kirkhart R, Ahuja N et al. Katatonia: a new conceptual understanding of catatonia and a new rating scale. Psychiatry (Edgmont). 2008;5:42–50. [PMC free article] [PubMed] [Google Scholar]
- 29. Sarkar S, Sakey S, Mathan K, Bharadwaj B, Kattimani S, Rajkumar RP. Assessing catatonia using four different instruments: inter-rater reliability and prevalence in inpatient clinical population. Asian J Psychiatr. 2016;23:27–31. [DOI] [PubMed] [Google Scholar]
- 30. Morrison JR. Catatonia: prediction of outcome. Compr Psychiatry. 1974;15:317–324. [DOI] [PubMed] [Google Scholar]
- 31. Stübner S, Rustenbeck E, Grohmann R et al. Severe and uncommon involuntary movement disorders due to psychotropic drugs. Pharmacopsychiatry. 2004;37(suppl 1):S54–S64. [DOI] [PubMed] [Google Scholar]
- 32. Stroup DF, Berlin JA, Morton SC et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 33. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. [DOI] [PubMed] [Google Scholar]
- 34. Wells GASB, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P: The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp2017. Accessed July 1, 2017.
- 35. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 37. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 39. Baran B, Bitter I, Ungvari GS, Gazdag G. The birth of convulsive therapy revisited: a reappraisal of László Meduna’s first cohort of patients. J Affect Disord. 2012;136:1179–1182. [DOI] [PubMed] [Google Scholar]
- 40. Flekkoy K. Changes of associative performance in hospitalized schizophrenics: a 16-year follow-up. Acta Psychiatr Scand. 1975;52:330–335. [DOI] [PubMed] [Google Scholar]
- 41. Guggenheim FG, Babigian HM. Catatonic schizophrenia: epidemiology and clinical course. A 7-year register study of 798 cases. J Nerv Ment Dis. 1974;158:291–305. [DOI] [PubMed] [Google Scholar]
- 42. Kimura S, Asai S, Wakeno M, Aoki N. On early and mid-adolescent schizophrenia. Part 1: phenomenological aspects. Folia Psychiatr Neurol Jpn. 1978;32:41–56. [DOI] [PubMed] [Google Scholar]
- 43. Kleinhaus K, Harlap S, Perrin MC et al. Catatonic schizophrenia: a cohort prospective study. Schizophr Bull. 2012;38:331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Petho B, Tolna J, Tusnády G et al. The predictive validity of the Leonhardean classification of endogenous psychoses: a 21–33-year follow-up of a prospective study (“BUDAPEST 2000”). Eur Arch Psychiatry Clin Neurosci. 2008;258:324–334. [DOI] [PubMed] [Google Scholar]
- 45. Bland RC. Demographic aspects of functional psychoses in Canada. Acta Psychiatr Scand. 1977;55:369–380. [DOI] [PubMed] [Google Scholar]
- 46. Scharfetter C. Schizophrenia’s classical subtypes. A family heredity study. Arch Psychiatr Nervenkr (1970). 1982;231:443–447. [DOI] [PubMed] [Google Scholar]
- 47. Serban G. Stress in schizophrenics and normals. Br J Psychiatry. 1975;126:397–407. [DOI] [PubMed] [Google Scholar]
- 48. Tsoi WF. First admission schizophrenia: clinical manifestation and subtypes. Singapore Med J. 1993;34:399–402. [PubMed] [Google Scholar]
- 49. Strian F, Klicpera C. Anxiety in schizophrenic psychoses. Arch Psychiatr Nervenkr (1970). 1983;233:347–357. [DOI] [PubMed] [Google Scholar]
- 50. Ihezue UH, Kumaraswamy N. A psychosocial study of schizophrenic patients treated at a Nigerian psychiatric hospital. J Natl Med Assoc. 1984;76:617–621. [PMC free article] [PubMed] [Google Scholar]
- 51. Beckmann H, Franzek E, Stöber G. Genetic heterogeneity in catatonic schizophrenia: a family study. Am J Med Genet. 1996;67:289–300. [DOI] [PubMed] [Google Scholar]
- 52. Northoff G, Wenke J, Demisch L, Eckert J, Gille B, Pflug B. Catatonia: short-term response to lorazepam and dopaminergic metabolism. Psychopharmacology (Berl). 1995;122:182–186. [DOI] [PubMed] [Google Scholar]
- 53. Beratis S, Gabriel J, Hoidas S. Gender differences in the frequency of schizophrenic subtypes in unselected hospitalized patients. Schizophr Res. 1997;23:239–244. [DOI] [PubMed] [Google Scholar]
- 54. Wing L, Shah A. Catatonia in autistic spectrum disorders. Br J Psychiatry. 2000;176:357–362. [DOI] [PubMed] [Google Scholar]
- 55. Peralta V, Cuesta MJ, Mata I, Serrano JF, Perez-Nievas F, Natividad MC. Serum iron in catatonic and noncatatonic psychotic patients. Biol Psychiatry. 1999;45:788–790. [DOI] [PubMed] [Google Scholar]
- 56. Lee JW. Serum iron in catatonia and neuroleptic malignant syndrome. Biol Psychiatry. 1998;44:499–507. [DOI] [PubMed] [Google Scholar]
- 57. Stein D, Kurtsman L, Stier S, Remnik Y, Meged S, Weizman A. Electroconvulsive therapy in adolescent and adult psychiatric inpatients—a retrospective chart design. J Affect Disord. 2004;82:335–342. [DOI] [PubMed] [Google Scholar]
- 58. Lykouras L, Oulis P, Daskalopoulou E, Psarros K, Christodoulou GN. Clinical subtypes of schizophrenic disorders: a cluster analytic study. Psychopathology. 2001;34:23–28. [DOI] [PubMed] [Google Scholar]
- 59. Stompe T, Ortwein-Swoboda G, Ritter K, Marquart B, Schanda H. The impact of diagnostic criteria on the prevalence of schizophrenic subtypes. Compr Psychiatry. 2005;46:433–439. [DOI] [PubMed] [Google Scholar]
- 60. Bräunig P, Krüger S, Shugar G. Prevalence and clinical significance of catatonic symptoms in mania. Compr Psychiatry. 1998;39:35–46. [DOI] [PubMed] [Google Scholar]
- 61. Northoff G, Pfennig A, Krug M et al. Delayed onset of late movement-related cortical potentials and abnormal response to lorazepam in catatonia. Schizophr Res. 2000;44:193–211. [DOI] [PubMed] [Google Scholar]
- 62. Lee JWY. Neuroleptic-induced catatonia. J Clin Psychopharmacol. 2010;30:3–10. [DOI] [PubMed] [Google Scholar]
- 63. Raffin M, Zugaj-Bensaou L, Bodeau N et al. Treatment use in a prospective naturalistic cohort of children and adolescents with catatonia. Eur Child Adolesc Psychiatry. 2015;24:441–449. [DOI] [PubMed] [Google Scholar]
- 64. Conca A, Bertsch E, Küng A et al. Zuclopenthixol-acetate treatment in catatonic patients: the implication of iron metabolism. Eur Psychiatry. 2003;18:28–31. [DOI] [PubMed] [Google Scholar]
- 65. Northoff G, Nagel D, Danos P, Leschinger A, Lerche J, Bogerts B. Impairment in visual-spatial function in catatonia: a neuropsychological investigation. Schizophr Res. 1999;37:133–147. [DOI] [PubMed] [Google Scholar]
- 66. Cohen D, Nicolas JD, Flament MF et al. Clinical relevance of chronic catatonic schizophrenia in children and adolescents: evidence from a prospective naturalistic study. Schizophr Res. 2005;76:301–308. [DOI] [PubMed] [Google Scholar]
- 67. Krüger S, Bräunig P, Höffler J, Shugar G, Börner I, Langkrär J. Prevalence of obsessive-compulsive disorder in schizophrenia and significance of motor symptoms. J Neuropsychiatry Clin Neurosci. 2000;12:16–24. [DOI] [PubMed] [Google Scholar]
- 68. Koch M, Chandragiri S, Rizvi S, Petrides G, Francis A. Catatonic signs in neuroleptic malignant syndrome. Compr Psychiatry. 2000;41:73–75. [DOI] [PubMed] [Google Scholar]
- 69. Bark R, Dieckmann S, Bogerts B, Northoff G. Deficit in decision making in catatonic schizophrenia: an exploratory study. Psychiatry Res. 2005;134:131–141. [DOI] [PubMed] [Google Scholar]
- 70. Stöber G. Genetic predisposition and environmental causes in periodic and systematic catatonia. Eur Arch Psychiatry Clin Neurosci. 2001;251(suppl 1):I21–I24. [DOI] [PubMed] [Google Scholar]
- 71. Tuerlings JH, van Waarde JA, Verwey B. A retrospective study of 34 catatonic patients: analysis of clinical care and treatment. Gen Hosp Psychiatry. 2010;32:631–635. [DOI] [PubMed] [Google Scholar]
- 72. Peralta V, Cuesta MJ. Motor features in psychotic disorders. II. Development of diagnostic criteria for catatonia. Schizophr Res. 2001;47:117–126. [DOI] [PubMed] [Google Scholar]
- 73. Consoli A, Raffin M, Laurent C et al. Medical and developmental risk factors of catatonia in children and adolescents: a prospective case–control study. Schizophr Res. 2012;137:151–158. [DOI] [PubMed] [Google Scholar]
- 74. Gazdag G, Kocsis-Ficzere N, Tolna J. The augmentation of clozapine treatment with electroconvulsive therapy. Ideggyogy Sz. 2006;59:261–267. [PubMed] [Google Scholar]
- 75. Krüger S, Cooke RG, Spegg CC, Bräunig P. Relevance of the catatonic syndrome to the mixed manic episode. J Affect Disord. 2003;74:279–285. [DOI] [PubMed] [Google Scholar]
- 76. Ungvari GS, Leung SK, Ng FS, Cheung HK, Leung T. Schizophrenia with prominent catatonic features (‘catatonic schizophrenia’): I. Demographic and clinical correlates in the chronic phase. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:27–38. [DOI] [PubMed] [Google Scholar]
- 77. Suzuki K, Awata S, Takano T, Ebina Y, Iwasaki H, Matsuoka H. Continuation electroconvulsive therapy for relapse prevention in middle-aged and elderly patients with intractable catatonic schizophrenia. Psychiatry Clin Neurosci. 2005;59:481–489. [DOI] [PubMed] [Google Scholar]
- 78. Benarous X, Consoli A, Raffin M et al. Validation of the Pediatric Catatonia Rating Scale (PCRS). Schizophr Res. 2016;176:378–386. [DOI] [PubMed] [Google Scholar]
- 79. Narayanaswamy JC, Tibrewal P, Zutshi A, Srinivasaraju R, Math SB. Clinical predictors of response to treatment in catatonia. Gen Hosp Psychiatry. 2012;34:312–316. [DOI] [PubMed] [Google Scholar]
- 80. Grover S, Ghosh A, Ghormode D. Do patients of delirium have catatonic features? An exploratory study. Psychiatry Clin Neurosci. 2014;68:644–651. [DOI] [PubMed] [Google Scholar]
- 81. Cavanna AE, Robertson MM, Critchley HD. Catatonic signs in Gilles de la Tourette syndrome. Cogn Behav Neurol. 2008;21:34–37. [DOI] [PubMed] [Google Scholar]
- 82. Chalasani P, Healy D, Morriss R. Presentation and frequency of catatonia in new admissions to two acute psychiatric admission units in India and Wales. Psychol Med. 2005;35:1667–1675. [DOI] [PubMed] [Google Scholar]
- 83. Dutt A, Grover S, Chakrabarti S, Avasthi A, Kumar S. Phenomenology and treatment of Catatonia: a descriptive study from north India. Indian J Psychiatry. 2011;53:36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ghaziuddin N, Dhossche D, Marcotte K. Retrospective chart review of catatonia in child and adolescent psychiatric patients. Acta Psychiatr Scand. 2012;125:33–38. [DOI] [PubMed] [Google Scholar]
- 85. Sayegh AA, Reid D. Prevalence of catatonic signs in acute psychiatric patients in Scotland. The Psychiatrist. 2010;34:479–484. [Google Scholar]
- 86. Cottencin O, Warembourg F, de Chouly de Lenclave MB et al. Catatonia and consultation-liaison psychiatry study of 12 cases. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1170–1176. [DOI] [PubMed] [Google Scholar]
- 87. Mustafa M, Bassim R, Meguid M, Sultan M, Al Dardiry M. Ethnic differences in the prevalence of catatonia among hospitalized psychiatric patients in Kuwait. Middle East Current Psychiatry. 2012;19:214–221. [Google Scholar]
- 88. Kruse JL, Lapid MI, Lennon VA et al. Psychiatric autoimmunity: N-Methyl-D-aspartate receptor IgG and beyond. Psychosomatics. 2015;56:227–241. [DOI] [PubMed] [Google Scholar]
- 89. Yoshimura B, Hirota T, Takaki M, Kishi Y. Is quetiapine suitable for treatment of acute schizophrenia with catatonic stupor? A case series of 39 patients. Neuropsychiatr Dis Treat. 2013;9:1565–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Peralta V, Campos MS, de Jalon EG, Cuesta MJ. DSM-IV catatonia signs and criteria in first-episode, drug-naive, psychotic patients: psychometric validity and response to antipsychotic medication. Schizophr Res. 2010;118:168–175. [DOI] [PubMed] [Google Scholar]
- 91. Peralta V, Campos MS, De Jalón EG, Cuesta MJ. Motor behavior abnormalities in drug-naïve patients with schizophrenia spectrum disorders. Mov Disord. 2010;25:1068–1076. [DOI] [PubMed] [Google Scholar]
- 92. Benzoni O, Fàzzari G, Marangoni C, Placentino A, Rossi A. Treatment of resistant mood and schizoaffective disorders with electroconvulsive therapy: a case series of 264 patients. J Psychopathol. 2015;21:266–268. [Google Scholar]
- 93. Zahid MA, Ohaeri JU. Schizophrenia psychopathology in a Kuwaiti Arab sample. Psychopathology. 2010;43:345–356. [DOI] [PubMed] [Google Scholar]
- 94. Guinchat V, Cravero C, Diaz L et al. Acute behavioral crises in psychiatric inpatients with autism spectrum disorder (ASD): recognition of concomitant medical or non-ASD psychiatric conditions predicts enhanced improvement. Res Dev Disabil. 2015;38:242–255. [DOI] [PubMed] [Google Scholar]
- 95. Menard ML, Thümmler S, Auby P, Askenazy F. Preliminary and ongoing French multicenter prospective naturalistic study of adverse events of antipsychotic treatment in naive children and adolescents. Child Adolesc Psychiatry Ment Health. 2014;8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Medda P, Toni C, Luchini F, Giorgi Mariani M, Mauri M, Perugi G. Catatonia in 26 patients with bipolar disorder: clinical features and response to electroconvulsive therapy. Bipolar Disord. 2015;17:892–901. [DOI] [PubMed] [Google Scholar]
- 97. Takahashi N, Takahashi M, Saito T et al. Randomized, placebo-controlled, double-blind study assessing the efficacy and safety of paliperidone palmitate in Asian patients with schizophrenia. Neuropsychiatr Dis Treat. 2013;9:1889–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Grover S, Kate N, Gupta G. Use of electroconvulsive therapy in an adolescent patient with catatonia. Indian J Psychol Med. 2014;36:195–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Jaimes-Albornoz W, Serra-Mestres J. Prevalence and clinical correlations of catatonia in older adults referred to a liaison psychiatry service in a general hospital. Gen Hosp Psychiatry. 2013;35:512–516. [DOI] [PubMed] [Google Scholar]
- 100. Nahar A, Kondapuram N, Desai G, Chandra PS. Catatonia among women with postpartum psychosis in a Mother-Baby inpatient psychiatry unit. Gen Hosp Psychiatry. 2017;45:40–43. [DOI] [PubMed] [Google Scholar]
- 101. Ishida T, Sakurai H, Watanabe K, Iwashita S, Mimura M, Uchida H. Incidence of deep vein thrombosis in catatonic patients: a chart review. Psychiatry Res. 2016;241:61–65. [DOI] [PubMed] [Google Scholar]
- 102. Kakooza-Mwesige A, Dhossche DM, Idro R, Akena D, Nalugya J, Opar BT. Catatonia in Ugandan children with nodding syndrome and effects of treatment with lorazepam: a pilot study. BMC Res Notes. 2015;8:825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Valencia M, Fresán A, Barak Y, Juárez F, Escamilla R, Saracco R. Predicting functional remission in patients with schizophrenia: a cross-sectional study of symptomatic remission, psychosocial remission, functioning, and clinical outcome. Neuropsychiatr Dis Treat. 2015;11:2339–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Espinola-Nadurille M, Ramirez-Bermudez J, Fricchione GL, Ojeda-Lopez MC, Perez-González AF, Aguilar-Venegas LC. Catatonia in Neurologic and Psychiatric Patients at a Tertiary Neurological Center. J Neuropsychiatry Clin Neurosci. 2016;28:124–130. [DOI] [PubMed] [Google Scholar]
- 105. Rajkumar RP. Recurrent unipolar mania: a comparative, cross-sectional study. Compr Psychiatry. 2016;65:136–140. [DOI] [PubMed] [Google Scholar]
- 106. Kaelle J, Abujam A, Ediriweera H, Macfarlane MD. Prevalence and symptomatology of catatonia in elderly patients referred to a consultation-liaison psychiatry service. Australas Psychiatry. 2016;24:164–167. [DOI] [PubMed] [Google Scholar]
- 107. Usman DM, Olubunmi OA, Taiwo O, Taiwo A, Rahman L, Oladipo A. Comparison of catatonia presentation in patients with schizophrenia and mood disorders in Lagos, Nigeria. Iran J Psychiatry. 2011;6:7–11. [PMC free article] [PubMed] [Google Scholar]
- 108. Cuevas-Esteban J, Iglesias-González M, Rubio-Valera M, Serra-Mestres J, Serrano-Blanco A, Baladon L. Prevalence and characteristics of catatonia on admission to an acute geriatric psychiatry ward. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:27–33. [DOI] [PubMed] [Google Scholar]
- 109. Smith JH, Smith VD, Philbrick KL, Kumar N. Catatonic disorder due to a general medical or psychiatric condition. J Neuropsychiatry Clin Neurosci. 2012;24:198–207. [DOI] [PubMed] [Google Scholar]
- 110. Fink M. Clinical practice to change with divorce of catatonia and schizophrenia. J Clin Psychopharmacol. 2013;33:287–288. [DOI] [PubMed] [Google Scholar]
- 111. Fink M, Taylor MA. Catatonia: subtype or syndrome in DSM?Am J Psychiatry. 2006;163:1875–1876. [DOI] [PubMed] [Google Scholar]
- 112. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160:1233–1241. [DOI] [PubMed] [Google Scholar]
- 113. Northoff G. Neuroleptic malignant syndrome and catatonia: one entity or two?Biol Psychiatry. 1996;40:431–433. [DOI] [PubMed] [Google Scholar]
- 114. Lin CC, Hung YY, Tsai MC, Huang TL. Relapses and recurrences of catatonia: 30-case analysis and literature review. Compr Psychiatry. 2016;66:157–165. [DOI] [PubMed] [Google Scholar]
- 115. Cornic F, Consoli A, Tanguy ML et al. Association of adolescent catatonia with increased mortality and morbidity: evidence from a prospective follow-up study. Schizophr Res. 2009;113:233–240. [DOI] [PubMed] [Google Scholar]
- 116. Sienaert P, Dhossche DM, Vancampfort D, De Hert M, Gazdag G. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Breen J, Hare DJ. The nature and prevalence of catatonic symptoms in young people with autism. J Intellect Disabil Res. 2017;61:580–593. [DOI] [PubMed] [Google Scholar]
- 118. Mazzone L, Postorino V, Valeri G, Vicari S. Catatonia in patients with autism: prevalence and management. CNS Drugs. 2014;28:205–215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.