Abstract
Pentylenetetrazole (PTZ) is a GABA-A receptor antagonist. An intraperitoneal injection of PTZ into an animal induces an acute, severe seizure at a high dose, whereas sequential injections of a subconvulsive dose have been used for the development of chemical kindling, an epilepsy model. A single low-dose injection of PTZ induces a mild seizure without convulsion. However, repetitive low-dose injections of PTZ decrease the threshold to evoke a convulsive seizure. Finally, continuous low-dose administration of PTZ induces a severe tonic-clonic seizure. This method is simple and widely applicable to investigate the pathophysiology of epilepsy, which is defined as a chronic disease that involves repetitive seizures. This chemical kindling protocol causes repetitive seizures in animals. With this method, vulnerability to PTZ-mediated seizures or the degree of aggravation of epileptic seizures was estimated. These advantages have led to the use of this method for screening anti-epileptic drugs and epilepsy-related genes. In addition, this method has been used to investigate neuronal damage after epileptic seizures because the histological changes observed in the brains of epileptic patients also appear in the brains of chemical-kindled animals. Thus, this protocol is useful for conveniently producing animal models of epilepsy.
Keywords: Neuroscience, Issue 136, Chemical kindling, seizure, epilepsy, neural plasticity, animal behavior, histopathology
Introduction
Epilepsy is a chronic neurological disorder that is characterized by recurrent seizures and affects approximately 1% of people. The underlying mechanisms of epileptogenesis and seizure generation in epilepsy patients cannot be fully clarified in clinical studies. Therefore, an appropriate animal model is required for the study of epilepsy1.
A variety of animal models of epilepsy have been used to investigate the physiology of epilepsy and to identify anti-epileptic drugs2,3. Among these models, pharmacological seizure induction is a common method used to generate an animal model for the investigation of the pathology of epilepsy4. This method is inexpensive and simple. Electrode-mediated kindling is also a commonly used method, but the costs of this procedure are higher, and the method requires surgical and electrical skills to induce repetitive seizures5.
Pharmacological induction is also advantageous because the timing and number of seizures are easily controlled. Genetic mouse models that exhibit spontaneous seizures are also used in the study of epilepsy. However, predicting when and how often the seizures arise in these genetic models may be impossible6. A monitoring system is required to observe the epileptic behavior of genetically modified mice6.
Kainic acid, pilocarpine and pentylenetetrazole (PTZ) are widely used as seizure-inducing drugs7. Kainic acid is an agonist for glutamate receptors, and pilocarpine activates cholinergic receptors. PTZ is a gamma aminobutyric acid (GABA)-A receptor antagonist8. PTZ suppresses the function of inhibitory synapses, leading to increased neuronal activity. This regulation causes generalized seizures in animals9. A single injection of kainic acid and pilocarpine can induce acute seizures, especially status epilepticus (SE)10,11 and kainic acid- or pilocarpine-mediated SE promotes chronic spontaneous and recurrent seizures12,13. Electroencephalographic (EEG) recordings and behavior analysis have indicated that spontaneous recurrent seizures are observed a month after a single injection12,13. A single injection of a convulsive dose of PTZ also induces acute seizure. However, chronic spontaneous seizures after a single injection of PTZ are difficult to promote. Chronic administration of PTZ is required to induce repetitive seizures14. In either method, the generation of repetitive seizures is able to induce a pathology more similar to that of human epilepsy than the generation of acute seizures. In the case of PTZ, each injection evokes a seizure, and seizure severity becomes more severe in a stepwise manner with each injection. Finally, a single low-dose PTZ injection induces a severe tonic-clonic seizure. In this phase, each injection evokes severe seizures. In addition, the seizure latency and duration also change over the course of the injections. The latency to tonic seizure often becomes shorter in the latter phase of kindling15. Furthermore, seizure aggravation is accompanied by a prolonged seizure duration16. Investigating the molecular mechanism regulating the seizure severity, latency, and duration is useful for screening anti-epileptic drugs17,18,19.
Seizures are commonly induced by a single systemic administration of PTZ, and the recovery is very fast, within 30 min4,5. Thus, the number of seizures is more controllable in the PTZ-kindling model. However, EEG monitoring has indicated that generalized spikes may be seen up to 12 h after PTZ-mediated seizure20. Therefore, animals should preferably remain under observation for 24 h after the myoclonic or tonic seizure21 for more precise analysis of the kindling mechanisms.
The administration of anti-epileptic drugs, such as ethosuximide, valproate, phenobarbital, vigabatrin, and retigabine3, before or after PTZ injection mitigates the aggravation of the seizure severity3,22,23. Similarly, knockout mice that lack genes involved in seizure exacerbation, such as matrix metalloproteinase-924, FGF-2225 and neuritin26, have been shown to exhibit reduced seizure severity after multiple PTZ injections. In addition, observing histopathological alterations after epileptic seizures is possible with this method. In patients with temporal lobe epilepsy, there are typical histological changes in the brain, such as mossy fiber sprouting27,28, abnormal granule neuron migration29, astrogliosis30, neuronal cell death in the hippocampus31,32, and hippocampal sclerosis33. Similar changes are observed in epileptic model animals. Among the available methods, PTZ-mediated chemical kindling is a good, reproducible and inexpensive method to produce an animal model of epilepsy. In a pilocarpine-mediated SE model, seizure control is difficult and many mice die or fail to develop SE34. In contrast, mortality and seizure severity are more controllable in the PTZ model. Additionally, PTZ is less expensive than kainic acid, and skills in mouse brain surgery are not required for drug administration.
Protocol
All experimental procedures were approved by the Animal Care and Use Committee of the Tokyo Metropolitan Institute of Medical Science. Postnatal 8 - 16-week-old mice are recommended. Any inbred strain is acceptable for the experiment. C57BL/6 mice are more resistant to PTZ, whereas BALB/c and Swiss albino mice are more sensitive to PTZ. C57BL/6 were used in this study. Vulnerability to PTZ also depends on the age of the mouse. Compared to younger mice, older mice are more refractory to PTZ35. The number of animals used for this method can vary, but at least 6 - 10 animals are required for each condition.
1. Preparation of PTZ
Dissolve 2 mg/mL PTZ in sterile 0.9% (w/v) NaCl. Prepare PTZ on the day of use.
2. Injection of PTZ
Perform all experiments between 9:00 a.m. and 12:00 p.m. (noon).
Measure the animal's body weight.
Place the animal in an observation chamber for habituation.
During the habituation period (3 min), calculate the volume of PTZ solution for the injection based on the body weight of the animal and the previously determined injection dose. NOTE: For example, when 35 mg/kg PTZ is used, 0.875 mg of PTZ is required for a mouse weighing 25 g (35 mg/kg x 0.025 kg = 0.875 mg). Therefore, 437.5 μL of a 2 mg/mL PTZ solution should be injected (0.875 mg / 2 mg/mL = 0.4375 mL). The injection dose of PTZ depends on the mouse genotype and strain. For wild-type C57BL/6 mice, a dose of 30-35 mg PTZ/kg body weight is recommended for the first trial. Sensitivity to PTZ depends on the mouse strain used36,37. The injection dose varies according to the purpose of the experiment.
- Inject PTZ intraperitoneally with a 1-mL syringe attached to a 27-gauge needle into the left or right quadrant of the abdomen of the animal. Avoid injecting at the midline.
- Avoid repeated injections at the same position.
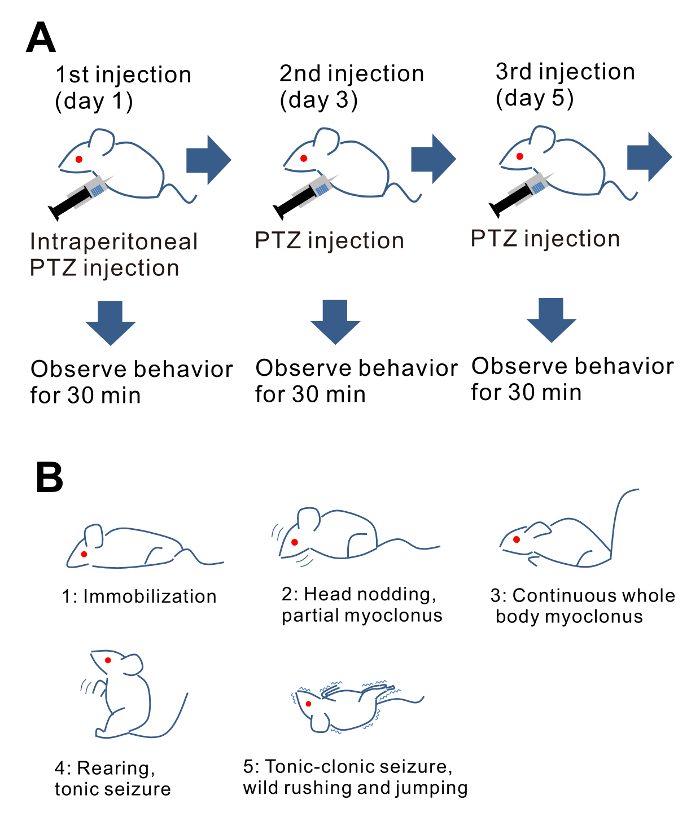
- Observe animal behaviors for 30 min after PTZ administration (Figure 1A), and classify and score the abnormal behavior as shown below. If possible, keep the animals under observation for 24 h, or at least an additional 6-10 h post-injection, especially once the seizure severity score reaches 3 or above.
- Note any mild seizures or behavioral changes in the animals beyond the 30-min observation period. This prolonged observation period may address a particularly important and complete effect of a subconvulsive dose of PTZ in the generation of chronic unprovoked seizures in epilepsy.
- In addition, measure the seizure duration of each observed seizure as changes in seizure duration relate to the seizure severity. Moreover, the latency to the first seizure after the PTZ injection is another important measure to collect. Monitoring the seizure frequency, duration, and latency is critical for any post-seizure molecular studies.
- Inject PTZ every other day (Figure 1A). The number of injections depends on the objective of the experiment. Representative examples are as follows.
- To generate completely kindled animals, once an animal experiences a seizure with a score of 5 (tonic-clonic seizure), finish the injection within additional three administrations. In this case, increase the injection dose if the seizure score does not increase for three consecutive administrations. Each animal can receive a different number and dose of PTZ injections.
- Fix the number and dose of PTZ injection in every condition to evaluate the vulnerability to PTZ, including in assessments of anti-epilepsy drugs and studies of the phenotype of genetically modified mice. Give all animals the same number of PTZ injections; a total of 8 to 12 injections is recommended. If an animal dies, the seizure score should be denoted as 6.
- Determine the injection dose necessary to maintain a high seizure score (4 or 5) for 10 or more injections to study the histopathological changes that occur epileptic seizures. In this case, the total injection number should range from 25 to 30. Decrease the injection dose if the seizure score reaches 5. If the seizure score decreases to 3 or lower, increase the injection dose.
3. Seizure Score
- Observe the animal behaviors and record the scores.
- Classify and score the epileptic behaviors as follows38,39 (Figure 1B): 0: normal behavior, no abnormality. 1: immobilization, lying on belly. 2: head nodding, facial, forelimb, or hindlimb myoclonus. 3: continuous whole-body myoclonus, myoclonic jerks, tail held up stiffly. 4: rearing, tonic seizure, falling down on its side. 5: tonic-clonic seizure, falling down on its back, wild rushing and jumping. 6: death.
Change the behavioral criteria based on the Racine score40, depending on the experimental conditions.
4. Post-Seizure Analysis
- Immunohistopathological analysis
- Perfusion fixation
- Preparation
- Dissolve 4% (w/v) paraformaldehyde (PFA) in 0.1 M phosphate buffer (pH 7.4). Heat the solution to approximately 70 °C with the addition of a drop of 1 M NaOH (approximately 1 µL of 1 M NaOH for 50 mL PFA solution).
- Prepare ice-cold 4% PFA and phosphate-buffered saline (PBS).
- Set up a peristaltic pump, with a tube and needle. Run approximately 20 mL of water through the tube to clear out residue. Then, place the open end of the tube in ice-cold PBS.
- Transcardial perfusion and preparation of the fixed brain
- Deeply anesthetize the mouse with 50% isoflurane/50% ethanol in a small jar.
- Keep the mouse in a supine position by pinning the tips of their forelimbs and hindlimbs.
- Cut the ventral midline of the abdomen and expose the diaphragm.
- Cut the diaphragm along the costal margin. Then, cut both lateral sides of the ribs and body. Pinch the xiphoid process by the locking forceps and evert the rib cage. Maintain the position of the xiphoid process by pinning it with a needle or pinching it with forceps. Expose the heart.
- Insert the needle into the left ventricle and make a cut in the right atrium using scissors. Be aware not to push the needle too far into the heart, as it can pierce an interior wall.
- Begin a steady flow of ice-cold PBS through the needle to wash out the blood (approximately 15 - 20 mL/min).
- When the blood has been cleared from the body, move the tube to a 4% paraformaldehyde solution (approximately 60 mL) without the addition of bubbles. Then, stop the perfusion.
- Cut the rear neck and spine and peel off the head skin and the dorsal part of the skull with dissecting scissors.
- Cut the premaxillary bone between the eye orbits and completely remove the dorsal portion of the skull.
- Create a gap between the brain and jugal bone from the posterior side and cut the trigeminal nerves and optic chiasm underneath the brain. Carefully remove the brain and place it in a vial containing 4% PFA. Post-fix the brain at 4°C for 12-16 h.
- Brain slice preparation
- Transfer the fixed brain to 20% sucrose/PBS until the brain sinks into the solution to allow for cryoprotection.
- Cut the fixed brain based on the required slice orientation and required part of the brain.
- Set the cryomicrotome. Set the blade in position and maintain the microtome stage at a temperature under -20 °C.
- Place the pre-cut brain on the stage and coat the brain with 20% sucrose/PBS. The sucrose/PBS solution will gradually freeze, covering the brain on the stage, until the brain is completely embedded in frozen 20% sucrose/PBS.
- Slice the brain (30 µm thickness) by sliding the blade through the tissue. Transfer the slices into PBS and keep them at 4 °C.
- Fluorescent immunohistochemistry
- Wash the necessary slices in 0.1% Triton X-100/PBS (PBST) for 10 min, 3 times at room temperature (RT).
- Block the slices by incubating them in PBST with 2% goat serum or 5% bovine serum albumin (BSA) for 1 - 2 h at RT.
- Incubate the slices in an antibody solution with the suitable concentration of primary antibody in PBST with 2% goat serum or 5% BSA for 1 to 7 overnights at 4 °C.
- Wash the slices in PBST for 10 min, 3 times at RT.
- Incubate the slices in a secondary antibody solution with the suitable concentration of secondary antibody in PBST for 1 - 2 h at RT, light-protected.
- Mount the slices onto glass slides and cover with mounting medium and cover glass.
- Observe the slices with fluorescence microscopy.
- Three-chamber test
- Preparation
- Prepare at least 2 2 - 6-month-old 129/Sv mice of the same sex as the subject mice to be the "Stranger mouse." Habituate all mice in the cage before the analysis. For each training session, place the mouse in the cage and leave the mouse for 15 min.
- Before each habituation phase with the subject mouse, clean the entire apparatus and both cages by wiping the surface with 70% ethanol.
- Place the empty cages in the two side chambers of the 3-chamber apparatus and open the doors between the chambers.
- To habituate the subject mouse, place a subject mouse in the center chamber and allow the mouse to freely move throughout the apparatus until the mouse has investigated both cages, plus an additional 5 min. Subject animals that may be fearful of the cage do not investigate the cage for 20 min and should not be used for this analysis. Such animals avoid the cage and rarely come close to the cage.
- After the animal moves into the center chamber, close the doors and let the mouse move freely in the center chamber for 5 min. During this period, put one of the 129/Sv mice in a cage to habituate to the cage.
- Sociality analysis
- Carry out this analysis immediately after the habituation period of the subject mouse.
- Place the cage containing a 129/Sv mouse, termed the "Stranger 1 cage", in one of the side chambers and place the empty cage, termed the "Object cage", in the other side chamber.
- Open the door and monitor the behavior of the subject mouse.
- Allow the subject mouse to move freely for 10 min and record the following behavioral parameters. If the subject mouse is afraid of the mouse in the Stranger 1 cage, as indicated by the mouse avoiding the Stranger 1 cage and remaining in the corner of one of the chambers, the animal should not be used for the analysis. a) Time spent in each chamber, the Stranger 1 chamber, the Object chamber, and center chamber. b) Time spent investigating each cage, the Stranger 1 cage and the Object cage. (The mouse is classified as investigating the cage if the mouse is sniffing or toughing the mouse or cage) c) The number of entrances into each chamber (optional) d) Total distance travelled (optional)
- After the mouse moves into the center chamber, close the doors.
- Social novelty analysis
- Carry out this analysis immediately after the sociality analysis.
- Wipe both cages with 70% ethanol.
- Place another 129/Sv mouse in one of the cages, termed the "Stranger 2 cage" and place the Stranger 2 cage in one of the chambers. Place the Stranger 1 mouse in the other cage, termed the "Stranger 1 cage", and place the Stranger 1 cage in the other chamber.
- Open the door and monitor the behavior of the subject mouse.
- Allow the subject mouse to move freely for 10 min, and measure the same behavioral parameters described for the sociality analysis. NOTE: The Stranger 1 and Stranger 2 mouse should be chosen randomly. The Stranger 1 chamber and Object chamber as well as the Stranger 1 chamber and Stranger 2 chamber should be randomly determined.
- Contextual fear discrimination
- Conditioning
- Before each conditioning trial, clean the experimental apparatus by wiping the surface with 70% ethanol.
- Place a mouse in a conditioning apparatus with specific conditions (apparatus shape, wall color, floor material, odor, lighting, and background noise volume should be pre-determined). For example, the conditioning apparatus used here was a square apparatus with clear plexiglass walls, a metal grid floor, an ethanol odor, 100 lux brightness, and 65-dB background white noise.
- Condition the mouse by foot-shock with pseudo-random timing of 0.1-mA electrical shocks for 2 s x 3 times over the course of 5 min.
- Return the mouse to the home cage after conditioning.
- Memory assessment
- Before each assessment, clean the experimental apparatus by wiping the surface with 70% ethanol.
- The next day following conditioning, place the mouse into the same apparatus as shock condition and measure the freezing time over the course of 5 min.
- Later the same day, place the mouse in a novel apparatus and measure the freezing time during a 5-min assessment. A triangular apparatus with white plexiglass walls, plate floor, no odor, 30 lux brightness, and 70-dB white noise was used here.
- Compare the freezing time between the same condition and novel condition. The mice with a normal memory ability will freeze more in the same condition than in the novel condition. Freezing is defined immobility of the animal for more than 2 s.
Representative Results
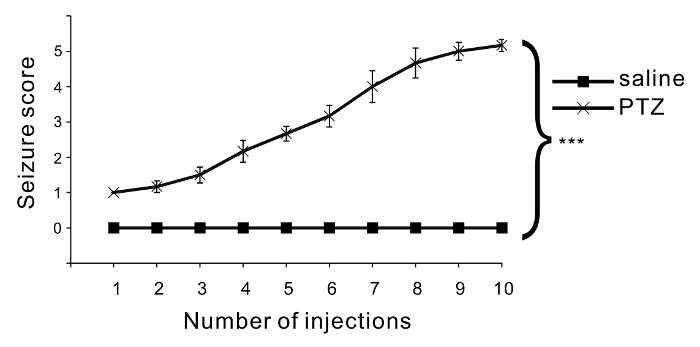
Repetitive injection of PTZ induces an increase in seizure severity. Six C57BL/6 mice were treated with PTZ, and another 6 mice were treated with saline as a control group. The PTZ dose was 35 mg/kg, and 10 injections were administered. The seizure score gradually increased with PTZ injections, whereas no seizures or abnormal behaviors were evoked by saline injections (Figure 2). ANOVA followed by Bonferroni test showed a significant difference between the PTZ-treated group and the saline-treated group.
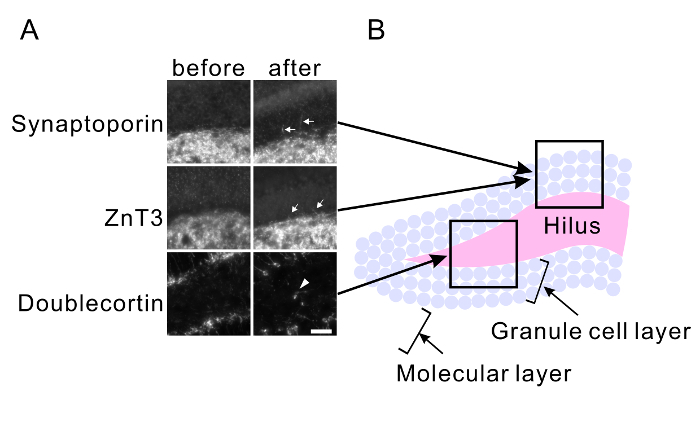
Repetitive seizure promotes aberrant axonal branch formation (mossy fiber sprouting) and abnormal migration of granule cells in the hippocampus. Mice were treated with PTZ for 25 injections (dose was adjusted between 24 mg/kg and 35 mg/kg to maintain severe seizure in mice without inducing death caused by a severe seizure). The mouse brains were fixed 3 weeks after the last injection. Control brains were fixed before PTZ injections. Brain slices were immunostained with anti-synaptoporin (x 500) and anti-ZnT3 (x 500) antibodies to observe the mossy fiber sprouting (Figure 3A) and with the anti-doublecortin antibody (x 200) to observe the abnormal migration of granule cells (Figure 3A). Mossy fiber sprouting in the granule cell layer was observed in PTZ-treated slices (Figure 3A). Newborn granule cells, which are immunoreactive for doublecortin, were observed within the hilus in the PTZ-treated slices (Figure 3A). The granule cell layer and hilus shown in Figure 3A is illustrated in Figure 3B.
Repetitive seizures also impair normal behavior of mice. Twelve C57BL/6 mice were treated with PTZ (35 mg/kg, 10 injections), and another 12 mice were treated with saline as a control group. Two weeks after the last injection, the mice were analyzed in a 3-chamber test (Figure 4A) and contextual fear discrimination test (Figure 5A). PTZ-treated mice showed normal sociality (Figure 4B). Mice spent more time in the Stranger 1 chamber than in the Object chamber (saline: p = 0.003, PTZ: p = 0.027) and investigated the Stranger 1 cage more than they investigated the Object cage (saline: p = 0.009, PTZ: p = 0.004). However, PTZ-treated mice showed abnormal social novelty (Figure 4C) indicative of impaired social memory. Control mice spent more time in the Stranger 2 chamber than in the Stranger 1 chamber and investigated the Stranger 2 cage more than they investigated the Stranger 1 cage, whereas the kindled mice did not show any significant difference in time spent in the chambers or time spent investigating the cages (Time in chamber: saline: p = 0.006, PTZ: p = 0.126. Time investigating: saline: p = 0.002, PTZ: p = 0.426). PTZ-treated mice also showed impaired memory in the contextual fear test (Figure 5B). Control mice showed a longer freezing time in the shock condition than in the novel condition, whereas PTZ-treated mice did not show any significant difference in freezing time (saline: p < 0.001, PTZ: p = 0.060). Unpaired t-tests were carried out for statistical analyses.
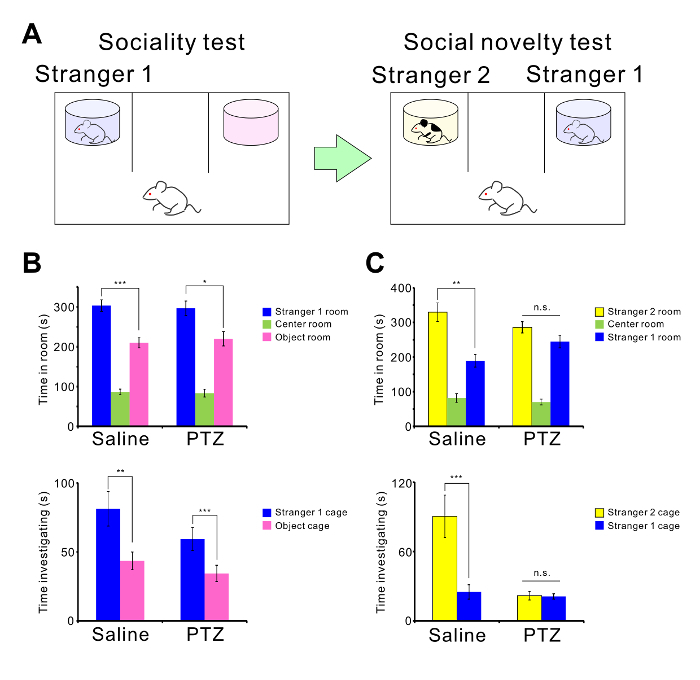
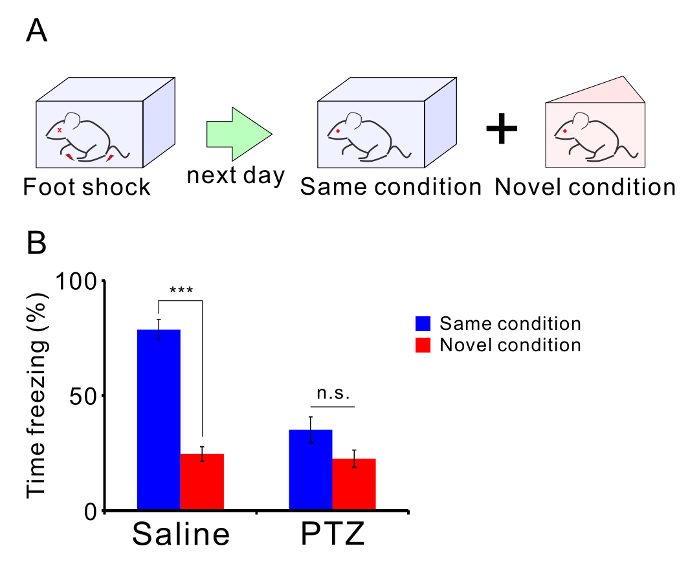
Figure 1: Brief description of the protocol. (A) Schematic illustration of PTZ-mediated kindling. (B) Illustrations of representative animal behaviors for respective seizure scores. Please click here to view a larger version of this figure.
Figure 2: Assessment of convulsive behavior. The mean seizure scores are indicated in the graph. Six mice were used in each condition, and one series of injections was carried out. After each injection, the seizure scores were monitored and scored. Compared to saline injections, PTZ injections significantly increased seizure severity (p < 0.001: repeated-measures ANOVA). Each seizure score is shown as the mean ± SEM. Please click here to view a larger version of this figure.
Figure 3: PTZ-mediated mossy fiber sprouting and abnormal migration of granule cells. (A) Maximum projected immunohistochemical images of the hilus, granular layer, and molecular layer of hippocampal slices of the mice before kindling (left) and after kindling (right). Anti-synaptoporin (top) and anti-ZnT3 antibodies (middle) were used to visualize the axons of granule neurons (mossy fibers). The anti-doublecortin antibody was used to visualize newborn granule neurons (bottom). PTZ-mediated repetitive seizures induce mossy fiber sprouting (arrows) and abnormal migration of granule cells into the hilus (arrowheads). Scale bar, 50 μm. The approximate position of each image is indicated in (B). Please click here to view a larger version of this figure.
Figure 4: PTZ-mediated abnormal social behavior.(A) Schematic illustration of the 3-chamber test. (B) The mean amount of time spent in each chamber and the mean amount of time spent investigating each cage in the sociality test are shown. (C) The mean amount of time spent in each chamber and the mean amount of time spent investigating each cage in the social novelty test are shown. There were twelve mice in both the PTZ- and saline-treated groups. PTZ-mediated seizures induced abnormal social behavior (***p < 0.001, n.s. = not significant). All times are shown as the mean ± SEM. Please click here to view a larger version of this figure.
Figure 5: PTZ-mediated memory impairment. (A) Schematic illustration of the contextual fear discrimination test. (B) The mean percentage of freezing times in each condition are shown. There were twelve mice in both the PTZ- and saline-treated groups. Graph shows the mean ± SEM. Please click here to view a larger version of this figure.
Discussion
Here, we present a widely accessible protocol for the establishment of a pharmacological animal model of epilepsy. PTZ-mediated chemical kindling has a long history and is a commonly accepted model for the study of the histopathology and cellular pathology of epilepsy41. The chemical kindling model of epilepsy has been reviewed previously by Suzdak and Jansen, 199542. Pharmacological seizure induction, especially with PTZ, is an easy and simple method for evoking severe seizures. Changes in the injection dose correlate with seizure severity. Thus, identifying the appropriate dose over several trials by changing the PTZ dose and examining the resulting behavior is very easy.
Many researchers have attempted to create knockout or knock-in mice as epilepsy models and have succeeded in producing mice that exhibit spontaneous seizures43,44. However, pharmacological seizure induction is still considered a good model for epilepsy. Another common method for seizure induction other than gene modification involves implanting electrodes into an animal's brain and inducing electroshock-mediated seizures. This method is costly, difficult, and requires surgical skill to implant the electrode at the precise locus in the brain5.
Chemical induction of convulsions enables the rapid investigation of epileptogenesis and anti-epileptic drug screening at low cost2,4. The frequency and severity of spontaneous seizures and SE vary depending on the drug used. Kainic acid and pilocarpine are mainly used to provoke SE and subsequent chronic spontaneous or recurrent seizures45,46,47. On the other hand, PTZ is used both to promote SE when given in a high-dose and to develop chemically kindled animals when given in a subconvulsive dose48,49. In addition, the mechanisms of convulsive drugs that induce seizures are well known. Thus, blocking the mechanism necessary to induce seizure may help to identify anti-epileptic drugs. On the other hand, genetic models are required to investigate the mechanisms that evoke epileptic seizures50. After the mechanisms by which seizures are induced are elucidated, the screening of anti-epileptic drugs may be started.
In the representative results shown here, animal behavior was observed for 30 min after PTZ administration. However, as mentioned in the protocol section, animal behavior is preferably observed for 24 h or at least 6-10 h after PTZ injection, especially once the seizure score reaches 3 or higher. Although an animal monitoring system may be required to observe the animal for the whole day, a prolonged observation is critical for obtaining a deep understanding of epilepsy. In addition, the seizure duration and seizure latency are useful measures to collect. These measurements are important when relating changes in seizure duration and latency to time and seizure severity.
The post-seizure histopathology of chemically kindled animals has been investigated, especially in the hippocampus. Repetitive seizures or SE induce mossy fiber sprouting27,28, abnormal migration of newborn granule neurons25,29, astrogliosis in the hippocampus30, and apoptosis of pyramidal neurons31,32. These histological changes in the brain disrupt neurological functions in epileptic patients. For example, the association of epilepsy with autism spectrum disorders (ASD) and intellectual disability (ID) is well recognized51,52,53. Whether epileptic seizures induce ASD and ID or ASD and ID induce epileptic symptoms is still a complicated question. Recent studies have shown that PTZ-mediated seizures induce ASD-like social-cognitive impairments54 and ID-like learning deficits 55,56,57. These findings indicate that the epilepsy-mediated histopathology elicits the neuronal dysfunction and psychiatric disorders.
Histological changes promoted by epilepsy take time to develop after seizure induction. In this regard, pharmacological seizure induction is advantageous because researchers can control the timing, number and severity of seizures in animals. Including chemical kindling, animal models of epileptogenesis will continue to promote the investigation of both the mechanism of epilepsy induction and related neurophysiological disorders.
Disclosures
The authors declare no conflicts of interest.
Acknowledgments
This work was partly supported by JSPS KAKENHI grant numbers 24700349, 24659093, 25293239, JP18H02536, and 17K07086, MEXT KAKENHI grant numbers 25110737 and 23110525, AMED Grant Number JP18ek0109311, and the SENSHIN Medical Research Foundation and the Japan Epilepsy Research Foundation.
References
- Löscher W, Brandt C. Prevention or Modification of Epileptogenesis after Brain Insults: Experimental Approaches and Translational Research. Pharmacological Reviews. 2010;62(4):668–700. doi: 10.1124/pr.110.003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher W. Animal Models of Seizures and Epilepsy: Past, Present, and Future Role for the Discovery of Antiseizure Drugs. Neurochem Res. 2017. [DOI] [PubMed]
- Löscher W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure. 2011;20(5):359–368. doi: 10.1016/j.seizure.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Kandratavicius L, et al. Animal models of epilepsy: use and limitations. Neuropsychiatr Dis Treat. 2014;10:1693–1705. doi: 10.2147/NDT.S50371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen A, Schwartzkroin PA, Moshé SL. Models of Seizures and Epilepsy. Academic Press; 2006. p. xvii. [Google Scholar]
- Yang Y, Frankel WN. Genetic approaches to studying mouse models of human seizure disorders. Adv Exp Med Biol. 2004;548:1–11. doi: 10.1007/978-1-4757-6376-8_1. [DOI] [PubMed] [Google Scholar]
- Leite JP, Garcia-Cairasco N, Cavalheiro EA. New insights from the use of pilocarpine and kainate models. Epilepsy Res. 2002;50(1-2):93–103. doi: 10.1016/s0920-1211(02)00072-4. [DOI] [PubMed] [Google Scholar]
- Squires RF, Saederup E, Crawley JN, Skolnick P, Paul SM. Convulsant potencies of tetrazoles are highly correlated with actions on GABA/benzodiazepine/picrotoxin receptor complexes in brain. Life Sci. 1984;35(14):1439–1444. doi: 10.1016/0024-3205(84)90159-0. [DOI] [PubMed] [Google Scholar]
- Tourov A, et al. Spike morphology in PTZ-induced generalized and cobalt-induced partial experimental epilepsy. Funct Neurol. 1996;11(5):237–245. [PubMed] [Google Scholar]
- Furtado Mde A, Braga GK, Oliveira JA, Del Vecchio F, Garcia-Cairasco N. Behavioral, morphologic, and electroencephalographic evaluation of seizures induced by intrahippocampal microinjection of pilocarpine. Epilepsia. 2002;43(Suppl 5):37–39. doi: 10.1046/j.1528-1157.43.s.5.41.x. [DOI] [PubMed] [Google Scholar]
- Hosford DA. Animal models of nonconvulsive status epilepticus. J Clin Neurophysiol. 1999;16(4):306–313. doi: 10.1097/00004691-199907000-00002. discussion 353. [DOI] [PubMed] [Google Scholar]
- Medina-Ceja L, Pardo-Pena K, Ventura-Mejia C. Evaluation of behavioral parameters and mortality in a model of temporal lobe epilepsy induced by intracerebroventricular pilocarpine administration. Neuroreport. 2014. [DOI] [PubMed]
- Bragin A, Azizyan A, Almajano J, Wilson CL, Engel J., Jr Analysis of chronic seizure onsets after intrahippocampal kainic acid injection in freely moving rats. Epilepsia. 2005;46(10):1592–1598. doi: 10.1111/j.1528-1167.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- Schmidt J. Changes in seizure susceptibility in rats following chronic administration of pentylenetetrazol. Biomed Biochim Acta. 1987;46(4):267–270. [PubMed] [Google Scholar]
- Angelatou F, Pagonopoulou O, Kostopoulos G. Changes in seizure latency correlate with alterations in A1 adenosine receptor binding during daily repeated pentylentetrazol-induced convulsions in different mouse brain areas. Neuroscience Letters. 1991;132(2):203–206. doi: 10.1016/0304-3940(91)90302-a. [DOI] [PubMed] [Google Scholar]
- Löscher W. Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs. A comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy. Epilepsy Research. 2002;50(1):105–123. doi: 10.1016/s0920-1211(02)00073-6. [DOI] [PubMed] [Google Scholar]
- Ilhan A, Iraz M, Kamisli S, Yigitoglu R. Pentylenetetrazol-induced kindling seizure attenuated by Ginkgo biloba extract (EGb 761) in mice. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2006;30(8):1504–1510. doi: 10.1016/j.pnpbp.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Emami S, Kebriaeezadeh A, Ahangar N, Khorasani R. Imidazolylchromanone oxime ethers as potential anticonvulsant agents: Anticonvulsive evaluation in PTZ-kindling model of epilepsy and SAR study. Bioorganic & Medicinal Chemistry Letters. 2011;21(2):655–659. doi: 10.1016/j.bmcl.2010.12.021. [DOI] [PubMed] [Google Scholar]
- Klitgaard H. Levetiracetam: The Preclinical Profile of a New Class of Antiepileptic Drugs? Epilepsia. 2001;42:13–18. [PubMed] [Google Scholar]
- Kellinghaus C, et al. Dissociation between in vitro and in vivo epileptogenicity in a rat model of cortical dysplasia. Epileptic Disord. 2007;9(1):11–19. doi: 10.1684/epd.2007.0061. [DOI] [PubMed] [Google Scholar]
- Koutroumanidou E, et al. Increased seizure latency and decreased severity of pentylenetetrazol-induced seizures in mice after essential oil administration. Epilepsy Res Treat. 2013;2013:532657. doi: 10.1155/2013/532657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall RL, Penry JK, White BG, Kupferberg HJ, Swinyard EA. Antiepileptic drug development: II. Anticonvulsant drug screening. Epilepsia. 1978;19(4):409–428. doi: 10.1111/j.1528-1157.1978.tb04507.x. [DOI] [PubMed] [Google Scholar]
- White HS. Preclinical development of antiepileptic drugs: past, present, and future directions. Epilepsia. 2003;44(Suppl 7):2–8. doi: 10.1046/j.1528-1157.44.s7.10.x. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, et al. Matrix metalloproteinase-9 contributes to kindled seizure development in pentylenetetrazole-treated mice by converting pro-BDNF to mature BDNF in the hippocampus. J Neurosci. 2011;31(36):12963–12971. doi: 10.1523/JNEUROSCI.3118-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Umemori H. Suppression of epileptogenesis-associated changes in response to seizures in FGF22-deficient mice. Front Cell Neurosci. 2013;7:43. doi: 10.3389/fncel.2013.00043. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Shimada T, Yoshida T, Yamagata K. Neuritin Mediates Activity-Dependent Axonal Branch Formation in Part via FGF Signaling. J Neurosci. 2016;36(16):4534–4548. doi: 10.1523/JNEUROSCI.1715-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, et al. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17(10):3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutula T, He XX, Cavazos J, Scott G. Synaptic reorganization in the hippocampus induced by abnormal functional activity. Science. 1988;239(4844):1147–1150. doi: 10.1126/science.2449733. [DOI] [PubMed] [Google Scholar]
- Parent JM, Elliott RC, Pleasure SJ, Barbaro NM, Lowenstein DH. Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Ann Neurol. 2006;59(1):81–91. doi: 10.1002/ana.20699. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32(12):638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzimanoglou A, et al. Epilepsy and neuroprotection: an illustrated review. Epileptic Disord. 2002;4(3):173–182. [PubMed] [Google Scholar]
- Represa A, Niquet J, Pollard H, Ben-Ari Y. Cell death, gliosis, and synaptic remodeling in the hippocampus of epileptic rats. Journal of Neurobiology. 1995;26(3):413–425. doi: 10.1002/neu.480260313. [DOI] [PubMed] [Google Scholar]
- Blumcke I. Neuropathology of focal epilepsies: a critical review. Epilepsy Behav. 2009;15(1):34–39. doi: 10.1016/j.yebeh.2009.02.033. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Haney MM. Factors affecting outcomes of pilocarpine treatment in a mouse model of temporal lobe epilepsy. Epilepsy Res. 2012;102(3):153–159. doi: 10.1016/j.eplepsyres.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nokubo M, et al. Age-dependent increase in the threshold for pentylenetetrazole induced maximal seizure in mice. Life Sci. 1986;38(22):1999–2007. doi: 10.1016/0024-3205(86)90147-5. [DOI] [PubMed] [Google Scholar]
- Ferraro TN, et al. Mapping Loci for Pentylenetetrazol-Induced Seizure Susceptibility in Mice. The Journal of Neuroscience. 1999;19(16):6733–6739. doi: 10.1523/JNEUROSCI.19-16-06733.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosobud AE, Cross SJ, Crabbe JC. Neural sensitivity to pentylenetetrazol convulsions in inbred and selectively bred mice. Brain Res. 1992;592(1-2):122–128. doi: 10.1016/0006-8993(92)91666-3. [DOI] [PubMed] [Google Scholar]
- Shimada T, Takemiya T, Sugiura H, Yamagata K. Role of Inflammatory Mediators in the Pathogenesis of Epilepsy. Mediators of Inflammation. 2014;2014:1–8. doi: 10.1155/2014/901902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, et al. Magnetic resonance and biochemical studies during pentylenetetrazole-kindling development: the relationship between nitric oxide, neuronal nitric oxide synthase and seizures. Neuroscience. 2004;129(3):757–766. doi: 10.1016/j.neuroscience.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32(3):281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Bialer M, White HS. Key factors in the discovery and development of new antiepileptic drugs. Nat Rev Drug Discov. 2010;9(1):68–82. doi: 10.1038/nrd2997. [DOI] [PubMed] [Google Scholar]
- Suzdak PD, Jansen JA. A review of the preclinical pharmacology of tiagabine: a potent and selective anticonvulsant GABA uptake inhibitor. Epilepsia. 1995;36(6):612–626. doi: 10.1111/j.1528-1157.1995.tb02576.x. [DOI] [PubMed] [Google Scholar]
- Seyfried TN, Glaser GH. A review of mouse mutants as genetic models of epilepsy. Epilepsia. 1985;26(2):143–150. doi: 10.1111/j.1528-1157.1985.tb05398.x. [DOI] [PubMed] [Google Scholar]
- Upton N, Stratton S. Recent developments from genetic mouse models of seizures. Current Opinion in Pharmacology. 2003;3(1):19–26. doi: 10.1016/s1471-4892(02)00010-3. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Reddy DS, Shetty AK. Hippocampal neurodegeneration, spontaneous seizures, and mossy fiber sprouting in the F344 rat model of temporal lobe epilepsy. J Neurosci Res. 2006;83(6):1088–1105. doi: 10.1002/jnr.20802. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Patrylo PR, Buckmaster PS, Dudek FE. Recurrent spontaneous motor seizures after repeated low-dose systemic treatment with kainate: assessment of a rat model of temporal lobe epilepsy. Epilepsy Res. 1998;31(1):73–84. doi: 10.1016/s0920-1211(98)00017-5. [DOI] [PubMed] [Google Scholar]
- Turski L, et al. Seizures produced by pilocarpine: neuropathological sequelae and activity of glutamate decarboxylase in the rat forebrain. Brain Res. 1986;398(1):37–48. doi: 10.1016/0006-8993(86)91247-3. [DOI] [PubMed] [Google Scholar]
- Itoh K, Watanabe M. Paradoxical facilitation of pentylenetetrazole-induced convulsion susceptibility in mice lacking neuronal nitric oxide synthase. Neuroscience. 2009;159(2):735–743. doi: 10.1016/j.neuroscience.2008.12.040. [DOI] [PubMed] [Google Scholar]
- Deng Y, Wang M, Wang W, Ma C, He N. Comparison and Effects of Acute Lamotrigine Treatment on Extracellular Excitatory Amino Acids in the Hippocampus of PTZ-Kindled Epileptic and PTZ-Induced Status Epilepticus Rats. Neurochemical Research. 2013;38(3):504–511. doi: 10.1007/s11064-012-0942-7. [DOI] [PubMed] [Google Scholar]
- Kupferberg H. Animal models used in the screening of antiepileptic drugs. Epilepsia. 2001;42(Suppl 4):7–12. [PubMed] [Google Scholar]
- Berg AT, Plioplys S. Epilepsy and autism: is there a special relationship? Epilepsy Behav. 2012;23(3):193–198. doi: 10.1016/j.yebeh.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SJ. Childhood epilepsy and autism spectrum disorders: psychiatric problems, phenotypic expression, and anticonvulsants. Neuropsychol Rev. 2012;22(3):271–279. doi: 10.1007/s11065-012-9212-3. [DOI] [PubMed] [Google Scholar]
- Tuchman R. Autism and Cognition Within Epilepsy: Social Matters. Epilepsy Curr. 2015;15(4):202–205. doi: 10.5698/1535-7511-15.4.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takechi K, Suemaru K, Kiyoi T, Tanaka A, Araki H. The alpha4beta2 nicotinic acetylcholine receptor modulates autism-like behavioral and motor abnormalities in pentylenetetrazol-kindled mice. Eur J Pharmacol. 2016;775:57–66. doi: 10.1016/j.ejphar.2016.02.021. [DOI] [PubMed] [Google Scholar]
- Abdel-Zaher AO, Farghaly HSM, Farrag MMY, Abdel-Rahman MS, Abdel-Wahab BA. A potential mechanism for the ameliorative effect of thymoquinone on pentylenetetrazole-induced kindling and cognitive impairments in mice. Biomed Pharmacother. 2017;88:553–561. doi: 10.1016/j.biopha.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Jia F, et al. Effects of histamine H(3) antagonists and donepezil on learning and mnemonic deficits induced by pentylenetetrazol kindling in weanling mice. Neuropharmacology. 2006;50(3):404–411. doi: 10.1016/j.neuropharm.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Pahuja M, Mehla J, Reeta KH, Tripathi M, Gupta YK. Effect of Anacyclus pyrethrum on pentylenetetrazole-induced kindling, spatial memory, oxidative stress and rho-kinase II expression in mice. Neurochem Res. 2013;38(3):547–556. doi: 10.1007/s11064-012-0947-2. [DOI] [PubMed] [Google Scholar]


