Abstract
Specific biomarker reflecting neurobiological substrates of schizophrenia (SZ) is required for its diagnosis and treatment selection of SZ. Evidence from neuroimaging has implicated disrupted functional connectivity in the pathophysiology. We aimed to develop and validate a method of disease definition for SZ by resting-state functional connectivity using radiomics strategy. This study included 2 data sets collected with different scanners. A total of 108 first-episode SZ patients and 121 healthy controls (HCs) participated in the current study, among which 80% patients and HCs (n = 183) and 20% (n = 46) were selected for training and testing in intra-data set validation and 1 of the 2 data sets was selected for training and the other for testing in inter-data set validation, respectively. Functional connectivity was calculated for both groups, features were selected by Least Absolute Shrinkage and Selection Operator (LASSO) method, and the clinical utility of its features and the generalizability of effects across samples were assessed using machine learning by training and validating multivariate classifiers in the independent samples. We found that the accuracy of intra-data set training was 87.09% for diagnosing SZ patients by applying functional connectivity features, with a validation in the independent replication data set (accuracy = 82.61%). The inter-data set validation further confirmed the disease definition by functional connectivity features (accuracy = 83.15% for training and 80.07% for testing). Our findings demonstrate a valid radiomics approach by functional connectivity to diagnose SZ, which is helpful to facilitate objective SZ individualized diagnosis using quantitative and specific functional connectivity biomarker.
Keywords: schizophrenia, functional connectivity, machine learning, radiomics
Introduction
Currently, schizophrenia (SZ) is considered as a heterogeneous clinical syndrome,1 whose diagnosis still depends on the psychiatrist’s assessment according to the patient’s behavior and reports of symptoms.2 Clinical practice requests guidance of some objective, quantitative and specific biomarker reflecting its neurobiological substrates for diagnosis and treatment selection.3 Functional magnetic resonance imaging (fMRI) studies have accumulated increasing evidence for neuroimaging basis of SZ,4–6 raising the possibility to understand the pathophysiology of this debilitating mental illness. Our previous studies have shown the dysconnectivity pattern of SZ patients by means of functional connectivity,7,8 effective connectivity,9,10 voxel-mirrored homotopic connectivity,11 and resting-state networks analyses.12 Since functional neuroimaging studies have suggested functional connectivity analysis derived from fMRI showing brain dysfunction in SZ resulting from impaired communication of the brain network,7,13 biologically based disease definition for SZ therefore seems to be critical and urgent, which also agrees the goal of “psychoradiology,” the frontier of neuroimaging in psychiatry.14
Most recently, resting-state functional connectivity has been proven useful for defining neurophysiological subtypes of depression and reliably facilitating personalized therapy.15 Skåtun et al also found a high overall accuracy in classifying SZ patients and controls using resting-state networks in independent training and test data sets.16 However, most routine studies identifying abnormal connections and examining their psychopathological correlates are unable to directly promote the translation of fMRI findings to diagnostic, predictive or prognostic biomarkers for SZ in clinical field. To this end, radiomics is an alternative approach, which has provided insights into several areas of medicine, particularly clinical oncology.17,18 The important process of radiomics analysis includes high-throughput extraction to converse medical images into high-dimensional data and detection of crucial features for supporting decision making.19 Radiomics allows the research of optimized combination of multiple functional connectivity features for discriminating SZ patients from healthy controls (HCs). In autism spectrum disorder patients, a radiomics study using hippocampus and amygdala biomarkers has provided an unprecedented opportunity to improve the individualized diagnosis and treatment by means of biologically based measurement for mental disorders.20
In this study, on the basis of the dysconnectivity theory of SZ and by means of radiomics approaches, we aimed to develop and validate a method of disease definition for SZ by resting-state functional connectivity using radiomics strategy in first-episode untreated patients, improving objective SZ individualized diagnosis using quantitative and specific biomarker in clinical practice.
Methods
Participants
This study was approved by the local Research Ethics Committee. All participants gave written informed consent after a full description of the aims and design of the study. Two samples of first-episode patients with SZ spectrum disorder were recruited in the Department of Psychiatry, Xijing Hospital; one between May 2011 and September 20137–12 and another between April 2015 and December 2016 from both inpatient department and outpatient clinic at the Xijing Hospital. Here, “first episode” refers to untreated patients identified by clinical psychiatrists after the onset of the disease without receiving any medication for the patients recruited between May 2011 and September 2013, including those inpatients undergoing their first hospitalization or help seeking outpatients for the first time. For the period between April 2015 and December 2016, “first episode” refers to inpatients during the first hospitalization and outpatients for the first help seeking, having no more than 2 weeks of cumulative exposure to antipsychotics. The inclusion criteria are listed as follows. (1) The Structural Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) and DSM, Fifth Edition (DSM-V) were used for these 2 groups, respectively, and consensus diagnoses were made using all the available information by 2 senior clinical psychiatrists. (2) All the patients were assessed by them using the Positive and Negative Syndrome Scale developed by Kay et al.21 (3) In addition, 2 groups of HCs were recruited from the local community by advertisement at the same period when SZ patients were recruited. (4) All the subjects were right handed and their biological parents were the Han Chinese ethnic group. The exclusion criteria included: (1) presence of another axis I or axis II psychiatric disorder; (2) history of receiving antipsychotics (or more than 2 wk of antipsychotic medication for the data between April 2015 and December 2016), repetitive transcranial magnetic stimulation, transcranial current stimulation, or behavioral treatment; (3) history of clinically significant neurological, neurosurgical or medical illnesses; (4) substance abuse within the prior 30 days or substance dependence within the prior 6 months; (5) pregnancy or MRI scanning contraindications, eg, cardiac pacemakers and other metallic implants. A total of 108 SZ patients and 121 HCs participated in this study (table 1).
Table 1.
Demographical and Clinical Data of Participants
| Data Set 1 | Data Set 2 | |||
|---|---|---|---|---|
| Characteristic | Patients (n = 52) | HCs (n = 66) | Patients (n = 56) | HCs (n = 55) |
| Age (y) | 26 ± 6 | 29 ± 7 | 23 ± 6 | 32 ± 11 |
| Gender (M/F) | 31/21 | 33/33 | 30/26 | 26/29 |
| Education level (y) | 13 ± 2 | 15 ± 2 | 11 ± 3 | 15 ± 4 |
| Scanner (T) | 3.0 | 3.0 | 3.0 | 3.0 |
| Inpatients/outpatients | 51/1 | — | 54/2 | — |
| Duration of illness (mon) | 11 ± 14 | — | 14 ± 20 | — |
| PANSS score | ||||
| Total score | 97 ± 17 | — | 85 ± 14 | — |
| Positive score | 24 ± 8 | — | 22 ± 5 | — |
| Negative score | 24 ± 7 | — | 20 ± 6 | — |
| General score | 49 ± 9 | — | 43 ± 9 | — |
Note: HCs, healthy controls; PANSS, Positive and Negative Syndrome Scale.
Image Acquisition
High-resolution T1-weighted anatomical imaging and resting-state blood oxygen level-dependent-fMRI were obtained for both data sets. Two data sets were acquired on a Siemens 3.0 T Magnetom Trio Tim MR scanner and GE Discovery MR750 3.0 T scanner in the Department of Radiology, Xijing Hospital, respectively. Specific scanning parameters varied by MRI scanner (supplementary table S1), as described elsewhere.7–12
Data Preprocessing
Functional image preprocessing was carried out using CONN software (http://web.mit.edu/swg/software.htm). Briefly, after excluding the first 10 images to ensure the signal to reach equilibrium, functional images were corrected for head motion and temporal differences. The subject was excluded if any translation or rotation parameters in this subject’s data set were exceeded ± 1 mm and/or ±1°. The corrected functional images were firstly co-registered to each subject’s T1 images without reslicing. Then, T1 images were normalized to the Montreal Neurological Institute (MNI) space, which generated a transformed matrix from native space to MNI space. Functional images were than transformed to the MNI space using this matrix and resampled at 2 × 2 × 2 mm3. Outlier detection was performed on the normalized images. Finally, all images were smoothed with a 6 mm full width at half maximum (FWHM) Gaussian kernel.22 Then, we randomly selected 80% of total subjects as training data set (total 183 subjects) and 20% as the validating data set (total 46 subjects) for 1000 times. Thus, the accuracy, sensitivity, and specificity in the following analyses were average values of results from random selection for 1000 times.
Network Constructed
Anatomical Automatic Labeling (AAL) cortical and subcortical atlas was used to segment the whole brain into 90 non-cerebellar anatomical regions of interest (ROI).23 For each subject, each ROI time series was extracted as the average time series across all voxels within that region. To remove spurious sources of variance, all of ROI time series were performed by the following steps: (1) linear detrending; (2) regressing out the 6 head motion parameters and their first-level derivative, the averaged cerebrospinal fluid and white matter signals, and the scrubbing signal from the time series; (3) 0.01–0.1 Hz band-pass filtering. All of these were done by using CONN software. Finally, Pearson correlation coefficients were calculated between each pair of preprocessed ROI time series, and a temporal correlation matrix (N × N, where N = 90 is the number of ROI in AAL atlas) was obtained for each subject. Additionally, in order to evaluate whether any between group differences could have been confounded by combining data from 2 different scanners, we compared the functional connectivity between patients on Siemens scanner and GE scanner, as well as between HCs on Siemens scanner and GE scanner. As a result, no difference was found (supplement table S2).
Feature Selection of Intra- and Inter-data Set Cross Validation
Two approaches were selected in our research, intra-data set and inter-data set cross validation (supplementary figure S1).24 Intra-data set cross validation includes randomly splitting the subjects for training and testing set. We randomly selected 80% of total subjects as training data set (a total of 183 subjects) and 20% as the testing data set (46 subjects). Inter-data set cross validation leaves out subjects on Siemens scanner as testing set.
1. Original features were selected as those edges which the connectivity coefficient were significantly differences using 2 sample t-tests (significant, P < .05, uncorrected for multiple comparisons). Considering the individual variation, leave-one out method was used to insure the stability of selected edges. Briefly, 183 times 2 sample t-tests were performed. Each time, one subject in order was excluded. Only those edges which had stably significantly at each time were selected. In this step, 117 original features were included.
2. LASSO method with 10-fold crossing validation using mean of square error (MSE) as cost function was performed to eliminate the multi-collinearity among 117 original features and shrink these features into few more important features according to MSE + 1SE Criteria. In this step, 32 features (intra-data set) and 43 features (inter-data set) (LASSO features) were remained.25
3. Support vector machine (SVM) method was used to estimate the status (SZ or not) of each subject based on the 32 (intra-data set) and 43 (inter-data set) features. The SVM procedures included t-test filtering to include only reliably different features, linear kernel separation and soft margin separation.26 In our research, 10-fold cross validation which is widely used in machine learning approaches and provides a good estimate of accuracy, was further used to estimate group classification and prediction accuracies, and repeated for 1000 times. Accuracy, sensitivity, and specificity were computed to quantify the classification performance. The receiver operating characteristic (ROC) curve is reported using R version 3.2.3 (R Foundation for Statistical Computing). The area under the ROC curve (AUC) represents the classification power of a classifier, and a larger AUC indicates a better classification power.26
The permutation test was applied to determine whether the accuracy obtained above were significantly higher than values expected by chance. Specifically, we permuted the class labels (1: SZ patients, 0: HCs) across the entire sample 1000 times without replacement, and the entire classification procedure was reapplied each time.27 The P value for the accuracy was calculated by dividing the number of permutations that showed a higher value than the actual value for the real sample by the total number of permutations.
Validating the Performance
To evaluate the classifier performance, we repeated the above processing and computed the accuracy, sensitivity and specificity in testing data set of 2 patterns (supplementary figure S1).
Results
Functional Connectivity Features Building
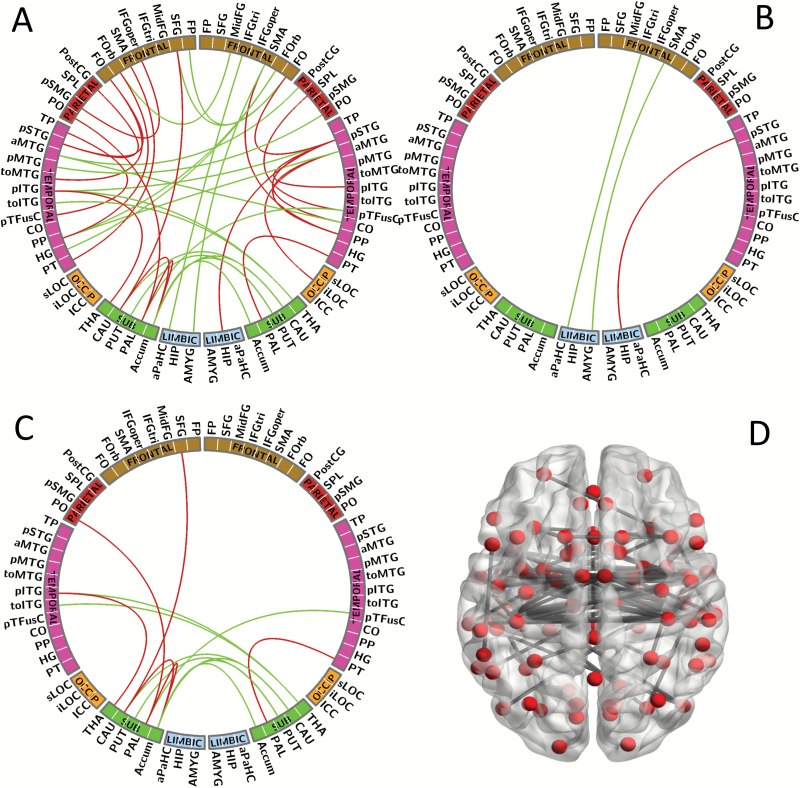
Figure 1 highlights all the 117 functional connectivity features with group differences used from SZ patients and HCs for classification analysis (P < .05, uncorrected for multiple comparisons). Here, for clear presentation, figures 1B and C show connectivity differences between cortical and limbic areas, and cortical and subcortical areas, respectively.
Fig. 1.
Functional connectivity features building. (A) All (117 connections) of the functional connectivity features between schizophrenia (SZ) patients and healthy controls (HCs) using 2-sample t test (P < .05, uncorrected for multiple comparisons). (B) Connections between cortical and limbic system areas. (C) Connections between cortical and subcortical areas. (D) Network schema displaying the nodes and connections of 117 features. Red line: connections between the same hemisphere; green line: connections between the different hemisphere.
Feature Selection
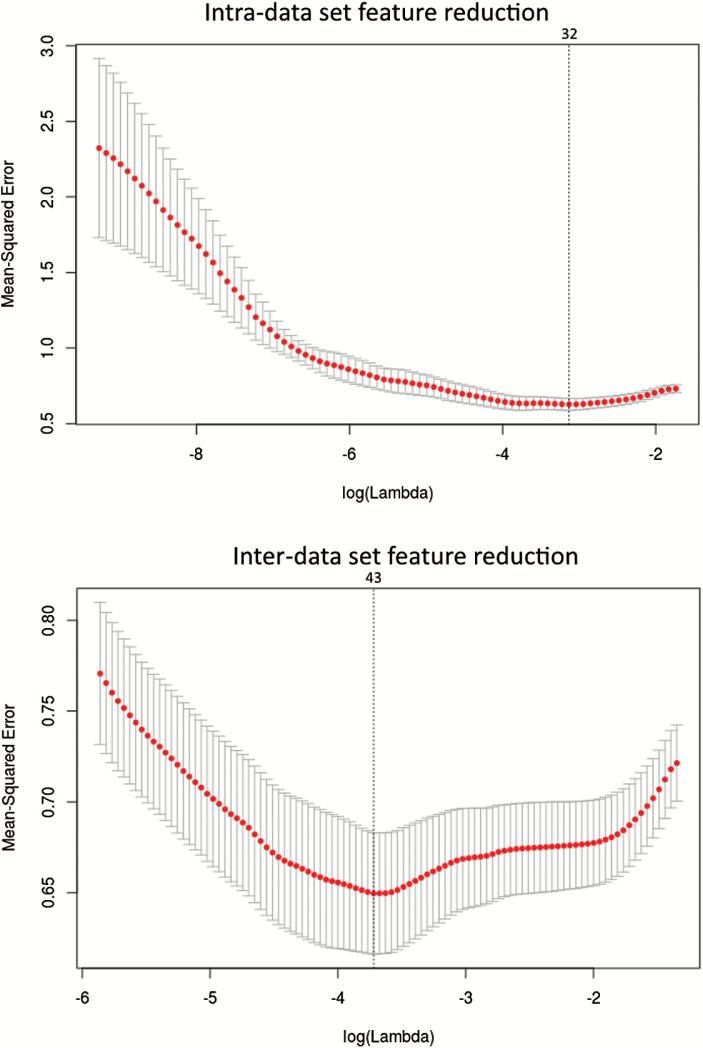
Of functional connectivity features, in the intra-data set cross validation, 117 features reduced to 32 potential predictors on the basis of 183 subjects in the training data set, and the 32 features were those with nonzero coefficients in the LASSO logistic regression model (figure 2). In the inter-data set cross validation, 117 features reduced to 43 potential predictors on the basis of 118 subjects in the training data set, and the 43 features were those with nonzero coefficients in the LASSO logistic regression model (figure 2).
Fig. 2.
Intra- and inter-data set feature reduction using the least absolute shrinkage and selection operator (LASSO) binary logistic regression model. In the upper panel, tuning parameter (lambda, λ) selection in the LASSO model used 10-fold cross validation via minimum criteria. Dotted vertical lines were drawn at the optimal values by using the minimum criteria (the value 32 means that the 117 features reduction to 32 features). In the lower panel, tuning parameter (lambda, λ) selection in the LASSO model used 10-fold cross validation via minimum criteria. Dotted vertical lines were drawn at the optimal values by using the minimum criteria (the value 43 means that the 117 features reduction to 43 features).
SVM Analysis and Intra- and Inter-data Set Cross Validations
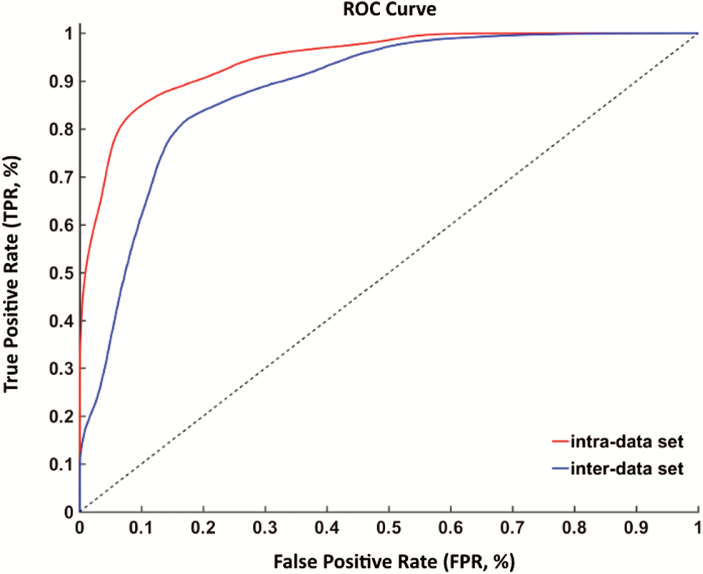
In the training of intra-data set, the SVM classifier accurately discriminated SZ patients from HCs on the basis of the ROC curve, with an accuracy of 87.09% (figure 3). The specificity and sensitivity was 86.79% and 87.22%, respectively. Thus, the prediction accuracy for SZ patients had a significantly higher level than that of chance. The functional connectivity features were further confirmed in the validating data set. In this cohort, the accuracy, specificity and sensitivity were 82.61%, 88.00% and 76.19%, respectively. As for the inter-data set cross validation, results are shown in table 2.
Fig. 3.
Classification results. Receiver operating characteristic (ROC) curve for the identification of schizophrenia (SZ) patients by functional connectivity features. The x-axis is the false positive rate (FPR); the y-axis is the true positive rate (TPR).
Table 2.
Results of Intra- and Inter-data Set Cross Validations
| Accuracy | Sensitivity | Specificity | |
|---|---|---|---|
| Intra-data set cross validation | |||
| Training | 87.09% | 86.79% | 87.22% |
| Testing | 82.61% | 88.00% | 76.19% |
| Inter-data set cross validation | |||
| Training | 83.15% | 80.79% | 83.63% |
| Testing | 80.07% | 78.65% | 81.33% |
Discussion
In the current study, we explored whether functional connectivity biomarker define SZ using radiomics strategy. Specifically, the accuracy was 87.09% for the purpose of diagnosing SZ patients by applying functional connectivity features, with a validation in the independent replication data set (accuracy = 82.61%). Our findings are helpful to facilitate objective SZ individualized diagnosis using quantitative and specific biomarker reflecting its neurobiological substrates in different sites.
Particularly, with the exception of presenting a full view of functional connectivity features, we also show connections between cortical and limbic system areas and connections between cortical and subcortical areas. As for the limbic system, abnormal connectivity between the parahippocampus and triangular part of inferior frontal gyrus, amygdala and supplemental motor area, and hippocampus and superior temporal gyrus was detected. Also, all the subcortical areas, including the thalamus, caudate, putamen, pallidum, and nucleus accumbens features, revealed aberrant connectivity pattern. Convergent findings based on functional connectivity provide support for these neuronal interaction deficits (eg, functional connectivity between the hippocampus and superior temporal gyrus28–30), suggesting that dysconnectivity among these regions is implicated in the pathophysiology of SZ. Obviously, this evidence is the fundamental basis for SZ definition by functional connectivity features.
Importantly, our study extends previous findings by 3 points. First, we studied first-episode SZ patients, thereby minimizing the treatment confounders of prior therapeutic exposure or the potential impact of chronicity, as we persistently insisted.7–12 Second, from the perspective of methodology, the newly developed radiomics strategy was used in the present study to push conventional investigation up to a translational level. Clearly, combining training and validating/replication data sets, the diagnostic performance of functional connectivity was proved liable and valid for differentiating individual SZ patients from HCs, yielding the practical value in both psychiatry and radiology. Third, the accuracy was from 73.0% to 77.91% in 2 previous similar studies.31,32 We obtained an accuracy of 87.09% and 82.61% in the training and validating data sets of our study, respectively, an identical level in contrast to a combined static and dynamic functional network connectivity approach showing an overall accuracy of 88.68% in classification of SZ and bipolar disorder patients.33 Our results further increase the diagnostic performance from an accuracy of 78.3% and 77.5% in principal and validating samples by resting-state networks features.16
Another issue needs to be concerned is combining data either from 2 scanners or of heterogeneous samples. Firstly, we performed comparison of functional connectivity for subjects between 2 scanners, but no difference was found in functional connectivity between scanners (supplementary table 2). As well, results of intra- and inter-data set cross validations confirmed the capacity of discriminating SZ from HCs by functional connectivity features from different scanners. It is feasible to combine data from 2 samples on different MRI scanners in the current study. Secondly, combining data with heterogeneity to analyze has been reported by previous studies. Drysdale et al combined data from various sites to investigate whether resting-state functional connectivity could be used to define neurophysiological subtypes of depression. Combing independent data of KaSP (Karolinska Schizophrenia Project) and HUBIN (Human Brain Informatics), the findings of a recent study supported “generalizability of connectivity alterations across … heterogeneous samples.” Likewise, we combined data on 2 scanners in our present study, following the routine and real clinical situation. Combination of data from different scanners could consolidate and promote the generalizability of fMRI findings in clinical practice.
We need to point out several limitations of the current study. The sample size (n = 229) was relatively small as compared with the multisite study by Drysdale et al, which included 711 subjects in the training data set and 477 subjects in the replication data set.15 We implemented feature reduction before classification due to the small sample size, otherwise a large number of features without reduction will induce overfitting during training. Hence, although the fact that feature reduction and training are done using the same sample may lead to overestimation of accuracy, we were still unable to repeat SVM analyses without feature reduction in this small sample. Clinically, the enrollment of first-episode medication-naïve SZ patients is still difficult due to a lack of high degree of their compliance. We could only depend on a longer time to accumulate a much larger sample size temporarily. Additionally, structural34 and functional35 brain features at baseline are important for predicting SZ patients’ subsequent response to antipsychotics, but our study just focus on the issue of SZ diagnosis. In the next step, longitudinal studies are needed to well establish the relationship between cerebral characteristics and future treatment response in SZ patients.
In conclusion, these limitations notwithstanding, our findings demonstrate a valid radiomics approach by functional connectivity to diagnose SZ with a high accuracy. The functional connectivity features could be applied in disease definition, which is promising to facilitate the guidance of clinical management for SZ.
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Funding
National Natural Science Foundation of China (81571651); State Scholarship Fund, China Scholarship Council (201603170143).
Acknowledgments
We thank Prof. Qingrong Tan at Department of Psychiatry, Xijing Hospital, for her ongoing support with the study, and Mr. Ziliang Xu at School of Life Sciences and Technology, Xidian University, for his help about the revision. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016;388:86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. APA. Diagnostic and Statistical Manual of Mental Disorders. 5th ed Washington, DC: American Psychiatric Association; 2013:87–118. [Google Scholar]
- 3. Tamminga CA, Pearlson GD, Stan AD et al. . Strategies for advancing disease definition using biomarkers and genetics: the bipolar and schizophrenia network for intermediate phenotypes. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:20–27. [DOI] [PubMed] [Google Scholar]
- 4. Sheffield JM, Barch DM. Cognition and resting-state functional connectivity in schizophrenia. Neurosci Biobehav Rev. 2016;61:108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Giraldo-Chica M, Woodward ND. Review of thalamocortical resting-state fMRI studies in schizophrenia. Schizophr Res. 2017;180:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hu ML, Zong XF, Mann JJ et al. . A review of the functional and anatomical default mode network in schizophrenia. Neurosci Bull. 2017;33:73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cui LB, Liu K, Li C et al. . Putamen-related regional and network functional deficits in first-episode schizophrenia with auditory verbal hallucinations. Schizophr Res. 2016;173:13–22. [DOI] [PubMed] [Google Scholar]
- 8. Chang X, Collin G, Xi Y et al. . Resting-state functional connectivity in medication-naïve schizophrenia patients with and without auditory verbal hallucinations: a preliminary report. Schizophr Res. 2017;188:75–81. [DOI] [PubMed] [Google Scholar]
- 9. Cui LB, Liu J, Wang LX et al. . Anterior cingulate cortex-related connectivity in first-episode schizophrenia: a spectral dynamic causal modeling study with functional magnetic resonance imaging. Front Hum Neurosci. 2015;9:589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li B, Cui LB, Xi YB et al. . Abnormal effective connectivity in the brain is involved in auditory verbal hallucinations in schizophrenia. Neurosci Bull. 2017;33:281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang X, Xi YB, Cui LB et al. . Distinct inter-hemispheric dysconnectivity in schizophrenia patients with and without auditory verbal hallucinations. Sci Rep. 2015;5:11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cui LB, Liu L, Guo F et al. . Disturbed brain activity in resting-state networks of patients with first-episode schizophrenia with auditory verbal hallucinations: a cross-sectional functional mr imaging study. Radiology. 2017;283:810–819. [DOI] [PubMed] [Google Scholar]
- 13. van den Heuvel MP, Sporns O, Collin G et al. . Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiatry. 2013;70:783–792. [DOI] [PubMed] [Google Scholar]
- 14. Lui S, Zhou XJ, Sweeney JA, Gong Q. Psychoradiology: the frontier of neuroimaging in psychiatry. Radiology. 2016;281:357–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Drysdale AT, Grosenick L, Downar J et al. . Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Skatun KC, Kaufmann T, Doan NT et al. . Consistent functional connectivity alterations in schizophrenia spectrum disorder: a multisite study. Schizophr Bull. 2017;43:914–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aerts HJ, Velazquez ER, Leijenaar RT et al. . Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang YQ, Liang CH, He L et al. . Development and validation of a radiomics nomogram for preoperative prediction of lymph node metastasis in colorectal cancer. J Clin Oncol. 2016;34:2157–2164. [DOI] [PubMed] [Google Scholar]
- 19. Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278:563–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chaddad A, Desrosiers C, Hassan L, Tanougast C. Hippocampus and amygdala radiomic biomarkers for the study of autism spectrum disorder. BMC Neurosci. 2017;18:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 22. Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. [DOI] [PubMed] [Google Scholar]
- 23. Tzourio-Mazoyer N, Landeau B, Papathanassiou D et al. . Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. [DOI] [PubMed] [Google Scholar]
- 24. Abraham A, Milham MP, Di Martino A et al. . Deriving reproducible biomarkers from multi-site resting-state data: an autism-based example. Neuroimage. 2017;147:736–745. [DOI] [PubMed] [Google Scholar]
- 25. Sauerbrei W, Royston P, Binder H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat Med. 2007;26:5512–5528. [DOI] [PubMed] [Google Scholar]
- 26. Smyser CD, Dosenbach NU, Smyser TA et al. . Prediction of brain maturity in infants using machine-learning algorithms. Neuroimage. 2016;136:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cui Z, Xia Z, Su M, Shu H, Gong G. Disrupted white matter connectivity underlying developmental dyslexia: a machine learning approach. Hum Brain Mapp. 2016;37:1443–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou Y, Shu N, Liu Y et al. . Altered resting-state functional connectivity and anatomical connectivity of hippocampus in schizophrenia. Schizophr Res. 2008;100:120–132. [DOI] [PubMed] [Google Scholar]
- 29. Samudra N, Ivleva EI, Hubbard NA et al. . Alterations in hippocampal connectivity across the psychosis dimension. Psychiatry Res. 2015;233:148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Duan HF, Gan JL, Yang JM et al. . A longitudinal study on intrinsic connectivity of hippocampus associated with positive symptom in first-episode schizophrenia. Behav Brain Res. 2015;283:78–86. [DOI] [PubMed] [Google Scholar]
- 31. Mikolas P, Melicher T, Skoch A et al. . Connectivity of the anterior insula differentiates participants with first-episode schizophrenia spectrum disorders from controls: a machine-learning study. Psychol Med. 2016;46:2695–2704. [DOI] [PubMed] [Google Scholar]
- 32. Yang H, He H, Zhong J. Multimodal MRI characterisation of schizophrenia: a discriminative analysis. Lancet. 2016;388(suppl 1):S36. [Google Scholar]
- 33. Rashid B, Arbabshirani MR, Damaraju E et al. . Classification of schizophrenia and bipolar patients using static and dynamic resting-state fMRI brain connectivity. Neuroimage. 2016;134:645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Crossley NA, Marques TR, Taylor H et al. . Connectomic correlates of response to treatment in first-episode psychosis. Brain. 2017;140:487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li F, Lui S, Yao L et al. . Longitudinal changes in resting-state cerebral activity in patients with first-episode schizophrenia: a 1-year follow-up functional mr imaging study. Radiology. 2016;279:867–875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.