Abstract
Introduction
Converging evidence suggests that hippocampal subregions subserve different functions, and are differentially affected by psychosis illness progression. Despite this fact, studies have not often studied subregions cross-sectionally across the psychosis spectrum. Furthermore, little is known about associations between subregion volumes and hippocampus-mediated cognition.
Methods
A total of 222 participants (61 ultra high risk [UHR], 91 schizophrenia [SCZ], and 70 healthy volunteers) underwent a 3T MRI scan, as well as structured clinical interviews and a cognitive battery. Hippocampal subfield analysis was conducted with Freesurfer. We compared subregion volumes across groups, controlling for age, gender, and intracranial volume. We also examined associations in the UHR and SCZ groups between hippocampal subregion volumes and verbal learning, visual learning, and working memory.
Results
We found a dose-dependent relationship such that the SCZ group showed significantly greater subfield volume reductions than the UHR group, which in turn showed significantly greater subfield volume reductions than the healthy volunteer group. We also found associations between subregion volume and cognitive performance in the visual memory, verbal memory, and working memory domains.
Discussion
Our study examined hippocampal subregion volumes cross-sectionally in a large sample across the psychosis spectrum, as well as links with hippocampus-mediated cognitive function. Our findings suggest that hippocampal abnormalities emerge before first psychosis episode onset, and may be etiologically informative.
Keywords: hippocampal subregions, psychosis spectrum, medial-temporal function, cognition
Introduction
The hippocampus modulates a range of functions including emotion, stress regulation, memory, and cognition.1 Given these roles, and the structure’s unique susceptibility to environmental insults and stressors,1–6 it is perhaps unsurprising that it has been widely hypothesized and reported to be altered in psychotic disorders,7–9: lower hippocampus volume is among the most consistently reported imaging findings in schizophrenia (SCZ).8,10–12 However, less is known about the pathogenic role that hippocampal abnormalities might play in the early progression of illness, or how this might relate to changes in cognitive function that characterize the prodromal (UHR) period, immediately preceding onset.
Previous studies have indicated that hippocampal abnormalities may be present in those at clinical and genetic high risk for developing SCZ, and that these may be predictive of illness progression.13–16 Despite this fact, recent meta analyses have converged on the consensus that there may not be overall volume differences between high risk and healthy volunteer (HV) groups.7,17–22 However, the literature in this area has been mixed, with some studies reporting smaller hippocampal volume16,23,24 and others larger volumes in these groups.25,26 Importantly, many questions also remain regarding the timing of hippocampal abnormalities across the progression to psychosis: as noted, some studies report smaller hippocampal volumes prior to psychosis onset,16,23,24 while others find hippocampal volume alterations only after first psychotic episode onset7,25,27 and yet others report no differences between SCZ and healthy volunteer groups.28,29
Compelling evidence suggests that more fine-grained analyses of subregion volumes or shape may be more sensitive to group differences than gross volumetric approaches,30 which may miss subtle developmental changes affecting different regions of the structure.8,19,21,31–33 Multiple landmark studies suggest that distinct areas within the hippocampus serve divergent functions, and are differentially affected by environmental and neuromaturational factors, as well as by psychosis illness progression.8,28,30,33–35 Several past groups have likewise found associations between abnormal hippocampal morphometry, asymmetry, and psychosis illness progression.7,28 A set of promising recent studies have compared hippocampal subregion volumes in psychotic disorders,36 and, more recently, hippocampal subregions and illness progression within individuals at high risk of developing psychosis.18
Thus, examining hippocampal subregions instead of studying the structure as a homogenous entity offers a promising avenue to clarifying questions about how it is affected during an important pathogenic period. Further, a cross-sectional approach would aid in isolating etiological factors from medication-mediated changes,37 and benefit power relative to longitudinal high-risk studies which rely on comparisons with significantly smaller converting subsamples.18 Cross-sectional studies comparing hippocampal subregions between UHR for psychosis, psychosis, and volunteer groups are needed in order to better understand psychosis progression.
Declarative memory, mediated by medial temporal structures, is among the most consistently impaired functions in SCZ.9,38 Cognitive domains heavily implicated in groups with SCZ and unaffected siblings include verbal memory, visual memory, processing speed, and working memory.38 In the prodrome, studies suggest that memory impairments predict likelihood of converting to a psychotic disorder.9 Examining relationships between specific subregions and cognitive domains is essential given that these subregions have divergent functions crucial for learning and memory, and are differentially affected by psychosis.34–37 For example, studies suggest SCZ populations exhibit reduced neurogenesis and glutamate transmission in the dentate gyrus, potentially impairing its function of fostering orthogonalization of hippocampal representations of similar but distinct events.8 Further, studies suggest this population may exhibit plasticity changes associated with long-term potentiation within CA3 specifically, which may ultimately lead to an enhanced production of incorrect/illogical associations.34 Some groups37 have begun to examine relationships between hippocampal subregion volumes and declarative memory, but these have lacked comparisons of more specific cognitive domains, as well as comparisons in groups at different stages of illness progression.
In the present study, we examine hippocampal subregion volumes cross-sectionally in a large sample across the psychosis spectrum, and examine links with hippocampus-mediated cognitive function. Given the functional specificity of the subregions,34 we predict that the CA2/3 and CA4/dentate gyrus regions will be particularly affected in a dose–response fashion, with healthy volunteers having significantly larger volumes than the UHR group, which will in turn be significantly less affected than the SCZ group. In addition, we predict that these regions will be associated with hippocampus-mediated cognition, specifically in the visual learning, verbal learning, and working memory domains.9,33
Methods
Participants
Our sample contained 222 subjects comprising groups of 70 HV, 61 UHR, and 91 SCZ. Participants were recruited as part of an ongoing collaboration between the Intermountain Neuroimaging Consortium sites at the University of Colorado Boulder and the Mind Research Network (Albuquerque, New Mexico). All procedures were reviewed and approved by the local institutional review board at each institution. In accordance with HIPAA, subject identifiable information was only shared among team members listed on the IRB protocol. Participants 18 years old or older gave written consent to participate. A parent gave written consent for participants younger than 18 years of age, who gave written assent as well. The Colorado sample comprised our HV (n = 70) and UHR (n = 61) samples. Adolescent and young adult HV and UHR subjects were recruited to the University of Colorado Boulder’s Adolescent Development and Preventive Treatment (ADAPT) research program. Exclusion criteria included head injury, presence of a neurological disorder, lifetime substance dependence, and the presence of any contraindication to the magnetic resonance imaging environment. The presence or lifetime history of an Axis I psychotic disorder were exclusion criteria for UHR participants. Exclusion criteria for healthy volunteers included presence of a psychotic disorder in a first-degree relative. SCZ patients (n = 91) were recruited as part of the Mind Research Network COBRE ongoing study based in Albuquerque, New Mexico. Patients were recruited from inpatient and outpatient psychiatric clinics, group homes, referrals from physicians, and advertisements. Patients met criteria for SCZ based on the Structured Clinical Interview for DSM-IV Axis I disorders (SCID)39 and were confirmed by review of the case file. Exclusion criteria for SCZ patients included head injury, presence of a neurological disorder, and the presence of any contraindication to the magnetic resonance imaging environment. In our SCZ sample, patients self-reported an average of 16.6 years since their first psychotic symptom emerged to the date of assessment. For our UHR sample, the average was 14.1 days between prodromal diagnoses during clinical assessment and the MRI scan. Of our initial sample, 4 HV, 11 UHR, and 15 SCZ were excluded from analyses due to inability to appropriately and successfully normalize and segment their neuroimaging scans (see below for imaging data processing). Medication status for SCZ and UHR was collected during the clinical interview; see table 1 for calculated chlorpromazine equivalent doses (CPZ)40 and percentage of subjects on antipsychotics. For those in the SCZ sample who were on antipsychotics (92%), 87.0% were on atypical antipsychotics and 15.4% were on typical antipsychotics; of the typical antipsychotics, 9 (10.7%) were on haloperidol, 2 (2.2%) were on perphenazine, and 2 (2.4%) were on fluphenazine. Of the atypical antipsychotics for our SCZ sample, 17 (20.2%) were on olanzapine, 7 (8.3%) were on quetiapine, 5 (6.0% were on ziprasidone, 16 (19.0%) were on clozapine, 14 (16.7%) were on aripiprazole, and 33 (39.3%) were on risperidone. Of our UHR subjects who were on antipsychotics (13%), all were on atypical antipsychotics, with 2 (25.0%) on aripiprazole, 3 (37.5%) on risperidone, 1 (12.5%) on quetiapine, and 2 (25.0%) on lurasidone.
Table 1.
Demographics by Diagnostic Group
| HV (1) (n = 70) | UHR (2) (n = 61) | SCZ (3) (n = 91) | Group Diff. | |
|---|---|---|---|---|
| Demographics | ||||
| Gender | 44% male | 61% male | 84% male | 3 > 1, 2; 1 = 2 |
| Age | 18.3 (2.7) | 18.7 (1.8) | 38.3 (14) | 3 > 1, 2*; 1 = 2 |
| % on antipsychotics, CPZ | 0% (0) | 13%, 118 mg (65 mg) | 92%, 382 mg (321 mg) | 3 > 2 > 1* |
| Cognition | ||||
| Working memorya | 54 (8.4) | 53 (7.6) | 38 (12.3) | 3 < 1, 2*; 1 = 2 |
| Verbal learningb | 50 (8.7) | 51 (10.6) | 38 (8.4) | 3 < 1, 2*; 1 = 2 |
| Visual learningc | 54 (7.8) | 54 (8.2) | 38 (12.3) | 3 < 1, 2*; 1 = 2 |
| IQ | 107 (14.9) d | 111 (12.7) d | 99 (17.6)e | 3 < 1, 2*; 1 = 2 |
| Hippocampus volume, mm3 | ||||
| Left | 4286 (509) | 4261 (442) | 3736 (480) | 3 < 1, 2*; 1 = 2 |
| Right | 4375 (495) | 4337 (372) | 3842 (528) | 3 < 1, 2*; 1 = 2 |
| Symptoms | ||||
| Positive | — | 13 (3.9)f | 15.31 (4.9)g | — |
| Negative | 11 (6.8)f | 15 (5)g | ||
Note: Mean (SD), 3 = schizophrenia (SCZ), 2 = UHR, 1 = HV. CPZ, chlorpromazine equivalent dose.
aMATRICS working memory domain t score.
bMATRICS verbal learning domain t score.
cMATRICS visual learning domain t score.
dEstimated by WRAT (Wide Range Achievement Test).
eEstimated by WASI (Wechsler Abbreviated Scale of Intelligence) composite score.
fMeasured by SIPS battery.
gMeasured by PANNS battery.
*P < .05.
Clinical Assessments
All subjects were administered the Structured Clinical Interview for DSM-IV Axis I disorders (SCID).39 The SCID was administered to diagnose psychosis disorder in the SCZ group, and to rule out a psychosis diagnosis for the UHR and HV groups, as well as to assess history of mood and anxiety disorders. For the SCZ group, symptom ratings were completed using the Positive and Negative Syndrome Scale (PANSS).41 The Structured Interview for Prodromal Syndromes (SIPS) was administered to UHR and HV participants in order to diagnose UHR subjects and rule out symptoms in healthy volunteers.42 Total sum scores for positive and negative symptoms are summarized in table 1.
Structural Imaging
Magnetic resonance imaging scans were acquired in both locations using matching parameters, with a 3-Tesla Siemens Tim Trio magnetic resonance imaging scanner (Siemens Healthineers, Erlangen, Germany) and a standard 12-channel head coil. This arrangement is part of a consortium between the Mind Research Network and University of Colorado Boulder, designed to facilitate multimodal imaging projects but adopting identical sequences, as well as careful between site reliability maintenance. Boulder scanner set up and quality testing was directed by New Mexico where the COBRE data were collected. Quality control scans were run daily and weekly in an identical manner following the exact same protocol, at both sites. Structural images were collected with a T1-weighted 3D magnetization prepared rapid gradient multi-echo sequence (sagittal plane; repetition time (TR) = 2530 ms; echo times (TE) = 1.64, 3.5, 5.36, 7.22, and 9.08 ms; GRAPPA parallel imaging factor of 2; 1 mm3 isomorphic voxels, 192 interleaved slices; FOV = 256 mm; flip angle = 7°, time = 6:03 min). A T2 weighted acquisition (axial oblique aligned with anterior commissure-posterior commissure line; TR = 3720 ms; TE = 89 ms; GRAPPA parallel imaging factor 2; 0.9 mm × 0.9 mm voxels; FOV = 240 mm; flip angle: 120°; 77 interleaved 1.5 mm slices; time = 5:14) was acquired in order to check for incidental pathology, whereby MRI technologists identified potential image quality issues, and forwarded images of concern to radiologists for a formal review. Data acquired at the Mind Research Network were downloaded from the Collaborative Informatics and Neuroimaging Suite data exchange tool (COINS; http://coins.mrn.org/dx43).
Consistent with earlier studies examining hippocampal subregions in psychosis,18,36,37 we processed our data using the 5.3 FreeSurfer software. Recent studies have confirmed the transplatform reliability across versions for this study’s subregions of interest.44 After processing and quality checking the structural images, hippocampal subregion segmentation was carried out using the FreeSurfer 5.3 software package.45 The FreeSurfer 5.3 hippocampal subregion segmentation package uses a Bayesian probabilistic model, and has been validated against manual morphometric measurements of ultra-high-resolution magnetic resonance imaging scans. Extracted volumes include CA1, 2/3, 4/dentate gyrus, presubiculum, subiculum, hippocampal fissure, and fimbria. Voxel subregion measurements were made on images interpolated to 0.5 × 0.5 × 0.5 mm3.
Cognitive Battery
All groups completed the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB).46 To limit the number of comparisons, we focused on cognitive domains most relevant to hippocampal function including performance in the visual learning, verbal learning, and working memory domains. Performance was converted to a t score for use in analyses. We examined relationships between subregion volumes and MATRICS battery working memory, visual learning, and verbal learning domain scores in the UHR and SCZ groups using partial correlations controlling for age, gender, and intracranial volume (ICV).
Statistical Analyses
One-way analysis of variance and chi-square tests, when appropriate, were used to test for demographic differences between diagnostic groups. To test the omnibus effect of stage of illness (diagnosis) on subregion volumes, we performed a 3 × 10 mixed model repeated measures ANCOVA. We then performed univariate ANCOVAs predicting subregion volumes with illness progression stage (HV, UHR, and SCZ groups), controlling for ICV, age, and gender. Regions assessed included left and right overall hippocampal volume, as well as CA1, 2/3, 4/dentate gyrus, presubiculum, and subiculum volumes. We did not include the fimbria, hippocampal fissure, and hippocampal tail due to reliability concerns in segmenting smaller subregion areas.45 Given possible confounding effects of antipsychotic medications on brain volumes,47 we examined correlations between regional volumes and CPZ equivalents in the UHR and SCZ groups. There were no significant correlations in either group (P > .1). Therefore, we presented results as originally planned.
Results
Our sample included 222 participants (70 HV, 61 UHR, and 91 SCZ; see table 1 for demographics and group differences). Preliminary analyses were conducted to determine if UHR youth treated with antipsychotics showed differences on target variables compared with those not on antipsychotics, and no significant differences were found. We found group differences in age, gender, antipsychotic usage, and ICV (treated as covariates in subsequent analyses). Consistent with the literature9,38 we also detected group differences in cognitive performance in the MATRICS domain scores for working memory, visual learning, and verbal learning, where the healthy volunteer group performed better than clinical groups in a dose dependent manner (table 1).
Hippocampal Subregions
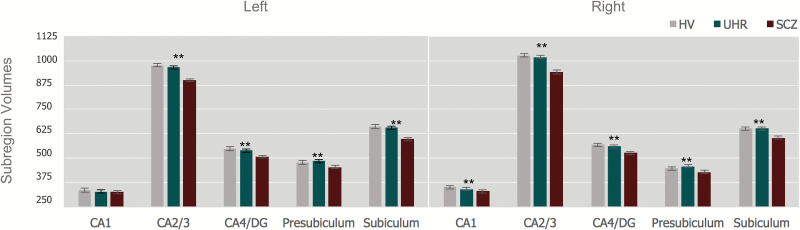
We found significantly smaller left and right hippocampal volumes in the SCZ group compared with HV and UHR groups. The HV and UHR groups did not significantly differ in hippocampal volume (table 1). The 3 × 10 mixed model repeated measures ANCOVA examining the omnibus effect with Greenhouse-Geisser correction of diagnosis on subregion volume was significant controlling for age, gender, and ICV, F(2, 221) = 6.104, P <.001. ANCOVAs predicting subregion volumes with group (HV, UHR, SCZ), controlling for age, gender, and ICV (figure 1), showed significant group effects for right [F(2, 221) = 11.48, P <.001, partial η2 = .1] but not left CA1 [F(2, 221) = 1.53, P = .22, partial η2 = .01], right [F(2, 221) = 17.94, P < .001, partial η2 = .14)] and left CA2/3 [F(2, 221) = 9.18, P < .001, partial η2 = .08], right [F(2, 221) = 16.82, P < .001, partial η2 = .14] and left CA 4/dentate gyrus [(F(2, 221) = 8.98, P < .001, partial η2 = .08], right [F(2, 221) = 10.75, P < .001, partial η2 = .09] and left presubiculum [F(2, 221) = 8.75, P < .001, partial η2 = .08], and right [F(2, 221) = 11.76, P < .001, partial η2 = .1] and left subiculum volumes [F(2, 221) = 9.35, P < .001, partial η2 = .08].
Fig. 1.
Diagnostic group comparisons of hippocampal subregion volumes (mm3). ** P < .001, ANCOVA comparing across groups controlling for age, gender, and intracranial volume. Error bars: ±1 SE.
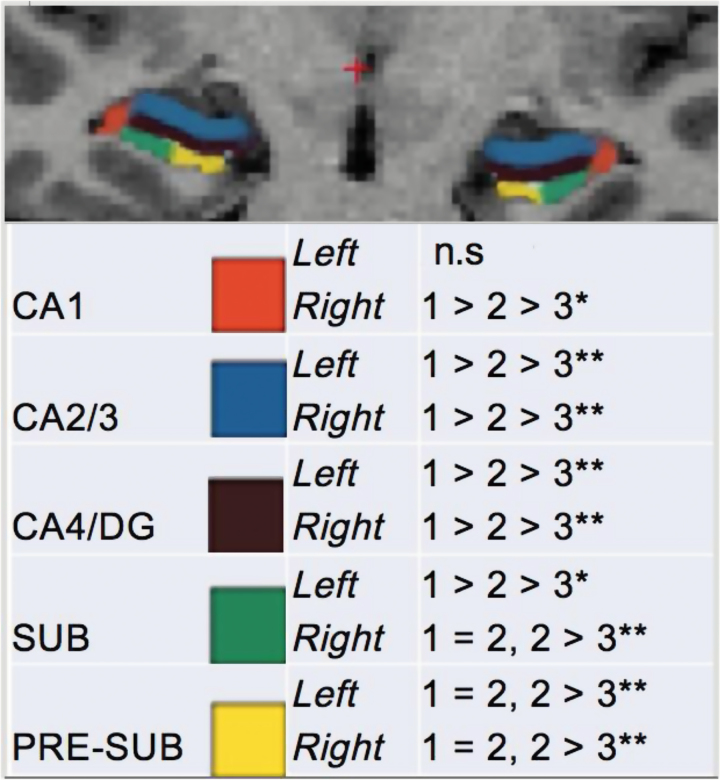
Post hoc analyses indicated that subregion volumes were significantly lower in the UHR group compared with the HV group bilaterally for CA1, 2/3, and 4/DG (figure 2). Compared with the UHR group, the SCZ group exhibited significantly reduced subregion volumes bilaterally for CA2/3, 4/DG, presubiculum, and subiculum. Our findings suggest a dose–response relationship whereby illness progression is significantly associated with lower subregion volumes cross-sectionally, when controlling for age, gender, and ICV (figure 2; supplementary table 1).
Fig. 2.
Post-hoc comparisons of subregion volumes by diagnostic group. *P < .05, **P < .01, controlling for intracranial volume, gender, and age; sub, subiculum; 1, healthy volunteer; 2, HR; 3, schizophrenia.
Correlations With Cognitive Performance
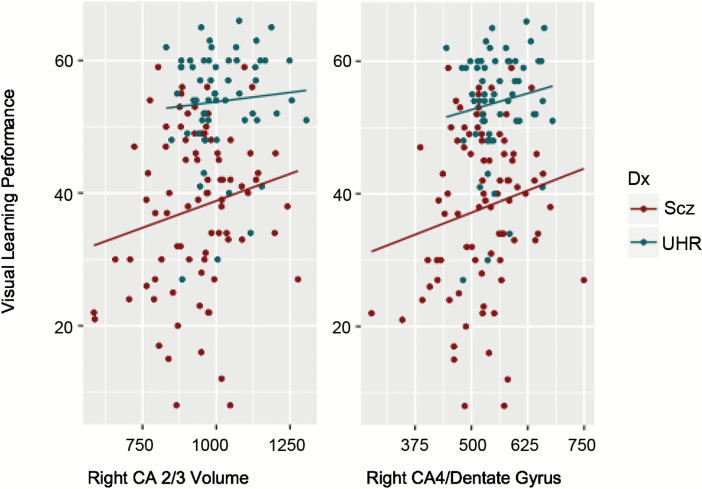
We examined relationships between subregion volumes and MATRICS battery working memory, visual learning, and verbal learning domain scores in the UHR and SCZ groups, controlling for age, gender, and ICV (table 2; figure 3). In the UHR group, we found significant positive correlations between bilateral CA2/3, 4/DG volume, and visual learning performance. We also found significant positive correlations between left CA1 and left subiculum volume and visual learning performance, as well as left presubiculum volume and working memory performance. In the SCZ group, we found significant positive correlations between right CA2/3 and 4/DG and performance for the visual learning, verbal learning, and working memory domains. We also found a positive association between right CA1 and left CA2/3 and working memory performance in the SCZ group, as well as a positive association between right subiculum and bilateral presubiculum volumes and working memory performance. The positive association between right CA2/3 and 4/DG and visual learning performance was found in both the UHR and SCZ groups independently (figure 3), as well as the association between working memory performance and left presubiculum volume.
Table 2.
Cognitive Performance and Subfield Volumes by Diagnostic Group
| Subfield | Left CA1 | Right CA1 | Left CA2/3 | Right CA2/3 | Left CA4/DG | Right CA4/DG | Left Sub | Right Sub | Left Presub | Right Presub |
|---|---|---|---|---|---|---|---|---|---|---|
| UHR | ||||||||||
| Visual learning | 0.23* | 0.17 | 0.27* | 0.23* | 0.25* | 0.31** | 0.26* | 0.04 | 0.08 | 0.02 |
| Verbal learning | −0.07 | −0.01 | 0.06 | 0.1 | 0.09 | 0.2 | −0.11 | −0.18 | −0.1 | −0.07 |
| Working memory | −0.06 | −0.02 | −0.1 | 0.03 | −0.01 | 0.12 | 0.11 | 0.17 | 0.25* | 0.002 |
| SCZ | ||||||||||
| Visual learning | 0.06 | 0.14 | 0.04 | 0.25** | 0.01 | 0.24* | 0.09 | 0.17 | 0.06 | 0.07 |
| Verbal learning | 0.1 | 0.14 | 0.18 | 0.23* | 0.1 | 0.23* | −0.02 | 0.16 | 0.1 | 0.1 |
| Working memory | 0.16 | 0.24* | 0.19* | 0.30** | 0.17 | 0.28** | 0.17 | 0.30** | 0.33** | 0.30** |
Note: Controlling for intracranial volume, age, and gender. SCZ, schizophrenia; sub, subiculum; presub, presubiculum.
*P < .05, **P < .01.
Fig. 3.
Visual learning performance* and subregion volumes (mm3) by diagnostic group. *t Scores, assessed by MATRICS battery Visual Learning Domain.46
Discussion
Our study examines hippocampal subregions cross-sectionally according to psychosis illness progression. We are also among the first to explore associations of subregion volumes with hippocampus-mediated cognitive performance. In regards to subregion volumes, we found a dose–response relationship whereby lower subregion volumes scaled with psychosis illness stage, even after controlling for age, gender, and ICV (figure 2). This was the case for all regions of interest except left CA1. Of particular interest is the fact that these findings were driven by differences between the HV and UHR group, as well as by differences between the UHR and SCZ group. That is, subregion volumes tended to be smallest in the SCZ group, which showed significantly smaller volumes relative to the UHR group, which in turn showed significant volume differences relative to the HV group. Our findings strongly suggest that hippocampal subregion abnormalities precede psychosis onset, and are present independently of third variable confounds typical to after-psychosis onset studies, such as medication status. These findings are consistent with a previous group who found lower subregion volumes across schizoaffective, SCZ, and psychotic bipolar disorder groups compared with healthy volunteers, with the CA 2/3 being the most affected.37 They are partially consistent with another group who found larger longitudinal reductions in CA1 volume in high risk individuals who converted to psychosis and in those whose symptoms persisted compared to high risk individuals whose symptoms remitted.18
In regards to overall hippocampal volume, consistent with a recent meta-analysis17 we did not find significant differences for overall hippocampus volume between the UHR and HV groups, controlling for age, gender, and ICV. In line with recent meta-analyses,11,12 we did find significant overall hippocampal volume differences between the SCZ group and the HV/UHR groups, with SCZ group having lower volume compared to the UHR and HV groups. These findings lend importance to the fact that given the region’s functional specificity, it is essential to look beyond overall volume, to further understand which specific areas may be differentially affected by illness progression, and how this maps on to symptoms and cognition. In our study, bilateral CA2/3 as well as CA4 and dentate gyrus structures showed significantly lower volumes in the UHR sample relative to healthy volunteers, with the SCZ sample showing significantly lower volumes relative to the UHR sample.
Further, we found that these subregion abnormalities were linked to critical functional abnormalities in hippocampus-mediated cognition, which is also critically affected in psychosis, and therefore highly informative to etiological conceptualization.9,38 Of note, we observed positive associations in both the SCZ and the UHR groups for the right CA2/3 and CA4/dentate gyrus regions across the visual memory and working memory domains. This is particularly fascinating given the role of the dentate-CA3 network in paired associations with space, spatial pattern separation, rapid encoding of new contextual memories, and spatial working memory.8,34 Previous studies have implicated abnormal CA3 function with exaggerated pattern completion memory activity and increased experience of incorrect or illogical associations, potentially including psychotic experiences.8,34 Impaired dentate gyrus function, in turn, is associated with impairments in pattern separation during memory encoding, which may contribute to illusory pattern completion and confer a reduced ability to discriminate between present and past experiences.8 Our study is among the first to draw this functionally relevant link between cross-sectional subregion volume abnormalities, and ensuing associations with cognitive function in groups with psychosis and high psychosis risk. The presence of less severe hippocampal subregion abnormalities in the UHR group (relative to the SCZ group) that are nonetheless associated with visual memory performance lends support to the notion that hippocampal subregion abnormalities (and their potential cognitive correlates) are endogenous to the psychosis spectrum.48 Our findings are consistent with a previous group who more broadly examined overall cognitive function associations with reduced subregion volumes.37
A neural diathesis-stress conception of psychosis posits that early insult (a first hit) confers a vulnerability, which then later interacts with environmental factors as well as both normative and pathological adolescent development (a second hit), ultimately driving the onset of psychosis.49 The hippocampus is central to this theory, as it is vulnerable to early insult50–52 and then later, serves as a critical nexus between environmental and developmental factors.53,54 Specifically, the structure is chiefly involved in the negative feedback system governing the biological response to stress,55 as well as in modulating a series of high-order cognitive functions.1 It is critical to consider the structure and function of the hippocampus also is significantly affected by persistent biological stress56 and this may confer increased risk and eventually psychosis.57 Disruption to hippocampal development in the UHR period might leave youth increasingly prone to stress, and also confer increasing cognitive deficits, thereby impacting skills that would be vital for facing an increasingly challenging environment. Our findings provide a new perspective on this model, indicating that specific hippocampal subregions are affected in the UHR period, and are indeed linked with cognitive dysfunction. Further, this cycle, wherein the hippocampus continues to be putatively affected, and reciprocally drives/maintains illness, appears to continue after formal onset. At the same time, this study highlights the relevance of prevention and intervention treatments targeting this structure, which has been shown to be highly amenable to neuroplasticity based efforts, such as exercise treatments and other interventions targeting lifetime changes.58–60
Although this is the largest subregion study that has been done in a high risk sample, future studies would benefit from incorporating an even larger sample, yielded from ongoing multisite studies and consortium. In addition, while a great majority of our UHR sample was medication free (89%), and we did not find medication effects for the UHR or for the SCZ samples, future studies in medication-naïve populations would be optimal. Although age was not of interest to this study’s aim, and was therefore controlled for in all analyses, studies modeling age and development in these populations would be informative. Given that there have not been any studies examining relationships with cognition and subregion volume, our correlations were exploratory. Future studies should develop directional hypotheses and correct for multiple comparisons. In addition, our SCZ data were collected at a separate site, and although protocols, scanners, and parameters were identical and we did everything we could to maximize between site reliability, it would have been ideal to obtain all data from the same site, or conduct mixed groups of patients and controls at each site, a matter that future investigations could address. Future studies benefiting from improved image resolution will also continue to improve the subregion analysis approach, as well as translational studies, informing our understanding of subregions from animal models, and then implementing these findings and emerging techniques in humans. Finally, it is important to note that while cross-sectional studies allow for a well powered perspective of brain characteristics across different stages (not relying on tracking converters, which typically represent a small heterogeneous group), the method does not characterize individual differences or disease course. As a result, we would recommend that future studies incorporate a mixed approach, including a longitudinal perspective, both in UHR and in SCZ participants.
Conclusion
We find that subregions of the hippocampus are smaller in UHR, even though the hippocampal volume as a whole is not significantly smaller than in the healthy volunteer group. In the SCZ group, the entire volume was smaller than in the UHR and healthy volunteer groups, suggesting additional effects with increasing disease progression. We also find associations between specific subregion volume and cognitive performance across both clinical groups, in the visual memory, verbal memory, and working memory domains. UHR participants on medications (13% of the sample) did not differ significantly on target variables compared with UHR participants not on medication. Our findings support the concept that regional hippocampal abnormalities emerge before the onset of psychosis, are independent of medication effects and functionally relevant, and are etiologically informative.
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Funding
This work was supported by National Institutes of Health [R01MH094650 and R21/R33MH103231 (VM)], as well as by the Centre of Biomedical Research Excellence (COBRE) (5P20RR021938/P20GM103472 and F32MH102898-01).
References
- 1. Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. [DOI] [PubMed] [Google Scholar]
- 2. Derks NA, Krugers HJ, Hoogenraad CC, Joëls M, Sarabdjitsingh RA. Effects of early life stress on synaptic plasticity in the developing hippocampus of male and female rats. PLoS One. 2016;11:e0164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iñiguez SD, Aubry A, Riggs LM et al. . Social defeat stress induces depression-like behavior and alters spine morphology in the hippocampus of adolescent male C57BL/6 mice. Neurobiol Stress. 2016;5:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sapolsky RM. Depression, antidepressants, and the shrinking hippocampus. Proc Natl Acad Sci U S A. 2001;98:12320–12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. [DOI] [PubMed] [Google Scholar]
- 6. Phillips LJ, McGorry PD, Garner B et al. . Stress, the hippocampus and the hypothalamic-pituitary-adrenal axis: implications for the development of psychotic disorders. Aust N Z J Psychiatry. 2006;40:725–741. [DOI] [PubMed] [Google Scholar]
- 7. Velakoulis D, Wood SJ, Wong MT et al. . Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of HRonic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63:139–149. [DOI] [PubMed] [Google Scholar]
- 8. Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167:1178–1193. [DOI] [PubMed] [Google Scholar]
- 9. Rasetti R, Mattay VS, White MG et al. . Altered hippocampal-parahippocampal function during stimulus encoding: a potential indicator of genetic liability for schizophrenia. JAMA Psychiatry. 2014;71:236–247. [DOI] [PubMed] [Google Scholar]
- 10. Haijma SV, Van Haren N, Cahn W et al. . Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39:1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Erp TG, Hibar DP, Rasmussen JM et al. . Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy volunteers via the ENIGMA consortium. Mol Psychiatry. 2016;21:547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Okada N, Fukunaga M, Yamashita F et al. . Abnormal asymmetries in subcortical brain volume in schizophrenia. Mol Psychiatry. 2016;21:1460–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tepest R, Wang L, Miller MI, Falkai P, Csernansky JG. Hippocampal deformities in the unaffected siblings of schizophrenia subjects. Biol Psychiatry. 2003;54:1234–1240. [DOI] [PubMed] [Google Scholar]
- 14. Lawrie SM, Whalley HC, Abukmeil SS et al. . Brain structure, genetic liability, and psychotic symptoms in subjects at high risk of developing schizophrenia. Biol Psychiatry. 2001;49:811–823. [DOI] [PubMed] [Google Scholar]
- 15. van Erp TG, Saleh PA, Huttunen M et al. . Hippocampal volumes in schizophrenic twins. Arch Gen Psychiatry. 2004;61:346–353. [DOI] [PubMed] [Google Scholar]
- 16. Hurlemann R, Jessen F, Wagner M et al. . Interrelated neuropsychological and anatomical evidence of hippocampal pathology in the at-risk mental state. Psychol Med. 2008;38:843–851. [DOI] [PubMed] [Google Scholar]
- 17. Walter A, Suenderhauf C, Harrisberger F et al. . Hippocampal volume in subjects at clinical high-risk for psychosis: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2016;71:680–690. [DOI] [PubMed] [Google Scholar]
- 18. Ho NF, Holt DJ, Cheung M et al. . Progressive decline in hippocampal CA1 volume in individuals at ultra-high-risk for psychosis who do not remit: findings from the longitudinal youth at risk study. Neuropsychopharmacology. 2017;42:1361–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Witthaus H, Mendes U, Brüne M et al. . Hippocampal subdivision and amygdalar volumes in patients in an at-risk mental state for schizophrenia. J Psychiatry Neurosci. 2010;35:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McDonald C, Marshall N, Sham PC et al. . Regional brain morphometry in patients with schizophrenia or bipolar disorder and their unaffected relatives. Am J Psychiatry. 2006;163:478–487. [DOI] [PubMed] [Google Scholar]
- 21. Johnson SL, Wang L, Alpert KI et al. . Hippocampal shape abnormalities of patients with childhood-onset schizophrenia and their unaffected siblings. J Am Acad Child Adolesc Psychiatry. 2013;52:527.e2–536.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mattai A, Hosanagar A, Weisinger B et al. . Hippocampal volume development in healthy siblings of childhood-onset schizophrenia patients. Am J Psychiatry. 2011;168:427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seidman LJ, Faraone SV, Goldstein JM et al. . Left hippocampal volume as a vulnerability indicator for schizophrenia: a magnetic resonance imaging morphometric study of nonpsychotic first-degree relatives. Arch Gen Psychiatry. 2002;59:839–849. [DOI] [PubMed] [Google Scholar]
- 24. Phillips LJ, Velakoulis D, Pantelis C et al. . Non-reduction in hippocampal volume is associated with higher risk of psychosis. Schizophr Res. 2002;58:145–158. [DOI] [PubMed] [Google Scholar]
- 25. Witthaus H, Kaufmann C, Bohner G et al. . Gray matter abnormalities in subjects at ultra-high risk for schizophrenia and first-episode schizophrenic patients compared to healthy controls. Psychiatry Res. 2009;173:163–169. [DOI] [PubMed] [Google Scholar]
- 26. Woods BT, Ward KE, Johnson EH. Meta-analysis of the time-course of brain volume reduction in schizophrenia: implications for pathogenesis and early treatment. Schizophr Res. 2005;73:221–228. [DOI] [PubMed] [Google Scholar]
- 27. Buehlmann E, Berger GE, Aston J et al. . Hippocampus abnormalities in at risk mental states for psychosis? A cross-sectional high resolution region of interest magnetic resonance imaging study. J Psychiatr Res. 2010;44:447–453. [DOI] [PubMed] [Google Scholar]
- 28. Csernansky JG, Wang L, Jones D et al. . Hippocampal deformities in schizophrenia characterized by high dimensional brain mapping. Am J Psychiatry. 2002;159:2000–2006. [DOI] [PubMed] [Google Scholar]
- 29. Fusar-Poli P, Perez J, Broome M et al. . Neurofunctional correlates of vulnerability to psychosis: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2007;31:465–484. [DOI] [PubMed] [Google Scholar]
- 30. Dean DJ, Orr JM, Bernard JA et al. . Hippocampal shape abnormalities predict symptom progression in neuroleptic-free youth at ultrahigh risk for psychosis. Schizophr Bull. 2016;42:161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thompson DK, Omizzolo C, Adamson C et al. . Longitudinal growth and morphology of the hippocampus through childhood: impact of prematurity and implications for memory and learning. Hum Brain Mapp. 2014;35:4129–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Narr KL, Thompson PM, Szeszko P et al. . Regional specificity of hippocampal volume reductions in first-episode schizophrenia. Neuroimage. 2004;21:1563–1575. [DOI] [PubMed] [Google Scholar]
- 33. O’Driscoll GA, Florencio PS, Gagnon D et al. . Amygdala-hippocampal volume and verbal memory in first-degree relatives of schizophrenic patients. Psychiatry Res. 2001;107:75–85. [DOI] [PubMed] [Google Scholar]
- 34. Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. [DOI] [PubMed] [Google Scholar]
- 35. Talati P, Rane S, Kose S et al. . Increased hippocampal CA1 cerebral blood volume in schizophrenia. Neuroimage Clin. 2014;5:359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Haukvik UK, Westlye LT, Mørch-Johnsen L et al. . In vivo hippocampal subfield volumes in schizophrenia and bipolar disorder. Biol Psychiatry. 2015;77:581–588. [DOI] [PubMed] [Google Scholar]
- 37. Mathew I, Gardin TM, Tandon N et al. . Medial temporal lobe structures and hippocampal subfields in psychotic disorders: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. JAMA Psychiatry. 2014;71:769–777. [DOI] [PubMed] [Google Scholar]
- 38. Dickinson D, Goldberg TE, Gold JM, Elvevåg B, Weinberger DR. Cognitive factor structure and invariance in people with schizophrenia, their unaffected siblings, and controls. Schizophr Bull. 2011;37:1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. First M, Spitzer RL, Gibbon M, Williams JBW.. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I). New York: Biometrics Research, New York State Psychiatric Institute; 2012. [Google Scholar]
- 40. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. [DOI] [PubMed] [Google Scholar]
- 41. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 42. Miller TJ, McGlashan TH, Woods SW et al. . Symptom assessment in schizophrenic prodromal states. Psychiatr Q. 1999;70:273–287. [DOI] [PubMed] [Google Scholar]
- 43. Scott A, Courtney W, Wood D et al. . COINS: an innovative informatics and neuroimaging tool suite built for large heterogeneous datasets. Front Neuroinform. 2011;5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Whelan CD, Hibar DP, van Velzen LS et al. ; Alzheimer’s Disease Neuroimaging Initiative. Heritability and reliability of automatically segmented human hippocampal formation subregions. Neuroimage. 2016;128:125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Van Leemput K, Bakkour A, Benner T et al. . Automated segmentation of hippocampal subfields from ultra-high resolution in vivo MRI. Hippocampus. 2009;19:549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. August SM, Kiwanuka JN, McMahon RP, Gold JM. The MATRICS Consensus Cognitive Battery (MCCB): clinical and cognitive correlates. Schizophr Res. 2012;134:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68:128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Greenspan KS, Arakelian CR, van Erp TGM. Heritability of hippocampal formation sub-region volumes. J Neurol Neurosci. 2016;7(6):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pruessner M, Cullen AE, Aas M, Walker EF. The neural diathesis-stress model of schizophrenia revisited: an update on recent findings considering illness stage and neurobiological and methodological complexities. Neurosci Biobehav Rev. 2017;73:191–218. [DOI] [PubMed] [Google Scholar]
- 50. Mittal VA, Walker EF. Minor physical anomalies and vulnerability in prodromal youth. Schizophr Res. 2011;129:116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Van Erp TG, Saleh PA, Rosso IM et al. . Contributions of genetic risk and fetal hypoxia to hippocampal volume in patients with schizophrenia or schizoaffective disorder, their unaffected siblings, and healthy unrelated volunteers. Am J Psychiatry. 2002;159:1514–1520. [DOI] [PubMed] [Google Scholar]
- 52. Szuran TF, Pliska V, Pokorny J, Welzl H. Prenatal stress in rats: effects on plasma corticosterone, hippocampal glucocorticoid receptors, and maze performance. Physiol Behav. 2000;71:353–362. [DOI] [PubMed] [Google Scholar]
- 53. Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol. 2008;4:189–216. [DOI] [PubMed] [Google Scholar]
- 54. Carol EE, Mittal VA. Resting cortisol level, self-concept, and putative familial environment in adolescents at ultra high-risk for psychotic disorders. Psychoneuroendocrinology. 2015;57:26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865–871. [DOI] [PubMed] [Google Scholar]
- 56. Sapolsky RM. Stress and plasticity in the limbic system. Neurochem Res. 2003;28:1735–1742. [DOI] [PubMed] [Google Scholar]
- 57. Corcoran C, Malaspina D, Goetz R, Gil R, Gorman J, Mcewen B. Elements of the stress cascade and the neurobiology of schizophrenia. Schizophr Res. 2003;60:13.12505134 [Google Scholar]
- 58. Mittal VA, Gupta T, Orr JM et al. . Physical activity level and medial temporal health in youth at ultra high-risk for psychosis. J Abnorm Psychol. 2013;122:1101–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kennedy S. Psychosocial stress, health, and the hippocampus. J Undergrad Neurosci Educ. 2016;15:R12–R13. [PMC free article] [PubMed] [Google Scholar]
- 60. Dean DJ, Bryan A, Newberry RE, Gupta T, Carol EE, & Mittal VA. A supervised exercise intervention for youth at risk for psychosis: an open-label pilot study. J Clin Psychiatry. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.