Abstract
Introduction:
The first human H7N9 avian influenza virus case was reported in Shanghai in 2013. Shortly thereafter, this virus spread to other regions in China. Molecular analysis indicated that the H7N9 virus is a reassortant virus containing internal genes from the H9N2 virus and previously described mammalian adaption markers, which could allow the virus to adapt efficiently to a mammalian host. Fortunately, there is no evidence of sustained person-to-person spread. Most of the human H7N9 cases have a history of exposure to live poultry markets (LPMs). The circulating H7N9 were low pathogenic viruses, however highly pathogenic H7N9 viruses were recently identified in human cases.
Areas covered:
In the present article, the circulation of H7N9 in LPMs of China, the five waves of H7N9 infection in humans, recently identified drug resistant mutants and potential antiviral drugs against H7N9 are discussed; this may provide further understanding of the evolution and pandemic potential of the H7N9 influenza viruses.
Expert commentary:
All the data reveal that the major source of H7N9 viruses is LPMs and the H7N9 virus is still circulating widely in China. It is concerning that the recent emergence of highly pathogenic H7N9 viruses may result in highly transmissible viruses in mammalian species.
Keywords: H7N9, drug resistant, highly pathogenic influenza virus, live poultry markets
1. Introduction
1.1. H7N9 avian influenza virus and live poultry markets
A new avian influenza A virus H7N9 was isolated from patients in China in March 2013; 1223 human cases have been reported to date (March 2017) with 339 fatalities in China [1]. Up to now, H7N9 infections of humans have been reported in China, Malaysia, and Canada [2–4]. Further genomic analysis showed that the novel H7N9 virus is a reassortant virus containing internal genes from the endemic avian H9N2 virus, and the surface genes from H7Nx and HxN9 viruses[5]. Most of the H7N9 viruses isolated from human cases carry mammalian adaptation signatures (e.g., PB2 with the 627K mutation and HA with the 226L mutation), which allows the virus to replicate more efficiently in mammalian cells [4–7]. The circulating H7N9 viruses were low pathogenic viruses carrying the KGR/G motif at the hemagglutinin cleavage site. Recently, a molecular change occurred and highly pathogenic H7N9 viruses are now being identified in human cases; these highly pathogenic viruses had insertions of multiple basic amino acids resulting in the KRKRTAR/G or KGKRIAR/G motifs [8]. It is a concern that continuous circulation of highly pathogenic H7N9 may generate highly transmissible virus in mammalian species.
Most of human H7N9 cases have been associated with previous exposure to birds, poultry, and/or to live poultry markets (LPMs)[9,10]. A majority of households in Southern China have a habit of purchasing live poultry in order to eat fresh poultry meat. This habit is the reason for the high numbers of poultry being sold in LPMs, allowing for a high contact frequency between people and poultry, and making LPMs an ideal place for influenza virus to jump across different avian and mammalian species[11]. Epidemiological surveillance revealed that H7N9 viruses can be isolated from pigeons, chicken, geese, and ducks sold in LPMs and therefore these birds are considered the major source of the H7N9 infection of humans[7,9,12–14].
From March 2013 to early 2014, the H7N9 virus was detected in LPMs in different provinces of China. It has been reported that 1.5% (131/8,900) of bird swabs and environmental samples were positive for H7N9 in Guangzhou, and 44.4% to 50 % of the surveilled LPMs were positive for H7N9 in the same province [15]. Also, 6,740 environmental samples collected and tested from LPMs in Zhejiang showed that 10.09% (680/6740) of the samples were H7N9-positive [16]. In April of 2013, 15 H7N9 viruses were isolated from 422 oral-pharyngeal and cloacal swabs collected from birds and environmental surfaces at five LPMs in Jiangsu [17]. In 2014, 6% (16/283; 4 chickens, 7 ducks, 1 goose, and 4 pigeons) of tracheal, cloacal, and fecal samples, collected from a variety of birds in poultry markets and farms in Jiangsu province, were positive for H7N9 viruses[18]. Additional surveillance data revealed that 1.47 % of samples collected in LPMs in Shanghai were positive for H7N9 markers by real-time RT-PCR [19]. Overall it was concluded, that LPMs are critical places where bird to human transmission of H7N9 viruses occurs. In order to control H7N9 virus spread in China, most of the LPMs in Southern China (Shanghai, Guangzhou, Zhejiang, and Jiangsu) were closed thus hopefully providing a control mechanisms for this epizootic and reduce the burden for human infections [20–22]. However, although 464 LPMs in Shanghai were closed on April 6, 2013, 4 additional human cases were found that year in Shanghai; nonetheless, in early 2014, Shanghai’s LPMs were reopened and subsequently, another 8 human cases were reported shortly thereafter [21]. This illustrates how culturally important LPMs in Southern China are and that besides closure of these markets, transmission of H7N9 to humans cannot be totally stopped.
To date (March 2017), partial or full genomic information on 1,356 strains of H7N9 virus are deposited in the GenBank database. Seven hundred twenty one H7N9 virus strains were isolated from humans so far, and molecular analysis showed that most of the H7N9 virus strains contain the mammalian adaptation markers PB2–627K (581/799) and HA-226L (721/799), while only a small number of these viruses contain neuraminidase inhibitor (NAI) resistant mutations (e.g., 18 strains NA292K, 1 strain NA222K, 4 strains NA119V). However, none of the 557 H7N9 strains isolated from poultry (535 from chicken, 13 from duck, 2 from goose and 7 from pigeon) contain known NAI resistant mutations, and only a few isolates (three out of 557 strains) contain the mammalian adaptation marker PB2–627K.
1.2. Five major waves of outbreaks of Human H7N9 infections since 2013
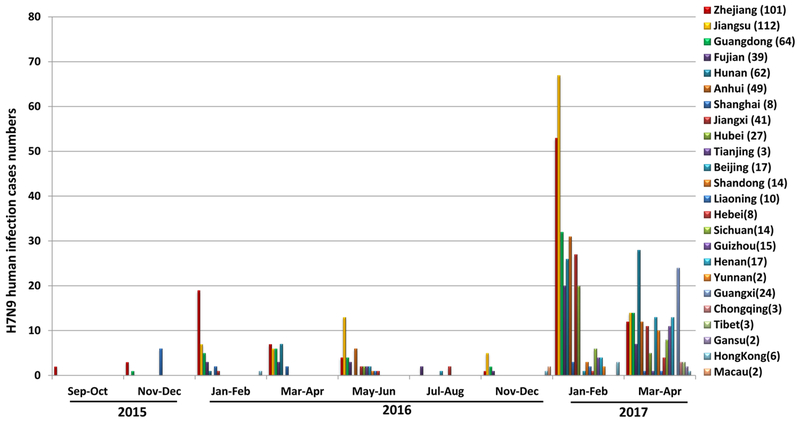
Outbreaks of the H7N9 virus generally follow a seasonal trend. Since the first H7N9 detection in humans in 2013, there were five major waves of outbreaks occurring which caused a continued public health threat in South-Eastern China. The first wave of human H7N9 infection took place from March 30th, 2013 to September 30th, 2013. At least 130 cases were confirmed in China with fatality rate of approximately 30%. The second wave happened in mainland China, Hong Kong, and Malaysia from October 1st, 2013 to September 30th, 2014. Over 260 additional cases were detected. The third wave was recorded from October 2014 to July 2015. A total of over 220 cases were reported in China, Hong Kong, and Canada. Human-to-human transmission has not been detected. All three waves embody similarity in disease symptoms, severity, and mortality rate, and no clear antigenic drift or shift was detected during wave 1 to 3 from 2013 to 2015 [23]. During the fourth wave of H7N9 infection (September 2015–August 2016[24]), 126 laboratory-confirmed human cases of influenza A (H7N9) virus infection have been reported, 35 of which were fatal. The rate of human infections dropped significantly when compared with the third wave (220 cases); however, the fifth wave of H7N9 virus infection of humans is occurring in China since October 2016; 315 cases have been reported in China during October, 2016 to February 14, 2017[25,26]. Notably, the number of reported cases in January to February of 2017 (305 cases) is higher than the number during the same time in the wave 1 to 4, suggesting that the H7N9 virus is still circulating widely in China; Moreover, the human cases reported during the fifth wave happened in multiple provinces in China, suggested that the H7N9 virus is circulating in an even larger geographic area in China (Figure 1).
Figure 1.
Numbers of H7N9 Influenza A virus infection in human from September 2015 to April 2017 in China. (No case was reported in September and October of 2016)
These data warrants continuous surveillance of LPMs and humans for the presence of H7N9 viruses and increased public health concerns regarding the extended geographical distribution of H7N9 viruses in China.
1.3. Novel drug-resistant substitutions in the NA protein of H7N9
Neuraminidase inhibitors (NAIs) have been used for the treatment of H7N9 virus infections, however, the NAI resistant substitution NA-R292K emerged soon after initial use of the drug[27]. Further laboratory studies showed that NA-R292K conferred resistance to NAIs such as oseltamivir, zanamivir and peramivir [27–30]. NA-R292K H7N9 virus shows compromised fitness in the ferret model as shown by Marjuki et al. [31,32]. Based on the available gene sequences of H7N9 isolates from humans, a limited number of viruses (13/651) contain the NA292K substitution, however no NA292K substitution was observed in any of the H7N9 viruses isolated from birds.
In addition to NA-R292K, additional NAI resistant substitutions, namely NA-E119V and NA-I222K/R, were found in patients after receiving oseltamivir treatment [31]. In vitro NAI assay indicated that the NA-E119V and NA-I222K/R substitutions conferred resistant to oseltamivir, zanamivir and peramivir to a different extent. NAI treatment of wild-type H7N9 infected ferrets resulted in a modest dose-dependent reduction of viral titers in nasal washes, while only a small reduction of viral titers was detected in nasal washes of ferrets infected with the NA-I222K virus [31]. However, NAI treatment failed to inhibit the replication of H7N9 viruses with the NA-R292K or NA-E119V substitutions [33,34]. Recent studies using NAIs and H7N9 virus infections were performed in ducks [28,35]. After treatment of H7N9 infected mallard ducks for 2 days with a low concentration (2.5ug/L) of oseltamivir carboxylate treatment in water, a NA-I222T variant could be isolated [35]. In the cynomolgus macaque model, a NA-I222T H7N9 variant was found in experimentally H7N9-infected macaques after treatment with NAI; further analysis indicated that the NA-I222T substitution conferred resistance to several NAIs, including oseltamivir and zanamivir[28,35].
Fortunately, so far no NAI resistant H7N9 viruses were isolated from birds, suggesting that the H7N9 viruses circulating in birds are still sensitive to NAIs; however, the repeated emergence of NAI-resistant viruses in humans increases the threat potential of the H7N9 viruses to public and global health.
1.4. Novel drug candidates against H7N9
Currently, two classes of drugs, M2 channel blockers (adamantanes) and neuraminidase inhibitors (zanamivir, oseltamivir, peramivi, and laninamivir) are approved for the treatment of influenza virus infections[36]. Adamantanes are commercial available; among the 4 NAIs, oseltamivir and zanamivir are approved in many countries; peramivir has been approved in USA, Japan, South Korea, and China; and laninamivir is approved only in Japan. However, most influenza A viruses including subtype H7N9 have adamantane-resistant mutations in their respective M2 proteins. NAIs are effective drugs for H7N9 treatment, however, the rapid occurrence of NAI-resistant H7N9 viruses highlights the need for new anti-influenza drugs. Several novel drug candidates were reported to have anti-viral activity against the H7N9 virus.
A novel antiviral drug, Favipiravir (T-705) has recently been approved in Japan for influenza treatment[37,38]. Favipiravir inhibits the RNA-dependent RNA polymerase of influenza viruses by preventing incorporation of natural ATP and GTP for viral RNA synthesis[39,40]. Both in vitro and in vivo data indicated that Favipiravir has antiviral effect against a broad range of influenza viruses, including highly pathogenic H5N1 and H7N9 viruses[39,41–44]. Watanabe et.al reported that Favipiravir has better therapeutic efficacy against NAI-resistant H7N9 viruses in mice compared to NA inhibitors, suggesting that Favipiravir could be a good treatment option for H7N9 viruses resistant to NAI[44]. Tharakaraman and coworkers found that a broad neutralizing human monoclonal antibody (VIS410), targeting a conserved epitope on the HA protein of influenza A, protected DBA mice from A/Anhui/2013 H7N9 virus infection[45]. Additionally, administration of a combination of VIS410 and oseltamivir significantly decreased virus loads and cytokine levels in the lungs [45]. Another human monoclonal antibody 81.39a was reported to effectively neutralize influenza A viruses with group 1 and 2 hemagglutinins, including the H7N9 virus[46]. A single injection (15 or 45 mg/kg) of 81.39a monoclonal antibody protected mice from a lethal challenge with the H7N9 virus and drastically reduced virus replication in mice lungs[46]. In addition, a murine monoclonal antibody (3c10–3) targeting the NA protein of H7N9 showed antiviral effects against the H7N9 virus in cultured cells and in a mouse model[47]. Other monoclonal antibodies with antiviral effects against the H7N9 were reported by different groups, e.g. HNIgGA6 and HNIgGB5 [48], 1B2 and 1H5 [49], and CR8020 [50]. Notably, Wu et al. reported the first successful treatment of an avian-origin H7N9 infection using convalescent plasma (with a neutralizing antibody titer of 1:80) from a recovered patient[51], which suggested that convalescent plasma or a combination of different monoclonal antibodies could present a potential treatment of H7N9 infections.
Another anti-viral drug candidate, DAS181 recombinant fusion protein with sialidase activity, was shown to inhibit the replication of H7N9 viruses by removing the cellular receptors for influenza viruses, thereby blocking the attachment of the influenza virus to its target cells[52]. In a mouse model, DAS181 protected mice from a lethal wild-type and oseltamivir-resistant H7N9 virus challenge [52]. Notably, DAS181 is now in clinical trials as an inhalation drug to treat seasonal influenza virus infections [52]. Similarly, another anti-viral drug candidate, Sp2CBMTD with sialic acid-binding property, has the ability to mask cellular receptors of influenza viruses and prevents viral attachment [53]. A single dose of 10 or 100 µg drug administration protected 80% or 100% of mice from a lethal H7N9 challenge, respectively. Repetitive Sp2CBMTD administration resulted in 100% survival of the mice even at a very low drug dose (0.1 µg). It could be shown that administration of Sp2CBMTD before challenge induced the expression of IL-6, IL-1, MCP-1, MIP-1 and recruitment of neutrophils to the respiratory tract, which resulted in rapid virus clearance from the mouse lungs [53].
In the face of emerging NAI resistant H7N9 viruses combinations of oseltamivir with other antiviral drugs are the choice for H7N9 treatment. For example, combinations of oseltamivir and fenofibrate inhibited virus replication and prolonged survival time of mice infected with a lethal dose of H7N9, by decreasing pulmonary inflammation and increasing the recruitment of CD4+ and CD8+ T lymphocytes. Thus, the combination of oseltamivir plus fenofibrate improved the outcomes of mice infected with H7N9 virus by simultaneously reducing viral replication and normalizing an aberrant immune response [54]. Similarly, Li et al. [55] reported that co-administration of the non-steroidal anti-inflammatory drug (celecoxib) in combination with zanamivir improved survival and decreased lung pathology of mice infected with H7N9, since celecoxib ameliorates pulmonary inflammation and thereby increases the chance of survival [55].
2. Expert commentary
Widespread human H7N9 infections in China indicate that this virus is circulating in poultry and LPMs in a wide geographic area of China. Continuous co-circulation with other subtype influenza A viruses may generate novel reassortant viruses with enhanced pathogenicity and mammalian transmissibility; Use of NAIs is critical to treat human H7N9 infections, however, the rapid occurrence of various NAI resistant substitutions in the NA protein makes the control and treatment of human H7N9 infections challenging.
3. Five-year view
Novel anti-viral drug development against influenza A virus in general is urgently needed. Vaccination is the best approach to control a variety of virus infections, however, so far, no commercial H7N9 vaccine is available, neither for birds nor humans. Given the ecology of the H7N9 viruses, it will be difficult to control this virus in poultry and humans without a well-designed and executed vaccination approach.
3. Key issues.
The LPMs are critical locations where bird to human transmission of H7N9 viruses occurs.
The H7N9 virus is still circulating widely in China; recent isolation of highly pathogenic H7N9 viruses warrants continuous surveillance of LPMs and humans for the emergence of H7N9 viruses transmissible in mammalian species.
Repeated isolation of novel NAI-resistant viruses in humans increases the threat potential of H7N9 viruses to public and global health.
Several novel drug candidates (e.g., Favipiravir, broad neutralizing monoclonal antibodies, Sp2CBMTD, etc.) were reported to have anti-viral activity against H7N9 viruses.
Favipiravir (T-705) has been approved for influenza treatment in Japan.
Acknowledgments
We would like to thank JP Gonzalez for his critical comments on the manuscript.
Funding
This study was supported by the National Key Research and Development Plan (YFD0500204), National Natural Science Foundation of China (no.31472206, no. 31402150 and no. 31572543), Chinese Academy of Agricultural Sciences Young Talent Scientist Program, and the Centers of Excellence for Influenza Research and Surveillance program.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
Papers of special note have been highlighted as:
• Of interest
• • Of considerable interest
- 1.http://www.who.int/csr/don/archive/year/2016/en/.).
- 2.Skowronski DM, Chambers C, Gustafson R et al. Avian Influenza A(H7N9) Virus Infection in 2 Travelers Returning from China to Canada, January 2015. Emerging infectious diseases, 22(1), 71–74 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.William T, Thevarajah B, Lee SF et al. Avian influenza (H7N9) virus infection in Chinese tourist in Malaysia, 2014. Emerging infectious diseases, 21(1), 142–145 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao R, Cao B, Hu Y et al. Human infection with a novel avian-origin influenza A (H7N9) virus. The New England journal of medicine, 368(20), 1888–1897 (2013).• • Detailed introduction of human infected by novel H7N9 in China
- 5.Zhou J, Wang D, Gao R et al. Biological features of novel avian influenza A (H7N9) virus. Nature, 499(7459), 500–503 (2013).• • Detailed description of biological features of H7N9 infection.
- 6.Zhang Q, Shi J, Deng G et al. H7N9 influenza viruses are transmissible in ferrets by respiratory droplet. Science, 341(6144), 410–414 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Su S, Bi Y, Wong G, Gray GC, Gao GF, Li S. Epidemiology, Evolution, and Recent Outbreaks of Avian Influenza Virus in China. Journal of virology, 89(17), 8671–8676 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang F, Bi Y, Wang J et al. Human infections with recently-emerging highly pathogenic H7N9 avian influenza virus in China. The Journal of infection, (2017).• • Report of a highly pathogenic H7N9 avian influenza virus in China
- 9.Zhou X, Li Y, Wang Y et al. The role of live poultry movement and live bird market biosecurity in the epidemiology of influenza A (H7N9): A cross-sectional observational study in four eastern China provinces. The Journal of infection, 71(4), 470–479 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Fan M, Huang B, Wang A et al. Human influenza A(H7N9) virus infection associated with poultry farm, Northeastern China. Emerging infectious diseases, 20(11), 1902–1905 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su S, Zhou P, Fu X et al. Virological and epidemiological evidence of avian influenza virus infections among feral dogs in live poultry markets, china: a threat to human health? Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 58(11), 1644–1646 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Wang C, Wang J, Su W et al. Relationship between domestic and wild birds in live poultry market and a novel human H7N9 virus in China. The Journal of infectious diseases, 209(1), 34–37 (2014).• • Detailed description of relationship between live poultry market and human H7N9 cases.
- 13.He F, Chen EF, Li FD, Wang XY, Wang XX, Lin JF. Human infection and environmental contamination with Avian Influenza A (H7N9) Virus in Zhejiang Province, China: risk trend across the three waves of infection. BMC public health, 15, 931 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun Y, Wang H, Lou X et al. Living poultry markets in rural area: Human infection with H7N9 virus re-emerges in Zhejiang Province, China, in winter 2014. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology, 70, 16–22 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Chen Z, Li K, Luo L et al. Detection of avian influenza A(H7N9) virus from live poultry markets in Guangzhou, China: a surveillance report. PloS one, 9(9), e107266 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Liu S, Mao H, Yu Z, Chen E, Chai C. Surveillance of Avian H7N9 Virus in Various Environments of Zhejiang Province, China before and after Live Poultry Markets Were Closed in 2013–2014. PloS one, 10(8), e0135718 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Huang XM, Zhao DM et al. Detection of Avian H7N9 Influenza A Viruses in the Yangtze Delta Region of China During Early H7N9 Outbreaks. Avian diseases, 60(1 Suppl), 118–125 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu W, Fan H, Raghwani J et al. Occurrence and reassortment of avian influenza A (H7N9) viruses derived from coinfected birds in China. Journal of virology, 88(22), 13344–13351 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ge FF, Ju HB, Yang DQ et al. Epidemiological situation and genetic analysis of H7N9 influenza viruses in Shanghai in 2013. Archives of virology, 159(11), 3029–3041 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Yuan J, Lau EH, Li K et al. Effect of Live Poultry Market Closure on Avian Influenza A(H7N9) Virus Activity in Guangzhou, China, 2014. Emerging infectious diseases, 21(10), 1784–1793 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He Y, Liu P, Tang S et al. Live poultry market closure and control of avian influenza A(H7N9), Shanghai, China. Emerging infectious diseases, 20(9), 1565–1566 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu H, Wu JT, Cowling BJ et al. Effect of closure of live poultry markets on poultry-to-person transmission of avian influenza A H7N9 virus: an ecological study. Lancet, 383(9916), 541–548 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang D, Yang L, Zhu W et al. Two Outbreak Sources of Influenza A (H7N9) Viruses Have Been Established in China. Journal of virology, 90(12), 5561–5573 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiang N, Li X, Ren R et al. Assessing Change in Avian Influenza A(H7N9) Virus Infections During the Fourth Epidemic - China, September 2015-August 2016. MMWR. Morbidity and mortality weekly report, 65(49), 1390–1394 (2016). [DOI] [PubMed] [Google Scholar]
- 25.http://www.who.int/csr/don/20-february-2017-ah7n9-china/en/.).
- 26.Iuliano AD, Jang Y, Jones J et al. Increase in Human Infections with Avian Influenza A(H7N9) Virus During the Fifth Epidemic - China, October 2016-February 2017. MMWR. Morbidity and mortality weekly report, 66(9), 254–255 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hay AJ, Hayden FG. Oseltamivir resistance during treatment of H7N9 infection. Lancet, 381(9885), 2230–2232 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Itoh Y, Shichinohe S, Nakayama M et al. Emergence of H7N9 Influenza A Virus Resistant to Neuraminidase Inhibitors in Nonhuman Primates. Antimicrobial agents and chemotherapy, 59(8), 4962–4973 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yen HL, McKimm-Breschkin JL, Choy KT et al. Resistance to neuraminidase inhibitors conferred by an R292K mutation in a human influenza virus H7N9 isolate can be masked by a mixed R/K viral population. mBio, 4(4) (2013).• • Detailed description of drug resistant H7N9 influenza viruses.
- 30.Sleeman K, Guo Z, Barnes J, Shaw M, Stevens J, Gubareva LV. R292K substitution and drug susceptibility of influenza A(H7N9) viruses. Emerging infectious diseases, 19(9), 1521–1524 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marjuki H, Mishin VP, Chesnokov AP et al. Characterization of drug-resistant influenza A(H7N9) variants isolated from an oseltamivir-treated patient in Taiwan. The Journal of infectious diseases, 211(2), 249–257 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yen HL, Zhou J, Choy KT et al. The R292K mutation that confers resistance to neuraminidase inhibitors leads to competitive fitness loss of A/Shanghai/1/2013 (H7N9) influenza virus in ferrets. The Journal of infectious diseases, 210(12), 1900–1908 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Q, Ma J, Strayer DR et al. Emergence of a novel drug resistant H7N9 influenza virus: evidence based clinical potential of a natural IFN-alpha for infection control and treatment. Expert review of anti-infective therapy, 12(2), 165–169 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Marjuki H, Mishin VP, Chesnokov AP et al. Neuraminidase Mutations Conferring Resistance to Oseltamivir in Influenza A(H7N9) Viruses. Journal of virology, 89(10), 5419–5426 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gillman A, Nykvist M, Muradrasoli S et al. Influenza A(H7N9) virus acquires resistance-related neuraminidase I222T substitution when infected mallards are exposed to low levels of oseltamivir in water. Antimicrobial agents and chemotherapy, 59(9), 5196–5202 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ison MG. Antivirals and resistance: influenza virus. Current opinion in virology, 1(6), 563–573 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Takashita E, Ejima M, Ogawa R et al. Antiviral susceptibility of influenza viruses isolated from patients pre- and post-administration of favipiravir. Antiviral research, 132, 170–177 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral research, 100(2), 446–454 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furuta Y, Takahashi K, Fukuda Y et al. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrobial agents and chemotherapy, 46(4), 977–981 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furuta Y, Takahashi K, Kuno-Maekawa M et al. Mechanism of action of T-705 against influenza virus. Antimicrobial agents and chemotherapy, 49(3), 981–986 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kiso M, Takahashi K, Sakai-Tagawa Y et al. T-705 (favipiravir) activity against lethal H5N1 influenza A viruses. Proceedings of the National Academy of Sciences of the United States of America, 107(2), 882–887 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sleeman K, Mishin VP, Deyde VM, Furuta Y, Klimov AI, Gubareva LV. In vitro antiviral activity of favipiravir (T-705) against drug-resistant influenza and 2009 A(H1N1) viruses. Antimicrobial agents and chemotherapy, 54(6), 2517–2524 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao RY, Xiao JH, Cao B, Li S, Kumaki Y, Zhong W. Inhibition of novel reassortant avian influenza H7N9 virus infection in vitro with three antiviral drugs, oseltamivir, peramivir and favipiravir. Antiviral chemistry & chemotherapy, 23(6), 237–240 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Watanabe T, Kiso M, Fukuyama S et al. Characterization of H7N9 influenza A viruses isolated from humans. Nature, 501(7468), 551–555 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tharakaraman K, Subramanian V, Viswanathan K et al. A broadly neutralizing human monoclonal antibody is effective against H7N9. Proceedings of the National Academy of Sciences of the United States of America, 112(35), 10890–10895 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marjuki H, Mishin VP, Chai N et al. Human Monoclonal Antibody 81.39a Effectively Neutralizes Emerging Influenza A Viruses of Group 1 and 2 Hemagglutinins. Journal of virology, 90(23), 10446–10458 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson JR, Guo Z, Reber A et al. An influenza A virus (H7N9) anti-neuraminidase monoclonal antibody with prophylactic and therapeutic activity in vivo. Antiviral research, 135, 48–55 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Z, Wang J, Bao L et al. Human monoclonal antibodies targeting the haemagglutinin glycoprotein can neutralize H7N9 influenza virus. Nature communications, 6, 6714 (2015). [DOI] [PubMed] [Google Scholar]
- 49.Tan GS, Leon PE, Albrecht RA et al. Broadly-Reactive Neutralizing and Non-neutralizing Antibodies Directed against the H7 Influenza Virus Hemagglutinin Reveal Divergent Mechanisms of Protection. PLoS pathogens, 12(4), e1005578 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tharakaraman K, Subramanian V, Cain D, Sasisekharan V, Sasisekharan R. Broadly neutralizing influenza hemagglutinin stem-specific antibody CR8020 targets residues that are prone to escape due to host selection pressure. Cell host & microbe, 15(5), 644–651 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu XX, Gao HN, Wu HB, Peng XM, Ou HL, Li LJ. Successful treatment of avian-origin influenza A (H7N9) infection using convalescent plasma. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases, 41, 3–5 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Marjuki H, Mishin VP, Chesnokov AP et al. An investigational antiviral drug, DAS181, effectively inhibits replication of zoonotic influenza A virus subtype H7N9 and protects mice from lethality. The Journal of infectious diseases, 210(3), 435–440 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Govorkova EA, Baranovich T, Marathe BM et al. Sialic acid-binding protein Sp2CBMTD protects mice against lethal challenge with emerging influenza A (H7N9) virus. Antimicrobial agents and chemotherapy, 59(3), 1495–1504 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu L, Bao L, Li F et al. Combinations of oseltamivir and fibrates prolong the mean survival time of mice infected with the lethal H7N9 influenza virus. The Journal of general virology, 96(Pt 1), 46–51 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Li C, Li C, Zhang AJ et al. Avian influenza A H7N9 virus induces severe pneumonia in mice without prior adaptation and responds to a combination of zanamivir and COX-2 inhibitor. PloS one, 9(9), e107966 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]