Abstract
The mevalonate-isoprenoid-cholesterol biosynthesis pathway plays a key role in human health and disease. The importance of this pathway is underscored by the discovery that two major isoprenoids, farnesyl and geranylgeranyl pyrophosphate, are required to modify an array of proteins through a process known as protein prenylation, catalyzed by prenyltransferases. The lipophilic prenyl group facilitates the anchoring of proteins in cell membranes, mediating protein-protein interactions and signal transduction. Numerous essential intracellular proteins undergo prenylation, including most members of the small GTPase superfamily as well as heterotrimeric G proteins and nuclear lamins, and are involved in regulating a plethora of cellular processes and functions. Dysregulation of isoprenoids and protein prenylation is implicated in various disorders, including cardiovascular and cerebrovascular diseases, cancers, bone diseases, infectious diseases, progeria, and neurodegenerative diseases including Alzheimer’s disease. Therefore, isoprenoids and/or prenyltransferases have emerged as attractive targets for developing therapeutic agents. Here, we provide a general overview of isoprenoid synthesis, the process of protein prenylation and the complexity of prenylated proteins, and pharmacological agents that regulate isoprenoids and protein prenylation. Recent findings that connect isoprenoids/protein prenylation with Alzheimer’s disease are summarized and potential applications of new prenylomic technologies for uncovering the role of prenylated proteins in the pathogenesis of Alzheimer’s disease are discussed.
Keywords: Isoprenoids, protein prenylation, small GTPases, statins, bisphosphonates, prenyltransferase inhibitors, prenylomics, Alzheimer’s disease
Introduction
In 1964, Konrad Bloch, together with Feodor Lynen, was awarded the Nobel Prize in Physiology or Medicine for discovering the pathway for the biological synthesis of cholesterol. In his Nobel Lecture, Bloch made it clear that the identification of mevalonic acid/mevalonate, produced from 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA), as the precursor of isoprenoids to squalene was one of the major breakthroughs in the elucidation of the cholesterol biosynthesis pathway (Bloch, 1965). Since then, remarkable advances have been achieved in understanding the regulation of isoprenoid and cholesterol metabolism, from basic biology to clinical applications (Goldstein and Brown, 2015). Brown and Goldstein were awarded the Nobel Prize in Physiology or Medicine in 1985 for discovering the LDL receptor and the genetic regulation of cholesterol metabolism (Brown and Goldstein, 1986). These discoveries, together with the discovery of natural fungal products that inhibit the activity of HMG-CoA reductase (Endo, Kuroda, and Tanzawa, 1976), the rate-limiting enzyme in the mevalonate-isoprenoid-cholesterol biosynthesis pathway, eventually led to the development of statins, one of the most widely prescribed medications for the management of plasma cholesterol levels and cardiovascular risks (Goldstein and Brown, 2015). In addition, the discovery of HMG-CoA reductase inhibitors, also facilitated the identification of an important post-translational modification process called protein prenylation, in which mevalonate-derived, short-chain isoprenoids are attached to specific proteins (Schmidt, Schneider, and Glomset, 1984), including oncogenic proteins. This early finding triggered the pursuit of identifying the prenyltransferases and developing prenyltransferase inhibitors as potential therapeutic agents for cancers (Berndt, Hamilton, and Sebti, 2011; Brown and Goldstein, 2012; Winter-Vann and Casey, 2005). In recent years, the interest in isoprenoids and protein prenylation has continued to grow. In addition to cardiovascular disease and cancer, prenylated proteins have been implicated in the development of cerebrovascular diseases (McTaggart, 2006), bone diseases (Luckman et al., 1998), infectious diseases (Charron et al., 2013; Gelb et al., 2003; Hast et al., 2011), progeria (Capell et al., 2008), and neurodegenerative diseases including Alzheimer’s disease (AD) (Cheng et al., 2013; Li et al., 2016; Li et al., 2012). This review attempts to provide a general overview of isoprenoid synthesis, the process of protein prenylation and the complexity of prenylated proteins, and pharmacological agents that regulate isoprenoid synthesis and protein prenylation. Further, it aims to summarize recent findings that connect isoprenoids/protein prenylation with AD and explore the potential application of new prenylomic technologies for uncovering the role of prenylated proteins in the pathogenesis of AD.
Isoprenoid Synthesis
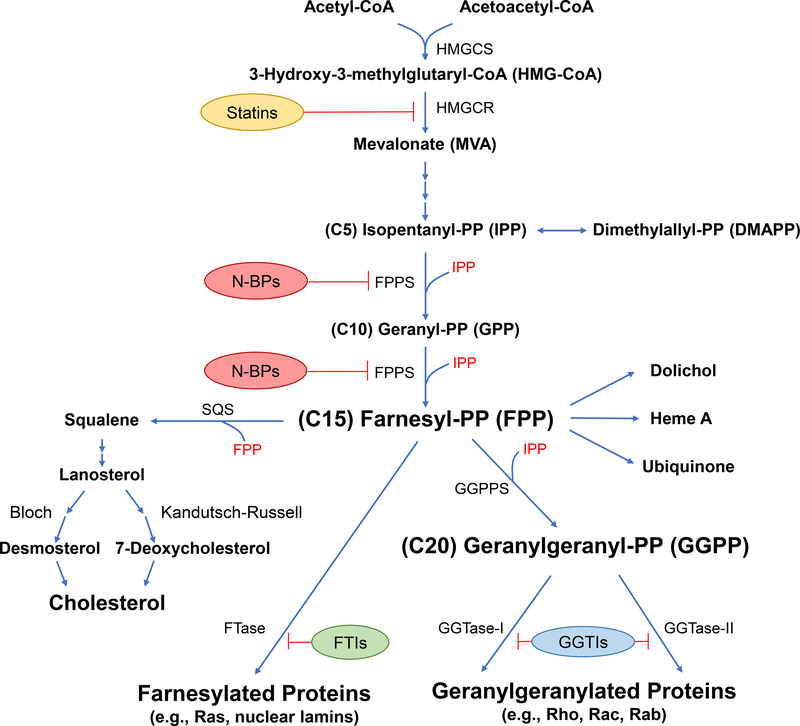
Isoprenoids, also known as terpenoids, are a diverse group of lipophilic molecules that are composed of five-carbon isoprene units. These lipids exist ubiquitously in all living organisms, from bacteria, fungi, plants, to mammals, and represent the largest and most diverse family of natural compounds (Sacchettini and Poulter, 1997). Isoprenoids play key structural and functional roles in a wide variety of biological processes, including formation of cell membranes, regulation of gene expression, modification of proteins, regulation of signal transduction pathways, participation in electron transport and photosynthesis, protection against infections, and biosynthesis of vitamins, cholesterol and related sterols, yeast mating pheromones, and mammalian reproductive hormones (Holstein and Hohl, 2004). Here we mainly focus on the short-chain isoprenoids produced in the mevalonate-isoprenoid-cholesterol biosynthesis pathway in mammalian cells (Figure 1).
Figure 1. The mevalonate-isoprenoid-cholesterol pathway and pharmacological agents that inhibit the synthesis of key intermediates.
HMGCS: 3-hydroxy-3-methylglutaryl-CoA synthase; HMGCR: 3-hydroxy-3-methylglutaryl-CoA reductase; FPPS: Farnesyl pyrophosphate synthase; N-BPs: Nitrogen-containing bisphosphonates; SQS: Squalene synthase; GGPPS: Geranylgeranyl pyrophosphate synthase; FTase: Farnesyltransferase; GGTase-I/II: Geranylgeranyltransferase-I/II; FTIs: Farnesyltransferase inhibitors; GGTIs: Geranylgeranyltransferase inhibitors. A color version of the figure is available online.
The synthesis of isoprenoids and ultimately cholesterol starts with “activated acetic acid”, acetyl Co-A, which is condensed with acetoacetyl-CoA (formed by self-condensation of two acetyl-CoA molecules) to produce 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) (Lynen, 1964). The HMG-CoA is converted to mevalonate in the rate-limiting step of the pathway catalyzed by HMG-CoA reductase (Bucher, Overath, and Lynen, 1960; Ferguson, Durr, and Rudney, 1959). Mevalonate is phosphorylated by mevalonate kinase (MK) to form 5-phosphomevalonate (Tchen, 1958). Through additional steps of phosphorylation and decarboxylation, phosphomevalonate is converted into the five-carbon isoprenoid compound, isopentenyl pyrophosphate (IPP) (Tada and Lynen, 1961). At this step, IPP can be converted to its isomer, dimethylallyl pyrophosphate (DMAPP), by the action of isopentenyl pyrophosphate isomerase (Agranoff et al., 1960). DMAPP serves as the isoprene donor in the synthesis of isopentenyl adenine, present in some tRNAs (Faust, Brown, and Goldstein, 1980; Hall, 1971). Continuing on the isoprenoid pathway, IPP and DMAPP are building blocks for all other isoprenoids. These two five-carbon molecules are condensed to produce the 10-carbon compound, geranyl pyrophosphate (GPP), followed by the addition of a second IPP unit to GPP forming the 15-carbon isoprenoid molecule, farnesyl pyrophosphate (FPP). Both of these reactions are catalyzed by FPP synthase (FPPS) (Poulter and Rilling, 1978; Poulter and Satterwhite, 1977). FPP resides at the important branch point between the non-sterol/isoprenoid and sterol/cholesterol synthetic pathways. In the isoprenoid pathway, the addition of a third IPP unit to FPP forms the 20-carbon isoprenoid molecule, geranylgeranyl pyrophosphate (GGPP), catalyzed by GGPP synthase (GGPPS) (Sagami, Ishi, and Ogura, 1981). Elongation via incorporation of additional IPP moieties leads to the formation of longer, biologically important isoprenoid molecules, including dolichols (for N-glycosylation of proteins), heme A, and ubiquinone (Goldstein and Brown, 1990). In the sterol pathway, the condensation of two FPP molecules forms the sterol precursor squalene, catalyzed by squalene synthase (Beytia, Qureshi, and Porter, 1973). Squalene undergoes cyclization to yield lanosterol, the first steroid molecule in the pathway (Bloch, 1965). Subsequently, lanosterol is transformed to cholesterol through two interrelated pathways, the Bloch pathway via desmosterol (Bloch, 1965) or the Kandutsch-Russell pathway via 7-dehydroxycholesterol (Kandutsch and Russell, 1960). The homeostasis of isoprenoids and cholesterol is maintained by multiple feedback regulations on HMG-CoA reductase to ensure normal cell growth and function (Brown and Goldstein, 1980; Brown and Goldstein, 1997; Brown, Radhakrishnan, and Goldstein, 2017). An additional layer of feedback regulation of the pathway has been discovered recently in which FPP binds to the allosteric site of FPPS and locks the enzyme in an inactive state (Park et al., 2017). The interest in isoprenoids and related pathways has been increased substantially in recent years following the discovery that FPP and GGPP could modify the structure and function of unique groups of proteins, which are implicated in cancer and other disorders, through the process of protein prenylation (see detailed discussion below).
Protein Prenylation
Protein prenylation consists of the addition of farnesyl or geranylgeranyl groups on specific cysteine residues located near the C-termini of various proteins. Prenylation was first discovered in 1979 by Kamiya in fungal mating factors (Kamiya et al., 1979), although it was not detected in mammalian cells until 1984 when the incorporation of mevalonate-derived species were discovered in 3T3 cells (Schmidt, Schneider, and Glomset, 1984). This modification is a three step process (Figure 2) (Zhang and Casey, 1996) that involves the initial attachment of an isoprenoid group derived from farnesyl (FPP) or geranylgeranyl pyrophosphate (GGPP) to a protein substrate catalyzed by a prenyltransferase. Farnesylated proteins usually undergo subsequent proteolysis to remove residues downstream of the prenylated cysteine, performed by specific proteases, followed by methylation mediated by a SAM-dependent methyl transferase to yield a protein containing a C-terminal farnesylcysteine methyl ester. However, some farnesylated proteins are not further processed post-prenylation including the yeast chaperone Ydj1p (Hildebrandt et al., 2016) and the brain-specific splice variant of Cdc42 (bCdc42) (Nishimura and Linder, 2013). Many geranylgeranylated proteins also undergo this same three-step process while others do not (Leung et al., 2007; Michaelson et al., 2005). Some mature prenylated proteins undergo additional lipid modifications including palmitoylation (Hancock et al., 1989). Ultimately, most prenylated proteins localize to the plasma membrane or internal membranes where they participate in various transduction pathways. The enzymes and biochemistry involved in protein prenylation are described in more detail as follows.
Figure 2. The three-step process of protein prenylation illustrated for farnesylation.
As noted in the text, the process is similar for geranylgeranylation of proteins containing CAAX-box sequences prenylated by GGTase-I.
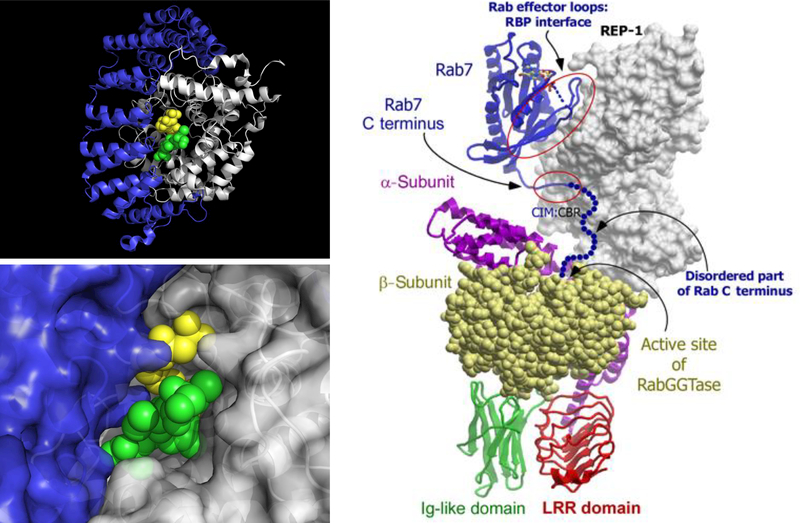
Three prenylating enzymes have been identified from a variety of eukaryotes ranging from yeast to mammals. Farnesyltransferase (FTase) and geranylgeranyltransferase-I (GGTase-I) are αβ heterodimeric proteins that share a common α-subunit (FNTA) and different β-subunits (FNTB and PGGT1B, respectively) (Casey et al., 1989; Casey, Thissen, and Moomaw, 1991), whereas geranylgeranyltransferase-II (GGTase-II), also a heterodimer, has distinct α and β subunits (RABGGTA and RABGGTB, respectively) along with a third subunit denoted as Rab escort protein (REP) (Seabra et al., 1992). All three of these enzymes are present in the cytosolic fraction of all tissues including the brain. The three-dimensional structures of FTase and GGTase-I are highly similar with active sites that completely envelop the isoprenoid and significant portions of the C-terminus from the substrate protein (Figure 3) (Strickland et al., 1998; Taylor et al., 2003). FTase and GGTase-I recognize a tetrapeptide sequence known as a CAAX-box where C is cysteine, A is typically but not exclusively an aliphatic amino acid and X controls whether the sequence is an FTase or GGTase-I substrate. Early work with a limited number of synthetic peptides demonstrated that FTase preferred Cys, Met, Ser, Ala and Gln residues at the X-position whereas GGTase-I showed a preference for Ile, Leu and Phe (Reiss et al., 1991; Yokoyama et al., 1991). However, more recent work with peptide libraries suggests that there are many exceptions to these rules (Hougland et al., 2010; Krzysiak et al., 2007; Wang and Distefano, 2012; Wang et al., 2014). Computational studies have also shown this to be true (London et al., 2011). Very recently, it has been reported that FTase can efficiently prenylate cysteines they are positioned five residues (instead of the usual four) from the C-terminus raising the possibility that the number of prenylated proteins is even larger than originally thought (Blanden et al., 2017). Interestingly, FTase appears to be more selective than GGTase-I since the latter accepts many FTase substrates while the converse is not generally true, although there are exceptions (Krzysiak et al., 2010). Given that more is known about FTase versus GGTase specificity, additional work is necessary to better define the substrate specificity of the latter enzyme. At this point, it is possible to predict with some certainty whether specific CAAX-box sequences will be prenylated although work remains to be done. GGTase-II recognizes C-terminal sequences including CC, CXC, CCX, CCXX, and CCXXX (Kinsella and Maltese, 1992; Pereira-Leal and Seabra, 2000). However, in contrast to the aforementioned enzymes, GGTase-II cannot prenylate short peptides due to the fact that the active site is somewhat more open; importantly, the escort protein REP serves to recruit substrate proteins and docks with GGTase-II to present them for prenylation (Figure 3) (Guo et al., 2008). Additionally, GGTase-II typically transfers geranylgeranyl groups to both Cys residues within the sequences noted above.
Figure 3. Key structural features of prenyltransferases.
Top Left: Overall αβ fold for prenyltransferases shown using the structure of FTase (pdb: 1TN8). Bottom Left: Active site structure of FTase showing envelopment of substrates. Color scheme: α subunit (blue), β subunit (white), FPP (yellow), peptide GCVLS (green). Right: Model for the structure of GGTase-II showing how Rab7 is presented to the transferase by REP-1. Adapted from Guo et al. (2008) with permission. A color version of the figure is available online.
Once proteins are prenylated, they typically localize to the endoplasmic reticulum (ER) for further processing although some such as Ydj1p do not (Hildebrandt et al., 2016). The “AAX” residues from the CAAX-box on farnesylated proteins are typically cleaved by one of two proteases that include Ras converting enzyme (Rce1) and Zinc metalloproteinase Ste24 (ZMPSte24) (Boyartchuk, Ashby, and Rine, 1997; Chen, Ma, and Rando, 1996); both of these enzymes have been detected in all tissues including the brain. To date, the human-derived Rce1 enzyme has not been purified to homogeneity in active form although it has been studied in partially purified form (Dolence et al., 2000; Schmidt et al., 1998); that enzyme is believed to be responsible for the processing of Ras proteins. In contrast, ZMPSte24 has been purified and its structure solved by x-ray crystallography (Fujimura-Kamada, Nouvet, and Michaelis, 1997; Quigley et al., 2013). This enzyme is particularly interesting since it appears to cleave two structurally unrelated sequences. In the processing of lamin A, ZMPSte24 cleaves the C-terminal “AAX” residues of the prenylated protein as well as at a site approximately 20 residues upstream of the C-terminus. The structure containing a short bound peptide and an appropriately positioned Zn atom provide a useful model for understanding how cleavage of the “AAX” sequence occurs but does not clarify how the upstream site is proteolyzed.
Following proteolysis of farnesylated CAAX-box sequences, the resulting C-terminal farnesylcysteine is methylated by the SAM-dependent methyl transferase Icmt (Hrycyna et al., 1991), located in the ER and also expressed in all tissues including the brain. While the structure of Icmt has not been determined, a structure of a bacterial homolog has been solved (Yang et al., 2011). To address this paucity of structural data, a variety of biochemical methods have been used to provide insight into the structure and activity of this enzyme (Anderson et al., 2005; Hrycyna and Clarke, 1992).
Complexity of Prenylated Proteins and Their Functions
Hundreds of proteins have been identified or predicted to undergo prenylation (Maurer-Stroh et al., 2007; McTaggart, 2006; Wang and Casey, 2016). They include many important intracellular proteins, ranging from heterotrimeric G protein subunits to nuclear lamins; however, the largest, and most extensively studied group of prenylated proteins is the Ras superfamily of small GTPases (McTaggart, 2006), which is comprised of over 150 known members. Based on sequence and functional similarities, this superfamily is divided into five subfamilies: Ras, Rho, Rab, Ran and Arf (Colicelli, 2004; Wennerberg, Rossman, and Der, 2005). These proteins serve as molecular switches, cycling between “on” and “off” states during signal transduction, and through interaction with their downstream effectors, they regulate a variety of cellular processes and functions, including cell growth and survival, differentiation and proliferation, cytoskeletal organization, vesicular trafficking, gene expression, and energy metabolism. The biological networks of small GTPases are complex. Each subfamily contains numerous members and each member interacts with multiple downstream effector proteins. Despite that complexity, one essential initiation step for almost all small GTPases to activate downstream signaling pathway is association with membranes, which relies on prenylation (and sometimes other lipid modifications) (Lane and Beese, 2006). Therefore, the prenylation status of small GTPases has a significant impact not only on the structure/function of small GTPases per se but also on the functions of their downstream effector proteins. Here we use the Ras subfamily as an example to illustrate the role of prenylation and the complexity of small GTPase biology briefly. For more extended discussions on small GTPases, the interested reader is referred to several excellent reviews (Cherfils and Zeghouf, 2013; Colicelli, 2004; Wennerberg, Rossman, and Der, 2005).
The Ras subfamily contains over 36 members and the best-characterized ones are the three Ras isoforms: H-Ras, K-Ras, and N-Ras, mainly because of their oncogenic roles (Wennerberg, Rossman, and Der, 2005). All three Ras isoforms undergo the three-step modification process, i.e., prenylation, proteolysis and carboxymethylation, at the ER membrane as described above (Figure 2). Subsequently, H-Ras and N-Ras undergo additional palmitoylation, trafficking through the Golgi and vesicular transport to the plasma membrane (Prior and Hancock, 2012). For K-Ras (referring to the major splice variant K-Ras4B), the presence of the basic hexalysine patch along with the farnesyl group is sufficient for anchoring into the plasma membrane via a Golgi-independent cytosolic route (Prior and Hancock, 2012) (Figure 4). Importantly, all biological functions of Ras occur at the plasma membrane (Simanshu, Nissley, and McCormick, 2017), including interactions with regulatory proteins, downstream effector proteins, and signal transduction.
Figure 4. Ras farnesylation and membrane trafficking.
Farnesyltransferase (FTase) adds farnesyl group to the cysteine residue of the CAAX motif of Ras proteins. The following modifications occur on the cytosolic surface of the ER: proteolysis of the terminal three amino acid residues of the CAAX motif by Ras-converting CAAX endopeptidase 1 (Rce1), then methylation by isoprenylcysteine carboxylmethyltransferase (Icmt). The fully processed prenylated Ras proteins are then either directly trafficked to the membrane (e.g. K-Ras) or further undergo palmitoylation at the Golgi complex prior to the membrane trafficking (e.g. N/H-Ras). FPP: farnesyl pyrophosphate; F: farnesyl; SAM: S‑adenosyl‑homocysteine. A color version of the figure is available online.
At the plasma membrane, Ras proteins exist in GDP-bound (inactive) or GTP-bound (active) forms and the switching between GDP/GTP states are highly regulated through sophisticated mechanisms (Cherfils and Zeghouf, 2013). Stimulated by extracellular signals through cell surface receptors, Ras proteins are activated or “turned on” by guanine nucleotide exchange factors (GEFs), which catalyze the release of GDP and the binding of GTP. In contrast, GTPase-activating proteins (GAPs) accelerate the intrinsic GTPase activity of Ras for hydrolysis of GTP and lead to the inactivation or “turning off” of Ras. This GDP/GTP switch is further regulated by guanine dissociation inhibitors (GDIs) and GDI-like proteins. The replacement of GDP for GTP induces a conformational change in Ras proteins that allows them to interact with their downstream effectors and execute their multiple signaling functions (Cherfils and Zeghouf, 2013) (Figure 4). Approximately 20 distinct Ras effector proteins have been identified (Prior and Hancock, 2012). Two of the major Ras-driven signaling pathways are the MAPK (Raf/MEK/ERK) and PI3K/Akt/mTOR pathways, which regulate a plethora of key cellular processes and functions under both physiological and pathological conditions (Nussinov, Tsai, and Jang, 2017; Stephen et al., 2014).
Finally, it is worth noting that, despite the fact that Ras isoforms share a high degree of sequence homology and interact with a common set of regulators/effectors, they are not functionally redundant. It is believed that the specific membrane/compartment localization for each of the isoforms, where there are different pools and concentrations of regulators and effectors, contribute to the lack of functional redundancy between them (Hancock, 2003; Prior and Hancock, 2012). Supporting this notion, recent work has demonstrated that K-Ras selectively interacts with membrane lipids and forms “nanoclusters” that determine its signaling output; subtle changes to its membrane anchor sequence or prenylation profoundly alter lipid specificity of K-Ras and its signaling output (Zhou et al., 2017). These findings further highlight the importance of prenylation in regulating the functions of Ras and render isoprenoids and prenyltransferases promising therapeutic targets for various disorders.
Pharmacological Agents that Regulate Isoprenoids and Protein Prenylation
Statins – HMG-CoA Reductase Inhibitors
Statins are a class of drugs that selectively inhibit the rate-limiting enzyme, HMG-CoA reductase, in the mevalonate-isoprenoid-cholesterol pathway (Figure 1) (Goldstein and Brown, 1990). Thus, these drugs reduce the biosynthesis of isoprenoid intermediates as well as the final product cholesterol. Statins have been successfully used to control plasma cholesterol levels and prevent cardiovascular disease (4S-group, 1994; Goldstein and Brown, 2015; Sacks et al., 1996). The first statin, ML-236B (later known as compactin or mevastatin), was discovered by Akira Endo in 1976 from work with a penicillium mold (Endo, Kuroda, and Tanzawa, 1976). This discovery stimulated the worldwide development of natural and synthetic HMG-CoA reductase inhibitors (Endo, 1992). At present, the US Food and Drug Administration (FDA) have approved the use of seven statins: lovastatin, pravastatin, simvastatin, fluvastatin, atorvastatin, rosuvastatin, and pitavastatin (Li et al., 2012). Each of the statins has a characteristic structure that competes with HMG-CoA binding to HMG-CoA reductase (Istvan and Deisenhofer, 2001) (Figure 5). As a result, the enzyme activity of HMG-CoA reductase is inhibited and the biosynthesis of downstream molecules, including isoprenoids and sterols, is blocked. Although all statins share a common mechanism for inhibiting HMG-CoA reductase, other unique structural features render them different in solubility (hydrophilic or lipophilic), potency, and permeability across the blood-brain barrier (BBB) (Li et al., 2012).
Figure 5. Structure of statins.
The structure in green indicates the HMG-CoA like unit that binds to the enzyme HMG-CoA reductase. A color version of the figure is available online.
While the power of statins to inhibit cholesterol synthesis has been harnessed successfully to manage plasma cholesterol levels and reduce the risk of cardiovascular disease, the clinical significance of statin-induced inhibition on isoprenoid production remains underexplored. Nevertheless, a growing body of evidence indicates that the benefits of statins in curtailing cardiovascular disease extend beyond their cholesterol-lowering property, and their ability to inhibit the synthesis of isoprenoids, in particular FPP and GGPP, and prenylation of small GTPases is responsible for their cholesterol-independent (pleiotropic) effects (Oesterle, Laufs, and Liao, 2017). In addition, owing to these pleotropic effects, there have been calls for repurposing statins for disorders other than cardiovascular disease, ranging from cancers, inflammatory diseases, infectious diseases, and neurodegenerative diseases including AD (Hennessy et al., 2016; Iannelli et al., 2017; Moutinho, Nunes, and Rodrigues, 2017; Walton et al., 2016).
However, the pleiotropic effects of statins have been difficult to quantitate because the inhibition of isoprenoids correlate with the inhibition of cholesterol biosynthesis. In addition, technically, it has been challenging to measure FPP and GGPP levels in tissues, including the brain (Wood et al., 2010; Wood, Mupsilonller, and Eckert, 2014). Recently, the development of an HPLC method has advanced the understanding of isoprenoid metabolism in vivo (Tong et al., 2008). This method was adapted to determine FPP and GGPP concentrations in the human brain frontal cortex (Hooff et al., 2008). It was found that GGPP levels were higher than FPP levels in both white and gray matter. There was approximately four times as much GGPP as FPP in the human frontal cortex. Consistently, GGPP was in greater abundance than FPP in brain homogenate of mice (Tong et al., 2008). Interestingly, FPP levels were slightly higher than GGPP levels in the kidney, liver, and heart. These findings suggest that regulation of FPP and GGPP may differ in the brain as compared with other organs, although the mechanisms accounting for differences in distribution and levels of FPP and GGPP in brain tissue are not known. As FPP serves as the precursor for other longer chain isoprenoids such as dolichol and ubiquinone in addition to GGPP and cholesterol (Figure 1), FPP utilization, turnover, and synthesis may be greater than GGPP. Importantly, with the establishment of a reliable quantitative method for isoprenoids, further studies in animals showed that chronic treatment with peripherally administered simvastatin could indeed significantly reduce the levels of FPP and GGPP in the brain (Eckert et al., 2009). Intriguingly, the effects of simvastatin on FPP and GGPP levels in the brain were not equal in magnitude. Simvastatin treatment had a much greater effect on brain FPP levels (48% reduction) as compared with GGPP (33% reduction) and cholesterol (22% reduction). The significance of such differential effects of statins on FPP and GGPP in the pathway warrants further investigation. It is also noteworthy that squalene synthase, GGPP synthase, and FTase, the three enzymes that use FPP as a substrate, have very different Km for FPP, in the order of 2 ¼M > 1 ¼M >>> 5 nM, respectively (Winter-Vann and Casey, 2005). Thus, statin-induced reduction of isoprenoids causes a reduction in sterol/cholesterol production first, followed by a reduction in GGPP synthesis (and therefore protein geranylgeranylation), whereas protein farnesylation is only affected when the level of FPP becomes extremely low. Nevertheless, it has been shown that treatment with simvastatin reduces both farnesylation and geranylgeranylation of specific Ras-related GTPases in mice and extends lifespan in aging flies (Spindler et al., 2012). In support of the brain bioavailability and target engagement of statins, a recent study has shown that treatment with clinically relevant doses of simvastatin decreases the prenylation of small GTPases in the brain of mice and rats (Ostrowski et al., 2016). These studies demonstrate that lipophilic and BBB-permeable statins could modulate isoprenoid metabolism and protein prenylation in the brain, and thus could potentially be used to treat relevant disorders of the central nervous system. The application of statins in AD is discussed in more detail in later sections of this review.
Bisphosphonates – FPP Synthase Inhibitors
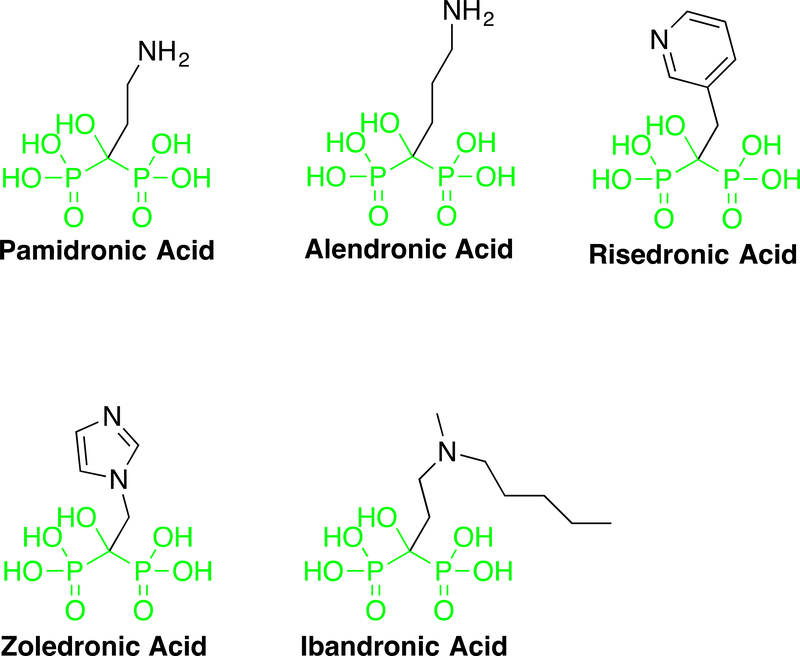
Bisphosphonates, specifically the nitrogen-containing bisphosphonates (N-BPs), are another class of drugs that act on the mevalonate-isoprenoid-cholesterol pathway. Different from statins, these drugs inhibit the activity of FPPS, downstream of HMG-CoA reductase in the pathway (Rogers et al., 2011) (Figure 1). N-BPs have been widely used to treat bone diseases such as osteoporosis for more than 40 years (Maraka and Kennel, 2015; Russell, 2011). The development of this class of drugs was based on early discoveries that natural pyrophosphate compounds could prevent calcification of soft tissues, regulate bone mineralization, and effectively inhibit bone resorption (Fleisch, Russell, and Francis, 1969; Fleisch et al., 1969; Fleisch, Russell, and Straumann, 1966). Currently, several FDA-approved N-BPs are available, including pamidronate, alendronate, risedronate, zoledronate, and ibandronate (Figure 6). The therapeutic application of this group of drugs relies on their backbone P-C-P structure and their ability to chelate calcium ions. Due to these properties, these drugs are targeted rapidly to the bone mineral surface in vivo, where they are taken up primarily by osteoclasts (bone-degrading cells) (Roelofs et al., 2010). Thus, the site of action for N-BPs is unique compared with that of statins, which are taken up predominantly by the liver (Sirtori, 2014). The intracellular mechanisms of action for all N-BPs depend on their ability to bind FPPS and inhibit its activity, limiting the synthesis of FPP and GGPP. Thus, similar to statins, N-BPs reduces protein prenylation, in particular the prenylation of small GTPases, disrupting the membrane association and intracellular trafficking of these proteins and consequently, blocking the interactions of GTPases with their effectors and downstream signaling pathways. Concomitantly, inhibition of FPPS by N-BPs also cause the accumulation of the upstream metabolite IPP (Figure 1), which leads to the formation of new metabolites such as ApppI (an ATP analog) that induces apoptosis (Monkkonen et al., 2006). Thus, inhibition of protein prenylation and accumulation of ApppI are two main mechanism by which N-BPs exert their anti-resorptive and apoptosis-inducing effects on osteoclasts (Rogers et al., 2011). Through these actions, N-BPs suppress bone degradation, increase bone mass and strength, and reduce the risk of fracture.
Figure 6. Structure of N-BPs.
Structure of N-BPs. The structure in green shows the conserved bisphosphonate moiety present in all approved drugs. A color version of the figure is available online.
One noteworthy aspect for bone metabolism is the relative importance of the two prenylation pathways. It appears that N-BPs work primarily through inhibition of protein geranylgeranylation rather than protein farnesylation, despite the fact that N-BPs depletes both GGPP and FPP. It has been shown that the effects of N-BPs on inhibiting osteoclast formation and bone resorption can be abolished by geranylgeraniol (precursor of GGPP for protein geranylgeranylation), but not by farnesol (precursor of FPP for protein farnesylation) (Fisher et al., 1999). This was further supported by studies where specific prenylation inhibitors were used. Use of a GGTI to suppress protein geranylgeranylation blocked bone resorption in osteoclasts, whereas the use of an FTI to block protein farnesylation had minimal effect (Coxon et al., 2000). Together, these findings suggest that the two prenylation pathways play differential roles in regulating the functions of osteoclasts, in which geranylgeranylated proteins are more crucial than farnesylated proteins.
Since N-BPs are rapidly targeted to the bone, their uses for other conditions are limited. However, the therapeutic potential of N-BPs has been explored for several disorders other than bone diseases in vitro and in vivo, including cancer, Hutchinson–Gilford progeria syndrome (HGPS), and AD (Li et al., 2012). In particular, for HGPS, which is caused by mutations in the nuclear lamin A protein and subsequent accumulation of a prenylated form of the mutant lamin A (Dechat et al., 2007; Young et al., 2006), human clinical trials have been conducted recently following the success in animal models with the treatment of N-BPs in combination with statins (Varela et al., 2008). Compared with the monotherapy with an FTI (lonafarnib), the combination therapy of an N-BP (zoledronate) with an FTI and a statin (pravastatin) provided additional bone mineral density benefit as well as attenuating the overall aging symptoms and increased survival (Gordon et al., 2016; Gordon et al., 2012; Gordon et al., 2014). The beneficial effects of N-BPs observed in animal and clinical studies of HGPS raise the possibility that these agents may have broader effects on normal aging processes. A recent study has showed that through its action on protein prenylation, zoledronate attenuates accumulation of DNA damage in stem cells and protects their function by inhibiting the mTOR signaling, a pathway involved in both aging and cancer (Misra et al., 2016). These findings suggest a potential relationship between isoprenoid metabolism/protein prenylation and normal aging processes, thus broadening the spectrum of potential applications of N-BPs.
In addition, a recent study indicates that N-BPs may be used to treat cerebral cavernous malformations (CCMs), common abnormal vascular formations that occur in the brain, spinal cord, and sometimes the retina (Nishimura et al., 2017). Through high-throughput screening, it was found that N-BPs and statins could act synergistically to reverse the phenotype of the disease in cell and fly models. Treatment with zoledronate in combination with fluvastatin effectively attenuated neural and vascular deficits in chronic and acute mouse models of CCMs. The combination treatment significantly reduced lesion burden and extended lifespan in these animals (Nishimura et al., 2017), suggesting inhibition of isoprenoids as a potential therapy for CCM disease.
The prospect of using N-BPs for AD was highlighted by a report that levels of FPP and GGPP are elevated significantly in the brain of Alzheimer’s patients (Eckert et al., 2009). Recently, the connection between AD and the isoprenoid pathway was further enhanced by findings that a polymorphic site in the promoter region of the human FPPS gene was significantly associated with the level of phosphorylated tau in the brains of AD patients, and that the mRNA expression of FPPS was markedly elevated in the brains of AD versus control subjects (De Schutter et al., 2014). These findings strongly suggest that inhibition of FPPS may produce beneficial effects against AD. However, minimal systemic exposure and poor BBB permeability of N-BPs limit the clinical use of these drugs in treating neurological disorders such as AD. In this regard, exciting advances have been made recently in developing novel non-BP inhibitors of FPPS that bind to an allosteric site on the enzyme (De Schutter et al., 2014; Gritzalis et al., 2015; Jahnke et al., 2010; Marzinzik et al., 2015). Interestingly, FPP, the natural product of FPPS, can bind to the allosteric site of the enzyme and inhibits its activity (Park et al., 2017). These new inhibitors lack the phosphonate groups of N-BPs and thus are not targeted to bone mineral, offering the hope that this new class of compounds may lead to much broader therapeutic applications of FPPS inhibitors.
Inhibitors for Prenyltransferases and Related enzymes
As discussed above, statins and N-BPs inhibit the biosynthesis of isoprenoids and reduce the availability of isoprenoid substrates for protein prenylation without directly acting on prenyltransferases themselves, and thus have limitations. By inhibiting isoprenoid synthesis, all downstream products are influenced; in contrast, specific inhibition of prenylation itself could potentially be more selective. The discovery that Ras proteins are farnesylated and the fact that farnesylation is required for membrane localization suggested that inhibition of FTase could serve as a strategy for shutting down the effects of constitutively activated mutant Ras proteins in cancer or related pathways that signal via Ras dependent processes. That hypothesis triggered an explosion of research into the development of prenyltransferase inhibitors leading to over 100 clinical trials. A number of excellent reviews on this topic have been previously published (Berndt, Hamilton, and Sebti, 2011; Gibbs, 2000; Leonard, 1997; Ochocki and Distefano, 2013; Wang, Yao, and Huang, 2017).
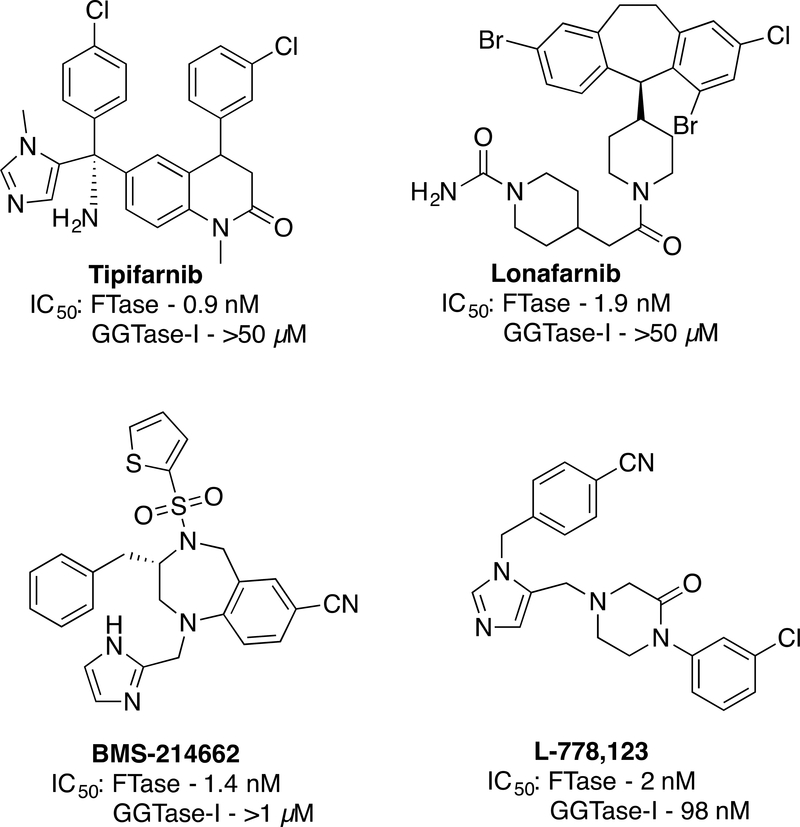
To date, three broad classes of FTase inhibitors (FTIs) have been developed (Figure 7, 8). Compounds such as α-hydroxyfarnesylphosphonate (HFP) (Pompliano et al., 1992) that mimic the substrate FPP and bind within the isoprenoid binding site of FTase (Strickland et al., 1998) have been found to be useful biochemical tools but lack specificity to be used as drugs. Bisubstrate inhibitors that combine elements from the isoprenoid and CAAX-box such as Schi-872 have also been prepared although none of them has progressed beyond experiments in cell culture (Manne et al., 1995; Patel et al., 1996; Schlitzer and Sattler, 1999). In contrast, peptidomimetic compounds that mimic the CAAX-box have proven to be the most specific and useful. Three general strategies have been employed in their development. Peptidomimetics based on CAAX-box peptides that have been modified by removal of labile amide bonds, exemplified by L-744,832, were among the first compounds studied in detail (Kohl et al., 1995). Rigid peptidomimetic scaffolds have also proved fruitful for the development of FTIs with FTI-277 being widely used in cell culture models (Lerner et al., 1995). However, the inhibitors Tipifarnib (Venet, End, and Angibaud, 2003) and Lonafarnib (Morgillo and Lee, 2006), obtained via optimization of hits identified from high throughput screening efforts have been the most extensively studied in a range of experiments including human clinical trials along with BMS-214662 (Rose et al., 2001) and L-778,123 (Lobell et al., 2002) (Figure 8). These compounds produced promising results in cell culture-based experiments and some animal models. Unfortunately, none of these compounds have shown significant efficacy in human trials. A central reason for this lies in the differential enzymology of Ras proteins. As discussed above, mammalian cells typically contain three prenylated isoforms including H-, N- and K-Ras. While H-Ras is only prenylated by FTase, N-Ras and K-Ras are also slow substrates for GGTase-I. In the presence of an FTI, They are alternatively prenylated by GGTase-I to yield proteins that retain signaling activity. This allows tumors driven by N- and K-Ras mutations to circumvent the effects of FTIs. In support of this notion, cancers specifically caused by overactive H-Ras are sensitive to treatment with FTIs (Chen et al., 2014; Kohl et al., 1995). In addition, FTIs may also be useful in some cases that involve prenylated proteins other than Ras but further study is required to determine precisely what proteins are involved in those processes. Highly potent inhibitors of GGTase-I (Kazi et al., 2009) or dual FTase/GGTase-I inhibitors (Lobell et al., 2002) have also been developed that work well in cell culture and some animal models but exhibit excessive toxicity in human trials; this is probably related to the fact that geranylgeranylation is more prevalent compared with farnesylation (Berndt, Hamilton, and Sebti, 2011; Reid et al., 2004). Inhibitors of GGTase-II including 3-PEHPC have also been developed and their use explored in biochemical and cell-based assays (Coxon et al., 2001). However, their use has been limited in the community since none is currently commercially available.
Figure 7.
Inhibitors of enzymes involved in protein prenylation.
Figure 8.
Structures of the four FTIs that have been studied in human clinical trials.
Beyond cancer, prenylation inhibitors are also being explored in other diseases. Positive results with Lonafarnib have recently been obtained in Phase II clinical trials for Hutchinson–Gilford progeria, a debilitating disease that causes premature aging and typically death before age 20 (Gordon et al., 2016; Gordon et al., 2012). In this case, the FTI inhibits prenylation of lamin A that is incorrectly processed due to an upstream mutation. Promising results were also obtained in a Phase II study using Lonafarnib to treat chronic hepatitis infection since farnesylation of the large delta hepatitis antigen is required for viral proliferation (Yurdaydin et al., 2017). More preliminary investigations using prenylation inhibitors for the treatment of other diseases including malaria, leishmania and fungal infections are also in progress (Gelb et al., 2006).
Limited progress has been made on inhibiting the proteases Rce1 and ZMPSte24 that act on prenylated proteins. Compounds that function in vitro or in cell-based assays have been developed although those studies have not been extended to animal models this far (Mohammed et al., 2016; Porter et al., 2007). More progress has been made in developing inhibitors of Icmt because genetic knockouts have demonstrated that this enzyme is essential for transformation by oncogenic K-Ras and B-Raf (Bergo et al., 2001; Bergo et al., 2000). A variety of inhibitors based on the structure of N-acetylfarnesylcysteine have been developed (Bergman et al., 2011; Bergman et al., 2012; Donelson et al., 2006). Also, since the development of Cysmethynil (a non-isoprenoid-containing inhibitor), which was shown to induce autophagy-mediated cell death in PC3 prostate cancer cells (Wang et al., 2008), a number of groups have developed Icmt inhibitors with improved pharmacological properties (Yang et al., 2017). However, those studies have not yet progressed into animals.
Connections between Isoprenoids/Protein Prenylation and Alzheimer’s Disease
As discussed above, abnormalities in prenylated proteins, such as Ras and nuclear lamins, cause cancers and premature aging. Recently, there has been an increased interest in understanding the role of isoprenoids and prenylated small GTPase in neurodegenerative diseases. AD is the most prevalent neurodegenerative disorder in the elderly. It destroys memory and other vital cognitive functions, eventually leading to dementia and death. Currently in the United States, over 5 million people live with AD, and the prevalence of this devastating disease is expected to triple by mid-century (Alzheimer’s Association, 2016). The pathological hallmarks of AD include the accumulation of amyloid-β peptide (Aβ) in neuritic plaques and cerebral vessels, and the formation of neurofibrillary tangles consisting of hyper-phosphorylated tau proteins in the brain (Selkoe, Mandelkow, and Holtzman, 2012). The pathogenic mechanisms that lead to the development of AD, particularly the sporadic form of AD, remain to be elucidated. Here we summarize multiple lines of evidence that suggest important roles of isoprenoids/protein prenylation in the pathogenesis of AD.
Epidemiological/Clinical Evidence
The link between isoprenoids/prenylation and AD has been suggested from studies with statins. Several cross-sectional, cohort, and case-control studies have found significantly lower incidence and slower progression of AD in statin users (Haag et al., 2009; Jick et al., 2000; Wolozin et al., 2000). However, clinical evidence remains inconclusive due to inconsistent results across randomized clinical trials and cohort studies (Richardson et al., 2013; Shepardson, Shankar, and Selkoe, 2011). A recent longitudinal cohort study that followed 20% of Medicare beneficiaries from 2006 to 2013 found significantly lower risk of AD in individuals who had high exposure to statins than those with low exposure to statins (Zissimopoulos et al., 2017). This suggests that the inconsistencies observed in previous studies may be partly attributable to insufficient doses of statins used in trials. The same study reported that a reduction of AD risk differs across statin types, gender, and ethnic groups suggesting the importance of choosing the right study population for a given statin type. Although the statin-induced cholesterol-lowering effects could contribute to favorable outcomes in individuals with hypercholesterolemia, the beneficial effect of statins has been also reported in AD patients with normal cholesterol levels (Simons et al., 2002). Moreover, other non-statin drugs that lower cholesterol levels do not produce the same benefits in AD patients (Haag et al., 2009). This indicates that mechanisms independent of the inhibition of cholesterol biosynthesis are involved in the neuroprotective effect of statins.
Compelling evidence suggests that the reduction of isoprenoid synthesis and protein prenylation could be a key mechanism mediating statin-induced neuroprotection (Hooff et al., 2010; Li et al., 2016; Ostrowski et al., 2007), although experimental data should be interpreted with caution as statin treatment affects a number of biomolecules and associated pathways/proteins beyond isoprenoids. Eckert et al. demonstrated elevated FPP and GGPP, and mRNA expression of their synthases (FPPS and GGPS) in the brain tissues of male AD patients compared with non-AD controls without any changes in brain cholesterol levels (Eckert et al., 2009), suggesting a specific dysregulation of isoprenoid homeostasis in AD. This notion is supported by a recent report that found a significant correlation between the levels of mRNA for FPPS and GGPPS and tau/tangle pathology in the frontal cortex of AD brains, as well as an association between high levels of FPPS and GGPPS mRNA and earlier age of onset in AD (Pelleieux et al., 2018). Consistent with an increase in isoprenoids, abnormal increase in membrane associated (prenylated) Ras and Rho GTPases in post-mortem brain tissues of AD patients were reported in other studies (Gärtner, Holzer, and Arendt, 1999; Zhu et al., 2000). Moreover, Ginsberg et al. reported selective upregulation of Rab5 and Rab7 in brain regions that are susceptible to vulnerability in AD such as the basal forebrain, frontal cortex, and hippocampus, but not in relatively spared regions such as the cerebellum and striatum in individuals with diagnosis of mild cognitive impairment (MCI) and AD (Ginsberg, 2011; Ginsberg et al., 2011).
It is also worth noting that dysregulation of isoprenoid synthesis and prenylated proteins are involved in pathological aging and other age-related neurodegenerative disorders. Hutchinson-Gilford progeria syndrome (HGPS), a laminopathy characterized by premature aging, is one of the most well studied diseases involving aberrant prenylation (Gordon et al., 2012; Reddy and Comai, 2012). Most HGPS cases are caused by a point mutation in the LMNA gene, which leads to the production and accumulation of a permanently farnesylated lamin A protein called progerin (Reddy and Comai, 2012). As discussed above, clinical trials have shown that suppression of protein farnesylation by an FTI inhibitor with or without a statin or N-BP drug improves vascular stiffness, bone structure, and audiological status, and significantly increases the survival of children with this fatal disease (Gordon et al., 2016; Gordon et al., 2012; Gordon et al., 2014). Intriguingly, lamin A‐dependent nuclear defects also occur during normal aging (Scaffidi and Misteli, 2006). Whether the modulation of protein prenylation modifies the course of normal aging is yet to be investigated. Interestingly, a recent study showed that dysfunction of lamin mediates neurodegeneration in AD and suggested that AD could be an acquired neurodegenerative laminopathy (Frost, 2016). Furthermore, a recent study showed a significant increase in the expression of PGGT-1B, which encodes the β subunit of GGTase-I, in motor neurons of individuals with another neurodegenerative disease, amyotrophic lateral sclerosis (ALS), especially in early-onset cases (Li et al., 2016). This suggests that upregulation of protein prenylation may play an important role in the pathogenesis of ALS.
Genetic Evidence
AD is a multifactorial disease with a substantial genetic component. Early onset Alzheimer’s disease (EOAD) is inherited in an autosomal-dominant pattern, and accounts for only 1–2% of total AD cases (Rosenthal and Kamboh, 2014). Mutations in three genes, amyloid-β precursor protein (APP), presenilin-1 (PS-1) and presenilin-2 (PS-2) are linked to EOAD (Rosenthal and Kamboh, 2014). On the other hand, late onset Alzheimer’s disease (LOAD) accounts for most AD cases, and is much more complex than EOAD. To date, the most important genetic risk factor for LOAD is the APOE-ε4 allele, which accounts for approximately 25% of heritability. Recent genome-wide association studies (GWASs) and an epigenome-wide association study have identified ~30 putative risk susceptibility genes (Lambert, 2013; Ridge et al., 2017; Rosenthal and Kamboh, 2014; Sims et al., 2017). Yet the functional consequences and individual contributions of these newly identified susceptibility genes are not currently well known.
Although several cholesterol-related genes (e.g., apoE, clusterin (apoJ), and ABCA7) have been identified as top genes associated with AD, genes that are directly involved in isoprenoid synthesis or prenylation have not been identified as AD susceptibility markers in large-scale GWASs. However, small-scale genetic association studies indicate altered dynamics in isoprenoid synthesis and protein prenylation in AD. A recent study, in which five single nucleotide polymorphisms (SNPs) in human FPPS gene loci were examined in the frontal cortex in AD brains and their age-matched controls, found an allele-dose dependent association between one SNP (rs4971072), located in the promoter region of human FPPS gene, and phosphorylated tau (p-tau) levels (De Schutter et al., 2014). The same study further reported an increase in human FPPS mRNA levels in AD cortical brain samples, consistent with findings from a previous study in which the expression of FPPS is upregulated in AD brains (Eckert et al., 2009). Moreover, microarray correlation analyses in hippocampal samples from AD subjects and control subjects identified upregulation of 6 out of 10 genes involved in isoprenoid metabolism in AD hippocampal samples (Blalock et al., 2004).
Emerging evidence also points toward the importance of the downstream small GTPases in the pathogenesis of AD. An exome sequencing study of neuropathologically defined 170 controls and 185 LOAD patients revealed a significant association between a genetic variant of RAB11A (rs117150201) and an increased risk of LOAD (Udayar et al., 2013). In addition, protein-protein interaction network analysis identified that Rab11A and Rab11B, and their effector proteins have significant interactions with bridging integrator 1 (BIN1), a risk gene strongly associated with LOAD that functions in clathrin-mediated endocytosis and endocytic recycling, which regulates β-secretase trafficking and Aβ production. A more recent study that investigated protective genetic variants in 232 cognitively-intact individuals over age of 75 with at least one APOE ε4 allele discovered a significant association of two loss-of-function genetic variants, one variant in RAB10 (rs142787485; odds ratio (OR) = 0.58), and the other in SAR1A (secretion associated Ras related GTPase 1A) (rs7653; OR = 0.35), with reduced risk of AD (Ridge et al., 2017). Since prenylation is a pre-requisite for the proper cellular localization and activation of these small GTPases, these genetic studies suggest that uncontrolled upregulation of small GTPase prenylation may play a significant role in the pathogenesis of AD.
Biochemical Evidence
Mounting biochemical evidence from in vitro and in vivo experiments indicates that isoprenoids and prenylated proteins are implicated in multiple processes pertinent to AD pathology, including APP processing and Aβ metabolism, tau phosphorylation, neuroinflammation, and synaptic plasticity and cognitive function as described below (Figure 9).
Figure 9. Implications of dysregulated isoprenoid synthesis and protein prenylation in processes pertinent to the pathogenesis of Alzheimer’s disease.
Dysregulations in isoprenoid biosynthesis and the prenylation of small GTPases have been linked to (i) alteration of APP trafficking/processing and the activity/localization of the secretases; (ii) increase in tau phosphorylation through the activation of GSK-3β and other kinases; (iii) microglial activation contributing to enhanced neuroinflammation; (iv) dendritic/synaptic loss leading to cognitive impairment. APP: amyloid-β precursor protein; Aβ: amyloid-β peptide; BACE1: β-secretase 1; sAPPα: α-secretase-cleaved soluble fragment of APP; sAPPβ: β-secretase-cleaved soluble fragment of APP; AICD: APP intracellular domain; P3: non-amyloidogenic peptide (~3 kDa); GSK-3β: glycogen synthase kinase-3β; NMDAR: N-methyl-D-aspartate receptor; AMPAR: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor. A color version of the figure is available online.
APP processing and Aβ metabolism:
Aβ is the major component of senile plaques, one of pathological hallmarks of AD, and is generated by sequential cleavages of amyloid-β precursor protein (APP) by β-secretase (BACE1) and γ-secretase. In contrast, α-secretase cleaves within the sequence of Aβ, thus precluding the formation of intact Aβ (non-amyloidogenic processing of APP), and produces the neurotrophic soluble fragment, sAPPα (Thinakaran and Koo, 2008). Subsequent cleavage of the C-terminal fragment by γ-secretase yields the small non-amyloidogenic peptide, P3 (Nhan, Chiang, and Koo, 2015). It has been shown that FPP and GGPP significantly elevate Aβ production by potentially stimulating γ-secretase in H4 neuroglioma cells expressing APP with the Swedish mutation (sweAPP) (Kukar et al., 2005), and in HEK 293 cells expressing sweAPP (Zhou et al., 2008). Interestingly, co-administration of a farnesyltransferase or geranylgeranyl transferase inhibitor with FPP and GGPP did not block the elevation of Aβ levels (Kukar et al., 2005), indicating that FPP and GGPP may increase Aβ level through pathways independent of protein prenylation. However, in this study the cells were treated with transferase inhibitors for 6 hours and the level of protein prenylation was not measured (Kukar et al., 2005). Therefore, it is possible that the short treatment duration did not sufficiently block protein prenylation to observe the effects on Aβ levels. The notion that statin-induced effects on Aβ levels are mediated through protein prenylation is supported by other studies described below, where the treatment duration was longer (24 hours) and the level of protein prenylation was measured (Ostrowski et al., 2007; Pedrini et al., 2005). It has also been demonstrated that statins may decrease Aβ production by reducing dimerization of BACE1, thereby blocking its co-localization with APP through both cholesterol-dependent and cholesterol-independent mechanisms (Parsons et al., 2006). Whereas another group showed that treatment with atorvastatin or simvastatin could stimulate α-secretase activity and shedding of non-amyloidogenic sAPPα by depleting FPP and inhibiting protein farnesylation in a murine neuroblastoma cell line (Pedrini et al., 2005). Furthermore, other in vitro studies have reported that treatment with lovastatin or simvastatin promotes intracellular accumulation of APP and Aβ, while causing a decrease of secreted Aβ, in a GGPP-dependent manner (Cole et al., 2005; Ostrowski et al., 2007; Zhou et al., 2008). Consistent with the findings from in vitro experiments, pharmacological or genetic inhibition of protein prenylation has been shown to reduce Aβ accumulation in vivo. For example, a high-dose simvastatin treatment in guinea pigs led to significant reduction of Aβ levels in the brain without affecting brain cholesterol levels (Fassbender et al., 2001). Similarly, atorvastatin reduced the brain Aβ by 38–52% with a minimal decrease in brain cholesterol levels in the APP/PS1 transgenic mouse model of AD (Petanceska et al., 2002). Recently, using genetically modified FTase and GGTase-I haplodeficient mice, we have demonstrated that reduction of protein prenylation (either farnesylation or geranylgeranylation) significantly decreases Aβ pathology in APP/PS1 transgenic mice (Cheng et al., 2013). We further showed that FTase haplodeficiency enhances non-amyloidogenic processing of APP and degradation of Aβ. These findings provide direct evidence that modulation of protein prenylation can modify the course of Aβ metabolism/deposition in the brain.
A growing body of evidence highlights the roles of small GTPase signaling pathways on amyloid pathology. For example, pitavastatin or atorvastatin treatment in APP-expressing rat primary cortical neurons resulted in concentration-dependent inhibition of Aβ, which was not mitigated by the co-administration of exogenous cholesterol (Hosaka et al., 2013). In the same study, immunoblot analysis of statin-treated primary cortical neurons showed not only a reduction in mature APP, but also a decrease in Thr668-phosphorylated APP. Phosphorylation of APP at Thr668 has been shown to enhance proteolysis by BACE1 in previous studies (Lee et al., 2003). A recent study demonstrated that neuronal exposure to oligomeric Aβ overactivates the Ras-MEK-ERK pathway which subsequently leads to hyperphosphorylation of APP at Thr668, establishing a vicious feed-forwarding cycle in sweAPP-expressing rat neuroblastoma B103 cells (Kirouac et al., 2017). Notably, both an MEK inhibitor and a farnesyltransferase inhibitor blocked this oligomeric Aβ-induced APP phosphorylation.
Similarly, the Rho/Rho-associated coiled-coil containing kinases (ROCK) pathway has also been shown to play a role in Aβ production. Constitutive activation of ROCK1 (CA-ROCK1) in murine N2a neuroblastoma cells overexpressing sweAPP was reported to diminish non-amyloidogenic α-secretase-type ectodomain shedding of APP, whereas dominant negative ROCK1 (DN-ROCK1) expression or pharmacological inhibition of ROCK1 increased sAPPα production (Pedrini et al., 2005). This indicates that the Rho/ROCK pathway can modulate α-secretase activity. However, a recent study showed that DN-ROCK1, CA-ROCK1, or ROCK1 knockdown had no effect on sAPPα or Aβ production in human cells (Herskowitz et al., 2013; Leuchtenberger et al., 2006). It was suggested that ROCK1 and ROCK2 might differentially affect Aβ production as selective ROCK2 inhibition significantly reduced Aβ along with the suppression of BACE1 activity, whereas the depletion of ROCK1 increased Aβ levels (Herskowitz et al., 2013). In the same study, a single hippocampal injection of a ROCK2-selective inhibitor to 3-month-old 5xFAD transgenic AD model mice also led to a significant reduction in Aβ levels as well as BACE1 activity. Notably, the effect of ROCK2 inhibition on Aβ generation could be partly mediated through phosphorylation of the lipoprotein receptor LR11/SorLA (Herskowitz et al., 2011), which is genetically associated with AD and has been shown to regulate intracellular transport and processing of APP (Andersen et al., 2005; Offe et al., 2006; Scherzer et al., 2004). Furthermore, a recent study showed that ROCK1 is increased in AD and reduction of ROCK1 or ROCK2 in neurons suppresses Aβ production, likely through induction of autophagy by Rho/ROCK signaling to enhance APP protein degradation (Henderson et al., 2016). These studies suggest that both ROCK1 and ROCK2 could serve as therapeutic targets to mitigate amyloidosis in AD.
In addition, the role of Rab GTPases has also been extensively studied in the context of APP processing. A multiplex high-throughput RNA interference screening of Rab GTPases revealed that knockdown of Rab3A and Rab3B reduced Aβ and sAPPβ levels along with overall APP levels in HeLa cells overexpressing sweAPP, suggesting a potential role for these Rab proteins in APP trafficking and processing (Udayar et al., 2013). The study further demonstrated consistent reduction in Aβ and sAPPβ in three different cell types after siRNA-mediated knockdown of Rab11A, potentially through the modulation of BACE1 trafficking and recycling. Moreover, a more recent study found that ~50% of Rab10 knockdown by RNA interference led to ~45% reduction in Aβ without affecting the level of full-length APP in APP-expressing SH-SY5Y neuroblastoma cells (Ridge et al., 2017). Taken together, these findings indicate that Rab proteins may play an important role in APP/Aβ metabolism.
Tau Phosphorylation:
Neurofibrillary tangles (NFTs) are another pathological hallmark of AD. During the course of AD, microtubule-associated tau proteins become hyper-phosphorylated, detached from microtubules, and aggregate to form intracellular NFTs. Although the role of isoprenoids/protein prenylation in tau pathology has not been studied as extensively as in β-amyloidosis, emerging evidence suggests a strong link between protein prenylation and tau phosphorylation, phospho-tau (p-tau) distribution, and tangle formation. An earlier study showed that prolonged treatment with a high concentration of lovastatin in primary neurons transiently increased p-tau while altering the intracellular distribution of tau, causing disruption of neuritic networks and apoptosis due to suppression of GGPP formation (Meske et al., 2003). In contrast, a later study showed that simvastatin treatment in vivo significantly reduced NFT burdens in a double-mutant tau transgenic mouse model (Boimel et al., 2009). Several in vitro studies also support the beneficial effects of statin treatment on tau pathology and the role of Rho GTPases. Treatment with pitavastatin was found to decrease total and p-tau in primary neurons, and these changes were correlated with decreases in membrane-associated (prenylated) Rho GTPases including RhoA, Rac1 and Cdc42, and inactivation of glycogen synthase kinase-3β (GSK-3β) (Hamano et al., 2012). Notably, GSK-3β is a major kinase driving tau phosphorylation thereby contributing to AD pathogenesis (Hooper, Killick, and Lovestone, 2008). The authors speculate that the hyperactivation of the RhoA/ROCK pathway leads to the overactivation of GSK-3β which then contributes to tauopathy. The study also pointed out that these statin-associated changes in tau and p-tau levels were dose-dependent. Only a low-to-moderate dose of pitavastatin reduced tau and p-tau levels, whereas a high-dose of pitavastatin activated caspase 3, and increased caspase3-cleaved tau, which could lead to negative effects. This may explain some of the detrimental effects with a high dose of lovastatin observed in an earlier study (Meske et al., 2003). The link between RhoA/ROCK pathway and tauopathy has been further demonstrated in a recent study (Gentry et al., 2016), which showed that ROCK1 and ROCK2 were elevated in the brains of patients with non-AD tauopathies, progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD). The study further showed that inhibition of Rho/ROCK signaling pathway significantly diminished total and p-tau levels in murine cortical neurons and in a Drosophila tauopathy model, through enhancing autophagy and reducing tau mRNA. The connection between isoprenoids and tau phosphorylation is supported by a study that reported a significant decrease in p-tau-to-total tau ratio in SH-SY5Y cells after N-BP-induced inhibition of FPPS (De Schutter et al., 2014). Furthermore, a high-throughput cell-based assay identified Ras GTPases as a putative pathway involved in tau phosphorylation (Cavallini et al., 2013). Intriguingly, Kirouac et al. reported that exposure to Aβ oligomers activated GSK-3β and increased p-tau levels in parallel with the elevation of APP hyperphosphorylation at Thr668 through the activation of Ras-ERK in rat neuroblastoma B103 cells expressing sweAPP (Kirouac et al., 2017). This suggests that the activated small GTPases may serve as an intersection between amyloid and tau metabolisms. However, further investigations are required to unravel the exact molecular link between small GTPase pathways and tau phosphorylation.
Neuroinflammation:
In parallel with misfolded aggregates of Aβ and hyperphosphorylated tau, emerging evidence indicates that microglia-mediated neuroinflammation plays an important role in AD pathogenesis (Akiyama et al., 2000; Heneka et al., 2015). The importance of microglia in AD is further underscored by recent discoveries that rare mutations in the triggering receptor expressed on myeloid cells 2 (TREM2), an innate immune receptor found in brain microglia, are associated with a significantly increased risk of AD (Colonna and Wang, 2016; Guerreiro et al., 2013; Jonsson et al., 2013). While the exact role of TREM2 in AD is being actively investigated (Jay, von Saucken, and Landreth, 2017; Ulrich et al., 2017; Yeh, Hansen, and Sheng, 2017), it is well established that aggregated Aβ leads to microglial activation by interacting with Toll-like receptors (TLRs) (Landreth and Reed-Geaghan, 2009; Su et al., 2016). In particular, it has been shown that TLR2 and TLR4 are required for fibrillar Aβ-stimulated microglial activation (Reed-Geaghan et al., 2009), and induce the release of proinflammatory cytokines such as IL-1β, IL-6, and TNFα upon binding of aggregated Aβ (Liu et al., 2012; Smith et al., 2012). Under physiological conditions, increases in TLR2/TLR4 activation promote microglial Aβ phagocytosis thereby facilitating Aβ clearance in the brain parenchyma. However, chronic exposure to toxic Aβ in AD results in the loss of the ability to suppress these immune responses thereby contributing to disease pathology (Wyss-Coray and Rogers, 2012). Emerging evidence suggest that small GTPases play an important role in TLR2 and TLR4 signal cascades. For example, the Rac1-dependent pathway is required for TLR2-mediated inflammatory signaling (Arbibe et al., 2000; Ruse and Knaus, 2006; Zolezzi and Inestrosa, 2017). In support of the role of isoprenoids/protein prenylation in inflammation, numerous studies have reported that statins exert anti-inflammatory activities, and these immunomodulatory properties of statins have been proposed to arise from the regulation of small GTPases (Cordle and Landreth, 2005; Liao and Laufs, 2009; Oesterle, Laufs, and Liao, 2017). Statin-mediated anti-inflammatory effects have been shown in various in vitro and in vivo models of AD. Treatment with simvastatin or lovastatin inhibited IL-1β production in primary microglia and THP-1 monocytes after the exposure to fibrillar Aβ peptides through a cholesterol-independent process that is prevented by GGPP (Cordle and Landreth, 2005). This effect was mimicked by treatment with a GGTase-I inhibitor or direct inactivation of Rho GTPases with Clostridium difficile toxin. Simvastatin or lovastatin treatment also prevented fibrillar Aβ-induced cytokine production and astrocyte death in a human BBB model that consists of a cerebral microvascular endothelial cell monolayer co-cultured with astrocytes (Griffin et al., 2016). In animal studies, long-term treatment (>5 months) with atorvastatin or pitavastatin significantly reduced monocyte chemoattractant protein-1 (MCP-1)-positive neurons, microglial activation, and TNF-α in Tg2576 AD model mice (Kurata et al., 2012). Furthermore, we have shown that direct reduction of protein prenylation by a genetic approach attenuates neuroinflammation in AD mice (Cheng et al., 2013). Heterozygous deletion of FTase or GGTase-I ameliorates Aβ-induced neuroinflammation in cortical and hippocampal areas in the APP/PS1 double transgenic mouse model of AD, providing direct evidence for the roles of protein prenylation and their downstream pathways in neuroinflammation in AD (Cheng et al., 2013).
Synaptic plasticity and cognitive function:
One of the key characteristics of AD brains is synaptic dysfunction demonstrated by a significant reduction of spine densities in neurons, consequently leading to cognitive impairment. It is well known that small GTPases play key roles in regulating synaptic plasticity under physiological conditions, and several lines of evidence suggest that the dysregulation of small GTPase signaling leads to synaptic dysfunction in AD (Hottman and Li, 2014; Stornetta and Zhu, 2010). Ras and Rab GTPases have been shown to regulate neurotransmitter receptor trafficking and recycling undergoing long-term potentiation in multiple studies (Ehlers, 2000; Stornetta and Zhu, 2010). The specific roles of Ras GTPases in neuronal plasticity and memory formation have been demonstrated in a number of studies (Ye and Carew, 2010). Rho GTPases, including Rac1, Cdc42, and RhoA, regulate neurite outgrowth and spine dynamics through their downstream effectors P21-activated kinases and ROCK (Briz et al., 2015; Haditsch et al., 2009; Ohashi et al., 2000). Tight spatiotemporal regulation of Rho GTPase signaling is critical in the neural circuit formation especially during neurodevelopment (Kimberly F. Tolias, 2011). Both hypo- and hyper-activation of these small GTPases and their downstream effectors have been shown to have detrimental effects on synaptic plasticity and cognitive functions, and have been implicated in learning and neurodegenerative disorders (Brambilla et al., 1997; Haditsch et al., 2009; Zhao et al., 2006). In AD, a recent study showed that differences in dendritic spine morphology distinguish individuals who had or did not have clinical dementia and proposed that dendritic spine plasticity offers cognitive resilience that protects people with AD pathology from developing dementia (Boros et al., 2017). Notably, pharmacologic inhibition of ROCK1 and ROCK2 has been shown to affect dendritic spine morphology in hippocampal neurons (Swanger et al., 2015), suggesting that the Rho/ROCK pathway may play a critical role in dendritic spine remodeling and that ROCK inhibitors could potentially be used to modulate dendritic spine plasticity in AD.
The function of the small GTPases described above depends on proper cellular localization, which is regulated by protein prenylation. Studies using post mortem human brain samples have provided evidence for dysregulation of isoprenoids, and small GTPase pathways in AD brains (De Schutter et al., 2014; Eckert et al., 2009; Gartner et al., 1995; Ginsberg, 2011). Consistently, multiple animal studies reported that statin treatment rescued synaptic plasticity and/or cognitive function in mice with or without AD pathology (Costa et al., 2002; Li et al., 2006; Mans et al., 2010; Mans, McMahon, and Li, 2012; Métais et al., 2014; Ye and Carew, 2010; Zhao et al., 2016). Together, these findings suggest that synaptic dysfunction and memory impairment in AD may be attributable to abnormal upregulation of isoprenoids or protein prenylation.
However, statins inhibit both protein farnesylation and geranylgeranylation by inhibiting upstream mevalonate/isoprenoid biosynthesis. Therefore, it is difficult to dissect the roles of different prenylation pathways in AD pathogenesis. Using a genetic approach, we have demonstrated recently that reduction of FTase, but not GGTase-I, ameliorates spatial learning and memory as well as attenuates Aβ deposition and neuroinflammation in APP/PS1 mice (Cheng et al., 2013). In a more recent study, we have observed that systemic or forebrain neuron specific deficiency of GGTase-I reduces dendritic spine density and impairs synaptic/cognitive function in wild type mice (Hottman et al., 2018), corroborating the critical role of GGTase-I and protein geranylgeranylation in neuronal structure and function suggested by previous studies (Gao, Yu, and Zhou, 2016; Zhou et al., 2008). These findings suggest that farnesylated and geranylgeranylated proteins play distinctive roles in the pathogenesis of AD. While inhibition of geranylgeranylation might be beneficial under pathological conditions with overactivation or upregulation of GGTase-I, the chronic use of GGTase-I inhibitors could potentially cause adverse effects on synaptic/cognitive function. In contrast, reduction/inhibition of FTase produces cognitive benefits without detectable side effects, which may serve as a novel therapeutic target for AD. Whether these benefits found in animals can be translated to humans awaits further investigation.
Prenylomic Approaches and Their Applications in Alzheimer’s Disease Research
Small Molecule Probes Used to Study Protein Prenylation
To improve understanding of protein prenylation, synthetic chemical probes have proven to be quite useful. Beyond the prenylation inhibitors discussed extensively above, a number of small molecule probes have proven useful in studying various aspect of protein prenylation. Photoactivatable forms of isoprenoid diphosphates such as DATFPGerOPP and BpGerOPP (Figure 10) were developed first and allowed the isoprenoid binding sites on different prenyltransferases to be identified (Bikhtiyarov, Omer, and Allen, 1995; Edelstein and Distefano, 1997; Gaon, Turek, and Distefano, 1996; Gaon et al., 1996; Kuang et al., 1996; Turek-Etienne, Strickland, and Distefano, 2003; Turek, Gaon, and Distefano, 1996; Turek et al., 2001; Yokoyama, McGeady, and Gelb, 1995). More recently, related probes have been used to study the active site of Icmt (a-FactorDiazGer) (Hahne et al., 2012). Photoactivatable probes such as BiotinCysBpGer have also been employed to study the interactions between prenylated proteins and their binding proteins (Alexander et al., 2009; Kale and Distefano, 2003; Kale et al., 2001). Examples of this include RhoGDI and PDEδ, two proteins that modulate the activity of prenylated proteins by binding directly to the isoprenoid group itself. These photoactive probes may prove to be useful in understanding the roles of prenylated proteins in Alzheimer’s disease since they can be potentially used to reveal specific interactions between prenylated proteins and their binding partners that participate in cellular signaling. Isoprenoid analogues that contain structural modifications comprise a second class of small molecule probes. These compounds include structures with different alkene stereochemistry (Z,E-FarOPP) as well as aromatic or bulky substitutions (BzAnalogOPP) (Adams et al., 2010; Gibbs et al., 1999; Shao, Eummer, and Gibbs, 1999). They are of particular interest since their incorporation into prenylated proteins can either modulate or eliminate their activity. Fluorescent analogues such as BdpyGerOPP have also proved to be useful for monitoring the binding of isoprenoids to prenyltransferases and binding partners of prenylated proteins (Nguyen et al., 2007). Others such as CouGerOPP have also proven to be useful for the development of convenient assays to measure prenyltransferase activity (Dozier et al., 2014). In general, these modified isoprenoids should be useful for biochemical analysis of the interactions of various prenylated proteins and other cellular components as they relate to Alzheimer’s disease. Small peptides equipped with fluorophores have been tremendously helpful in the development of enzyme assays for prenyltransferase activity as well as for monitoring the proteolytic activity of Ste24 and Rce1 that do not rely on radiolabeled substrates. Prenyltransferase activity is conveniently followed by monitoring the increase in dansyl group fluorescence that occurs upon farnesylation of the synthetic peptide Dansyl-GCVIA (Bond, Dolence, and Poulter, 1995; Pompliano, Gomez, and Anthony, 1992). Ste24 and Rce1 activity can be followed by measuring the increase in Abz fluorescence as a Dnp quencher is removed upon proteolysis of peptides such as Abz-FWDPAC(far)VIK(Dnp) (Hollander, Frommer, and Mallon, 2000). However, radiochemical methods are the method of choice for monitoring Icmt-catalyzed methylation. Cell penetrating peptides have also been used study localization and processing of prenylated molecules in cells (Ochocki, Wattenberg, and Distefano, 2010; Wollack et al., 2009; Wollack et al., 2010); such peptides are capable of entering astrocytic cell lines making them attractive tools for studying brain-related processes involving prenylated molecules. Prenylated peptides have also been used to prepare full-length forms of Ras for a range of experiments (Chen et al., 2010; Zhang et al., 2017). More recently, isoprenoid analogues functionalized with different “handles” including biotin (Nguyen et al., 2009), antigenic moieties (AnGerOPP) (Onono et al., 2010) and biorthogonal groups (C15AzOPP and C15AlkOPP) (Hosokawa et al., 2007; Rose et al., 2005) have been prepared and employed in pull-down and enrichment experiments designed to identify prenylated proteins and measure how their levels change. Those containing biorthogonal azides and alkynes have been the most widely used since they can be metabolically incorporated in live cells and then linked to biotin or fluorophores for analysis. This last topic is discussed in more detail below.
Figure 10.
Small molecule probes used for studying protein prenylation.
Proteomic and Prenylomic Approaches
The growing interest in understanding the roles of prenylated proteins in biology has led to the development of proteomic strategies to characterize the members of the prenylome (prenylomics), the total complement of prenylated proteins. Earlier methods to identify prenylated proteins involved the use of radiolabeled forms of the isoprenoids, [3H]-FPP and [3H]-GGPP (Yalovsky, Loraine, and Gruissem, 1996), or [3H]-mevalonate (Laezza, Wolff, and Bifulco, 1997) and detection through fluorography. However, this approach is limited by its low sensitivity and exacerbated by its cost, arduousness, and the length of time required for the entire process. Gel-based separations were employed to improve sensitivity where protein mixtures are resolved in a gel and digested with trypsin. The peptides are then analyzed on a mass spectrometer to identify the prenylated proteins mapped through their peptide mass fingerprints (Cenedella, 1998).
In the past decade, higher throughput profiling of lipid-modified proteins including the prenylome have emerged as a powerful tool to identify the set of post-translationally lipidated proteins (Tate et al., 2015). This so-called chemical proteomics is a mass spectrometry-based technique that requires an analogue of the lipid functionalized with a bioorthogonal functional group, typically an azide or alkyne. These cell-penetrating probes are introduced into a biological system of interest wherein the cell’s machinery metabolically incorporates them into the substrate proteins. The tagged proteins are then ligated via a bioorthogonal reaction (e.g. click reaction) with complementary chemical reporters or affinity handles that allow visualization or enrichment of these labeled proteins (Figure 11A). For visualization of labeled proteins, labeled lysates are subjected to derivatization with a fluorophore and resolved via SDS-PAGE. In-gel fluorescence is then performed where labeled proteins appear as bands owing to the fluorescence conferred by the conjugated fluorophore. For enrichment of the labeled proteins, biotin is commonly used to derivatize the labeled proteins followed by pulldown using avidin beads. The enriched proteins are then either digested on-bead or eluted, resolved in an SDS-PAGE gel and digested in-gel. The resulting peptides are then subjected to LC-MS/MS methods for proteomic analysis.
Figure 11. A general chemical proteomic approach to label and enrich proteins of interest via click reaction (A) and the isoprenoid probes used to profile prenylated proteins (B).
A color version of the figure is available online.
Metabolic labeling strategies for chemical proteomic profiling of prenylated proteins have relied on the use of functionalized isoprenoids to identify the prenylome of a given system of interest (Figure 11B). The first report using this approach employed an azide-modified farnesol (C15AzOH) into COS-1 cells followed by Staudinger ligation with triphenylphosphine-modified biotin and labeled prenylated proteins were enriched through avidin pulldown (Kho et al., 2004). This resulted in the identification of 17 bona fide prenylated proteins. A subsequent study used azide-functionalized geranylgeraniol (C20AzOH) to metabolically label proteins followed by copper-catalyzed azide-alkyne cycloaddition (click reaction) with an alkyne-modified fluorophore in lysate. Proteins were then resolved through 2D gel electrophoresis and labeled protein bands were subjected to MS analysis (Chan et al., 2009). A total of 10 geranylgeranylated proteins were identified. However, the use of azide-modified isoprenoids followed by conjugation with an excess of alkyne-modified reporter presents significant background labeling problems (Speers and Cravatt, 2004). For this reason, azide-containing probes have been slowly replaced by alkyne-modified isoprenoids that usually result in lower nonspecific labeling. The first report on the use of alkyne-modified isoprenoids C10AlkOH, C15AlkOH and their diphosphate analogues, C10AlkOPP and C15AlkOPP, were evaluated in HeLa cells (DeGraw et al., 2010). Proteomic analysis using C10AlkOH has led to the identification of seven prenylated proteins in that study. Metabolic labeling with alkyne-funtionalized farnesol (C15AlkOH) profiled more than 20 prenylated proteins in macrophages, which included an unannotated zinc-finger antiviral protein that was shown to be indispensable for host defense against viral infection (Charron et al., 2013). A combination of C15AlkOPP metabolic labeling and 2D gel electrophoresis identified 19 prenylated proteins that are sensitive to the presence of farnesyltransferase inhibitors (FTIs) (Palsuledesai et al., 2014). Recently, the prenylome of Plasmodium falciparum—the causal agent of malaria—was defined in two independent studies using C15AlkOPP (Suazo et al., 2016) and C15AlkOH (Gisselberg et al., 2017). It was found that this parasite has a limited set of prenylated proteins that are mainly involved in protein trafficking, and thus serves as a good target to develop parasite-specific FTIs. While the incorporation of these functionalized isoprenoids may be of concern in terms of altering protein function, it was recently reported that prenylated peptides using these probes are able to undergo the processes downstream of prenylation and that they retain signaling activity (Diaz-Rodriguez et al., 2017).
A plethora of proteomic studies on actual AD post-mortem tissue have been reported wherein differentially expressed proteins in AD brains compared to healthy controls have been identified (Brinkmalm et al., 2015; Korolainen et al., 2010). In particular, the use of high mass accuracy, label-free quantitative techniques (Andreev et al., 2012) and multiplexing strategies (Musunuri et al., 2014) have been successful. A number of proteins have been identified to be differentially expressed in AD but only a few were found common among the studies conducted (Moya-Alvarado et al., 2016). For example, GAPDH, a protein involved in glycolysis, has been consistently found to have altered levels in AD. Differential expression of some mitochondrial proteins and those that catalyze redox and phosphorylation processes such as peroxiredoxins and kinases, respectively, were also observed. Overall, it has been suggested that proteins associated with the oxidation and phosphorylation of glycolytic intermediates and processes in the mitochondria are drivers of the early progression of AD (Moya-Alvarado et al., 2016). Recently, quantitative label-free proteomics combined with systems biology has allowed networks of proteins to be defined that are associated with the neuropathology of AD with high specificity (Seyfried et al., 2017). Both the proteome and transcriptome analysis converged on networks involved in inflammation and glial dysfunction and thus are implicated in the etiology of AD. These results reflect the significance of non-neuronal drivers in the pathology of AD.
From the proteomic studies conducted, a number of known prenylated proteins were found to have altered expression in AD brain tissues compared to the healthy controls. For example, decreased levels of 2’,3’-cyclic nucleotide-3’-phosphodiesterase (CNP) in the late-myelinating region (frontal cortex) of AD patient brains were determined using 2D gel electrophoresis and MALDI-MS (Vlkolinský et al., 2001). It was earlier suggested that the presence of the prenyl group is essential for the interaction of CNP with the hydrophobic components of myelin (De Angelis and Braun, 1996). The same proteomic technique led to the identification of UCHL1 as a major target of oxidative damage in AD (Castegna et al., 2002; Choi et al., 2004). Although it was shown that the farnesylation of UCHL1 is not involved in its association to neuronal membranes (Bishop et al., 2014), inhibition of this process using farnesyltransferase inhibitors (FTIs) reduced the accumulation and neurotoxicity of α-synuclein-transfected cell cultures (Liu et al., 2009). Hence, targeting the UCHL1 farnesylation may be an avenue as a therapeutic strategy for the treatment of Parkinson’s disease and other related synucleinopathies such as AD. Label-free quantitative LC-MS proteomic experiments have shown that lamin-B, Rap-2A, Rap-2B and NAP1L1 are increased in AD compared to normally aged human brains (Andreev et al., 2012). The same approach combined with systems biology identified that the levels of RHOB and GNAI1, a G protein subunit that is potentially prenylated (Palsuledesai et al., 2014), were decreased exclusively in the symptomatic stage of AD (Seyfried et al., 2017). Furthermore, a quantitative proteomic study conducted on APP-expressing neuronal-like B103 cells that recapitulate the AD phenotype has found increased levels of the prenylated proteins CNP and Di-Ras (Chaput et al., 2012). Taken together, these studies demonstrate that prenylated proteins indeed have potential implications in AD and that proteomic methods could be an advantageous technique to analyze AD brain samples and models. Thus, proteomic methods such as chemical proteomic profiling may be useful for specifically identifying prenylated proteins involved in AD pathogenesis.
While the above mentioned proteomic analyses have been useful in identifying putative prenylated proteins involved in AD, they do not establish that the prenylated form is relevant nor do they allow changes in the levels of prenylation to be studied since lipid-modified peptides themselves produced upon tryptic digestion of whole proteins are rarely identified in proteomic experiments. In contrast, metabolic labeling studies with probes such as C15AlkOPP allow enrichment of the actual prenylated forms of proteins since the enrichment process itself relies on the presence of the modified isoprenoid. A recent report employed the use of C15AlkOH to image and quantify levels of prenylated proteins in astrocytes using confocal microscopy and flow cytometry (Palsuledesai et al., 2016), suggesting that metabolic labeling could be used to profile the prenylome in AD cell culture models. It should also be noted that metabolic labeling has been used to incorporate probes containing bioorthogonal functionality in whole animal models raising the possibility that similar experiments could be used to identify and study prenylated proteins involved in AD (Baskin et al., 2007). Overall, further efforts are needed to explore the role of prenylated proteins in AD using prenylomic strategies employing these new probes and tools.
Concluding Remarks/Perspectives
Understanding the biological role of isoprenoids and protein prenylation is of central importance from both a basic scientific and a clinical perspective. Fundamental scientific discoveries often provide critical insights that catalyze progress in the clinic. Remarkable advances have been achieved in the last century in understanding the regulation of isoprenoid and cholesterol metabolism in the peripheral organs, and successful drugs (e.g., statins and N-BPs) have been developed to treat cardiovascular and bone diseases, respectively. The recognition that isoprenoids can modify the structure and function of many important proteins involved in human diseases has invigorated the prenylation field with the goal of developing effective therapies for a range of disorders, from cancer, premature aging, infectious disease, to neurodegenerative diseases including Alzheimer’s disease.
To reach this goal in the context of Alzheimer’s disease, advances in several areas must occur. First, while statins that cross the blood brain barrier are available, bisphosphonates and related prodrugs that are able to do this have not yet been developed. More work needs to be done with prenyltransferase inhibitors to increase their brain bioavailability as well. Next, more global and systematic data regarding protein prenylation in the brain must be obtained. This means that the prenylome within the brain must be defined. The new developments in metabolic labeling described herein should be instrumental in accomplishing this goal. In concert with genetically modified animal models, in which prenyltransferase activities can be manipulated in a tissue specific manner coupled with expression of disease-specific genes, these tools should provide unique in vivo model systems to identify disease-specific prenylated proteins from the entire prenylome. However, the identification of specific prenylated proteins in the brain must be coupled with methods that allow both changes in prenylation levels and overall protein expression to be measured. For this, new advances in quantitative mass spectrometric methods should be particularly helpful. Ultimately, applying these methods to brain tissue samples from humans with a spectrum of cognition from normal to Alzheimer’s dementia should enable a quantitative proteome-wide analysis of prenylated proteins during the progression of the disease. Such data could reveal the underlying roles of prenylated proteins in the pathogenesis of Alzheimer’s disease and facilitate the identification of new targets for developing novel therapeutic interventions against Alzheimer’s disease. Finally, a better understanding of which prenylated proteins are critical for pathogenesis will catalyze efforts to develop inhibitors that interfere with specific prenylated proteins. Such agents would be inherently more selective than the current generation of prenyltransferase inhibitors that act on a global level. Overall, it is clear that the intersection of research in Alzheimer’s disease and protein prenylation is an exciting and accelerating area that holds great promise for therapeutic development.
Acknowledgments
This work was supported in part by the National Institute on Aging of the National Institutes of Health grant (RF1AG056976) to LL and MD and by the National Science Foundation grant (CHE-1308655) to MD. AJ is supported by the Kwanjeong Educational Foundation Overseas Scholarship from South Korea.
Footnotes
Disclosure of interest
The authors report no declarations of interest.
References
- 1.4S-group. (1994).Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet, 344, 1383–9 [PubMed] [Google Scholar]
- 2.Adams VR, Deremer DL, Stevich B, Mattingly CA, Gallt B, Subramanian T, Troutman JM, Spielmann HP. (2010).Anticancer Activity of Novel Unnatural Synthetic Isoprenoids. Anticancer Res, 30, 2505–12 [PubMed] [Google Scholar]
- 3.Agranoff BW, Eggerer H, Henning U, Lynen F. (1960).Biosynthesis of terpenes. VII. Isopentenyl pyrophosphate isomerase. J Biol Chem, 235, 326–32 [PubMed] [Google Scholar]
- 4.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WST, Hampel H, Hull M, Landreth G, Lue LF, Mrak R, MacKenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. (2000). Inflammation and Alzheimer’s diseaseed.^eds., 383–421 [DOI] [PMC free article] [PubMed]
- 5.Alexander M, Gerauer M, Pechlivanis M, Popkirova B, Dvorsky R, Brunsveld L, Waldmann H, Kuhlmann J. (2009).Mapping the isoprenoid binding pocket of PDEdelta by a semisynthetic, photoactivatable N-Ras lipoprotein. ChemBioChem, 10, 98–108 [DOI] [PubMed] [Google Scholar]
- 6.Alzheimer’s Association. (2016).2016 Alzheimer’s disease facts and figures. Alzheimers Dement, 12, 459–509 [DOI] [PubMed] [Google Scholar]
- 7.Andersen OM, Reiche J, Schmidt V, Gotthardt M, Spoelgen R, Behlke J, von Arnim CA, Breiderhoff T, Jansen P, Wu X, Bales KR, Cappai R, Masters CL, Gliemann J, Mufson EJ, Hyman BT, Paul SM, Nykjaer A, Willnow TE. (2005).Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc Natl Acad Sci U S A, 102, 13461–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson JL, Frase H, Michaelis S, Hrycyna CA. (2005).Purification, functional reconstitution, and characterization of the Saccharomyces cerevisiae isoprenylcysteine carboxylmethyltransferase Ste14p. J Biol Chem, 280, 7336–45 [DOI] [PubMed] [Google Scholar]
- 9.Andreev VP, Petyuk VA, Brewer HM, Karpievitch YV, Xie F, Clarke J, Camp D, Smith RD, Lieberman AP, Albin RL, Nawaz Z, El Hokayem J, Myers AJ. (2012).Label-Free Quantitative LC–MS Proteomics of Alzheimer’s Disease and Normally Aged Human Brains. J Proteome Res, 11, 3053–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arbibe L, Mira JP, Teusch N, Kline L, Guha M, Mackman N, Godowski PJ, Ulevitch RJ, Knaus UG. (2000).Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nature immunology, 1, 533–40 [DOI] [PubMed] [Google Scholar]
- 11.Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzi CR. (2007).Copper-free click chemistry for dynamic in vivo imaging. Proc Natl Acad Sci U S A, 104, 16793–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergman JA, Hahne K, Hrycyna CA, Gibbs RA. (2011).Lipid and sulfur substituted prenylcysteine analogs as human Icmt inhibitors. Bioorganic & medicinal chemistry letters, 21, 5616–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergman JA, Hahne K, Song J, Hrycyna CA, Gibbs RA. (2012).S-Farnesyl-Thiopropionic Acid (FTPA) Triazoles as Potent Inhibitors of Isoprenylcysteine Carboxyl Methyltransferase. ACS medicinal chemistry letters, 3, 15–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergo MO, Leung GK, Ambroziak P, Otto JC, Casey PJ, Gomes AQ, Seabra MC, Young SG. (2001).Isoprenylcysteine carboxyl methyltransferase deficiency in mice. J Biol Chem, 276, 5841–5 [DOI] [PubMed] [Google Scholar]
- 15.Bergo MO, Leung GK, Ambroziak P, Otto JC, Casey PJ, Young SG. (2000).Targeted inactivation of the isoprenylcysteine carboxyl methyltransferase gene causes mislocalization of K-Ras in mammalian cells. J Biol Chem, 275, 17605–10 [DOI] [PubMed] [Google Scholar]
- 16.Berndt N, Hamilton AD, Sebti SM. (2011).Targeting protein prenylation for cancer therapy. Nat Rev Cancer, 11, 775–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beytia E, Qureshi AA, Porter JW. (1973).Squalene synthetase. 3. Mechanism of the reaction. J Biol Chem, 248, 1856–67 [PubMed] [Google Scholar]
- 18.Bikhtiyarov YE, Omer CA, Allen CM. (1995).Photoreactive Analogues of Prenyl Diphosphates as Inhibitors and Probes of Human Protein Farnesyltransferase and Geranylgeranyltransferase Type I. J Biol Chem, 270, 19035–40 [DOI] [PubMed] [Google Scholar]
- 19.Bishop P, Rubin P, Thomson AR, Rocca D, Henley JM. (2014).The Ubiquitin C-Terminal Hydrolase L1 (UCH-L1) C Terminus Plays a Key Role in Protein Stability, but Its Farnesylation Is Not Required for Membrane Association in Primary Neurons. J Biol Chem, 289 36140–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blalock EM, Geddes JW, Chen KC, Porter NM, Markesbery WR, Landfield PW. (2004).Incipient Alzheimer’s disease: Microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proceedings of the National Academy of Sciences, 101, 2173–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanden MJ, Suazo KF, Hildebrandt ER, Hardgrove DS, Patel M, Saunders WP, Distefano MD, Schmidt WK, Hougland JL. (2017).Efficient farnesylation of an extended C-terminal C(x)3X sequence motif expands the scope of the prenylated proteome. J Biol Chem, 10.1074/jbc.M117.805770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bloch K (1965).The biological synthesis of cholesterol. Science, 150, 19–28 [DOI] [PubMed] [Google Scholar]
- 23.Boimel M, Grigoriadis N, Lourbopoulos A, Touloumi O, Rosenmann D, Abramsky O, Rosenmann H. (2009).Statins reduce the neurofibrillary tangle burden in a mouse model of tauopathy. Journal of Neuropathology and Experimental Neurology, 68, 314–25 [DOI] [PubMed] [Google Scholar]
- 24.Bond PD, Dolence JM, Poulter CD. (1995).A Continuous Fluorescence Assay for Protein:Prenyl Transferases. Meth Enzymol, 250, 30–43 [DOI] [PubMed] [Google Scholar]
- 25.Boros BD, Greathouse KM, Gentry EG, Curtis KA, Birchall EL, Gearing M, Herskowitz JH. (2017).Dendritic spines provide cognitive resilience against Alzheimer’s disease. Ann Neurol, 82, 602–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyartchuk VL, Ashby MN, Rine J. (1997).Modulation of Ras and a-Factor Function by Carboxyl-Terminal Proteolysis. Science (New York, NY), 275, 1796–800 [DOI] [PubMed] [Google Scholar]
- 27.Brambilla R, Gnesutta N, Minichiello L, Klein R, Sturani E, White G, Chapman PF, Roylance AJ, Herron CE, Grant SGN, Ramsey M, Wolfer DP, Lipp HP, Cestari V, Rossi-Arnaud C. (1997).A role for the Ras signalling pathway in synaptic transmission and long-term memory. Nature, 390, 281–86 [DOI] [PubMed] [Google Scholar]
- 28.Brinkmalm A, Portelius E, Öhrfelt A, Brinkmalm G, Andreasson U, Gobom J, Blennow K, Zetterberg H. (2015).Explorative and targeted neuroproteomics in Alzheimer’s disease. Biochim Biophys Acta, Proteins Proteomics, 1854, 769–78 [DOI] [PubMed] [Google Scholar]
- 29.Briz V, Zhu G, Wang Y, Liu Y, Avetisyan M, Bi X, Baudry M. (2015).Activity-Dependent Rapid Local RhoA Synthesis Is Required for Hippocampal Synaptic Plasticity. Journal of Neuroscience, 35, 2269–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown MS, Goldstein JL. (1980).Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J Lipid Res, 21, 505–17 [PubMed] [Google Scholar]
- 31.Brown MS, Goldstein JL. (1986).A receptor-mediated pathway for cholesterol homeostasis. Science, 232, 34–47 [DOI] [PubMed] [Google Scholar]
- 32.Brown MS, Goldstein JL. (1997).The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell, 89, 331–40 [DOI] [PubMed] [Google Scholar]
- 33.Brown MS, Goldstein JL. (2012).Scientific side trips: six excursions from the beaten path. J Biol Chem, 287, 22418–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown MS, Radhakrishnan A, Goldstein JL. (2017).Retrospective on Cholesterol Homeostasis: The Central Role of Scap. Annu Rev Biochem [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bucher NL, Overath P, Lynen F. (1960).beta-Hydroxy-beta-methyl-glutaryl coenzyme A reductase, cleavage and condensing enzymes in relation to cholesterol formation in rat liver. Biochim Biophys Acta, 40, 491–501 [DOI] [PubMed] [Google Scholar]
- 36.Capell BC, Olive M, Erdos MR, Cao K, Faddah DA, Tavarez UL, Conneely KN, Qu X, San H, Ganesh SK, Chen X, Avallone H, Kolodgie FD, Virmani R, Nabel EG, Collins FS. (2008).A farnesyltransferase inhibitor prevents both the onset and late progression of cardiovascular disease in a progeria mouse model. Proc Natl Acad Sci U S A, 105, 15902–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casey PJ, Solski PA, Der CJ, Buss JE. (1989).p21ras is Modified by a Farnesyl Isoprenoid. Proc Nat Acad Sci USA, 86, 8323–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casey PJ, Thissen JA, Moomaw JF. (1991).Enzymatic modification of proteins with a geranylgeranyl isoprenoid. Proc Nat Acad Sci USA, 88, 8631–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castegna A, Aksenov M, Aksenova M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR, Butterfield DA. (2002).Proteomic identification of oxidatively modified proteins in alzheimer’s disease brain. part I: creatine kinase BB, glutamine synthase, and ubiquitin carboxy-terminal hydrolase L-1. Free Rad Biol Med, 33, 562–71 [DOI] [PubMed] [Google Scholar]
- 40.Cavallini A, Brewerton S, Bell A, Sargent S, Glover S, Hardy C, Moore R, Calley J, Ramachandran D, Poidinger M, Karran E, Davies P, Hutton M, Szekeres P, Bose S. (2013).An unbiased approach to identifying tau kinases that phosphorylate tau at sites associated with alzheimer disease. Journal of Biological Chemistry, 288, 23331–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cenedella RJ. (1998).Prenylation of proteins by the intact lens. Investig Ophthalmol Vis Sci, 39, 1276–80 [PubMed] [Google Scholar]
- 42.Chan LN, Hart C, Guo L, Nyberg T, Davies BSJ, Fong LG, Young SG, Agnew BJ, Tamanoi F. (2009).A novel approach to tag and identify geranylgeranylated proteins. Electrophoresis, 30, 3598–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaput D, Kirouac LH, Bell-Temin H, Stevens SM, Padmanabhan J. (2012).SILAC-based proteomic analysis to investigate the impact of amyloid precursor protein expression in neuronal-like B103 cells. Electrophoresis, 33, 3728–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Charron G, Li MM, MacDonald MR, Hang HC. (2013).Prenylome profiling reveals S-farnesylation is crucial for membrane targeting and antiviral activity of ZAP long-isoform. Proc Natl Acad Sci U S A, 110, 11085–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen X, Makarewicz JM, Knauf JA, Johnson LK, Fagin JA. (2014).Transformation by Hras(G12V) is consistently associated with mutant allele copy gains and is reversed by farnesyl transferase inhibition. Oncogene, 33, 5442–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Y, Ma Y-t, Rando RR. (1996).Solubilization, Partial Purfication, and Affinity Labeling of the Membrane-Bound Isoprenylated Protein Endoprotease. Biochemistry, 35, 3227–37 [DOI] [PubMed] [Google Scholar]
- 47.Chen YX, Koch S, Uhlenbrock K, Weise K, Das D, Gremer L, Brunsveld L, Wittinghofer A, Winter R, Triola G, Waldmann H. (2010).Synthesis of the Rheb and K-Ras4B GTPases. Angewandte Chemie (International ed in English), 49, 6090–5 [DOI] [PubMed] [Google Scholar]
- 48.Cheng S, Cao D, Hottman DA, Yuan L, Bergo MO, Li L. (2013).Farnesyltransferase haplodeficiency reduces neuropathology and rescues cognitive function in a mouse model of Alzheimer disease. J Biol Chem, 288, 35952–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cherfils J, Zeghouf M. (2013).Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev, 93, 269–309 [DOI] [PubMed] [Google Scholar]
- 50.Choi J, Levey AI, Weintraub ST, Rees HD, Gearing M, Chin L-S, Li L. (2004).Oxidative Modifications and Down-regulation of Ubiquitin Carboxyl-terminal Hydrolase L1 Associated with Idiopathic Parkinson’s and Alzheimer’s Diseases. J Biol Chem, 279 13256–64 [DOI] [PubMed] [Google Scholar]
- 51.Cole SL, Grudzien A, Manhart IO, Kelly BL, Oakley H, Vassar R. (2005).Statins cause intracellular accumulation of amyloid precursor protein, beta-secretase-cleaved fragments, and amyloid beta-peptide via an isoprenoid-dependent mechanism. J Biol Chem, 280, 18755–70 [DOI] [PubMed] [Google Scholar]
- 52.Colicelli J (2004).Human RAS superfamily proteins and related GTPases. Science’s STKE : signal transduction knowledge environment, 2004, RE13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Colonna M, Wang Y. (2016).TREM2 variants: new keys to decipher Alzheimer disease pathogenesis. Nat Rev Neurosci, 17, 201–7 [DOI] [PubMed] [Google Scholar]
- 54.Cordle A, Landreth G. (2005).3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors attenuate beta-amyloid-induced microglial inflammatory responses. The Journal of neuroscience : the official journal of the Society for Neuroscience, 25, 299–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Costa RM, Federov NB, Kogan JH, Murphy GG, Stern J, Ohno M, Kucherlapati R, Jacks T, Silva AJ. (2002).Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature, 415, 526–30 [DOI] [PubMed] [Google Scholar]
- 56.Coxon FP, Helfrich MH, Larijani B, Muzylak M, Dunford JE, Marshall D, McKinnon AD, Nesbitt SA, Horton MA, Seabra MC, Ebetino FH, Rogers MJ. (2001).Identification of a novel phosphonocarboxylate inhibitor of Rab geranylgeranyl transferase that specifically prevents Rab prenylation in osteoclasts and macrophages. J Biol Chem, 276, 48213–22 [DOI] [PubMed] [Google Scholar]
- 57.Coxon FP, Helfrich MH, Van’t Hof R, Sebti S, Ralston SH, Hamilton A, Rogers MJ. (2000).Protein geranylgeranylation is required for osteoclast formation, function, and survival: inhibition by bisphosphonates and GGTI-298. J Bone Miner Res, 15, 1467–76 [DOI] [PubMed] [Google Scholar]
- 58.De Angelis DA, Braun PE. (1996).Binding of 2′,3′-Cyclic Nucleotide 3′-Phosphodiesterase to Myelin: An In Vitro Study. J Neurochem, 66, 2523–31 [DOI] [PubMed] [Google Scholar]
- 59.De Schutter JW, Park J, Leung CY, Gormley P, Lin YS, Hu Z, Berghuis AM, Poirier J, Tsantrizos YS. (2014).Multistage screening reveals chameleon ligands of the human farnesyl pyrophosphate synthase: implications to drug discovery for neurodegenerative diseases. J Med Chem, 57, 5764–76 [DOI] [PubMed] [Google Scholar]
- 60.Dechat T, Shimi T, Adam SA, Rusinol AE, Andres DA, Spielmann HP, Sinensky MS, Goldman RD. (2007).Alterations in mitosis and cell cycle progression caused by a mutant lamin A known to accelerate human aging. Proc Natl Acad Sci U S A, 104, 4955–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DeGraw AJ, Palsuledesai C, Ochocki JD, Dozier JK, Lenevich S, Rashidian M, Distefano MD. (2010).Evaluation of alkyne-modified isoprenoids as chemical reporters of protein prenylation. Chem Biol Drug Des, 76, 460–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diaz-Rodriguez V, Hsu ET, Ganusova E, Werst ER, Becker JM, Hrycyna CA, Distefano MD. (2017).a-Factor Analogues Containing Alkyne- and Azide-Functionalized Isoprenoids Are Efficiently Enzymatically Processed and Retain Wild-Type Bioactivity. Bioconjugate chemistry [DOI] [PMC free article] [PubMed]
- 63.Dolence JM, Steward LE, Dolence EK, Wong DH, Poulter CD. (2000).Studies with Recombinant Saccharomyces cerevisiae CaaX Prenyl Protease Rce1p. Biochemistry, 39, 4096–104 [DOI] [PubMed] [Google Scholar]
- 64.Donelson JL, Hodges HB, Macdougall DD, Henriksen BS, Hrycyna CA, Gibbs RA. (2006).Amide-substituted farnesylcysteine analogs as inhibitors of human isoprenylcysteine carboxyl methyltransferase. Bioorganic & medicinal chemistry letters, 16, 4420–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dozier JK, Khatwani SL, Wollack JW, Wang Y-C, Schmidt-Dannert C, Distefano MD. (2014).Engineering Protein Farnesyltransferase for Enzymatic Protein Labeling Applications. Bioconj Chem, 25, 1203–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eckert GP, Hooff GP, Strandjord DM, Igbavboa U, Volmer DA, Muller WE, Wood WG. (2009).Regulation of the brain isoprenoids farnesyl- and geranylgeranylpyrophosphate is altered in male Alzheimer patients. Neurobiol Dis, 35, 251–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Edelstein RL, Distefano MD. (1997).Photoaffinity labeling of yeast farnesyl protein transferase and enzymic synthesis of a Ras protein incorporating a photoactive isoprenoid. Biochem Biophys Res Comm, 235, 377–82 [DOI] [PubMed] [Google Scholar]
- 68.Ehlers MD. (2000).Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron, 28, 511–25 [DOI] [PubMed] [Google Scholar]
- 69.Endo A (1992).The Discovery and Development of Hmg-Coa Reductase Inhibitors. Journal of Lipid Research, 33, 1569–82 [PubMed] [Google Scholar]
- 70.Endo A, Kuroda M, Tanzawa K. (1976).Competitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase by ML-236A and ML-236B fungal metabolites, having hypocholesterolemic activity. FEBS Lett, 72, 323–6 [DOI] [PubMed] [Google Scholar]
- 71.Fassbender K, Simons M, Bergmann C, Stroick M, Lutjohann D, Keller P, Runz H, Kuhl S, Bertsch T, von Bergmann K, Hennerici M, Beyreuther K, Hartmann T. (2001).Simvastatin strongly reduces levels of Alzheimer’s disease -amyloid peptides A 42 and A 40 in vitro and in vivo. Proceedings of the National Academy of Sciences, 98, 5856–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Faust JR, Brown MS, Goldstein JL. (1980).Synthesis of delta 2-isopentenyl tRNA from mevalonate in cultured human fibroblasts. J Biol Chem, 255, 6546–8 [PubMed] [Google Scholar]
- 73.Ferguson JJ, Durr IF, Rudney H. (1959).The Biosynthesis of Mevalonic Acid. Proc Natl Acad Sci U S A, 45, 499–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fisher JE, Rogers MJ, Halasy JM, Luckman SP, Hughes DE, Masarachia PJ, Wesolowski G, Russell RG, Rodan GA, Reszka AA. (1999).Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proc Natl Acad Sci U S A, 96, 133–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fleisch H, Russell RG, Francis MD. (1969).Diphosphonates inhibit hydroxyapatite dissolution in vitro and bone resorption in tissue culture and in vivo. Science, 165, 1262–4 [DOI] [PubMed] [Google Scholar]
- 76.Fleisch H, Russell RG, Simpson B, Muhlbauer RC. (1969).Prevention by a diphosphonate of immobilization “osteoporosis” in rats. Nature, 223, 211–2 [DOI] [PubMed] [Google Scholar]
- 77.Fleisch H, Russell RG, Straumann F. (1966).Effect of pyrophosphate on hydroxyapatite and its implications in calcium homeostasis. Nature, 212, 901–3 [DOI] [PubMed] [Google Scholar]
- 78.Frost B (2016).Alzheimer’s disease: An acquired neurodegenerative laminopathy. Nucleus, 7, 275–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fujimura-Kamada K, Nouvet FJ, Michaelis S. (1997).A novel membrane-associated metalloprotease, Ste24p, is required for the first step of NH2-terminal processing of the yeast a-factor precursor. J Cell Biol, 136, 271–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gao S, Yu R, Zhou X. (2016).The Role of Geranylgeranyltransferase I-Mediated Protein Prenylation in the Brain. Mol Neurobiol, 53, 6925–37 [DOI] [PubMed] [Google Scholar]
- 81.Gaon I, Turek TC, Distefano MD. (1996).Farnesyl and geranylgeranyl pyrophosphate analogs incorporating benzoylbenzyl ethers: synthesis and inhibition of yeast protein farnesyltransferase. Tetrahedron Lett, 37, 8833–36 [Google Scholar]
- 82.Gaon I, Turek TC, Weller VA, Edelstein RL, Singh SK, Distefano MD. (1996).Photoactive Analogs of Farnesyl Pyrophosphate Containing Benzoylbenzoate Esters: Synthesis and Application to Photoaffinity Labeling of Yeast Farnesyltransferase. J Org Chem, 61, 7738–45 [DOI] [PubMed] [Google Scholar]
- 83.Gärtner U, Holzer M, Arendt T. (1999).Elevated expression of p21(ras) is an early event in Alzheimer’s disease and precedes neurofibrillary degeneration. Neuroscience, 91, 1–5 [DOI] [PubMed] [Google Scholar]
- 84.Gartner U, Holzer M, Heumann R, Arendt T. (1995).Induction of p21ras in Alzheimer pathology. Neuroreport, 6, 1441–4 [DOI] [PubMed] [Google Scholar]
- 85.Gelb MH, Brunsveld L, Hrycyna CA, Michaelis S, Tamanoi F, Van Voorhis WC, Waldmann H. (2006).Therapeutic intervention based on protein prenylation and associated modifications. Nat Chem Biol, 2, 518–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gelb MH, Van Voorhis WC, Buckner FS, Yokoyama K, Eastman R, Carpenter EP, Panethymitaki C, Brown KA, Smith DF. (2003).Protein farnesyl and N-myristoyl transferases: piggy-back medicinal chemistry targets for the development of antitrypanosomatid and antimalarial therapeutics. Molecular and biochemical parasitology, 126, 155–63 [DOI] [PubMed] [Google Scholar]
- 87.Gentry EG, Henderson BW, Arrant AE, Gearing M, Feng Y, Riddle NC, Herskowitz JH. (2016).Rho Kinase Inhibition as a Therapeutic for Progressive Supranuclear Palsy and Corticobasal Degeneration. J Neurosci, 36, 1316–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gibbs BS, Zahn TJ, Mu YQ, Sebolt-Leopold JS, Gibbs RA. (1999).Novel farnesol and geranylgeraniol analogues: A potential new class of anticancer agents directed against protein prenylation. Journal of Medicinal Chemistry, 42, 3800–08 [DOI] [PubMed] [Google Scholar]
- 89.Gibbs RA. (2000).Farnesyltransferase inhibitors: novel anticancer mechanisms and new therapeutic applications. Curr Opin Drug Discovery Dev, 3, 585–96 [PubMed] [Google Scholar]
- 90.Ginsberg SD. (2011).Regional selectivity of rab5 and rab7 protein up regulation in mild cognitive impairment and Alzheimer’s disease. Journal of Alzheimer ‘s Disease, 22, 631–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ginsberg SD, Mufson EJ, Alldred MJ, Counts SE, Wuu J, Nixon RA, Che S. (2011).Upregulation of select rab GTPases in cholinergic basal forebrain neurons in mild cognitive impairment and Alzheimer’s disease. Journal of Chemical Neuroanatomy, 42, 102–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gisselberg JE, Zhang L, Elias JE, Yeh E. (2017).The Prenylated Proteome of Plasmodium falciparum Reveals Pathogen-specific Prenylation Activity and Drug Mechanism-of-action. Mol Cell Proteomics, 16, S54–S64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goldstein JL, Brown MS. (1990).Regulation of the mevalonate pathway. Nature, 343, 425–30 [DOI] [PubMed] [Google Scholar]
- 94.Goldstein JL, Brown MS. (2015).A century of cholesterol and coronaries: from plaques to genes to statins. Cell, 161, 161–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gordon LB, Kleinman ME, Massaro J, D’Agostino RB Sr, Shappell H, Gerhard-Herman M, Smoot LB, Gordon CM, Cleveland RH, Nazarian A, Snyder BD, Ullrich NJ, Silvera VM, Liang MG, Quinn N, Miller DT, Huh SY, Dowton AA, Littlefield K, Greer MM, Kieran MW. (2016).Clinical Trial of the Protein Farnesylation Inhibitors Lonafarnib, Pravastatin, and Zoledronic Acid in Children With Hutchinson-Gilford Progeria Syndrome. Circulation, 134, 114–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gordon LB, Kleinman ME, Miller DT, Neuberg DS, Giobbie-Hurder A, Gerhard-Herman M, Smoot LB, Gordon CM, Cleveland R, Snyder BD, Fligor B, Bishop WR, Statkevich P, Regen A, Sonis A, Riley S, Ploski C, Correia A, Quinn N, Ullrich NJ, Nazarian A, Liang MG, Huh SY, Schwartzman A, Kieran MW. (2012).Clinical trial of a farnesyltransferase inhibitor in children with Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A, 109, 16666–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gordon LB, Massaro J, D’Agostino RB Sr., Campbell SE, Brazier J, Brown WT, Kleinman ME, Kieran MW, Progeria Clinical Trials C. (2014).Impact of farnesylation inhibitors on survival in Hutchinson-Gilford progeria syndrome. Circulation, 130, 27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Griffin JM, Kho D, Graham ES, Nicholson LFB, O’Carroll SJ. (2016).Statins inhibit fibrillary β-amyloid induced inflammation in a model of the human blood brain barrier. PLoS ONE, 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gritzalis D, Park J, Chiu W, Cho H, Lin YS, De Schutter JW, Lacbay CM, Zielinski M, Berghuis AM, Tsantrizos YS. (2015).Probing the molecular and structural elements of ligands binding to the active site versus an allosteric pocket of the human farnesyl pyrophosphate synthase. Bioorganic & medicinal chemistry letters, 25, 1117–23 [DOI] [PubMed] [Google Scholar]
- 100.Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert JC, Amouyel P, Goate A, Rademakers R, Morgan K, Powell J, St George-Hyslop P, Singleton A, Hardy J, Alzheimer Genetic Analysis G. (2013).TREM2 variants in Alzheimer’s disease. N Engl J Med, 368, 117–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guo Z, Wu YW, Das D, Delon C, Cramer J, Yu S, Thuns S, Lupilova N, Waldmann H, Brunsveld L, Goody RS, Alexandrov K, Blankenfeldt W. (2008).Structures of RabGGTase-substrate/product complexes provide insights into the evolution of protein prenylation. EMBO J, 27, 2444–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Haag MDM, Hofman A, Koudstaal PJ, Stricker BHC, Breteler MMB. (2009).Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity. The Rotterdam Study. Journal of neurology, neurosurgery, and psychiatry, 80, 13–7 [DOI] [PubMed] [Google Scholar]
- 103.Haditsch U, Leone DP, Farinelli M, Chrostek-Grashoff A, Brakebusch C, Mansuy IM, McConnell SK, Palmer TD. (2009).A central role for the small GTPase Rac1 in hippocampal plasticity and spatial learning and memory. Molecular and Cellular Neuroscience, 41, 409–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hahne K, Vervacke JS, Shrestha L, Donelson JL, Gibbs RA, Distefano MD, Hrycyna CA. (2012).Evaluation of Substrate and Inhibitor Binding to Yeast and Human Isoprenylcysteine Carboxyl Methyltransferases (Icmts) using Biotinylated Benzophenone-containing Photoaffinity Probes. Biochem Biophys Res Comm, 423, 98–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hall RH. (1971).The Modified Nucleotides in Nucleic Acids. New York, Columbia University Press [Google Scholar]
- 106.Hamano T, Yen SH, Gendron T, Ko Lw, Kuriyama M. (2012).Pitavastatin decreases tau levels via the inactivation of Rho/ROCK. Neurobiology of Aging, 33, 2306–20 [DOI] [PubMed] [Google Scholar]
- 107.Hancock JF. (2003).Ras proteins: different signals from different locations. Nat Rev Mol Cell Biol, 4, 373–84 [DOI] [PubMed] [Google Scholar]
- 108.Hancock JF, Magee AI, Childs JE, Marshall CJ. (1989).All ras proteins are polyisoprenylated but only some are palmitoylated. Cell, 57, 1167–77 [DOI] [PubMed] [Google Scholar]
- 109.Hast MA, Nichols CB, Armstrong SM, Kelly SM, Hellinga HW, Alspaugh JA, Beese LS. (2011).Structures of Cryptococcus neoformans protein farnesyltransferase reveal strategies for developing inhibitors that target fungal pathogens. J Biol Chem, 286, 35149–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Henderson BW, Gentry EG, Rush T, Troncoso JC, Thambisetty M, Montine TJ, Herskowitz JH. (2016).Rho-associated protein kinase 1 (ROCK1) is increased in Alzheimer’s disease and ROCK1 depletion reduces amyloid-beta levels in brain. J Neurochem, 138, 525–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Heneka MT, Carson MJ, Khoury JE, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA, Breitner JC, Cole GM, Golenbock DT, Kummer MP. (2015). Neuroinflammation in Alzheimer’s diseaseed.^eds., 388–405 [DOI] [PMC free article] [PubMed]
- 112.Hennessy E, Adams C, Reen FJ, O’Gara F. (2016).Is There Potential for Repurposing Statins as Novel Antimicrobials? Antimicrob Agents Ch, 60, 5111–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Herskowitz JH, Feng Y, Mattheyses AL, Hales CM, Higginbotham LA, Duong DM, Montine TJ, Troncoso JC, Thambisetty M, Seyfried NT, Levey AI, Lah JJ. (2013).Pharmacologic Inhibition of ROCK2 Suppresses Amyloid- Production in an Alzheimer’s Disease Mouse Model. Journal of Neuroscience, 33, 19086–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Herskowitz JH, Seyfried NT, Gearing M, Kahn RA, Peng J, Levey AI, Lah JJ. (2011).Rho kinase II phosphorylation of the lipoprotein receptor LR11/SORLA alters amyloid-beta production. J Biol Chem, 286, 6117–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hildebrandt ER, Cheng M, Zhao P, Kim JH, Wells L, Schmidt WK. (2016).A shunt pathway limits the CaaX processing of Hsp40 Ydj1p and regulates Ydj1p-dependent phenotypes. eLife, 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hollander I, Frommer E, Mallon R. (2000).Human ras-converting enzyme (hRCE1) endoproteolytic activity on K-ras-derived peptides. Anal Biochem, 286, 129–37 [DOI] [PubMed] [Google Scholar]
- 117.Holstein SA, Hohl RJ. (2004).Isoprenoids: remarkable diversity of form and function. Lipids, 39, 293–309 [DOI] [PubMed] [Google Scholar]
- 118.Hooff GP, Peters I, Wood WG, Müller WE, Eckert GP. (2010).Modulation of cholesterol, farnesylpyrophosphate, and geranylgeranylpyrophosphate in neuroblastoma SH-SY5Y-APP695 cells: Impact on amyloid beta-protein production. Molecular Neurobiology, 41, 341–50 [DOI] [PubMed] [Google Scholar]
- 119.Hooff GP, Volmer DA, Wood WG, Muller WE, Eckert GP. (2008).Isoprenoid quantitation in human brain tissue: a validated HPLC-fluorescence detection method for endogenous farnesyl- (FPP) and geranylgeranylpyrophosphate (GGPP). Analytical and bioanalytical chemistry, 392, 673–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hooper C, Killick R, Lovestone S. (2008). The GSK3 hypothesis of Alzheimer’s diseaseed.^eds., 1433–39 [DOI] [PMC free article] [PubMed]
- 121.Hosaka A, Araki W, Oda A, Tomidokoro Y, Tamaoka A. (2013).Statins reduce amyloid β-peptide production by modulating amyloid precursor protein maturation and phosphorylation through a cholesterol- independent mechanism in cultured neurons. Neurochemical Research, 38, 589–600 [DOI] [PubMed] [Google Scholar]
- 122.Hosokawa A, Wollack JW, Zhang Z, Chen L, Barany G, Distefano MD. (2007).Evaluation of an alkyne-containing analogue of farnesyl diphosphate as a dual substrate for protein-prenyltransferases. Int J Peptide Res, 13, 345–54 [Google Scholar]
- 123.Hottman D, Cheng S, Gram A, LeBlanc K, Yuan LL, Li L. (2018).Systemic or Forebrain Neuron-Specific Deficiency of Geranylgeranyltransferase-1 Impairs Synaptic Plasticity and Reduces Dendritic Spine Density. Neuroscience, 373, 207–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hottman DA, Li L. (2014).Protein Prenylation and Synaptic Plasticity: Implications for Alzheimer???s Disease. Molecular Neurobiology, 50, 177–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hougland JL, Hicks KA, Hartman HL, Kelly RA, Watt TJ, Fierke CA. (2010).Identification of novel peptide substrates for protein farnesyltransferase reveals two substrate classes with distinct sequence selectivities. J Mol Biol, 395, 176–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hrycyna CA, Clarke S. (1992).Maturation of isoprenylated proteins in Saccharomyces cerevisiae. Multiple activities catalyze the cleavage of the three carboxyl-terminal amino acids from farnesylated substrates in vitro. The Journal of biological chemistry, 267, 10457–64 [PubMed] [Google Scholar]
- 127.Hrycyna CA, Sapperstein SK, Clarke S, Michaelis S. (1991).The Saccharomyces cervisiae STE14 Gene Encodes a Methyltransferase that Mediates C-terminal Methylation of a-Factor and RAS Proteins. EMBO J, 10, 1699–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Iannelli F, Lombardi R, Milone MR, Pucci B, De Rienzo S, Budillon A, Bruzzese F. (2017).Targeting Mevalonate Pathway in Cancer Treatment: Repurposing of Statins. Recent patents on anti-cancer drug discovery [DOI] [PubMed]
- 129.Istvan ES, Deisenhofer J. (2001).Structural mechanism for statin inhibition of HMG-CoA reductase. Science, 292, 1160–4 [DOI] [PubMed] [Google Scholar]
- 130.Jahnke W, Rondeau JM, Cotesta S, Marzinzik A, Pelle X, Geiser M, Strauss A, Gotte M, Bitsch F, Hemmig R, Henry C, Lehmann S, Glickman JF, Roddy TP, Stout SJ, Green JR. (2010).Allosteric non-bisphosphonate FPPS inhibitors identified by fragment-based discovery. Nat Chem Biol, 6, 660–6 [DOI] [PubMed] [Google Scholar]
- 131.Jay TR, von Saucken VE, Landreth GE. (2017).TREM2 in Neurodegenerative Diseases. Mol Neurodegener, 12, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. (2000).Statins and the risk of dementia. Lancet, 356, 1627–31. [DOI] [PubMed] [Google Scholar]
- 133.Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, Rujescu D, Hampel H, Giegling I, Andreassen OA, Engedal K, Ulstein I, Djurovic S, Ibrahim-Verbaas C, Hofman A, Ikram MA, van Duijn CM, Thorsteinsdottir U, Kong A, Stefansson K. (2013).Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med, 368, 107–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kale TA, Distefano MD. (2003).Diazotrifluoropropionamido-Containing Prenylcysteines: Syntheses and Applications for Studying Isoprenoid-Protein Interactions. Org Lett, 5, 609–12 [DOI] [PubMed] [Google Scholar]
- 135.Kale TA, Raab C, Yu N, Dean DC, Distefano MD. (2001).A Photoactivatable Prenylated Cysteine Designed to Study Isoprenoid Recognition. J Am Chem Soc, 123, 4373–81 [DOI] [PubMed] [Google Scholar]
- 136.Kamiya Y, Sakurai A, Tamura S, Takahashi N, Tsuchiya E, Abe K, Fukui S. (1979).Structure of Rhodotorucine A, a Peptidyl Factor, Inducing Mating Tube Formation in Rhodospotidium toruloides. Agric Biol Chem, 43, 363–69 [Google Scholar]
- 137.Kandutsch AA, Russell AE. (1960).Preputial gland tumor sterols. 3. A metabolic pathway from lanosterol to cholesterol. J Biol Chem, 235, 2256–61 [PubMed] [Google Scholar]
- 138.Kazi A, Carie A, Blaskovich MA, Bucher C, Thai V, Moulder S, Peng H, Carrico D, Pusateri E, Pledger WJ, Berndt N, Hamilton A, Sebti SM. (2009).Blockade of Protein Geranylgeranylation Inhibits Cdk2-Dependent p27(Kip1) Phosphorylation on Thr187 and Accumulates p27(Kip1) in the Nucleus: Implications for Breast Cancer Therapy. Mol Cell Biol, 29, 2254–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kho Y, Kim SC, Jiang C, Barma D, Kwon SW, Cheng J, Jaunbergs J, Weinbaum C, Tamanoi F, Falck J, Zhao Y. (2004).A tagging-via-substrate technology for detection and proteomics of farnesylated proteins. Proc Natl Acad Sci USA, 101, 12479–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kimberly F Tolias JGDKU. (2011).Control of synapse development and plasticity by RhoGTPase regulatory proteins. Prog Neurobiol, 94, 133–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kinsella BT, Maltese WA. (1992).rab GTP-binding Proteins with Three Different Carboxyl-terminal Cysteine Motifs are Modified in Vivo by 20-Carbon Isoprenoids. J Biol Chem, 267, 3940–45 [PubMed] [Google Scholar]
- 142.Kirouac L, Rajic AJ, Cribbs DH, Padmanabhan J. (2017).Activation of Ras-ERK Signaling and GSK-3 by Amyloid Precursor Protein and Amyloid Beta Facilitates Neurodegeneration in Alzheimer’s Disease. Eneuro, 4, ENEURO.0149–16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kohl NE, Omer CA, Conner MW, Anthony NJ, Davide JP, deSolms SJ, Giuliani EA, Gomez RP, Graham SL, Hamilton K, et al. (1995).Inhibition of farnesyltransferase induces regression of mammary and salivary carcinomas in ras transgenic mice. Nat Med, 1, 792–7 [DOI] [PubMed] [Google Scholar]
- 144.Korolainen MA, Nyman TA, Aittokallio T, Pirttilä T. (2010).An update on clinical proteomics in Alzheimer’s research. J Neurochem, 112, 1386–414 [DOI] [PubMed] [Google Scholar]
- 145.Krzysiak AJ, Aditya AV, Hougland JL, Fierke CA, Gibbs RA. (2010).Synthesis and screening of a CaaL peptide library versus FTase reveals a surprising number of substrates. Bioorganic & medicinal chemistry letters, 20, 767–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Krzysiak AJ, Scott SA, Hicks KA, Fierke CA, Gibbs RA. (2007).Evaluation of protein farnesyltransferase substrate specificity using synthetic peptide libraries. Bioorg Med Chem Lett, 17, 5548–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kuang H, Brown ML, Davies RR, Young EC, Distefano MD. (1996).Enantioselective Reductive Amination of a-Keto Acids to a-Amino Acids by a Pyridoxamine Cofactor in a Protein Cavity. Journal of the American Chemical Society, 118, 10702–06 [Google Scholar]
- 148.Kukar T, Murphy MP, Eriksen JL, Sagi SA, Weggen S, Smith TE, Ladd T, Khan MA, Kache R, Beard J, Dodson M, Merit S, Ozols VV, Anastasiadis PZ, Das P, Fauq A, Koo EH, Golde TE. (2005).Diverse compounds mimic Alzheimer disease-causing mutations by augmenting A??42 production. Nature Medicine, 11, 545–50 [DOI] [PubMed] [Google Scholar]
- 149.Kurata T, Miyazaki K, Kozuki M, Morimoto N, Ohta Y, Ikeda Y, Abe K. (2012).Atorvastatin and pitavastatin reduce senile plaques and inflammatory responses in a mouse model of Alzheimer’s disease. Neurological Research, 34, 601–10 [DOI] [PubMed] [Google Scholar]
- 150.Laezza C, Wolff J, Bifulco M. (1997).Identification of a 48-kDa prenylated protein that associates with microtubules as 2′,3′-cyclic nucleotide 3′-phosphodiesterase in FRTL-5 cells. FEBS Lett, 413, 260–64 [DOI] [PubMed] [Google Scholar]
- 151.Lambert J-C. (2013).Meta-Analysis of 74,046 Individuals Identifies 11 New Susceptibility Loci for Alzheimer’s Disease. Nature Genetics, 45, 1452–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Landreth GE, Reed-Geaghan EG. (2009).Toll-like receptors in Alzheimer’s disease. Curr Top Microbiol Immunol, 336, 137–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lane KT, Beese LS. (2006).Thematic review series: lipid posttranslational modifications. Structural biology of protein farnesyltransferase and geranylgeranyltransferase type I. J Lipid Res, 47, 681–99 [DOI] [PubMed] [Google Scholar]
- 154.Lee MS, Kao SC, Lemere CA, Xia W, Tseng HC, Zhou Y, Neve R, Ahlijanian MK, Tsai LH. (2003).APP processing is regulated by cytoplasmic phosphorylation. Journal of Cell Biology, 163, 83–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Leonard DM. (1997).Ras Farnesyltransferase: A New Therapeutic Target. J Med Chem, 40, 2971–90 [DOI] [PubMed] [Google Scholar]
- 156.Lerner EC, Qian Y, Blaskovich MA, Fossum RD, Vogt A, Sun J, Cox AD, Der CJ, Hamilton AD, Sebti SM. (1995).Ras CAAX Peptidomimetic FTI-277 Selectively Blocks Oncogenic Ras Signaling by Inducing Cytoplasmic Accumulation of Inactive Ras-Raf Complexes. J Biol Chem, 270, 26802–06 [DOI] [PubMed] [Google Scholar]
- 157.Leuchtenberger S, Kummer MP, Kukar T, Czirr E, Teusch N, Sagi SA, Berdeaux R, Pietrzik CU, Ladd TB, Golde TE, Koo EH, Weggen S. (2006).Inhibitors of Rho-kinase modulate amyloid-β (Aβ) secretion but lack selectivity for Aβ42. Journal of Neurochemistry, 96, 355–65 [DOI] [PubMed] [Google Scholar]
- 158.Leung KF, Baron R, Ali BR, Magee AI, Seabra MC. (2007).Rab GTPases containing a CAAX motif are processed post-geranylgeranylation by proteolysis and methylation. J Biol Chem, 282, 1487–97 [DOI] [PubMed] [Google Scholar]
- 159.Li H, Kuwajima T, Oakley D, Nikulina E, Hou J, Yang WS, Lowry ER, Lamas NJ, Amoroso MW, Croft GF, Hosur R, Wichterle H, Sebti S, Filbin MT, Stockwell B, Henderson CE. (2016).Protein Prenylation Constitutes an Endogenous Brake on Axonal Growth. Cell reports, 16, 545–58 [DOI] [PubMed] [Google Scholar]
- 160.Li L, Cao D, Kim H, Lester R, Fukuchi K. (2006).Simvastatin enhances learning and memory independent of amyloid load in mice. Ann Neurol, 60, 729–39 [DOI] [PubMed] [Google Scholar]
- 161.Li L, Zhang W, Cheng S, Cao D, Parent M. (2012).Isoprenoids and related pharmacological interventions: potential application in Alzheimer’s disease. Mol Neurobiol, 46, 64–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liao JK, Laufs U. (2009).Pleiotropic Effects of Statins. Annual Reviews of Pharmacology and Toxicology, 45, 89–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu S, Liu Y, Hao W, Wolf L, Kiliaan AJ, Penke B, Rube CE, Walter J, Heneka MT, Hartmann T, Menger MD, Fassbender K. (2012).TLR2 Is a Primary Receptor for Alzheimer’s Amyloid Peptide To Trigger Neuroinflammatory Activation. The Journal of Immunology, 188, 1098–107 [DOI] [PubMed] [Google Scholar]
- 164.Liu Z, Meray RK, Grammatopoulos TN, Fredenburg RA, Cookson MR, Liu Y, Logan T, Lansbury PT. (2009).Membrane-associated farnesylated UCH-L1 promotes α-synuclein neurotoxicity and is a therapeutic target for Parkinson’s disease. Proc Natl Acad Sci USA, 106, 4635–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lobell RB, Liu D, Buser CA, Davide JP, DePuy E, Hamilton K, Koblan KS, Lee Y, Mosser S, Motzel SL, Abbruzzese JL, Fuchs CS, Rowinsky EK, Rubin EH, Sharma S, Deutsch PJ, Mazina KE, Morrison BW, Wildonger L, Yao SL, Kohl NE. (2002).Preclinical and clinical pharmacodynamic assessment of L-778,123, a dual inhibitor of farnesyl:protein transferase and geranylgeranyl:protein transferase type-I. Mol Cancer Ther, 1, 747–58 [PubMed] [Google Scholar]
- 166.London N, Lamphear CL, Hougland JL, Fierke CA, Schueler-Furman O. (2011).Identification of a novel class of farnesylation targets by structure-based modeling of binding specificity. PLoS Comput Biol, 7, e1002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Luckman SP, Coxon FP, Ebetino FH, Russell RG, Rogers MJ. (1998).Heterocycle-containing bisphosphonates cause apoptosis and inhibit bone resorption by preventing protein prenylation: evidence from structure-activity relationships in J774 macrophages. J Bone Miner Res, 13, 1668–78 [DOI] [PubMed] [Google Scholar]
- 168.Lynen F (1964). The pathway from “activated acetic acid” to the terpenes and fatty acidsed.^eds. Nobel Lecture, Nobelprize.org, https://www.nobelprize.org/nobel_prizes/medicine/laureates/1964/lynen-lecture.html
- 169.Manne V, Yan N, Carboni JM, Tuomai AV, Ricca CS, Brown JG, Andahazy ML, Schmidt RJ, Patel D, et al. (1995).Bisubstrate inhibitors of farnesyltransferase: a novel class of specific inhibitors of ras transformed cells. Oncogene, 10, 1763–79 [PubMed] [Google Scholar]
- 170.Mans RA, Chowdhury N, Cao D, McMahon LL, Li L. (2010).Simvastatin enhances hippocampal long-term potentiation in C57BL/6 mice. Neuroscience, 166, 435–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mans RA, McMahon LL, Li L. (2012).Simvastatin-mediated enhancement of long-term potentiation is driven by farnesyl-pyrophosphate depletion and inhibition of farnesylation. Neuroscience, 202, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Maraka S, Kennel KA. (2015).Bisphosphonates for the prevention and treatment of osteoporosis. Bmj, 351, h3783. [DOI] [PubMed] [Google Scholar]
- 173.Marzinzik AL, Amstutz R, Bold G, Bourgier E, Cotesta S, Glickman JF, Gotte M, Henry C, Lehmann S, Hartwieg JC, Ofner S, Pelle X, Roddy TP, Rondeau JM, Stauffer F, Stout SJ, Widmer A, Zimmermann J, Zoller T, Jahnke W. (2015).Discovery of Novel Allosteric Non-Bisphosphonate Inhibitors of Farnesyl Pyrophosphate Synthase by Integrated Lead Finding. ChemMedChem, 10, 1884–91 [DOI] [PubMed] [Google Scholar]
- 174.Maurer-Stroh S, Koranda M, Benetka W, Schneider G, Sirota FL, Eisenhaber F. (2007).Towards complete sets of farnesylated and geranylgeranylated proteins. PLoS Comput Biol, 3, e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.McTaggart SJ. (2006).Isoprenylated proteins. Cell Mol Life Sci, 63, 255–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Meske V, Albert F, Richter D, Schwarze J, Ohm TG. (2003).Blockade of HMG-CoA reductase activity causes changes in microtubule-stabilizing protein tau via suppression of geranylgeranylpyrophosphate formation: Implications for Alzheimer’s disease. European Journal of Neuroscience, 17, 93–102 [DOI] [PubMed] [Google Scholar]
- 177.Métais C, Brennan K, Mably AJ, Scott M, Walsh DM, Herron CE. (2014).Simvastatin treatment preserves synaptic plasticity in AβPPswe/PS1dE9 mice. Journal of Alzheimer’s Disease, 39, 315–29 [DOI] [PubMed] [Google Scholar]
- 178.Michaelson D, Ali W, Chiu VK, Bergo M, Silletti J, Wright L, Young SG, Philips M. (2005).Postprenylation CAAX processing is required for proper localization of Ras but not Rho GTPases. Mol Biol Cell, 16, 1606–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Misra J, Mohanty ST, Madan S, Fernandes JA, Hal Ebetino F, Russell RG, Bellantuono I. (2016).Zoledronate Attenuates Accumulation of DNA Damage in Mesenchymal Stem Cells and Protects Their Function. Stem cells, 34, 756–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mohammed I, Hampton SE, Ashall L, Hildebrandt ER, Kutlik RA, Manandhar SP, Floyd BJ, Smith HE, Dozier JK, Distefano MD, Schmidt WK, Dore TM. (2016).8-Hydroxyquinoline-based inhibitors of the Rce1 protease disrupt Ras membrane localization in human cells. Bioorg Med Chem, 24, 160–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Monkkonen H, Auriola S, Lehenkari P, Kellinsalmi M, Hassinen IE, Vepsalainen J, Monkkonen J. (2006).A new endogenous ATP analog (ApppI) inhibits the mitochondrial adenine nucleotide translocase (ANT) and is responsible for the apoptosis induced by nitrogen-containing bisphosphonates. Br J Pharmacol, 147, 437–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Morgillo F, Lee HY. (2006).Lonafarnib in cancer therapy. Expert Opin Investig Drugs, 15, 709–19 [DOI] [PubMed] [Google Scholar]
- 183.Moutinho M, Nunes MJ, Rodrigues E. (2017).The mevalonate pathway in neurons: It’s not just about cholesterol. Exp Cell Res, 360, 55–60 [DOI] [PubMed] [Google Scholar]
- 184.Moya-Alvarado G, Gershoni-Emek N, Perlson E, Bronfman FC (2016).Neurodegeneration and Alzheimer’s disease (AD). What Can Proteomics Tell Us About the Alzheimer’s Brain? Mol Cell Proteomics, 15, 409–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Musunuri S, Wetterhall M, Ingelsson M, Lannfelt L, Artemenko K, Bergquist J, Kultima K, Shevchenko G. (2014).Quantification of the Brain Proteome in Alzheimer’s Disease Using Multiplexed Mass Spectrometry. J Proteome Res, 13, 2056–68 [DOI] [PubMed] [Google Scholar]
- 186.Nguyen UTT, Cramer J, Gomis J, Reents R, Gutierrez-Rodriguez M, Goody RS, Alexandrov K, Waldmann H. (2007).Exploiting the substrate tolerance of farnesyltransferase for site-selective protein derivatization. ChemBioChem, 8, 408–23 [DOI] [PubMed] [Google Scholar]
- 187.Nguyen UTT, Guo Z, Delon C, Wu Y, Deraeve C, Fraenzel B, Bon RS, Blankenfeldt W, Goody RS, Waldmann H, Wolters D, Alexandrov K. (2009).Analysis of the eukaryotic prenylome by isoprenoid affinity tagging. Nat Chem Biol 5, 227–35 [DOI] [PubMed] [Google Scholar]
- 188.Nhan HS, Chiang K, Koo EH. (2015).The multifaceted nature of amyloid precursor protein and its proteolytic fragments: friends and foes. Acta Neuropathol, 129, 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nishimura A, Linder ME. (2013).Identification of a novel prenyl and palmitoyl modification at the CaaX motif of Cdc42 that regulates RhoGDI binding. Mol Cell Biol, 33, 1417–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nishimura S, Mishra-Gorur K, Park J, Surovtseva YV, Sebti SM, Levchenko A, Louvi A, Gunel M. (2017).Combined HMG-COA reductase and prenylation inhibition in treatment of CCM. Proc Natl Acad Sci U S A, 114, 5503–08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nussinov R, Tsai CJ, Jang H. (2017).Oncogenic Ras Isoforms Signaling Specificity at the Membrane. Cancer Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ochocki JD, Distefano MD. (2013).Prenyltransferase inhibitors: treating human ailments from cancer to parasitic infections. Med Chem Commun, 4, 476–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ochocki JD, Wattenberg EV, Distefano MD. (2010).Enlarging the scope of cell penetrating prenylated peptides to include farnesylated “CAAX” box sequences and diverse cell types. Chem Biol Drug Des, 76, 107–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Oesterle A, Laufs U, Liao JK. (2017).Pleiotropic Effects of Statins on the Cardiovascular System. Circ Res, 120, 229–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Offe K, Dodson SE, Shoemaker JT, Fritz JJ, Gearing M, Levey AI, Lah JJ. (2006).The lipoprotein receptor LR11 regulates amyloid beta production and amyloid precursor protein traffic in endosomal compartments. J Neurosci, 26, 1596–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ohashi K, Nagata K, Maekawa M, Ishizaki T, Narumiya S, Mizuno K. (2000).Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. Journal of Biological Chemistry, 275, 3577–82 [DOI] [PubMed] [Google Scholar]
- 197.Onono FO, Morgan MA, Spielmann HP, Andres DA, Subramanian T, Ganser A, Reuter CWM. (2010).A tagging-via-substrate approach to detect the farnesylated proteome using two-dimensional electrophoresis coupled with Western blotting. Mol Cell Proteomics 9, 742–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ostrowski SM, Johnson K, Siefert M, Shank S, Sironi L, Wolozin B, Landreth GE, Ziady AG. (2016).Simvastatin inhibits protein isoprenylation in the brain. Neuroscience, 329, 264–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ostrowski SM, Wilkinson BL, Golde TE, Landreth G. (2007).Statins reduce amyloid-beta production through inhibition of protein isoprenylation. J Biol Chem, 282, 26832–44 [DOI] [PubMed] [Google Scholar]
- 200.Palsuledesai CC, Ochocki JD, Kuhns MM, Wang Y-C, Warmka JK, Chernick DS, Wattenberg EV, Li L, Arriaga EA, Distefano MD. (2016).Metabolic Labeling with an Alkyne-modified Isoprenoid Analog Facilitates Imaging and Quantification of the Prenylome in Cells. ACS Chem Biol, 11, 2820–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Palsuledesai CC, Ochocki JD, Markowski TW, Distefano MD. (2014).A combination of metabolic labeling and 2D-DIGE analysis in response to a farnesyltransferase inhibitor facilitates the discovery of new prenylated proteins. Mol Biosys, 10, 1094–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Park J, Zielinski M, Magder A, Tsantrizos YS, Berghuis AM. (2017).Human farnesyl pyrophosphate synthase is allosterically inhibited by its own product. Nature communications, 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Parsons RB, Price GC, Farrant JK, Subramaniam D, Adeagbo-Sheikh J, Austen BM. (2006).Statins inhibit the dimerization of beta-secretase via both isoprenoid- and cholesterol-mediated mechanisms. The Biochemical journal, 399, 205–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Patel DV, Young MG, Robinson SP, Hunihan L, Dean BJ, Gordon EM. (1996).Hydroxamic Acid-Based Bisubstrate Analog Inhibitors of Ras Farnesyl Protein Transferase. J Med Chem, 39, 4197–210 [DOI] [PubMed] [Google Scholar]
- 205.Pedrini S, Carter TL, Prendergast G, Petanceska S, Ehrlich ME, Gandy S. (2005).Modulation of statin-activated shedding of Alzheimer APP ectodomain by ROCK. PLoS Medicine, 2, 0069–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Pelleieux S, Picard C, Lamarre-Theroux L, Dea D, Leduc V, Tsantrizos YS, Poirier J. (2018).Isoprenoids and tau pathology in sporadic Alzheimer’s disease. Neurobiol Aging, 65, 132–39 [DOI] [PubMed] [Google Scholar]
- 207.Pereira-Leal JB, Seabra MC. (2000).The mammalian Rab family of small GTPases: definition of family and subfamily sequence motifs suggests a mechanism for functional specificity in the Ras superfamily11Edited by M. Yaniv. J Mol Bio, 301, 1077–87 [DOI] [PubMed] [Google Scholar]
- 208.Petanceska SS, Derosa S, Olm V, Diaz N, Sharma A, Thomas-bryant T, Duff K, Pappolla M, Refolo LM. (2002).Statin Therapy for Alzheimer ‘ s Disease. Journal of Molecular Neuroscience, 19, 155–61 [DOI] [PubMed] [Google Scholar]
- 209.Pompliano DL, Gomez RP, Anthony NJ. (1992).Intramolecular Fluorescence Enhancement: A Continuous Assay of Ras Farnesyl:Protein Transferase. J Am Chem Soc, 114, 7945–46 [Google Scholar]
- 210.Pompliano DL, Rands E, Schaber MD, Mosser SD, Anthony NJ, Gibbs JB. (1992).Steady-State Kinetic Mechanism of Ras Farnesyl:Protein Transferase. Biochemistry, 31, 3800–07 [DOI] [PubMed] [Google Scholar]
- 211.Porter SB, Hildebrandt ER, Breevoort SR, Mokry DZ, Dore TM, Schmidt WK. (2007).Inhibition of the CaaX proteases Rce1p and Ste24p by peptidyl (acyloxy)methyl ketones. Biochim Biophys Acta, 1773, 853–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Poulter CD, Rilling HC. (1978).Prenyl Transfer-Reaction - Enzymatic and Mechanistic Studies of 1’−4 Coupling Reaction in Terpene Biosynthetic-Pathway. Accounts Chem Res, 11, 307–13 [Google Scholar]
- 213.Poulter CD, Satterwhite DM. (1977).Mechanism of Prenyl-Transfer Reaction - Studies with (E)-3-Trifluoromethyl-2-Buten-1-Yl and (Z)-3-Trifluoromethyl-2-Buten-1-Yl Pyrophosphate. Biochemistry, 16, 5470–78 [DOI] [PubMed] [Google Scholar]
- 214.Prior IA, Hancock JF. (2012).Ras trafficking, localization and compartmentalized signalling. Semin Cell Dev Biol, 23, 145–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Quigley A, Dong YY, Pike AC, Dong L, Shrestha L, Berridge G, Stansfeld PJ, Sansom MS, Edwards AM, Bountra C, von Delft F, Bullock AN, Burgess-Brown NA, Carpenter EP. (2013).The structural basis of ZMPSTE24-dependent laminopathies. Science (New York, NY), 339, 1604–7 [DOI] [PubMed] [Google Scholar]
- 216.Reddy S, Comai L. (2012). Lamin A, farnesylation and aginged.^eds., 1–7 [DOI] [PMC free article] [PubMed]
- 217.Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. (2009).CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. J Neurosci, 29, 11982–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Reid TS, Terry KL, Casey PJ, Beese LS. (2004).Crystallographic analysis of CaaX prenyltransferases complexed with substrates defines rules of protein substrate selectivity. J Mol Biol, 343, 417–33 [DOI] [PubMed] [Google Scholar]
- 219.Reiss Y, Stradley SJ, Gierasch LM, Brown MS, Goldstein JL. (1991).Sequence Requirement for Peptide Recognition by the Rat Brain p21ras Protein Farnesyltransferase. Procedings of the National Academy of Sciences USA, 88, 732–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Richardson K, Schoen M, French B, Umscheid CA, Mitchell MD, Arnold SE, Heidenreich PA, Rader DJ, deGoma EM. (2013).Statins and cognitive function: a systematic review. Annals of internal medicine, 159, 688–97 [DOI] [PubMed] [Google Scholar]
- 221.Ridge PG, Karch CM, Hsu S, Arano I, Teerlink CC, Ebbert MTW, Gonzalez Murcia JD, Farnham JM, Damato AR, Allen M, Wang X, Harari O, Fernandez VM, Guerreiro R, Bras J, Hardy J, Munger R, Norton M, Sassi C, Singleton A, Younkin SG, Dickson DW, Golde TE, Price ND, Ertekin-Taner N, Cruchaga C, Goate AM, Corcoran C, Tschanz J, Cannon-Albright LA, Kauwe JSK. (2017).Linkage, whole genome sequence, and biological data implicate variants in RAB10 in Alzheimer’s disease resilience. Genome Medicine, 9, 100–00 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Roelofs AJ, Thompson K, Ebetino FH, Rogers MJ, Coxon FP. (2010).Bisphosphonates: molecular mechanisms of action and effects on bone cells, monocytes and macrophages. Curr Pharm Des, 16, 2950–60 [DOI] [PubMed] [Google Scholar]
- 223.Rogers MJ, Crockett JC, Coxon FP, Monkkonen J. (2011).Biochemical and molecular mechanisms of action of bisphosphonates. Bone, 49, 34–41 [DOI] [PubMed] [Google Scholar]
- 224.Rose MW, Rose ND, Boggs J, Lenevich S, Xu J, Barany G, Distefano MD. (2005).Evaluation of geranylazide and farnesylazide diphosphate for incorporation of prenylazides into a CAAX box-containing peptide using protein farnesyltransferase. J Peptide Res, 65, 529–37 [DOI] [PubMed] [Google Scholar]
- 225.Rose WC, Lee FYF, Fairchild CR, Lynch M, Monticello T, Kramer RA, Manne V. (2001).Preclinical Antitumor Activity of BMS-214662, a Highly Apoptotic and Novel Farnesyltransferase Inhibitor. Cancer Res, 61, 7507. [PubMed] [Google Scholar]
- 226.Rosenthal SL, Kamboh MI. (2014).Late-Onset Alzheimer’s Disease Genes and the Potentially Implicated Pathways. Current Genetic Medicine Reports, 2, 85–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ruse M, Knaus UG. (2006). New players in TLR-mediated innate immunity: PI3K and small Rho GTPasesed.^eds., 33–48 [DOI] [PubMed]
- 228.Russell RG. (2011).Bisphosphonates: the first 40 years. Bone, 49, 2–19 [DOI] [PubMed] [Google Scholar]
- 229.Sacchettini JC, Poulter CD. (1997).Creating isoprenoid diversity. Science, 277, 1788–9 [DOI] [PubMed] [Google Scholar]
- 230.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E. (1996).The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med, 335, 1001–9 [DOI] [PubMed] [Google Scholar]
- 231.Sagami H, Ishi K, Ogura K. (1981).Occurrence and Unusual Properties of Geranylgeranyl Pyrophosphate Synthetase of Pig-Liver. Biochemistry International, 3, 669–75 [Google Scholar]
- 232.Scaffidi P, Misteli T. (2006).Lamin A-dependent nuclear defects in human aging. Science, 312, 1059–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Scherzer CR, Offe K, Gearing M, Rees HD, Fang G, Heilman CJ, Schaller C, Bujo H, Levey AI, Lah JJ. (2004).Loss of apolipoprotein E receptor LR11 in Alzheimer disease. Arch Neurol, 61, 1200–5 [DOI] [PubMed] [Google Scholar]
- 234.Schlitzer M, Sattler I. (1999).Design, synthesis, and evaluation of novel modular bisubstrate analogue inhibitors of farnesyltransferase. Angew Chem, Int Ed, 38, 2032–34 [DOI] [PubMed] [Google Scholar]
- 235.Schmidt RA, Schneider CJ, Glomset JA. (1984).Evidence for post-translational incorporation of a product of mevalonic acid into Swiss 3T3 cell proteins. J Biol Chem, 259, 10175–80 [PubMed] [Google Scholar]
- 236.Schmidt WK, Tam A, Fujimura-Kamada K, Michaelis S. (1998).Endoplasmic reticulum membrane localization of Rce1p and Ste24p, yeast proteases involved in carboxyl-terminal CAAX protein processing and amino-terminal a-factor cleavage. ProcNatl Acad Sci USA, 95, 11175–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Seabra MC, Goldstein JL, Sudhof TC, Brown MS. (1992).Rab geranylgeranyl transferase. A multisubunit enzyme that prenylates GTP-binding proteins terminating in Cys-X-Cys or Cys-Cys. J Biol Chem, 267, 14497–503 [PubMed] [Google Scholar]
- 238.Selkoe D, Mandelkow E, Holtzman D. (2012).Deciphering Alzheimer disease. Cold Spring Harbor perspectives in medicine, 2, a011460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Seyfried NT, Dammer EB, Swarup V, Nandakumar D, Duong DM, Yin L, Deng Q, Nguyen T, Hales CM, Wingo T, Glass J, Gearing M, Thambisetty M, Troncoso JC, Geschwind DH, Lah JJ, Levey AI. (2017).A Multi-network Approach Identifies Protein-Specific Co-expression in Asymptomatic and Symptomatic Alzheimer’s Disease. Cell Sys, 4, 60–72.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Shao Y, Eummer JT, Gibbs RA. (1999).Stereospecific Synthesis and Biological Evaluation of Farnesyl Diphosphate Isomers. Organic Letters, 1, 627–30 [DOI] [PubMed] [Google Scholar]
- 241.Shepardson NE, Shankar GM, Selkoe DJ. (2011).Cholesterol Level and Statin Use in Alzheimer Disease: II. Review of Human Trials and Recommendations. Archives of neurology, 68, 1385–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Simanshu DK, Nissley DV, McCormick F. (2017).RAS Proteins and Their Regulators in Human Disease. Cell, 170, 17–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Simons M, Schwärzler F, Lütjohann D, Von Bergmann K, Beyreuther K, Dichgans J, Wormstall H, Hartmann T, Schulz JB. (2002).Treatment with simvastatin in normocholesterolemic patients with Alzheimer’s disease: A 26-week randomized, placebo-controlled, double-blind trial. Annals of Neurology, 52, 346–50 [DOI] [PubMed] [Google Scholar]
- 244.Sims R, van der Lee SJ, Naj AC, Bellenguez C, Badarinarayan N, Jakobsdottir J, Kunkle BW, Boland A, Raybould R, Bis JC, Martin ER, Grenier-Boley B, Heilmann-Heimbach S, Chouraki V, Kuzma AB, Sleegers K, Vronskaya M, Ruiz A, Graham RR, Olaso R, Hoffmann P, Grove ML, Vardarajan BN, Hiltunen M, Nöthen MM, White CC, Hamilton-Nelson KL, Epelbaum J, Maier W, Choi S-H, Beecham GW, Dulary C, Herms S, Smith AV, Funk CC, Derbois C, Forstner AJ, Ahmad S, Li H, Bacq D, Harold D, Satizabal CL, Valladares O, Squassina A, Thomas R, Brody JA, Qu L, Sánchez-Juan P, Morgan T, Wolters FJ, Zhao Y, Garcia FS, Denning N, Fornage M, Malamon J, Naranjo MCD, Majounie E, Mosley TH, Dombroski B, Wallon D, Lupton MK, Dupuis J, Whitehead P, Fratiglioni L, Medway C, Jian X, Mukherjee S, Keller L, Brown K, Lin H, Cantwell LB, Panza F, McGuinness B, Moreno-Grau S, Burgess JD, Solfrizzi V, Proitsi P, Adams HH, Allen M, Seripa D, Pastor P, Cupples LA, Price ND, Hannequin D, Frank-García A, Levy D, Chakrabarty P, Caffarra P, Giegling I, Beiser AS, Giedraitis V, Hampel H, Garcia ME, Wang X, Lannfelt L, Mecocci P, Eiriksdottir G, Crane PK, Pasquier F, Boccardi V, Henández I, Barber RC, Scherer M, Tarraga L, Adams PM, Leber M, Chen Y, Albert MS, Riedel-Heller S, Emilsson V, Beekly D, Braae A, Schmidt R, Blacker D, Masullo C, Schmidt H, Doody RS, Spalletta G Jr WTL, Fairchild TJ, Bossù P, Lopez OL, Frosch MP, Sacchinelli E, Ghetti B, Yang Q, Huebinger RM, Jessen F, Li S, Kamboh MI, Morris J, Sotolongo-Grau O, Katz MJ, Corcoran C, Dunstan M, Braddel A, Thomas C, Meggy A, Marshall R, Gerrish A, Chapman J, Aguilar M, Taylor S, Hill M, Fairén MD, Hodges A, Vellas B, Soininen H, Kloszewska I, Daniilidou M, Uphill J, Patel Y, Hughes JT, Lord J, Turton J, Hartmann AM, Cecchetti R, Fenoglio C, Serpente M, Arcaro M, Caltagirone C, Orfei MD, Ciaramella A, Pichler S, Mayhaus M, Gu W, Lleó A, Fortea J, Blesa R, Barber IS, Brookes K, Cupidi C, Maletta RG, Carrell D, Sorbi S, Moebus S, Urbano M, Pilotto A, Kornhuber J, Bosco P, Todd S, Craig D, Johnston J, Gill M, Lawlor B, Lynch A, Fox NC, Hardy J, Albin RL, Apostolova LG, Arnold SE, Asthana S, Atwood CS, Baldwin CT, Barnes LL, Barral S, Beach TG, Becker JT, Bigio EH, Bird TD, Boeve BF, Bowen JD, Boxer A, Burke JR, Burns JM, Buxbaum JD, Cairns NJ, Cao C, Carlson CS, Carlsson CM, Carney RM, Carrasquillo MM, Carroll SL, Diaz CC, Chui HC, Clark DG, Cribbs DH, Crocco EA, DeCarli C, Dick M, Duara R, Evans DA, Faber KM, Fallon KB, Fardo DW, Farlow MR, Ferris S, Foroud TM, Galasko DR, Gearing M, Geschwind DH, Gilbert JR, Graff-Radford NR, Green RC, Growdon JH, Hamilton RL, Harrell LE, Honig LS, Huentelman MJ, Hulette CM, Hyman BT, Jarvik GP, Abner E, Jin L-W, Jun G, Karydas A, Kaye JA, Kim R, Kowall NW, Kramer JH, LaFerla FM, Lah JJ, Leverenz JB, Levey AI, Li G, Lieberman AP, Lunetta KL, Lyketsos CG, Marson DC, Martiniuk F, Mash DC, Masliah E, McCormick WC, McCurry SM, McDavid AN, McKee AC, Mesulam M, Miller BL, Miller CA, Miller JW, Morris JC, Murrell JR, Myers AJ, O’Bryant S, Olichney JM, Pankratz VS, Parisi JE, Paulson HL, Perry W, Peskind E, Pierce A, Poon WW, Potter H, Quinn JF, Raj A, Raskind M, Reisberg B, Reitz C, Ringman JM, Roberson ED, Rogaeva E, Rosen HJ, Rosenberg RN, Sager MA, Saykin AJ, Schneider JA, Schneider LS, Seeley WW, Smith AG, Sonnen JA, Spina S, Stern RA, Swerdlow RH, Tanzi RE, Thornton-Wells TA, Trojanowski JQ, Troncoso JC, Van Deerlin VM, Van Eldik LJ, Vinters HV, Vonsattel JP, Weintraub S, Welsh-Bohmer KA, Wilhelmsen KC, Williamson J, Wingo TS, Woltjer RL, Wright CB, Yu C-E, Yu L, Garzia F, Golamaully F, Septier G, Engelborghs S, Vandenberghe R, De Deyn PP, Fernadez CM, Benito YA, Thonberg H, Forsell C, Lilius L, Kinhult-Stählbom A, Kilander L, Brundin R, Concari L, Helisalmi S, Koivisto AM, Haapasalo A, Dermecourt V, Fievet N, Hanon O, Dufouil C, Brice A, Ritchie K, Dubois B, Himali JJ, Keene CD, Tschanz J, Fitzpatrick AL, Kukull WA, Norton M, Aspelund T, Larson EB, Munger R, Rotter JI, Lipton RB, Bullido MJ, Hofman A, Montine TJ, Coto E, Boerwinkle E, Petersen RC, Alvarez V, Rivadeneira F, Reiman EM, Gallo M, O’Donnell CJ, Reisch JS, Bruni AC, Royall DR, Dichgans M, Sano M, Galimberti D, St George-Hyslop P, Scarpini E, Tsuang DW, Mancuso M, Bonuccelli U, Winslow AR, Daniele A, Wu C-K, Peters O, Nacmias B, Riemenschneider M, Heun R, Brayne C, Rubinsztein DC, Bras J, Guerreiro R, Al-Chalabi A, Shaw CE, Collinge J, Mann D, Tsolaki M, Clarimón J, Sussams R, Lovestone S, O’Donovan MC, Owen MJ, Behrens TW, Mead S, Goate AM, Uitterlinden AG, Holmes C, Cruchaga C, Ingelsson M, Bennett DA, Powell J, Golde TE, Graff C, De Jager PL, Morgan K, Ertekin-Taner N, Combarros O, Psaty BM, Passmore P, Younkin SG, Berr C, Gudnason V, Rujescu D, Dickson DW, Dartigues J-F, DeStefano AL, Ortega-Cubero S, Hakonarson H, Campion D, Boada M, Kauwe JK, Farrer LA, Van Broeckhoven C, Ikram MA, Jones L, Haines JL, Tzourio C, Launer LJ, Escott-Price V, Mayeux R, Deleuze J-F, Amin N, Holmans PA, Pericak-Vance MA, Amouyel P, van Duijn CM, Ramirez A, Wang L-S, Lambert J-C, Seshadri S, Williams J, Schellenberg GD. (2017).Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer’s disease. Nature Genetics [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sirtori CR. (2014).The pharmacology of statins. Pharmacol Res, 88, 3–11 [DOI] [PubMed] [Google Scholar]
- 246.Smith JA, Das A, Ray SK, Banik NL. (2012). Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseasesed.^eds., 10–20 [DOI] [PMC free article] [PubMed]
- 247.Speers AE, Cravatt BF. (2004).Profiling Enzyme Activities In Vivo Using Click Chemistry Methods. Chem Biol, 11, 535–46 [DOI] [PubMed] [Google Scholar]
- 248.Spindler SR, Li R, Dhahbi JM, Yamakawa A, Mote P, Bodmer R, Ocorr K, Williams RT, Wang Y, Ablao KP. (2012).Statin treatment increases lifespan and improves cardiac health in Drosophila by decreasing specific protein prenylation. PLoS One, 7, e39581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Stephen AG, Esposito D, Bagni RK, McCormick F. (2014).Dragging ras back in the ring. Cancer cell, 25, 272–81 [DOI] [PubMed] [Google Scholar]
- 250.Stornetta RL, Zhu JJ. (2010).Ras and Rap signaling in synaptic plasticity and mental disorders. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry, 17, 54–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Strickland CL, Windsor WT, Syto R, Wang L, Bond R, Wu Z, Schwartz J, Le HV, Beese LS, Weber PC. (1998).Crystal Structure of Farnesyl Protein Transferase Complexed with a CaaX Peptide and Farnesyl Diphosphate Analogue. Biochemistry, 37, 16601–11 [DOI] [PubMed] [Google Scholar]
- 252.Su F, Bai F, Zhou H, Zhang Z. (2016).Microglial toll-like receptors and Alzheimer’s disease. Brain Behav Immun, 52, 187–98 [DOI] [PubMed] [Google Scholar]
- 253.Suazo KF, Schaber C, Palsuledesai CC, Odom John AR, Distefano MD. (2016).Global proteomic analysis of prenylated proteins in Plasmodium falciparum using an alkyne-modified isoprenoid analogue. Sci Rep, 6, 38615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Swanger SA, Mattheyses AL, Gentry EG, Herskowitz JH. (2015).ROCK1 and ROCK2 inhibition alters dendritic spine morphology in hippocampal neurons. Cellular logistics, 5, e1133266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Tada M, Lynen F. (1961).[On the biosynthesis of terpenes. XIV. On the determination of phosphomevalonic acid kinase and pyrophosphomevalonic acid decarboxylase in cell extracts]. Journal of biochemistry, 49, 758–64 [DOI] [PubMed] [Google Scholar]
- 256.Tate EW, Kalesh KA, Lanyon-Hogg T, Storck EM, Thinon E. (2015).Global profiling of protein lipidation using chemical proteomic technologies. Curr Opin Chem Biol, 24, 48–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Taylor JS, Reid TS, Terry KL, Casey PJ, Beese LS. (2003).Structure of mammalian protein geranylgeranyltransferase type-I. EMBO J, 22, 5963–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Tchen TT. (1958).Mevalonic kinase: purification and properties. J Biol Chem, 233, 1100–3 [PubMed] [Google Scholar]
- 259.Thinakaran G, Koo EH. (2008).Amyloid precursor protein trafficking, processing, and function. J Biol Chem, 283, 29615–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Tong H, Wiemer AJ, Neighbors JD, Hohl RJ. (2008).Quantitative determination of farnesyl and geranylgeranyl diphosphate levels in mammalian tissue. Anal Biochem, 378, 138–43 [DOI] [PubMed] [Google Scholar]
- 261.Turek-Etienne TC, Strickland CL, Distefano MD. (2003).Biochemical and Structural Studies with Prenyl Diphosphate Analogues Provide Insights into Isoprenoid Recognition by Protein Farnesyl Transferase. Biochemistry, 42, 3716–24 [DOI] [PubMed] [Google Scholar]
- 262.Turek TC, Gaon I, Distefano MD. (1996).Analogs of farnesyl pyrophosphate incorporating internal benzoylbenzoate esters: Synthesis, inhibition kinetics and photoinactivation of yeast protein farnesyltransferase. Tetrahedron Lett, 37, 4845–48 [DOI] [PubMed] [Google Scholar]
- 263.Turek TC, Gaon I, Distefano MD, Strickland CL. (2001).Synthesis of Farnesyl Diphosphate Analogues Containing Ether-Linked Photoactive Benzophenones and Their Application in Studies of Protein Prenyltransferases. J Org Chem, 66, 3253–64 [DOI] [PubMed] [Google Scholar]
- 264.Udayar V, Buggia-Prévot V, Guerreiro RL, Siegel G, Rambabu N, Soohoo AL, Ponnusamy M, Siegenthaler B, Bali J, Simons M, Ries J, Puthenveedu MA, Hardy J, Thinakaran G, Rajendran L. (2013).A Paired RNAi and RabGAP overexpression screen identifies Rab11 as a regulator of??-amyloid production. Cell Reports, 5, 1536–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Ulrich JD, Ulland TK, Colonna M, Holtzman DM. (2017).Elucidating the Role of TREM2 in Alzheimer’s Disease. Neuron, 94, 237–48 [DOI] [PubMed] [Google Scholar]
- 266.Varela I, Pereira S, Ugalde AP, Navarro CL, Suarez MF, Cau P, Cadinanos J, Osorio FG, Foray N, Cobo J, de Carlos F, Levy N, Freije JM, Lopez-Otin C. (2008).Combined treatment with statins and aminobisphosphonates extends longevity in a mouse model of human premature aging. Nat Med, 14, 767–72 [DOI] [PubMed] [Google Scholar]
- 267.Venet M, End D, Angibaud P. (2003).Farnesyl Protein Transferase Inhibitor ZARNESTRA™ R115777 - History of a Discovery. Curr Top Med Chem, 3, 1095–102 [DOI] [PubMed] [Google Scholar]
- 268.Vlkolinský R, Cairns N, Fountoulakis M, Lubec G. (2001).Decreased brain levels of 2′,3′-cyclic nucleotide-3′-phosphodiesterase in Down syndrome and Alzheimer’s disease. Neurobiol Aging, 22, 547–53 [DOI] [PubMed] [Google Scholar]
- 269.Walton GM, Stockley JA, Griffiths D, Sadhra CS, Purvis T, Sapey E. (2016).Repurposing Treatments to Enhance Innate Immunity. Can Statins Improve Neutrophil Functions and Clinical Outcomes in COPD? Journal of clinical medicine, 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wang J, Yao X, Huang J. (2017).New tricks for human farnesyltransferase inhibitor: cancer and beyond. MedChemComm, 8, 841–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Wang M, Casey PJ. (2016).Protein prenylation: unique fats make their mark on biology. Nat Rev Mol Cell Biol, 17, 110–22 [DOI] [PubMed] [Google Scholar]
- 272.Wang M, Tan W, Zhou J, Leow J, Go M, Lee HS, Casey PJ. (2008).A small molecule inhibitor of isoprenylcysteine carboxymethyltransferase induces autophagic cell death in PC3 prostate cancer cells. The Journal of biological chemistry, 283, 18678–84 [DOI] [PubMed] [Google Scholar]
- 273.Wang Y-C, Distefano MD. (2012).Solid-Phase Synthesis of C-Terminal Peptide Libraries for Studying the Specificity of Enzymatic Protein Prenylation. Chem Comm, 1359–7345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Wang YC, Dozier JK, Beese LS, Distefano MD. (2014).Rapid analysis of protein farnesyltransferase substrate specificity using peptide libraries and isoprenoid diphosphate analogues. ACS Chem Biol, 9, 1726–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Wennerberg K, Rossman KL, Der CJ. (2005).The Ras superfamily at a glance. J Cell Sci, 118, 843–6 [DOI] [PubMed] [Google Scholar]
- 276.Winter-Vann AM, Casey PJ. (2005).Post-prenylation-processing enzymes as new targets in oncogenesis. Nat Rev Cancer, 5, 405–12 [DOI] [PubMed] [Google Scholar]
- 277.Wollack JW, Zeliadt NA, Mullen DG, Amundson G, Geier S, Falkum S, Wattenberg EV, Barany G, Distefano MD. (2009).Multifunctional prenylated peptides for in vivo analysis. J Am Chem Soc, 131, 7293–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Wollack JW, Zeliadt NA, Mullen DG, Wattenberg EV, Barany G, Distefano MD. (2010).Investigation of a Minimal Sequence for Cell-Penetrating Prenylated Peptides. Bioorg Med Chem Lett, 20, 161–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G. (2000).Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3- methyglutaryl coenzyme A reductase inhibitors. Arch Neurol, 57, 1439–43. [DOI] [PubMed] [Google Scholar]
- 280.Wood WG, Eckert GP, Igbavboa U, Muller WE. (2010).Statins and neuroprotection: a prescription to move the field forward. Ann N Y Acad Sci, 1199, 69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Wood WG, Mupsilonller WE, Eckert GP. (2014).Statins and neuroprotection: basic pharmacology needed. Mol Neurobiol, 50, 214–20 [DOI] [PubMed] [Google Scholar]
- 282.Wyss-Coray T, Rogers J. (2012).Inflammation in Alzheimer disease-A brief review of the basic science and clinical literature. Cold Spring Harbor Perspectives in Medicine, 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Yalovsky S, Loraine AE, Gruissem W. (1996).Specific Prenylation of Tomato Rab Proteins by Geranylgeranyl Type-II Transferase Requires a Conserved Cysteine-Cysteine Motif. Plant physiology, 110, 1349–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Yang J, Kulkarni K, Manolaridis I, Zhang Z, Dodd Roger B, Mas-Droux C, Barford D. (2011).Mechanism of Isoprenylcysteine Carboxyl Methylation from the Crystal Structure of the Integral Membrane Methyltransferase ICMT. Molec Cell, 44, 997–1004 [DOI] [PubMed] [Google Scholar]
- 285.Yang WS, Yeo SG, Yang S, Kim KH, Yoo BC, Cho JY. (2017).Isoprenyl carboxyl methyltransferase inhibitors: a brief review including recent patents. Amino acids, 49, 1469–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Ye X, Carew TJ. (2010).Small G protein signaling in neuronal plasticity and memory formation: the specific role of ras family proteins. Neuron, 68, 340–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Yeh FL, Hansen DV, Sheng M. (2017).TREM2, Microglia, and Neurodegenerative Diseases. Trends Mol Med, 23, 512–33 [DOI] [PubMed] [Google Scholar]
- 288.Yokoyama K, Goodwin GW, Ghomashchi F, Glomset JA, Gelb MH. (1991).A protein geranylgeranyltransferase from bovine brain: implications for protein prenylation specificity. Proc Natl Acad Sci USA, 88, 5302–06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Yokoyama K, McGeady P, Gelb MH. (1995).Mammalian Protein Geranylgeranyltransferase-I: Substrate Specificity, Kinetic Mechanism, Metal Requirements, and Affinity Labeling. Biochemistry, 34, 1344–54 [DOI] [PubMed] [Google Scholar]
- 290.Young SG, Meta M, Yang SH, Fong LG. (2006).Prelamin A farnesylation and progeroid syndromes. J Biol Chem, 281, 39741–5 [DOI] [PubMed] [Google Scholar]
- 291.Yurdaydin C, Keskin O, Kalkan C, Karakaya F, Caliskan A, Karatayli E, Karatayli S, Bozdayi AM, Koh C, Heller T, Idilman R, Glenn JS. (2017).Optimizing lonafarnib treatment for the management of chronic delta hepatitis: The lowr HDV - 1 study. Hepatology (Baltimore, Md) [DOI] [PubMed] [Google Scholar]
- 292.Zhang FL, Casey PJ. (1996).Protein prenylation: Molecular mechanisms and functional consequences. Annu Rev Biochem, 65, 241–69 [DOI] [PubMed] [Google Scholar]
- 293.Zhang S-Y, Sperlich B, Li F-Y, Al-Ayoubi S, Chen H-X, Zhao Y-F, Li Y-M, Weise K, Winter R, Chen Y-X. (2017).Phosphorylation Weakens but Does Not Inhibit Membrane Binding and Clustering of K-Ras4B. ACS Chem Biol, 12, 1703–10 [DOI] [PubMed] [Google Scholar]
- 294.Zhao L, Chen T, Wang C, Li G, Zhi W, Yin J, Wan Q, Chen L. (2016).Atorvastatin in improvement of cognitive impairments caused by amyloid β in mice: Involvement of inflammatory reaction. BMC Neurology, 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Zhao L, Ma QL, Calon F, Harris-White ME, Yang F, Lim GP, Morihara T, Ubeda OJ, Ambegaokar S, Hansen JE, Weisbart RH, Teter B, Frautschy SA, Cole GM. (2006).Role of p21-activated kinase pathway defects in the cognitive deficits of Alzheimer disease. Nature Neuroscience, 9, 234–42 [DOI] [PubMed] [Google Scholar]
- 296.Zhou XP, Wu KY, Liang B, Fu XQ, Luo ZG. (2008).TrkB-mediated activation of geranylgeranyltransferase I promotes dendritic morphogenesis. Proc Natl Acad Sci U S A, 105, 17181–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Zhou Y, Prakash P, Liang H, Cho KJ, Gorfe AA, Hancock JF. (2017).Lipid-Sorting Specificity Encoded in K-Ras Membrane Anchor Regulates Signal Output. Cell, 168, 239–51 e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Zhou Y, Suram A, Venugopal C, Prakasam A, Lin S, Su Y, Li B, Paul SM, Sambamurti K. (2008).Geranylgeranyl pyrophosphate stimulates gamma-secretase to increase the generation of Abeta and APP-CTFgamma. Faseb J, 22, 47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Zhu X, Raina AK, Boux H, Simmons ZL, Takeda A, Smith MA. (2000).Activation of oncogenic pathways in degenerating neurons in Alzheimer disease. International Journal of Developmental Neuroscience, 18, 433–37 [DOI] [PubMed] [Google Scholar]
- 300.Zissimopoulos JM, Barthold D, Brinton RD, Joyce G. (2017).Sex and Race Differences in the Association Between Statin Use and the Incidence of Alzheimer Disease. JAMA Neurology, 74, 225–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Zolezzi JM, Inestrosa NC. (2017). Wnt/TLR dialog in neuroinflammation, relevance in Alzheimer’s diseaseed.^eds. [DOI] [PMC free article] [PubMed]