Abstract
The human tongue has a complex architecture, consistent with its complex roles in eating, speaking and breathing. Tongue muscle architecture has been depicted in drawings and photographs, but not quantified volumetrically. This paper aims to fill that gap by measuring the muscle architecture of the tongue for 14 people captured in high-resolution 3D MRI volumes. The results show the structure, relationships and variability among the muscles, as well as the effects of age, gender and weight on muscle volume. Since the tongue consists of partially interdigitated muscles, we consider the muscle volumes in two ways. The functional muscle volume encompasses the region of the tongue served by the muscle. The structural volume halves the volume of the muscle in regions where it interdigitates with other muscles. Results show similarity of scaling across subjects, and speculate on functional effects of the anatomical structure.
Keywords: Tongue, anatomy, muscles, MRI, 3D, volumes
1. Introduction
The tongue plays a crucial role in three critical human functions: speech, swallowing and breathing. It forms speech sounds; it contains and propels food; it maintains airway opening. The tongue is ideally located to perform these functions because of its location at the entrance to the gastrointestinal system and the respiratory system. It is ideally constructed to perform these functions because its complex architecture allows it to form markedly complex shapes. The tongue is virtually unique in the body as it is a deformable structure, with no bones, joints or air filled chambers, which nonetheless, must move about within the oral cavity. The tongue is part of a group of biological structures, including tentacles and the elephant's trunk, which preserve volume and consist of muscles that are orthogonal in three dimensions (Kier & Smith 1985). These structures, called muscular hydrostats, move by deforming local regions, which changes their surface shapes and positions. The human tongue has a complex muscle architecture; it is anisotropic in the anterior–posterior direction, and its two sides are mirror images (Stone 1990). Its muscle fibres are oriented in three orthogonal directions and they are extensively interdigitated. As a result, a sample of tissue from any region will often contain two fibre orientations and multiple muscles, and may include intrinsic and extrinsic, protrusor and retrusor, elevator and depressor muscles.
Although tongue muscle structure can aid in our knowledge of disease and normal tongue function, little is known regarding the relationship between tongue structure and function. Even knowledge about the variability of tongue structure among different persons does not yet exist. This is partly because dissections of cadavers result in pictures of tongue muscles and fibres, but interdigitation makes it virtually impossible to dissect out a single tongue muscle in its entirety, unlike the limb muscles. Although muscle interdigitation also poses challenges to imaging techniques (Gaige et al. 2007), the present study is able to overcome these limitations using in vivo magnetic resonance imaging (MRI) recordings and extracting tongue muscle volumes from high-resolution MRI data. Two recent technological developments allow us to extract and characterise the muscle volumes of the tongue. (1) The ability to create 3D MRI super-resolution volumes of the tongue (Woo et al. 2012). (2) The creation of a 3D anatomical MRI tongue atlas, which has been segmented to define the muscles and glands of the tongue, and which can be deformed to individual subjects' tongues (Woo et al. 2015). This study provides baseline knowledge to confirm quantitatively the muscle relationships that are assumed generally, and to provide new information as well.
Relative muscle volume across subjects is important in determining normal anatomical variability and the relationship between muscle structure and function. Handsfield et al. (2014) compared relative muscle volumes and lengths in the muscles of the ankle.Holzbaur et al. (2007) examined upper limb muscle volumes. The present study determines how tongue muscles scale in volume relative to tongue size, and relative to subject-based parameters such as age, gender and weight. In addition, muscles are grouped functionally according to neuromuscular organisation (retrusors vs. protrusors), type of origin (extrinsic vs. intrinsic vs. floor of the mouth) and structure (bundled, interdigitated, both). If proportional muscle volume is consistent across subjects, it will allow prediction of many muscles from measurement of only a few.
Anatomical data are badly needed to inform tongue models. There is no gold standard tongue model to date, and current models can all be improved by replacing estimated muscle sizes, locations and shapes with measured anatomical data. Models would not have to assume the percentage of the tongue encompassed by a muscle. Moreover, the muscle volumes can be used in the generation of forces needed to drive modelled muscles. Anatomical variation is always an issue in modelling. The present study measured volumes from 14 subjects, consistency among subjects indicate that the muscle proportions can be applied to models of new subjects.
Methodologically, this paper measures muscle volume in two ways. The ‘functional’ muscle volumes extract the region of the tongue served by that muscle, without respect to the density of the interdigitated fibres. The ‘structural’ muscle volumes halve the size of the muscle in regions where voxels are shared between two muscles; there are not regions with three orthogonal muscles. These two methods provide meaningfully different information. The functional volume indicates the proportion of the tongue controlled by the muscle. The structural volume is a better representation of actual volume and force.
Finally, these measurements allow us to answer three questions of interest. What is the anatomical variability across subjects? What is the proportionality of functional muscle groups, such as antagonists, or extrinsic/intrinsic muscles? What is the extent of the interdigitation within the tongue, and how important is it?
2. Methods
2.1. Subject and data acquisition
Fourteen high-resolution MRI data-sets were included in this study. The data were collected following a protocol approved by the IRB of the University of Maryland Baltimore (IRB# 42060). Data-sets were selected based on the criteria that no pathology existed, and that the MRI data-set allowed the septum and muscle fibres to be easily visualised within the body of the tongue. All MRI scanning was performed on a Siemens 3.0T Tim Trio system (Siemens Healthcare, Inc., Malvern, PA, USA) with a 12-channel head and a 4-channel neck coil using a segmented gradient echo sequence. Each data-set starts with a sagittal, coronal and axial stack of images encompassing the tongue and surrounding structures. The spatial resolution of each slice in the stack is 256 × 256 pixels by z slices (where z ranges from 10 to 24) with 0.94 mm × 0.94 mm in-plane resolution and 3 mm slice thickness. The data-sets were acquired in a supine rest position, and the subjects were required to remain still from 1.5 to 3 min for each orientation (Woo et al. 2015). Subject demographics are identified in Table 1.
Table 1.
subject demographics.
| ID# | Age | Gender | Weight | Handedness | Functional pooled tongue vol (mm3) | Structural pooled tongue vol (mm3) | Tng vol/weight (lbs) |
|---|---|---|---|---|---|---|---|
| 1 | 22 | F | 160 | R | 141,309 | 89,310 | 883 |
| 2 | 26 | F | 126 | R | 101,238 | 76,925 | 803 |
| 3 | 27 | F | 125 | 88,762 | 54,904 | 710 | |
| 4 | 27 | F | 180 | R | 143,207 | 90,350 | 796 |
| 5 | 31 | F | 150 | AMBI | 127,340 | 92,830 | 849 |
| 6 | 41 | F | 217 | R | 141,475 | 90,913 | 652 |
| 7 | 45 | F | 180 | R | 124,760 | 90,520 | 693 |
| 8 | 59 | F | 180 | R | 159,747 | 101,099 | 887 |
| Mean | 35 | 165 | 128,480 | 85,856 | 784 | ||
| SD | 13 | 31 | 23,491 | 14,133 | 90 | ||
| 9 | 22 | m | 130 | R | 161,652 | 104,256 | 1243 |
| 10 | 23 | m | 155 | R | 156,658 | 97,402 | 1011 |
| 11 | 27 | m | 250 | 202,717 | 126,947 | 811 | |
| 12 | 33 | m | 210 | R | 162,691 | 101,217 | 775 |
| 13 | 43 | m | 180 | R | 165,602 | 108,802 | 920 |
| 14 | 52 | m | 156 | R | 122,751 | 78,108 | 787 |
| Mean | 33 | 180 | 162,012 | 102,789 | 924 | ||
| SD | 12 | 44 | 25,458 | 15,891 | 181 |
2.2. Creation of super-resolution volumes (supervolumes) and segmentation of the 3D anatomical atlas
MRI of the tongue has been used widely in scientific research studies to analyse tongue structure, function and its relation to the vocal tract. often to allow for better visualisation, three orthogonal image volumes are acquired, e.g. axial, sagittal and coronal. Each stack of images is acquired with an in-plane resolution that is much better than the through-plane resolution (0.9 × 0.9 × 3 mm). This is because acquisition of a stack with an isotropic voxel size would require the subject to refrain from swallowing for an unreasonably long time. The anisotropic voxel size helps to minimise data collection time while maintaining low noise, high visual detail and minimal blurring due to involuntary motion artefacts. As a result, any one stack, by itself, is not ideal for automatic volumetric analyses such as segmentation, registration, and atlas building or even for visualisation when oblique slices are required. In particular, it is difficult to observe 3D tongue muscles clearly in any one of the volumes (Woo et al. 2012). Therefore, a super-resolution technique is used to reconstruct a single high-resolution volume with an isotropic resolution from three orthogonal stacks. In brief, the method uses pre-processing steps including registration and intensity matching, followed by a data combination approach with the edge-preserving property using Markov random field optimisation.
To perform the initial muscle segmentation, a 3D muscle atlas was created, (Woo et al. 2015). The atlas was built using a difeomorphic groupwise registration with a cross-correlation similarity metric. The 3D high-resolution supervolumes for 20 subjects were deformably registered to a single subject, and averaged to create a 3D, high-resolution, anatomical atlas. From this 3D atlas, each muscle of the tongue was manually extracted by two of the investigators independently (MS and EZM) using custom software (Lee et al. 2013, 2014), such that the outer boundary of each muscle was represented by a 3D ‘mask’.
Several tongue dissections and anatomical models were studied to determine the fine details of each muscle's location (Abd-el-Malek 1939; Miyawaki 1974; Takemoto 2001; Sanders & Mu 2013). Ten muscles were segmented in the tongue and floor of the mouth: (1) superior longitudinal (SL), (2) inferior longitudinal (IL), (3) styloglossus (SG), (4) hyoglossus (HG), (5) verticalis (V), (6) genioglossus (GG), (7) transversus (T), (8) anterior belly of digastric (ABD), (9) mylohyoid (MH) and (10) geniohyoid (GH). In order to visualise all the muscles of the tongue without obscuration by other muscles, three separate muscle mask files were needed. Each mask file depicts muscles that do not overlap, allowing accurate segmentation and volume calculation (see Figure 1).
Figure 1.
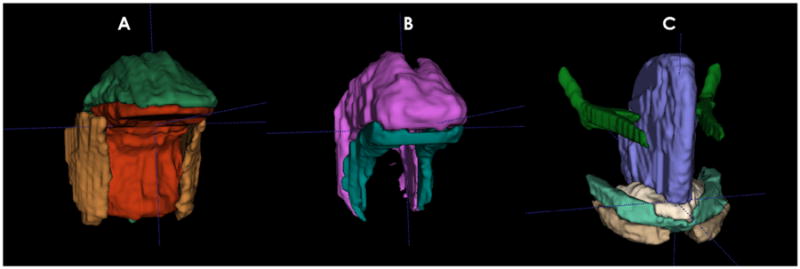
tongue muscles. (a) superior longitudinal (green), transverse (orange), hyoglossus (yellow). (B) Verticalis (pink), inferior longitudinal (aqua). (C) genioglossus (blue), styloglossus (green), geniohyoid (white), mylohyoid (aqua), anterior belly of digastric (tan).
Although our definition of the muscles segmented here was consistent with known tongue anatomy found in research papers and anatomy textbooks, three methodological aspects of this study need to be detailed further. First, there were three muscles which could not be completely measured because of image resolution limitations. (1) The hyoglossus fibres, after they entered the tongue and began coursing anteriorly, could not be distinguished from those of the styloglossus. Therefore, the lateral longitudinal fibres were all attributed to styloglossus, whose extra-lingual and intra-lingual fibres were visible and linked. (2) The styloglossus could not be traced to its origin. Although the styloid process of the temporal bone was visible, the styloglossus fibres could not be distinguished from the fibres of the neighbouring muscles until almost halfway to its insertion into the tongue body. Therefore, styloglossus measurements omit the origin and under-represent the muscle length by about 20%. (3) The insertion of the palatoglossus into the tongue was not visible in the images. Even in dissection it is challenging to find the lingual insertion of this muscle (cf. Kuehn & Azzam 1978; Miller et al. 2002). Therefore, the palatoglossus muscle was not segmented in this project. The second methodological aspect of note was our treatment of the verticalis muscle. The verticalis and transverse muscles alternate, in repeating laminae, from the front to the back of the tongue. However, it has been observed during dissection, that the posterior tongue contains only surface fibres for verticalis (Miyawaki 1974; Takemoto 2001). Therefore, the verticalis masks included only voxels at the surface of the posterior tongue.
The third methodological point of interest was our treatment of voxels as containing muscle fibres in one or two directions, but not three. Although it has not been mentioned previously, observation of published anatomical drawings and photographs of dissections indicates that fibre-crossing occurs in two, but not three, directions in (Abd-el-Malek 1939; Miyawaki 1974; Sanders & Mu 2013). Although unexpected, there are no published data showing more than two fibre directions in the same location. Thus, our muscle masks were constructed so that voxels contained one or two orthogonal muscle directions, but not three. The implications of this architecture are considered in the discussion.
2.3. Using the atlas to segment muscles of individual subjects
The 3D MRI atlas was deformed to 14 high resolution 3D supervolumes of the tongue using Deformable Registration (Woo et al. 2015). The initial deformation was performed automatically by deformably registering each voxel in the atlas volume to the closest voxel in the subject's tongue, using pixel intensity of the MRI images. After deformation, the voxels were still linked to the specific muscle masks segmented in the atlas. That is, the voxels of each subject's tongue volume, retained the labels and the allegiances to the same muscles in the atlas. Thus, the atlas mask for each muscle was deformed automatically to the muscles of each subject. The second level of registration was manual refinement of these automatically generated muscle masks using IT-SNAP (Yushkevich et al. 2006), to correct for errors. ITK-SNAP displays the 3D volume and each plane, allow scrolling through the stack in all three directions, and labels voxels with user-defined names. It was used to modify the atlas generated muscle masks. The manual refinement of the masks included smoothing of the muscle boundaries, assuring that adjacent muscles did not overlap and visual comparison to the three original MR stacks to make up for any image quality lost during the supervolume construction. To maximise measurement consistency, a single muscle was manually refined by a single experimenter across all subjects, with portions checked by others. This refinement method allowed disambiguation of any muscle boundaries in a supervolume.
Volume calculations were made and recorded for the muscles in two ways, using ITK-SNAP. In the first method, the entire tongue region covered by each muscle was determined by adding all the voxels with the muscle's label. In this method, the muscles were measured in the tongue, and their volumes summed to create a pooled tongue volume, which was used to calculate the relative volume of each muscle. This first method was used because each tongue muscle serves an entire tongue region, despite being interdigitated with other muscles. Thus, this is the functional size of the muscle. However, because most voxels of the tongue contain fibres from two muscles, the pooled volume was close to double the size of the natural tongue. Therefore, a second method was used to calculate muscle volume, which more closely measured the structural size of the muscle. In this method, the portions of each muscle, which contained interdigitated fibres, were assigned half volume. no volume was subtracted from any voxel for fat, connective tissue, nerves or blood vessels, because they are distributed throughout the tongue. For example, the volume of the transverse muscle is entirely interdigitated with the volumes of the genioglossus and verticalis muscles. Therefore, all of the transverse voxels were assigned half volume. The tongue muscles that do not contain interdigitated fibres are: the anterior portion of the inferior longitudinal muscle, the portions of the extrinsic muscles that are outside the tongue and the floor of the mouth muscles (FOM). Figure 2 below shows the differences in these calculations. The lingual tonsils and salivary glands were segmented but not included in this analysis.
Figure 2.
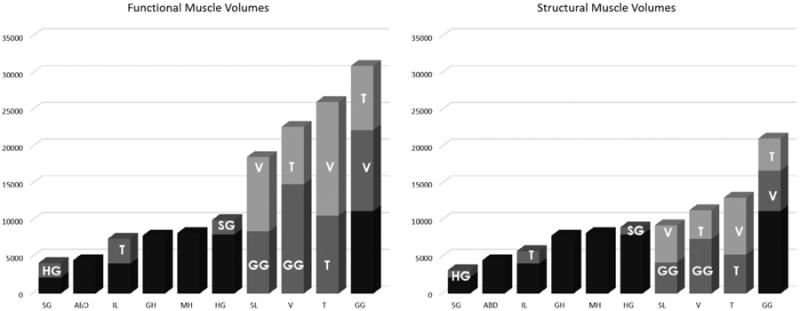
Functional and structural muscle volumes arranged by size. Bars include bundled fibres (black), interdigitated fibres (grey and light grey) with interdigitated muscles indicated on bar.
2.4. Data analysis
Means and standard deviations were used to determine the relative scaling of subjects for each muscle relative to the pooled tongue volume and within muscle groups based on anatomy and function: elevator versus depressor; extrinsic versus intrinsic; retrusor versus protrusor; agonist versus antagonist, These muscle group volumes were also compared to tongue size, subject weight, age and gender. An analysis of variance compared the effects of gender on tongue size and correlations were used to compare tongue size to age and weight.
3. Results
The goals of this study were (1) to determine how tongue muscles scale in volume relative to tongue size, and relative to subject-based parameters such as age, gender and weight. (2) To determine how muscle volume relates to function (retrusor vs. protrusor, agonist vs. antagonist), muscle origin (extrinsic vs. intrinsic) and neuromuscular organisation (extrinsic vs. intrinsic vs. FOM). (3) To consider the origins and effects of bundled versus interdigitated fibres.
3.1. Scaling individual muscle volumes relative to tongue size
Figure 2 and Table 2 show the average volume and standard deviation of each muscle in the tongue. Figure 2 shows the per cent of the muscle that is bundled (black), and the per cent that interdigitates with one or more other muscles. There is no interdigitation at all in the FOM: ABD, MH, GH. The extrinsic muscles are bundled at their origin and interdigitate as they enter the tongue: SG, HG, GG. The intrinsic muscles mostly interdigitate throughout all their fibres: SL, V, T. However, one intrinsic muscle, the IL, is bundled and surrounded by septa anteriorly and interdigitates posteriorly (Abd-el-Malek 1939). Table 2 shows the per cent of the muscle that is interdigitated, and the per cent of the tongue accounted for by each muscle. Both the functional and structural muscle volumes follow the same scaling order, however, the interdigitated muscles naturally have less volume in the structural calculation. Structurally, the muscles form a fairly linear size continuum (Figure 2 right). Functionally, there are four large muscles (Figure 2 left), who by themselves serve almost 70% of the tongue and represent all three directions of motion available to the tongue. The largest muscle is the genioglossus, which accounts for over 20% of the pooled tongue volume in both data-sets. The transverse is the second largest at 18.5% of the functional and 14% of the structural volume. The verticalis and superior longitudinal also have more volume in the functional than the structural data-set. These four large muscles are highly interdigitated, in fact the last three muscles are entirely interdigitated. Therefore, their calculated volumes are reduced by 50% in the structural calculation. However, they are still among the largest muscles even when halved. The standard deviations are fairly small (∼20%) for all muscles in both methods, reflecting similarity of muscle volume scaling across subjects.
Table 2.
Means and standard deviations of functional and structural muscle volumes (mm3).
| Functional volume | Structural volume | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Per cent interdigitated mean (SD) | Mean | SD | % of whole tongue | Mean | SD | % of whole tongue | |
| SG | 47.3 (3.0) | 4147 | 665 | 3.0 | 3167 | 501 | 3.4 |
| ABD | 0 | 4380 | 1130 | 3.2 | 4380 | 1130 | 4.7 |
| IL | 45.7 (11) | 7459 | 1295 | 5.3 | 5766 | 1153 | 6.2 |
| GH | 0 | 7861 | 1799 | 5.6 | 7861 | 1799 | 8.4 |
| MH | 0 | 8221 | 1875 | 5.9 | 8221 | 1875 | 8.8 |
| HG | 19.7 (1.1) | 9990 | 1818 | 7.1 | 9008 | 1648 | 9.7 |
| SL | 100 | 18,556 | 3429 | 13.2 | 9278 | 1714 | 10.0 |
| Vert | 100 | 22,659 | 4148 | 16.1 | 11,329 | 2074 | 12.2 |
| T | 100 | 26,045 | 5774 | 18.5 | 13,023 | 2887 | 14.0 |
| GG | 63.4 (4.3) | 30,966 | 6847 | 22.1 | 21,079 | 4393 | 22.6 |
| pooled vol | 140,284 | 100.0 | 93,113 | 100.0 | |||
Table 3 also shows how similar the subjects are in proportionality of muscle volume. The correlations among subjects are high between the individual muscles and the pooled tongue volume, both for the functional muscle size (all inclusive) and the structural muscle size (volume halved for shared voxels).
Table 3.
Correlations between muscle volume and total tongue volume across all subjects.
| Muscle volume × pooled tongue volume | Muscle volume × subject weight | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Functional size | Structural size | Functional size | Structural size | |||||
|
|
|
|
|
|||||
| Pearson's r | p value | Pearson's r | p value | Pearson's r | p value | Pearson's r | p value | |
| T | 0.95 | 0.000 | 0.93 | 0.000 | 0.71 | 0.007 | 0.71 | 0.007 |
| V | 0.90 | 0.000 | 0.88 | 0.000 | 0.51 | 0.077 | 0.51 | 0.077 |
| SL | 0.93 | 0.000 | 0.92 | 0.000 | 0.51 | 0.073 | 0.51 | 0.073 |
| GG | 0.96 | 0.000 | 0.93 | 0.004 | 0.73 | 0.004 | 0.71 | 0.006 |
| SG | 0.93 | 0.000 | 0.94 | 0.000 | 0.69 | 0.009 | 0.70 | 0.007 |
| HG | 0.90 | 0.000 | 0.90 | 0.000 | 0.62 | 0.024 | 0.62 | 0.025 |
| IL | 0.63 | 0.022 | 0.74 | 0.000 | 0.38 | 0.154 | 0.42 | 0.200 |
| GH | 0.86 | 0.000 | 0.88 | 0.000 | 0.70 | 0.008 | 0.70 | 0.008 |
| MH | 0.75 | 0.003 | 0.79 | 0.001 | 0.35 | 0.239 | 0.35 | 0.239 |
| Abd | 0.73 | 0.005 | 0.72 | 0.006 | 0.43 | 0.144 | 0.43 | 0.144 |
The relationship between muscle volume and subject weight was also considered as larger people were expected to have larger tongues.
3.2. Muscle volume across subject-related factors
Table 1 above shows the demographic data for each subject including pooled tongue volume. The male tongues were slightly larger, on average, than the female tongues. This difference showed trends between gender and polled tongue volume (structural, F = 4.433, p = 0.057; functional F = 3.745, p = 0.77), but not when tongue volume was controlled for total body weight (structural: F = 1.378, p = 0.263; functional: F = 1.172, p = 0.30). Pearson's product–moment correlations were used to compare age and body weight with tongue volume. Age did not show a correlation with pooled tongue volume (structural r = 0.025, p = 0.933, functional r = 0.016, p = 0.956). However, positive correlations were found between body weight and tongue volume (functional r = 0.674, p = 0.008; structural r = 0.67, p = 0.009). The individual muscles also correlated very highly to total tongue volume and somewhat with body weight (Table 3). This supports the consistency of muscle scaling across subjects relative to tongue volume, even more than the standard deviations of the mean data (Table 2) which are confounded by variations in tongue size due to weight.
3.3. Categorisation of muscles relative to anatomy and function
Considering the tongue muscle volumes relative to anatomical features, Tables 4 and 5, respectively, present the muscle volumes as a percentage of the pooled tongue for the functional and structural muscle volumes, Tables 4(A) and 5(A) show proportionality of the intrinsic, extrinsic and FOM. The intrinsic muscles originate and insert within the tongue, whereas the extrinsic muscles originate on bones external to the tongue, enter the tongue, interdigitate with other muscles and finally insert within the tongue. The FOM muscles, originate and insert immediately below the tongue, and are contiguous with it. A secondary effect of FOM muscle shortening is to elevate the tongue body. Of these three groups, the largest was the intrinsic muscles, accounting for 53% of the functional tongue volume and 42% of the structural tongue volume. The smallest group was the FOM muscles at 15.9 and 21.9% of the volume, respectively.
Table 4.
Functional proportion of the tongue for grouped muscles.
| (A) | Extrinsic (%) | Intrinsic (%) | FOM (%) | (B) | Protrusor (%) | Retrusor (%) | (C) | Elevator (%) | Depressor (%) |
|---|---|---|---|---|---|---|---|---|---|
| SL | 13.2 | GG | 22.1 | SL | 13.2 | ||||
| IL | 5.3 | V | 16.1 | SG | 3.0 | ||||
| V | 16.1 | T | 18.5 | T | 18.5 | ||||
| T | 18.5 | SL | 13.2 | MH | 7.1 | ||||
| SG | 3.0 | IL | 5.3 | GH | 5.6 | ||||
| HG | 5.9 | SG | 3.0 | ABD | 3.2 | ||||
| GG | 22.1 | HG | 5.9 | GG | 22.1 | ||||
| MH | 7.1 | MH | IL | 5.3 | |||||
| GH | 5.6 | GH | HG | 5.9 | |||||
| ABD | 3.2 | ABD | V | 16.1 | |||||
| total | 30.9 | 53.2 | 15.9 | total | 56.7 | 27.3 | total | 72.7 | 49.4 |
Table 5.
structural proportion of the tongue for grouped muscles.
| (A) | Extrinsic (%) | Intrinsic (%) | FOM (%) | (B) | Protrusor (%) | Retrusor (%) | (C) | Elevator (%) | Depressor (%) |
|---|---|---|---|---|---|---|---|---|---|
| SL | 10.0 | GG | 22.6 | SL | 10.0 | ||||
| IL | 6.2 | V | 12.2 | SG | 3.4 | ||||
| V | 12.2 | T | 14.0 | T | 14.0 | ||||
| T | 14.0 | SL | 10.0 | MH | 8.8 | ||||
| SG | 3.4 | IL | 6.2 | GH | 8.4 | ||||
| HG | 9.7 | SG | 3.4 | ABD | 4.7 | ||||
| GG | 22.6 | HG | 9.7 | GG | 22.6 | ||||
| MH | 8.8 | MH | IL | 6.2 | |||||
| GH | 8.4 | GH | HG | 9.7 | |||||
| ABD | 4.7 | ABD | V | 12.2 | |||||
| TOTAL | 35.7 | 42.4 | 21.9 | TOTAL | 48.8 | 29.3 | TOTAL | 70.2 | 28.1 |
Tables 4(B) and 5(B) group the muscles into protrusors and retrusors. The protrusors extend the tongue forward, and the retrusors return it inside the mouth. The genioglossus is often grouped with the protrusors, but the anterior portion actually lowers the tongue tip acting more like a retrusor. Therefore, since we could not subdivide function based on static anatomy data, the protrusor volume was considered with and without the GG; the first overestimated its role as a protrusor and the second underestimated it. In the functional data-set, there was more tongue volume served by the protrusors than the retrusors; with the GG protrusor muscles accounted for 2.1 times the volume of the retrusors; without the GG protrusors were 1.3 times greater. When considering the structural muscle volume, the protrusors were 1.7 times the retrusors with the GG, and without the GG they were the same volume (1.0).
Tables 4(C) and 5(C) group the muscles into elevators and depressors. Elevation and depression of the tongue is largely accomplished by the jaw, whose muscles are not considered here. Considering only the tongue and FOM muscles, there was more muscle volume devoted to elevation than depression; the posterior GG can assist elevation. Without the GG, the elevator muscle group had greater structural volume, but the same functional volume as the depressor group.
4. Discussion
Although this paper does not concern innervation of the tongue, it is worthwhile to remember that the tongue is highly innervated. There are at least 8,000 motor units in the tongue, and all the tongue muscles have many local innervation sites (Wozniak & Young 1969; Atsumi & Miyatake 1987; O'Kusky & norman 1995). Thus, all the tongue muscles are quite capable of independently controlled local motion (Sokolof 2000).
4.1. Muscle volume relative to tongue anatomy: extrinsic/intrinsic
It once was thought that the extrinsic muscles moved the tongue and the intrinsic muscles shaped it (Perkell 1969; Hardcastle 1976). This was reasonable for two reasons, first, other structures, such as the hands, use extrinsic muscles for power and intrinsic muscles for precision (Long et al. 1970). Second, early theories were based on X-ray movies. X-ray, a through transmission projection, collapsed the 3D tongue into a 2D image which was unable to visualise the rich shape changes occurring at midline and the non-uniformity of those changes along the tongue's length. once 3D shapes became available through ultrasound in the 1980s and MRI in the 1990s, thinking changed dramatically about how the mechanics of the tongue worked (cf. Smith & Kier 1989). Moreover, fine muscle dissection and perfusion showed innervation of the tongue muscles and fibre locations that were not consistent with dividing fine and coarse control along the lines of intrinsic and extrinsic muscles (Mu & Sanders 2010). Thus, we need to rethink whether there are task-based differences, extrinsic for position and intrinsic for shape, or whether each has different biomechanical advantages that can be exploited for both shaping and moving the tongue. First, what is the advantage of being intrinsic or extrinsic? Extrinsic muscles originate on an immobile rigid bone, which guarantees that activation will cause only the insertion end of the muscle to move. Biomechanically, this would create a fairly direct response to muscle activation. Whereas with intrinsic muscles, a second muscle is often needed to stiffen part of the tongue, as in bending (Smith & Kier 1989), or to provide a platform to support local tongue motion, as when trilling the tongue tip. Theoretically, no additional muscle co-activation is necessary with extrinsic muscles. However, several features of the extrinsic muscles suggest they are as likely to create local deformations in conjunction with other muscles as they are to position the tongue. They interdigitate when they enter the tongue, and their proportion of the tongue mass is smaller than that devoted to the intrinsic muscles (Tables 4(A) and 5(A)). In addition, their insertion at multiple locations throughout the tongue, and their extensive innervation suggests finer local control than required for coarse positioning of the tongue. Thus, it is possible that the extrinsic/intrinsic difference provides biomechanical advantages rather than task-dependent functional differences.
4.2. Muscle volume relative to tongue function
The only function reflected in the tongue's neuromuscular organisation is protrusion and retrusion. The protrusor and retrusor muscles are represented separately in the ventral versus dorsal regions of the hypoglossal nucleus (HGN), as well as the lateral versus medial branches of the hypoglossal nerve, consistent with antagonistic behaviour of these two sets of muscles (McClung & Goldberg 1999, 2000). However, these regionalised sections of the HGN are embryologically based, and not functionally important as co-contraction of the tongue musculature develops early for speech. Protrusion is used by newborns who suckle using a tongue-thrust motion. In infancy, however, tongue motion rapidly becomes differentiated for speech (Davis & Macneilage 1995). In adults, even the simplest speech gestures utilise co-contraction of the tongue muscles (cf. Macneilage & Sholes 1964; vowels – Miyawaki 1975; Baer et al. 1988). Thus, muscle innervation and muscle function are not tightly linked due to co-activation.
4.3. Muscle volume relative to other factors: age, gender weight
The lack of correlation between age and tongue muscle volume is interesting as one might expect atrophy in the muscles of older subjects. However, our measurements of volume represent the volume of the tongue covered by the muscle, not the size of the muscle fibres. Even our structural measure cannot determine what per cent of the muscle might be replaced with fat. Thus, we may simply be unable to capture muscle atrophy in the form of substitution of fat for muscle. Gender also had no significant effect on tongue size. Body weight, however, correlated highly with total tongue volume both for functional (r = 0.67), and structural (r = 0.70) data. In addition, correlations were fairly high between body weight and most muscles, though not as high as between pooled tongue volume and the muscles. Body weight could be useful in estimating tongue size.
4.4. Functional versus structural muscle volume calculations
The two methods of volume calculation, functional versus structural, present different perspectives on tongue muscle proportionality. Due to our limited spatial resolution, voxels are 2 × 2 × 2 mm, it is impossible to accurately estimate fibre dimensions or density in a voxel. our estimates are not meant to be perfectly accurate, as might occur in a dissection, but rather to represent the gross muscle anatomy. We considered using other percentages, such as estimating muscle and connective tissue proportionality. However, the resolution makes it impossible to accurately do this. Therefore, we settled for a rough estimate in which we ignored ‘passive’ tissue and allocated 50% of each voxel volume to each of the two muscles occupying it.
Similarities between the two data-sets include muscle volume scaling, which showed the same order of relative muscle size in both methods. In addition, the correlation between individual muscle volume and pooled tongue volume was similar with both methods, though stronger in the functional data-set (Table 3). differences result from the inconsistent size reduction across muscles in the structural data-sets. After halving the interdigitated regions, a volume difference emerged between the relative size of the muscle and the size of the tongue region it served. The functional measures might overestimate the strength attributed to an interdigitated muscle. However, both methods showed interesting muscle relationships, especially between the four largest muscles (see section 4.5). Depending on the goals of future studies, one might prefer to represent the tongue region served by the muscle, or calculate a more representative measure of the muscle's volume.
4.5. Muscle size and how size affects function
If one considers the top four functional muscle volumes in Figure 2/Table 2, one observes that they serve 70% of the tongue volume, and encompass all three directions of compression and expansion. Because the volume of the tongue served by these muscles is so large, it implies that they are the major tongue movers, that is, the workhorses of the tongue. They would be able to move the most tissue with the least effort. Takemoto (2001) grouped GG, V and T together as a structural unit due to their overlapping fibres and repeating lamina. The addition of SL makes this a functional unit which is able to grossly deform the tongue in all the ways needed to create 3D motions and to return from them. A secondary role for these muscles, and indeed all muscles of the tongue, is to stabilise one region to facilitate motion of another. The soft tissue composition of the tongue and its position in a restricted oral cavity, means that its motions are small, that it deforms as it moves, and that it receives considerable feedback from contact with other structures. In addition, the tongue can use contact with other structures to help ‘brace’ local regions as other regions move (cf. Stone 1991; Stone & Lundberg 1996). However, rapid motions of the tongue tip, for example, suggest that muscles internal to the tongue also need to activate to stiffen regions used in, or adjacent to, a specific deformation (Kier & Smith 1985; Smith & Kier 1989). While any muscle can serve this role, the large volume served by these muscles makes them well suited to supporting local motions.
If one considers the structural volumes, the GG emerges as the biggest and the most pervasive muscle. (1) It covers the largest proportion of the tongue and most crucially, the midline, which is the most important region of the vocal tract during speech. (2) Local muscle contraction would stiffen that region and assist local movements. Global activation would increase rigidity and thus tongue speed during speech. (3) The GG also is critical in non-speech functions, such as bolus propulsion during swallowing, and maintenance of a patent airway during inhalation (Sauerland & Mitchell 1975; Sauerland & Harper 1976). Although GG has been labelled a protrusor, it has been shown that in the rat and the dog, the GG muscle is divided into two subdivisions, with the posterior horizontal fibres receiving innervation from the lateral subnucleus of the ventral HGN and the radial fibres innervated from the central subnucleus juxtaposed between the dorsal and ventral subdivisions of the HGN (McClung & Goldberg 2000). Thus, the GG is well positioned, sized and shaped to be one of the most important muscles of the tongue.
4.6. Features of interdigitation
This is the first study to quantify the extent of interdigitation in the tongue. Tongue muscle interdigitation is well known, but not well explored. It may be worth considering the role of interdigitation more than has been done previously. Although the tongue itself is almost entirely interdigitated, only the IL has a bundled region within the tongue, the muscles that influence the tongue contain three levels of interdigitation. The FOM muscles, mylohyoid, geniohyoid, anterior digastric, are entirely bundled and not interdigitated at all. The extrinsic muscles, the genioglossus, styloglossus, hyoglossus, are bundled at their origins and interdigitate when they enter the tongue. The intrinsic muscles, transverse, verticalis and superior longitudinal, are entirely interdigitated. The inferior longitudinal muscle is bundled anteriorly, where it is surrounded by septa, and interdig-itates posteriorly with transverse (cf. Abd-el-Malek 1939). An interesting observation from dissection data is that there are many regions where two muscle directions interdigitate, but none with three directions of interdigitation (cf. Abd-el-Malek 1939; Miyawaki 1974). For example, the vertical fibres of genio-glossus and verticalis interdigitate, in very thin sheets, with the horizontal fibres of transverse. These same vertical fibres also interdigitate superiorly with the lengthwise fibres of superior longitudinal. However, the transverse fibres stop before reaching the superior longitudinal, thus preventing three orthogonal directions.
In the rest of the human body, almost all muscles are bundled and unidirectional. The exceptions are other soft structures, such as, the lips, velum and heart. This overwhelming use of bundled muscles suggests that it is the preferred architecture for muscles. Perhaps interdigitation is disadvantageous in some way. one potential disadvantage is the difficulty of separate innervation for even two orthogonal and possibly antagonistic muscles. The second disadvantage is the reduction in tissue flexibility; the more fibre directions there are the more difficult it is to bend. The third is increased friction occurring when the muscle shortens and each fibre slides past an orthogonal one. In fact, the tongue is replete with fat and a grid of connective tissue through which the fibres pass and which reduce friction and potential heat (Sokoloff p.c.). With three fibre directions additional disadvantages would arise. All lingual tissue must be locally volume preserving, and if muscles in three orthogonal directions activated simultaneously there would be no direction to expand. The final disadvantage is that the more tightly packed a region is with tissue the more difficult it is for the muscle to expand laterally when shortened, and the more likely it is to compress local blood vessels. Thus, 2D orthogonality of the muscle architecture of the tongue is advantageous to its motion requirements, but also reduces the disadvantages of multiple fibre directions as much as possible.
5. Conclusions
This paper calculated the muscle volumes of the tongue to highlight two perspectives, the region of the tongue served by the muscle (functional) and a closer representation of the true volume (structural). Each method revealed relationships between the muscles that might influence interpretation of tongue data and should be considered in future measurement decisions. This study also considered the value and effects of interdigitation, innervation pattern (protrusion/retrusion) and muscle origin (extrinsic/intrinsic) on the subtlety of tongue motion, to show that muscle architecture complexity is essential for creating complex tongue motion, and also appears to maximise motion effciency with co-activation and only two fibre directions.
Acknowledgments
Funding: This work was supported in part by the national Cancer Institute (NIH) [grant number R01CA133015].
Footnotes
Disclosure statement: No potential conflict of interest was reported by the authors.
References
- Abd-el-Malek S. Observations on the morphology of the human tongue. J Anat. 1939;73:201–210. [PMC free article] [PubMed] [Google Scholar]
- Atsumi T, Miyatake T. Morphometry of the degenerative process in the hypoglossal nerves in amyotrophic lateral sclerosis. Acta Neuropathol. 1987;73:25–31. doi: 10.1007/BF00695498. [DOI] [PubMed] [Google Scholar]
- Baer T, Alfonso PJ, Honda K. Electromyography of the tongue muscle during vowels in /pvp/ environment. Ann Bull RILP. 1988;7:7–10. [Google Scholar]
- Davis BL, Macneilage PF. The articulatory basis of babbling. J Speech, Lang Hear Res. 1995;38:1199–1211. doi: 10.1044/jshr.3806.1199. [DOI] [PubMed] [Google Scholar]
- Gaige TA, Benner T, Wang R, Wedeen VJ, Gilbert RJ. Three dimensional myoarchitecture of the human tongue determined in vivo by diffusion tensor imaging with tractography. J Magn Reson Imaging. 2007;26:654–661. doi: 10.1002/jmri.21022. [DOI] [PubMed] [Google Scholar]
- Handsfield G, Meyer C, Hart J, Abel M, Blemker SS. Relationships of 35 lower limb muscles to height and body mass quantified using MRI. J Biomech. 2014;47:631–638. doi: 10.1016/j.jbiomech.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Hardcastle WJ. Physiology of speech production. London: Academic Press; 1976. [Google Scholar]
- Holzbaur KRS, Murray WM, Gold GE, Delp SL. Upper limb muscle volumes in adult subjects. J Biomech. 2007;40:742–749. doi: 10.1016/j.jbiomech.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Kier WM, Smith KK. Tongues, tentacles and trunks: the biomechanics of movement in muscular-hydrostats. Zool J Linn Soc. 1985;83:307–324. [Google Scholar]
- Kuehn DP, Azzam NA. Anatomical characteristics of palatoglossus and the anterior faucial pillar. Cleft Palate J. 1978;15:349–359. [PubMed] [Google Scholar]
- Lee J, Woo J, Xing F, Murano EZ, Stone M, Prince JL. IEEE International Symposium on Biomedical Imaging (ISBI) San Francisco (CA): IEEE; 2013. Semi-automatic segmentation of tongue for 3D motion analysis with dynamic MRI; pp. 1465–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Woo J, Xing F, Murano EZ, Stone M, Prince JL. Semi-automatic segmentation for 3D motion analysis of the tongue with dynamic MRI. Comput Med Imaging Graph. 2014;38:714–724. doi: 10.1016/j.compmedimag.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long C, Conrad PW, hall EA, Furler SL. Intrinsic-extrinsic muscle control of the hand in power grip and precision handling. J Bone Joint Surg Am. 1970;52:853–867. [PubMed] [Google Scholar]
- MacNeilage P, Sholes G. An electromyographic study of the tongue during vowel production. J Speech Hear Res. 1964;7:209–232. doi: 10.1044/jshr.0703.209. [DOI] [PubMed] [Google Scholar]
- McClung JR, Goldberg SJ. Organization of motoneurons in the dorsal hypoglossal nucleus that innervate the retrusor muscles of the tongue in the rat. Anat Rec. 1999;254:222–230. doi: 10.1002/(SICI)1097-0185(19990201)254:2<222::AID-AR8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- McClung JR, Goldberg SJ. Functional anatomy of the hypoglossal innervated muscles of the rat tongue: a model for elongation and protrusion of the mammalian tongue. Anat Rec. 2000;260:378–386. doi: 10.1002/1097-0185(20001201)260:4<378::AID-AR70>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Miller JL, Watkin KL, Chen MF. Muscle, adipose and connective tissue variations in intrinsic musculature of the adult human tongue. J Speech Lang Hear Res. 2002;45:51–65. doi: 10.1044/1092-4388(2002/004). [DOI] [PubMed] [Google Scholar]
- Miyawaki K. A study on the musculature of the human tongue. Ann Bull RILP. 1974;8:23–49. [Google Scholar]
- Miyawaki K. A prelimilary report on the electromyographic study of the activity of lingual muscles. Ann Bull RILP. 1975;9:91–106. [Google Scholar]
- Mu L, Sanders I. Human tongue neuroanatomy: nerve supply and motor endplates. Clin Anat. 2010;23:777–791. doi: 10.1002/ca.21011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Kusky JR, norman MG. Sudden infant death syndrome: increased number of synapses in the hypoglossal nucleus. J Neuropathol Exp Neurol. 1995;54:627–634. doi: 10.1097/00005072-199509000-00003. [DOI] [PubMed] [Google Scholar]
- Perkell JS. MIT Research Monograph No 53. Cambridge (MA): MIT Press; 1969. Physiology of speech production: results and implications of a quantitative cineradiographic study; p. 104. [Google Scholar]
- Sanders I, Mu L. A three-dimensional atlas of human tongue muscles. Anat Rec. 2013;296:1102–1114. doi: 10.1002/ar.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerland EK, Harper RM. The human tongue during sleep: electromyographic activity of the genioglossus muscle. Exp Neurol. 1976;51:160–170. doi: 10.1016/0014-4886(76)90061-3. [DOI] [PubMed] [Google Scholar]
- Sauerland EK, Mitchell SP. Electromyographic activity of intrinsic and extrinsic muscles of the human tongue. Tex Rep Biol Med. 1975;33:444–455. [PubMed] [Google Scholar]
- Smith KK, Kier WM. Trunks, tongues and tentacles: moving with skeletons of muscle. Am Sci. 1989;77:29–35. [Google Scholar]
- Sokolof AJ. Localization and contractile properties of intrinsic longitudinal motor units of the rat tongue. J Neurophysiol. 2000;84:827–835. doi: 10.1152/jn.2000.84.2.827. [DOI] [PubMed] [Google Scholar]
- Stone M. A three-dimensional model of tongue movement based on ultrasound and x-ray microbeam data. J Acoust Soc Am. 1990;87:2207–2217. doi: 10.1121/1.399188. [DOI] [PubMed] [Google Scholar]
- Stone M. Imaging the tongue and vocal tract. Int J Lang Comm Dis. 1991;26:11–23. doi: 10.3109/13682829109011990. [DOI] [PubMed] [Google Scholar]
- Stone M, Lundberg A. Three-dimensional tongue surface shapes of English consonants and vowels. J Acoust Soc Am. 1996;99:3728–3737. doi: 10.1121/1.414969. [DOI] [PubMed] [Google Scholar]
- Takemoto H. Morphological analyses of the human tongue musculature for three-dimensional modeling. J Speech Lang Hear Res. 2001;44:95–107. doi: 10.1044/1092-4388(2001/009). [DOI] [PubMed] [Google Scholar]
- Woo J, Murano E, Stone M, Prince JL. Reconstruction of high-resolution tongue volumes from MRI. IEEE Trans Biomed Eng. 2012;59:3511–3524. doi: 10.1109/TBME.2012.2218246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J, Lee J, Murano E, Xing F, Al-Talib M, Stone M, Prince J. A high-resolution atlas and statistical model of the vocal tract from structural MRI. Comput Methods Biomech Biomed Eng: Imaging Visualization. 2015 doi: 10.1080/21681163.2014.933679. [DOI] [PMC free article] [PubMed]
- Wozniak W, Young PA. Further observations on human hypoglossal nerve. Anat Anz. 1969;125:203–205. [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]


