Abstract
Alamethicin (ALA), a voltage-gated, ion channel-forming peptide mixture from Trichoderma viride, is a potent elicitor of the biosynthesis of volatile compounds in lima bean (Phaseolus lunatus). Unlike elicitation with jasmonic acid or herbivore damage, the blend of substances emitted comprises only the two homoterpenes, 4,11-dimethylnona-1,3,7-triene and 4,8,12-trimethyltrideca-1,3,7,11-tetraene, and methyl salicylate. Inhibition of octadecanoid signaling by aristolochic acid and phenidone as well as mass spectrometric analysis of endogenous jasmonate demonstrate that ALA induces the biosynthesis of volatile compounds principally via the octadecanoid-signaling pathway (20-fold increase of jasmonic acid). ALA also up-regulates salicylate biosynthesis, and the time course of the production of endogenous salicylate correlates well with the appearance of the methyl ester in the gas phase. The massive up-regulation of the SA-pathway (90-fold) interferes with steps in the biosynthetic pathway downstream of 12-oxophytodienoic acid and thereby reduces the pattern of emitted volatiles to compounds previously shown to be induced by early octadecanoids. ALA also induces tendril coiling in various species like Pisum, Lathyrus, and Bryonia, but the response appears to be independent from octadecanoid biosynthesis, because inhibitors of lipoxygenase and phospholipase A2 do not prevent the coiling reaction.
In their natural environment, plants permanently encounter the attack of a plethora of phytopathogens and herbivores, but nevertheless, the majority of them withstand without obvious damage. The defense strategies are generally organized as a network of responses including the initially infected/damaged cell, surrounding cells, as well as the entire plant referred to as the systemic response. The primary leaf damages or infestations are often mediated by specific fungal/herbivore- or plant cell wall-derived elicitors that may bind to specific receptors in the plant plasma membrane (Nürnberger et al., 1997; Nürnberger, 1999). Low-molecular-weight compounds that bypass the endogenous signaling cascades by mimicking endogenous signals of the plant are also known and represent another class of highly effective elicitors of microbial, fungal, and insect origin (Weiler et al., 1994; Greulich et al., 1995; Alborn et al., 1997; Koch et al., 1999). A few hours after elicitation the plant begins to express a subset of pathogenesis-related genes locally, at the point of infection, and systemically, throughout the whole plant (Keen, 1990; De Wit, 1997) contributing to so called systemic acquired resistance (SAR, based on salicylate-signaling) and wound response (mediated by octadecanoids). In many plants the SAR is preceded by a strong increase of the endogenous level of salicylate (SA; Raskin, 1992). Depending on the type of elicitors also the octadecanoid signaling path may be stimulated triggering the network of wound responses such as accumulation of proteinase inhibitors (Green and Ryan, 1972; Farmer and Ryan, 1992), phytoalexin synthesis (Blechert et al., 1995), and jasmonate (JA)-induced proteins (for review, see Weiler, 1997; Wasternack et al., 1998). Another effect of JA, in a great variety of plants, is the induction of the biosynthesis of volatile compounds (Boland et al., 1995; Dicke et al., 1999), mainly terpenoids, eventually contributing to plant-plant, plant-fungi, and/or plant-insect communication (Dicke et al., 1990; Turlings et al., 1990). 12-Oxo-phytodienoic acid (OPDA), an early intermediate of the JA-signaling cascade, has been recently recognized as an early but independent center of biological activity within the octadecanoids (Blechert et al., 1999; Koch et al., 1999). In addition to the defense-related complex, JA and OPDA proved to be potent inducers of tendril coiling in Bryonia dioica and accumulated after mechanical stimulation in internodes of B. dioica with OPDA being the more potent compound in arresting growth (Falkenstein et al., 1991; Weiler et al., 1993; Blechert et al., 1999). Significant qualitative differences between early and late octadecanoids concerning the induction of volatile compounds were observed for the first time in the lima bean (Phaseolus lunatus). Linolenic acid, at high concentrations, induced only the biosynthesis of two tetranor terpenes, namely 4,8-dimethylnona-1,3,7-triene (DMNT) and, to a much higher extent, that of the degraded diterpene 4,8,11-trimethyltrideca-1,3,7,11-tetraene (TMTT), whereas JA induced other terpenoids such as ocimene, linalool, and caryophyllene (Koch et al., 1999). The events following the increase of endogenous OPDA or JA, but preceding gene expression, are not known as yet.
The presence of different receptors for the great variety of elicitors from microorganisms in the plasma membrane of plants has been clearly demonstrated. In this context the non-host resistance response of cultured parsley (Petroselinum crispum) cells to a Phytophthora sojae-derived elicitor proved to be a particularly valuable model system (Parker et al., 1991). Isolated protoplasts from this cell culture retained their sensitivity to elicitor preparations from P. sojae (Dangl et al., 1987). Recognition of this elicitor by a plasma membrane receptor was accompanied by activation of ion channels, resulting in transient influxes of Ca2+ and H+ and effluxes of K+ and Cl− (Jabs et al., 1997). This pattern of ion fluxes was essential for oxidative burst, gene activation, and phytoalexin production (Hahlbrock et al., 1995). Furthermore, a specific kinase, related to animal mitogen-activated protein kinases, was activated by the same events (Likterink et al., 1997). Anion channel inhibitors blocked the elicitor responses, suggesting a dominant role for these channels in the network leading to defense reactions (Jabs et al., 1997). Similar effects have also been observed in response to the Phytophthora hepta-β-glucoside elicitor (Ebel and Cosio, 1994).
Cellulysin, a crude cellulase from the plant parasitic fungus Trichoderma viride, recently has been demonstrated to be a potent general elicitor of plant volatile synthesis. This mixture of several cell wall-degrading enzymes induced a blend of volatile compounds in different plant species such as Zea mays, Nicotiana plumbaginifolia, and P. lunatus resembling that of a JA treatment or herbivore damage (Piel et al., 1997). In fact, it could be shown that the action of cellulysin was followed by a rapid but transient increase of endogenous JA (Koch et al., 1999). In addition to the production of proteinaceous elicitors this fungus is known to produce a number of ion channel-forming peptides belonging to the class of peptaibols. One of the major compounds among these peptides is alamethicin (ALA) that contains a high proportion of the unusual amino acid α,α-dimethylisobutyric acid (Brewer et al., 1987). Although being obtained as crystalline material, it was established that ALA is a mixture of at least 12 compounds each containing 20 amino acid residues. There are two major forms of ALAs that differ only in the nature of the residue at position 18; Glu in one is replaced by Gln in the other (Sansom, 1993). Interest arose from their effectiveness as antimicrobial agents toward gram-positive bacteria. This ability was due to the specific property of ALA to build up voltage-dependent three-dimensional structures in membranes resulting in an ion channel specific for monovalent cations with a moderate selectivity for H+ over other cations (Cafiso, 1994). These modifications of the membrane structures led to uncoupling of oxidative phosphorylation, release of catecholamines from adrena chromaffin cells, inhibition of amoeba cell multiplication, and at high concentration, cell lysis (Huang et al., 1995). The origin of ALA from a plant parasitic fungus and its capability to form voltage-dependent ion channels prompted us to start an investigation about a possible role of these compounds in plant signaling. In this work we present data on the effect of ALA on plant physiology with respect to volatile production and tendril coiling. The simultaneous involvement of octadecanoid and salicylic acid signaling and the consequences of their mutual interactions will be discussed.
RESULTS
Induction of Volatile Biosynthesis after Elicitation with ALA
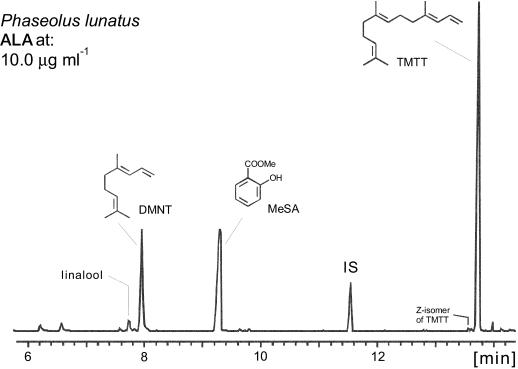
When freshly detached plantlets of lima bean were placed into a solution of ALA at 10 μg/mL a pronounced emission of volatile compounds synthesized de novo started several hours after addition of the chemical stimulus (Fig. 1). In contrast to the rather complex pattern of volatiles, induced by elicitation with jasmonic acid (Hopke et al., 1994; Boland et al., 1995; Dicke et al., 1999), spider mite infestation (Dicke et al., 1990), or treatment with cellulysin (Fig. 2), the ALA-responsive blend exhibited only three major compounds, namely the two homoterpenes, DMNT (19% ± 9) and TMTT (61% ± 14), and methyl salicylate (MeSA, 20% ± 10) as shown in Figure 1. Linalool occurs as a trace compound (≤3%).
Figure 1.
Gas chromatographic profile of volatile compounds after treatment of lima bean leaves with a solution of ALA (10 μg mL−1). The chromatogram is normalized to the main constituent (100%). Composition of the blend: DMNT, MeSA, TMTT, and small amounts of linalool.
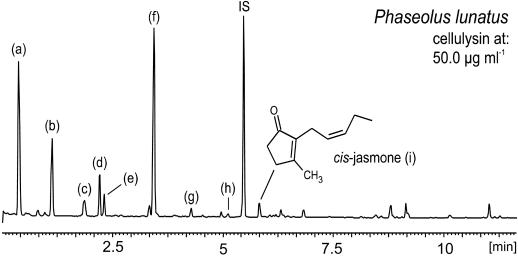
Figure 2.
Typical spectrum of volatile compounds after treatment of lima bean leaves with cellulysin (50 μg mL−1). Identification of compounds: a, (3Z)-hexenyl acetate; b, β-ocimene; c, linalool; d, DMNT; e, C10H14; f, C10H16O; g, indole; h, (3Z)-hexenyl methylbutanoate; IS, internal standard (1-bromodecane); and i, cis-jasmone.
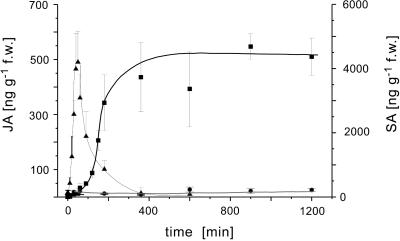
Both homoterpenes represent homologous products of an oxidative degradation of either nerolidol (C15 → C11) or geranyllinalool (C20 → C16) (Gäbler et al., 1991; Boland et al., 1998) and are produced de novo approximately 4 to 8 h, respectively, after the onset of the stimulus (Donath, 1994; Paré and Tumlinson, 1997; Piel et al., 1998). As shown previously, the biosynthesis of the two homoterpenes can be selectively triggered by early octadecanoids (Koch et al., 1999) and the plant's response to ALA is largely comparable with the previous findings, except that, in addition, significant amounts of MeSA were released to the gas phase. The appearance of the volatile MeSA in the gas phase correlated well with the time course of the endogenous level of salicylate, which started to increase approximately 1.5 h after the ALA-treatment to approximately 4.5 to 5.0 μg g−1 fresh weight (Fig. 3), corresponding to an approximately 90-fold increase over the resting level (average approximately 50 ng g−1 fresh weight). The induction of volatile biosynthesis by ALA was dose dependent with a minimum required concentration in the range of 0.1 μg mL−1, approximately 50 pMol, demonstrating the high biological activity of the substance.
Figure 3.
Quantification and time course of the endogenous JA and SA levels after treatment of lima bean leaves with aqueous solutions of ALA at 10 μg mL−1. ▪, Salicylate (SA); ▴, JA; ●, control of SA. Data represent results of at least four individual replicates (mean ± sd).
To determine whether or not ALA acts via activation of the octadecanoid pathway, generally involved in the induction of the biosynthesis of volatile compounds (Boland et al., 1995; Baldwin, 1997; Buonaurio and Servili, 1999) lima bean plantlets were pretreated with well-established inhibitors of the pathway. Phenidone interferes with the production of fatty acid hydroperoxides (Cucurou et al., 1991), whereas aristolochic acid, a potent inhibitor of phospholipase A2, prevents the release of linolenic acid from phospholipids (Rosenthal et al., 1989; Scherer and Arnold, 1997). The efficiency of the block after phenidone, (at 1 mm) or aristolochic acid pretreatment (0.3 mm), was demonstrated by the complete lack of emission of volatile compounds following elicitation with cellulysin shown to act through activation of the octadecanoid pathway (Piel et al., 1998; Koch et al., 1999). Subsequent addition of JA clearly by-passed the block and induced the characteristic blend of volatiles indicating that the plant's signaling system was fully functional.
When ALA was added to phenidone- or aristolochic acid-pretreated lima bean leaves, no induction of volatile biosynthesis was observed, supporting the involvement of the octadecanoid pathway in the induction process. Table I gives a synopsis of the different combinations of inhibitors/elicitors and the resulting biological effects.
Table I.
Effect of inhibitors of the octadecanoid pathway on biosynthesis of volatile compounds or tendril coiling stimulated by different elicitors
| Inhibitor | Biosynthesis of Volatile
Compounds
|
Tendril-Coiling | ||
|---|---|---|---|---|
| P. lunatus ALA | P. lunatus JA | P. lunatus cellulysin | B. dioica ALA | |
| Phenidone (1.0 mm) | − | + | − | + |
| Aristolochic acid (0.3 mm) | − | + | − | + |
| Control | + | + | + | + |
Volatiles were collected and identified as described in “Materials and Methods.”
The ALA-induced up-regulation and the kinetics of the formation of the two phytohormones JA and SA in lima bean were independently demonstrated by quantification of the endogenous levels of both compounds by mass spectrometry. As shown in Figure 3 the level of endogenous JA started to raise within the first 10 min after the onset of the experiment, reached a transient maximum of approximately 450 to 500 ng g−1 fresh weight after 40 min and then dropped off within 4 to 5 h to the starting concentration of approximately 20 to 40 ng g−1 fresh weight.
Based on the resting level, the amount of endogenous JA was increased by the ALA-treatment by a factor of 20. Control experiments using tap water also exhibited a moderate increase of endogenous JA, but the maximum level was much lower (approximately 70 ng g−1 fresh weight) and could be attributed to wounding by cutting the stem (Koch et al., 1999). The rather low level of damage-induced JA was, however, not sufficient to trigger the biosynthesis of volatile compounds. In contrast to JA the production of free SA started later (60–90 min) and reached a steady state level at 4.5 μg g−1 fresh weight after 6 to 7 h, which did not significantly decrease during the next 15 h. The SA-level of untreated lima bean leaves proved to be rather constant and was found around 50 ng g−1 fresh weight over the test period. Based on this ground level the response to ALA corresponded to a 90-fold increase of endogenous SA. Accordingly ALA elicited the biosynthesis of SA much more efficiently (90-fold) than that of JA (20-fold), and unlike JA, the level of SA remained high over a longer period (Fig. 3). If the bean leaves were first elicited with cellulysin followed by a treatment with phenidone after 24 h, the production of volatile compounds ceased, indicating that a permanent flow through the octadecanoid signaling cascade is essential to maintain metabolic activities.
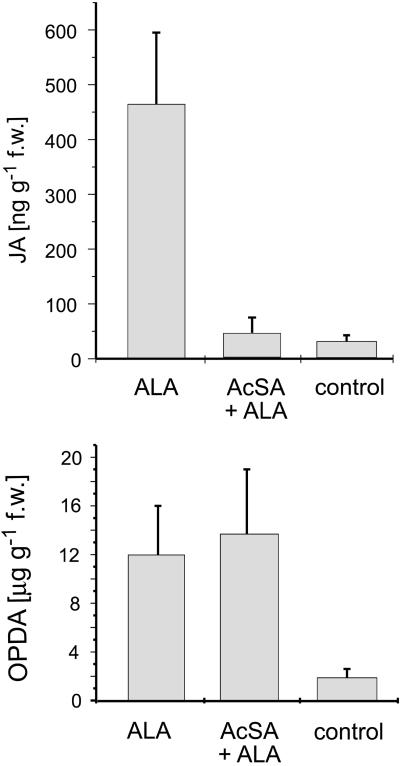
To study the influence of endogenous SA upon the octadecanoid signaling pathway (Bostok, 1999), freshly cut shoots of the lima beans were pre-incubated with acetyl salicylate (AcSA; 0.5 mm) for 13 h. The pretreated plants were transferred into an aq. solution of ALA (10 μg mL−1) for 40 min and, then, the production of the endogenous octadecanoids OPDA and JA was monitored (Fig. 4; compare with time course of Fig. 3). Without pretreatment, application of ALA up-regulated the entire octadecanoid pathway exemplified by high amounts of OPDA (approximately 12 μg g−1 fresh weight) and significant quantities of JA (approximately 450 ng g−1 fresh weight). Pretreatment with AcSA apparently had no influence on the level of induced endogenous OPDA (13.5 μg g−1 fresh weight), but clearly blocked steps downstream of OPDA because in this case the amount of JA did not exceed the resting level of JA (20–40 ng g−1 fresh weight). A pretreatment with free salicylic acid proved to be less effective probably due to a restricted transport of the polar compound through membranes.
Figure 4.
Quantification of endogenous OPDA and JA in AcSA (0.5 mm)-pretreated lima bean leaves. Samples were taken at maximum concentration of endogenous JA (40 min). Induction: ALA at 10 μg mL−1. Data represent results of at least four individual replicates (mean ± sd).
Despite a strongly enhanced emission of MeSA, the pretreatment with AcSA followed by elicitation with ALA resulted in a volatile pattern comparable with Figure 1. More evidence for a direct interaction between the ALA-enhanced endogenous SA-level and the octadecanoid signaling pathway was obtained by elicitation of ALA-pretreated bean leaves with cellulysin. One hour pretreatment with ALA followed by elicitation with cellulysin (50 μg mL−1) and collection of volatile compounds (48 h) resulted in a moderate reduction of the terpenoids shown in Figure 2 (compounds b, c, e, and f). If the pretreatment was extended to 2 h prior to the addition of cellulysin (50 μg mL−1) and volatile collection (48 h), the emission of the terpenoids was completely suppressed and a volatile profile corresponding to Figure 1 was obtained.
Tendril Coiling
The involvement of the octadecanoid signaling in the production of volatile compounds and perception of mechanical stimuli prompted us to investigate whether or not ALA could induce tendril coiling in B. dioica and Lathyrus sp. The tendrils of B. dioica react to exogenously applied JA and OPDA by showing a coiling reaction comparable with that of the free coiling reaction of the mechanically stimulated plant (Weiler et al., 1993). When ALA was applied to Bryonia tendrils at a concentration sufficient to induce the production of volatile compounds in lima beans (2.5 μg mL−1), the compound also turned out to be a potent elicitor of tendril coiling. The coiling response started within the first 2 h in the biotest using floating tendrils in a Petri dish and between 3 and 5 h after the onset of the stimulus in the shoot test system. The response interval in Pisum and Bryonia shoots was comparable. However, in contrast to a JA-induced tendril coiling, ALA failed to induce lignification of the Bianconi plate. The typical touch-induced free coiling response of B. dioica tendrils is accompanied by the differentiation of supporting tissue at the ventral side of the organ, becoming the inner (concave) side of the coiled tendril. As part of this process, the Bianconi plate, a continuous sclerenchyma sheath stretching along the ventral face of the five bicollateral vascular bundles, becomes strongly lignified. This remarkable difference suggested a response pathway different from the octadecanoid route. In line with this result is the observation that a pretreatment of B. dioica tendrils with an inhibitor of the octadecanoid pathway (phenidone) did not hamper the ALA-responsive tendril coiling. Also, the rapid induction of tendril coiling in Lathyrus and Pisum spp. by treatment with ALA required for an alternative mode of signal transduction, since both species proved to be insensitive to JA concerning tendril coiling.
DISCUSSION
ALA is a mixture of highly homologous peptides of 20 amino acids produced by the fungus T. viride (Brewer et al., 1987). The fungal origin of ALA along with its well-documented ability to form α-helical structures producing voltage-dependent ion channels within biological membranes prompted us to investigate its capability to induce (defense) responses in plants. Unlike cellulysin from the same fungus ALA induced the biosynthesis of only very few volatile compounds albeit in high quantities. Besides very small amounts of hexenyl acetate and linalool emitted during the first few hours after application of ALA, only the two homoterpenes DMNT, in particular TMTT, along with MeSA contributed to the blend of emitted volatile compounds (compare Figs. 1 and 2). With the exception of MeSA this pattern closely resembled the previously reported profile of volatile compounds induced by linolenic acid or OPDA (Koch et al., 1999). MeSA was never observed after induction with OPDA or cellulysin (Fig. 2). As the kinetics of the emission of MeSA to the gas phase and the kinetics of internal SA-accumulation resemble each other, it is reasonable to assume that the SA produced de novo is, at least in part, methylated and emitted to the gas phase (Fig. 3).
According to Shulaev et al. (1997) the emission of MeSA may benefit the plant population by prophylactic induction of defense genes in neighbored, uninfested or undamaged plants. However, SA has been claimed to interfere with some early steps of the JA biosynthesis. Peña-Cortés et al. (1993) postulated an interference with JA biosynthesis upstream of OPDA, whereas Laudert and Weiler (1998) provided evidence for inhibition of the export of OPDA from the plastid to the cytosol, thus, preventing further processing of OPDA to JA. According to Doares et al. (1995) SA may interfere with gene expression downstream of JA and, consequently, the high internal level of ALA-induced SA could account for the reduced number of volatiles. In the present study the biosynthesis of JA was apparently not inhibited (Fig. 3) but exhibited the typical transient accumulation within the first 90 min. However, during the first 2 h the amount of endogenous SA was still low and, therefore, may have been insufficient to interfere with JA production (Fig. 3). Very high levels of SA accumulated after 4 to 5 h and, hence, the biosynthesis of JA may be effectively blocked only in the later phase. This differential view considering also the metabolic dynamics of SA and JA production was strongly supported by the pre-incubation experiments with AcSA. Due to the presence of large amounts of AcSA already at the onset of ALA-treatment, the effect of SA on the octadecanoid pathway became more obvious. The lipid-based signaling pathway was clearly up-regulated but terminated with significant accumulation of OPDA. Further processing and oxidative degradation to JA did not occur (Fig. 4). The accumulation of early octadecanoids such as linolenic acid and OPDA as the only bioactive octadecanoids then easily accounts for the type of volatiles shown in Figure 1 (Koch et al., 1999). If the block of AcSA was bypassed by externally added JA, the full spectrum of JA-responsive volatiles was induced demonstrating that SA did not interfere with gene expression. The occasionally observed transient production of small amounts of JA-responsive volatiles such as hexenyl acetate or ocimene (Hopke et al., 1994) during the first 4 h after stimulation with ALA is, thus, in line with the kinetics of JA- and SA-production (Fig. 3). This finding, once more, demonstrates clearly the different roles of early and late octadecanoids in the signaling texture of the lima bean. However, more data are needed concerning local subcellular concentrations of octadecanoids, salicylates, and the kinetics of their accumulation along with information on the sensitivity and localization of their receptors.
As tendrils of B. dioica were known to respond to JA, MeJA, and OPDA with a coiling reaction comparable with the free coiling reaction of mechanically stimulated tendrils (Weiler et al., 1993), the successful induction of a coiling response by ALA was not surprising. All of the plant species tested exhibited a rapid coiling after treatment with ALA. However, unlike the induction of volatile compounds via the octadecanoid cascade, phenidone and aristolochic acid did not hamper the coiling reaction, indicating that signaling systems independent of the lipid-based pathway must exist. Membrane depolarization, probably inducing rapid auxin-translocation has to be considered as alternatives in mechanotransduction (Pickard and Ding, 1993; Klüsener et al., 1995). A further hint for octadecanoids not necessarily involved in the ALA-induced tendril-coiling reaction is the lack of lignification reaction of the Bianconi-plate, previously reported to depend on JA. This specific tissue of tendrils from B. dioica responds to mechanical stimulation and MeJA with a remarkable lignification starting approximately 20 h after the onset of the stimulus (Kaiser et al., 1994). Thus, ALA seems to induce a reaction comparable with contact-induced coiling but does not proceed further to free coiling as indicated by the absence of lignification.
Mutual interferences between the wound response- and SAR-pathways at different levels and targets have been shown to occur in other plants (Felton et al., 1999; Malek and Dietrich, 1999; Thaler, 1999). For example, in tomato the expression of a gene encoding for one of the enzymes of JA biosynthesis was repressed by SA (Peña-Cortés et al., 1993). Felton et al. (1999) have shown that silencing the expression of Phe ammonia lyase (PAL) reduces SAR against tobacco mosaic virus in tobacco, but at the same time enhanced grazing-induced systemic resistance to larvae of Heliothis virescens. However, overexpression of PAL-enhanced SAR and larval resistance was reduced. For the understanding of the early events of induction of plant defenses after pathogen or herbivore attack and their mutual interactions, the effect of channel-forming peptides like ALA (membrane depolarization) causing volatile production via induction of the octadecanoid pathway might represent a significant finding probably often involved in plant-pathogen and plant-insect interactions. The massive up-regulation of the SA pathway, which finally interferes with the signaling of the simultaneously induced octadecanoid pathway, represents a regulatory key element to modulate the plants response into the direction of SAR or wound response. As both signaling-pathways are claimed to exhibit mutually inhibitory effects (Felton et al., 1999; Malek and Dietrich, 1999; Preston et al., 1999), the relative amount (spatial and temporal) of the phytohormones eventually determine whether the typical wound responses (lipid-signaling) or the effects of a SAR will dominate the response reaction. Summarizing the above results we postulate that the elicitor-active compounds of an attacking organism, specifically up-regulating the relative internal levels of JA and SA in conjunction with the time course of their production, may represent the key elements that finally determine the expression of a subset of defense related genes and their products, respectively. Together with the presence of different receptors for different octadecanoids and SA in different tissues (Weiler, 1997; Koch et al., 1999), this multitude of signal-processing pathways and their mutual interaction via locally and spatially different concentrations of phytohormones may determine the pathogen- and herbivore-characteristic responses of plants. To date, the early events following leaf damage leading to the up-regulation of signal transduction pathways are not well known. Channel-forming peptides such as ALA and related compounds may be therefore used as valuable, organism-independent, tools to unravel the early events of plant defense reactions under well-defined conditions. Current analyses of insect salivary secretions will help to clarify whether or not channel-forming peptides and/or proteins, functionally related to ALA, have to be also considered as insect-characteristic elicitors for the induction of plant defense reactions. The first encouraging results in this direction will be published in due course.
MATERIALS AND METHODS
Plant Material
Volatile induction experiments were carried out using the lima bean Phaseolus lunatus (cv Ferry Morse var Jackson Wonder Bush). Individual plants were grown from seed in a plastic pot (⊘ = 5.5 cm) with sterilized potting soil at 23°C and 60% humidity using daylight fluorescent tubes at approximately 270 μE m−2 s−1 with a photophase of 16 h. Experiments were conducted with 12- to 16-d-old seedlings showing two fully developed primary leaves. Tendril coiling experiments were performed with Bryonia dioica, Pisum sativum (cv Gloriosa), and Lathyrus spp. Seeds of B. dioica were collected from plants at local habitats and stored at 4°C for 3 months. Germination was achieved by sowing in commercial soil and incubation at the above-described conditions. Seeds of P. sativum were purchased from a local garden center and seeds were treated as described for B. dioica. Tendrils or shoots were taken from several-month-old B. dioica or from 4- to 6-week-old Pisum plants. Tendrils of Lathyrus sp. were collected at local habitats.
Volatile Induction Experiments
Plantlets of P. lunatus with two fully developed primary leaves were cut with razor blades and immediately transferred into vials containing a solution of the test substance in tap water. ALA was dissolved in methanol at a concentration of 10 mg mL−1; 10 μL of the stock solution was added to 10 mL of tap water resulting in a final concentration of 10 μg mL−1 ALA in the test solution. To achieve a high concentration of emitted volatiles in the headspace, the vials with the cut plantlets were enclosed in a small desiccator (750 mL). The experimental set-up was kept at 25°C and illuminated during the first 12 h followed by a dark period of 8 h and another 4 h with illumination. The inhibitors phenidone and aristolochic acid were used at 1 and 0.3 mm, respectively. Freshly cut plantlets were pre-incubated with the solutions of the inhibitors for 24 h prior to induction experiments. Pre-incubation experiments with AcSA (0.5 mm) were carried out overnight (13 h) followed by transfer of the plantlet into a solution of ALA (10 μg mL−1). Pre-incubation with AcSA was significantly more effective than with free SA.
To demonstrate the effect of a high endogenous level of SA on the octadecanoid signaling pathway, plants were pre-incubated with ALA (5 μg mL−1) for 1 or 2 h and subsequently stimulated by application of cellulysin (50 μg mL−1) in the same vial. Volatile compounds were collected for 48 h as described (vide infra). Control experiments were run by placing freshly cut plantlets into tap water. Experiments were generally carried out in triplicate.
Collection and Analysis of Headspace Volatiles
The volatile compounds emitted from the pretreated plants were continuously collected over a period of 24 h on charcoal traps (1.5 mg of charcoal, CLSA-Filter, Le Ruisseau de Montbrun, F-09350 Daumazan sur Arize, France) using air circulation as described (Donath and Boland, 1995). After desorption of the volatiles from the carbon trap with dichloromethane (2 × 15 μL), the extracts were directly analyzed by gas chromatography (GC)/mass spectrometry (MS). GC-conditions consisted of a fused-silica capillary column Optima-5 (15 m × 0.25 mm, Macherey and Nagel, Düren Germany). Helium at 40 cm min−1 served as carrier gas. Separation of the compounds was achieved under programmed conditions (50°C for 1 min, then at 10°C min−1 to 180°C, then at 35°C min−1 to 280°C). MS: Finnigan GCQ; EI (70 eV). GC interface ran at 265°C with an ion source of 180°C and a scan range of 35 to 300 amu.
Quantification of Endogenous JA and SA
The quantification of endogenous JA and SA followed the protocol of McCloud and Baldwin (1997) originally developed for determination of endogenous jasmonic acid. Treated leaves (1.0 g of tissue) were frozen and ground under liquid nitrogen. The resulting powder was suspended in a solution of acetone and 50 mm citric acid (70:30 [v:v]). As internal standards [9,10-2H2]-9,10-dihydro-JA (146 ng) and [3,4,5,6-2H4]SA (500 ng) were added. The solvents were allowed to evaporate overnight at room temperature to avoid losses of volatile fatty acids. The resulting aqueous solutions were filtered and extracted with 3 × 10 mL of diethyl ether. The pooled extracts were then loaded onto a solid-phase extraction cartridge (500 mg of sorbent, aminopropyl, Varian, Darmstadt, Germany). After loading, the cartridges were washed with 7.0 mL of a solvent mixture of trichloromethane:2-propanol (2:1 [v/v]). Bound JA, SA, and the corresponding standards were eluted with 10 mL of diethyl ether:acetic acid (98:2 [v/v]). After evaporation of solvents and esterification of the residue with excess diazomethane, the sample was adjusted to 50 μL with dichloromethane. The solutions were analyzed by GC/MS without further purification. To enhance the sensitivity of the method, spectra were recorded in the selected ion mode, in case of JA-determination monitoring only the fragment ion at m/z = 83 amu corresponding to the base peaks of JA and [9,10-2H2]-9,10-dihydro-JA (Koch et al., 1999), and in case of SA-determination at m/z = 120 and 124 amu corresponding to the base peaks of SA and [3,4,5,6-2H4]SA, respectively. The amounts of endogenous JA and SA were calculated from the peak areas of JA and SA in comparison with the corresponding standards using calibration curves determined independently.
Quantification of Endogenous OPDA
Leaves (1.0 g of tissue) were frozen, ground under liquid nitrogen, and the resulting powder was extracted two times with 25 mL of peroxide-free ether. The pooled extracts were loaded onto a solid-phase extraction cartridge (500 mg of sorbent, aminopropyl, Varian). After loading, the cartridges were washed with 5.0 mL of a solvent mixture of trichloromethane:2-propanol (2:1 [v/v]). Bound OPDA was eluted with 10 mL of diethyl ether:acetic acid (98:2 [v/v]). After evaporation of solvents the residue was dissolved in 100 μL of methanol. An aliquot (60 μL) was analyzed by HPLC (Kontron System, autosampler 560, pump 525, DAD 440; column CC 250/4 Nucleosil 100-5 C-18; Macherey Nagel, Düren), flow rate 1 mL min−1, UV detection at 221 nm). For the mobile phase, acetonitrile (+0.1% [v/v] TFA) and water (+0.2% [v/v] TFA) were used in a binary gradient flow starting with 40% (v/v) CH3CN (5 min), 50% (v/v; 20 min), 60% (v/v; 55 min), and ending with 98% (v/v) CH3CN (5 min); RT(PDA) = 17.5 min. The quantification of endogenous OPDA was achieved by comparison of the peak area with a calibration curve determined independently with authentic material.
Tendril Coiling
To test the ability of ALA to induce tendril coiling, two different procedures were followed. a) Three tendrils of B. dioica were cut and immediately placed for 20 h into a Petri dish containing diluted solutions of the peptide. Controls were performed by using tap water with the solvent used for the stock solution of ALA (Falkenstein et al., 1991). b) Shoots with the youngest, well-developed tendrils were cut and immediately placed into vials with tap water. After regeneration from cutting stress the test solution with ALA was added and the extent of coiling was followed over a period of 20 h.
Chemicals
ALA, phenidone, and aristolochic acid were purchased from Sigma (St. Louis). Cellulysin was obtained from Calbiochem. Free JA was prepared from the methyl ester by saponification.
ACKNOWLEDGMENTS
We thank BASF (Ludwigshafen, Germany) and Bayer AG (Leverkusen, Germany) for generously supplying us with chemicals and solvents. We thank Dr. R. Kaiser (Givaudan-Roure, Dübendorf, Switzerland) for his generous supply of methyl jasmonate.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (Bonn) and the Fonds der Chemischen Industrie (Frankfurt).
LITERATURE CITED
- Alborn HT, Turlings TCJ, Jones TH, Stenhagen G, Loughrin JH, Tumlinson JH. An elicitor of plant volatiles from beet armyworm oral secretions. Science. 1997;276:945–949. [Google Scholar]
- Blechert S, Bockelmann M, Füβlein M, von Schrader T, Stelmach B, Niesel U, Weiler EW. Structure-activity analyses reveal the existence of two separate groups of active octadecanoids in elicitation of the tendril-coiling response of Bryonia dioica Jacq. Planta. 1999;207:470–479. [Google Scholar]
- Blechert S, Brodschelm W, Hölder S, Kammerer L, Kutchan TM, Müller MJ, Xia ZQ, Zenk MH. The octadecanoid pathway: signal molecules for the regulation of secondary pathways. Proc Natl Acad Sci USA. 1995;92:4099–4105. doi: 10.1073/pnas.92.10.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland W, Gäbler A, Gilbert M, Feng Z. Biosynthesis of C11 and C16 homoterpenes in higher plants: stereochemistry of the C-C-bond cleavage reaction. Tetrahedron. 1998;54:14725–14736. [Google Scholar]
- Boland W, Hopke J, Donath J, Nüske F, Bublitz F. Jasmonic acid and coronatine induce volatile biosynthesis in plants. Angew Chem Int Ed Engl. 1995;34:1600–1602. [Google Scholar]
- Bostok RM. Signal conflicts and synergies in induced resistance to multiple attackers. Physiol Mol Plant Pathol. 1999;55:99–109. [Google Scholar]
- Brewer D, Mason FG, Taylor A. The production of alamethicins by Trichoderma spp. Can J Microbiol. 1987;33:619–625. doi: 10.1139/m87-108. [DOI] [PubMed] [Google Scholar]
- Buonaurio R, Servili M. Involvement of lipoxygenase, lipoxygenase pathway volatiles, and lipid peroxidation during the hypersensitive reaction of pepper leaves to Xanthomonas campestris pv. vesicatoria. Physiol Mol Plant Pathol. 1999;54:155–169. [Google Scholar]
- Cafiso DS. Alamethicin: a peptide model for voltage gating and protein-membrane interactions. Annu Rev Biophys Biomol Struct. 1994;23:141–165. doi: 10.1146/annurev.bb.23.060194.001041. [DOI] [PubMed] [Google Scholar]
- Cucurou C, Battioni JP, Thang DC, Nam NH, Mansuy D. Mechanisms of inactivation of lipoxygenase by phenidone and BW755C. Biochemistry. 1991;30:8964–8970. doi: 10.1021/bi00101a008. [DOI] [PubMed] [Google Scholar]
- Dangl JL, Hauffe KD, Lipphart S, Hahlbrock K, Scheel D. Parsley protoplasts retain differential responsiveness to UV light and fungal elicitor. EMBO J. 1987;6:2551–2556. doi: 10.1002/j.1460-2075.1987.tb02543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit PJGM. Pathogen avirulence and plant resistance: a key role for recognition. Trends Plant Sci. 1997;2:452–458. [Google Scholar]
- Dicke M, Gols R, Ludeking D, Posthumus MA. Jasmonic acid and herbivory differentially induce carnivore-attracting plant volatiles in lima bean plants. J Chem Ecol. 1999;25:1907–1922. [Google Scholar]
- Dicke M, Sabelis MW, Takabayashi J, Bruin J, Posthumus MA. Plant strategies of manipulating predator-prey interactions through allelochemicals: prospects for application in pest control. J Chem Ecol. 1990;16:3091–3118. doi: 10.1007/BF00979614. [DOI] [PubMed] [Google Scholar]
- Doares SH, Narvaez-Vasques J, Conconi A, Ryan CA. Salicylic acid inhibits synthesis of proteinase inhibitors in tomato leaves induced by systemin and jasmonic acid. Plant Physiol. 1995;108:1741–1746. doi: 10.1104/pp.108.4.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donath J. Oxidativer Abbau von Nerolidol zu 4,8-Dimethylnona-1,3,7-trien in höheren Pflanzen: mechanismus und induktion. PhD thesis. Germany: University of Karlsruhe; 1994. [Google Scholar]
- Donath J, Boland W. Biosynthesis of acyclic homoterpenes: enzyme selectivity and absolute configuration of the nerolidol precursor. Phytochemistry. 1995;39:785–790. [Google Scholar]
- Ebel J, Cosio EG. Elicitors of plant defense responses. Int Rev Cytol. 1994;148:1–36. [Google Scholar]
- Falkenstein E, Groth B, Mithöfer A, Weiler EW. Methyl jasmonate and α-linolenic acid are potent inducers of tendril coiling. Planta. 1991;185:316–322. doi: 10.1007/BF00201050. [DOI] [PubMed] [Google Scholar]
- Farmer EE, Ryan CA. Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell. 1992;4:129–134. doi: 10.1105/tpc.4.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felton GW, Korth KL, Bi JL, Wesley SV, Huhmann DV, Methews MC, Murphy JB, Lamb C, Dixon RA. Inverse relationship between systemic resistance of plants to microorganisms and to insect herbivory. Curr Biol. 1999;9:317–320. doi: 10.1016/s0960-9822(99)80140-7. [DOI] [PubMed] [Google Scholar]
- Gäbler A, Boland W, Preiss U, Simon H. Stereochemical studies on homoterpene biosynthesis in higher plants: phylogenetic, ecological and mechanistic aspects. Helv Chim Acta. 1991;74:1773–1789. [Google Scholar]
- Green TR, Ryan CA. Wound-induced proteinase inhibitor in plant leaves: a possible defense against insects. Science. 1972;175:776–777. doi: 10.1126/science.175.4023.776. [DOI] [PubMed] [Google Scholar]
- Greulich F, Yoshihara T, Ichihara A. Coronatine, a bacterial phytotoxin, acts as a stereospecific analog of jasmonate type signals in tomato cells and potato tissue. J Plant Physiol. 1995;147:359–366. [Google Scholar]
- Hahlbrock K, Scheel D, Logeman E, Nürnberger T, Parniske M, Reinhold S. Oligopeptide elicitor-mediated defense gene activation in cultured parsley cells. Proc Natl Acad Sci USA. 1995;92:4150–4157. doi: 10.1073/pnas.92.10.4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopke J, Donath J, Blechert S, Boland W. Herbivore-induced volatiles: the emission of acyclic homoterpenes from leaves of Phaseolus lunatus and Zea mays can be triggered by a β-glucosidase and jasmonic acid. FEBS Lett. 1994;352:146–150. doi: 10.1016/0014-5793(94)00948-1. [DOI] [PubMed] [Google Scholar]
- Huang Q, Tezuka Y, Hatanaka Y, Kikuchi T, Nishi A, Tubaki K. Studies on metabolites of mycoparasitic fungi: IV. Minor peptaibols of Trichoderma koningii. Chem Pharmacol Bull. 1995;34:1035–1038. doi: 10.1248/cpb.43.1663. [DOI] [PubMed] [Google Scholar]
- Jabs T, Tschöpe M, Colling C, Hahlbrock K, Scheel D. Elicitor-stimulated ion fluxes and O2- from the oxidative burst are essential components in triggering defense gene activation and phytoalexin synthesis in parsley cells. Proc Natl Acad Sci USA. 1997;92:4150–4157. doi: 10.1073/pnas.94.9.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser I, Engelberth J, Groth B, Weiler EW. Touch- and methyl jasmonate-induced lignification in tendrils of Bryonia dioica Jacq. Bot Acta. 1994;107:24–29. [Google Scholar]
- Keen NT. Gene-for-gene complementarity in plant-pathogen interactions. Annu Rev Genet. 1990;24:447–463. doi: 10.1146/annurev.ge.24.120190.002311. [DOI] [PubMed] [Google Scholar]
- Klüsener B, Boheim G, Liβ H, Engelberth J, Weiler EW. Gadolinium-sensitive, voltage-dependent calcium release channels in the endoplasmatic reticulum of a higher plant mechanoreptor organ. EMBO J. 1995;14:2708–2714. doi: 10.1002/j.1460-2075.1995.tb07271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch T, Krumm T, Jung V, Engelberth J, Boland W. Differential induction of plant volatile biosynthesis in the lima bean by early and late intermediates of the octadecanoid signaling pathway. Plant Physiol. 1999;121:153–162. doi: 10.1104/pp.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudert D, Weiler EW. Allene oxide synthase: a major control point in Arabidopsis thaliana octadecanoid signaling. Plant J. 1998;15:675–684. doi: 10.1046/j.1365-313x.1998.00245.x. [DOI] [PubMed] [Google Scholar]
- Likterink W, Kroj T, zur Nieden U, Hirt H, Scheel D. Receptor-mediated activation of a MAP kinase in pathogen defense of plants. Science. 1997;276:2054–2057. doi: 10.1126/science.276.5321.2054. [DOI] [PubMed] [Google Scholar]
- Malek K, Dietrich RA. Defense on multiple fronts: how do plants cope with diverse enemies? Trends Plant Sci. 1999;4:215–219. doi: 10.1016/s1360-1385(99)01415-6. [DOI] [PubMed] [Google Scholar]
- McCloud ES, Baldwin IT. Herbivory and caterpillar regurgitants amplify the wound-induced increases in jasmonic acid but not nicotine in Nicotiana sylvestris. Planta. 1997;203:430–435. [Google Scholar]
- Nürnberger T. Signal perception in plant pathogen defense. Cell Mol Life Sci. 1999;55:167–182. doi: 10.1007/s000180050283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nürnberger T, Wirtz W, Nennstiel D, Hahlbrock K, Jabs T, Zimmermann S, Scheel D. Signal perception and intracellular signal transduction in plant pathogen defense. J Recept Sign Trans Res. 1997;17:127–136. doi: 10.3109/10799899709036598. [DOI] [PubMed] [Google Scholar]
- Paré PW, Tumlinson JH. Induced synthesis of plant volatiles. Nature. 1997;385:30–31. [Google Scholar]
- Parker JE, Schulte W, Hahlbrock K, Scheel D. An extracellular glycoprotein from Phytophthora megasperma f.sp. glycinea elicits phytoalexin synthesis in cultured parsley cells and protoplasts. Mol Plant Microbe Interact. 1991;4:19–27. [Google Scholar]
- Peña-Cortés H, Albrecht T, Prat S, Weiler EW, Willmitzer L. Aspirin prevents wound-induced gene expression in tomato leaves by blocking jasmonic acid biosynthesis. Planta. 1993;191:123–128. [Google Scholar]
- Pickard BG, Ding JP. The mechanosensory calcium-selective ion channnel: key component of a plasmalemmal control center? Aust J Plant Physiol. 1993;20:439–459. doi: 10.1071/pp9930439. [DOI] [PubMed] [Google Scholar]
- Piel J, Atzorn R, Gäbler R, Kühnemann F, Boland W. Cellulysin from the plant parasitic fungus Trichoderma viride elicits volatile biosynthesis in higher plants via the octadecanoid signaling cascade. FEBS Lett. 1997;416:143–148. doi: 10.1016/s0014-5793(97)01169-1. [DOI] [PubMed] [Google Scholar]
- Piel J, Donath J, Bandemer K, Boland W. Mevalonate-independent biosynthesis of terpenoid volatiles in plants: induced and constitutive emission of volatiles. Angew Chem Int Ed Engl. 1998;37:2478–2481. doi: 10.1002/(SICI)1521-3773(19981002)37:18<2478::AID-ANIE2478>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Preston CA, Lewandowski C, Enyedi AJ, Baldwin IT. Tobacco mosaic virus inoculation inhibits wound-induced jasmonic acid-mediated responses within but not between leaves. Planta. 1999;209:87–95. doi: 10.1007/s004250050609. [DOI] [PubMed] [Google Scholar]
- Raskin I. Role of salicylic acid in plants. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:439–463. [Google Scholar]
- Rosenthal MD, Vishwanath BS, Franson RC. Effects of aristolochic acid on phospholipase-A2 activity and arachidonate metabolism of human-neutrophils. Biochim Biophys Acta. 1989;1001:1–8. doi: 10.1016/0005-2760(89)90299-3. [DOI] [PubMed] [Google Scholar]
- Sansom MSP. Structure and function of channel-forming peptaibols. Q Rev Biophys. 1993;26:365–421. doi: 10.1017/s0033583500002833. [DOI] [PubMed] [Google Scholar]
- Scherer GFE, Arnold B. Inhibitors of animal phospholipase A2 enzymes are selective inhibitors of auxin-dependent growth: implications of for auxin-induced signal transduction. Planta. 1997;202:462–469. [Google Scholar]
- Shulaev V, Silverman P, Raskin I. Airborne signalling by methyl salicylate in plant pathogen resistance. Nature. 1997;385:718–721. [Google Scholar]
- Thaler JA. Jasmonate-inducible plant defenses cause increased parasitism of herbivores. Nature. 1999;399:686–688. [Google Scholar]
- Turlings TCJ, Tumlinson JH, Lewis WJ. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science. 1990;250:1251–1253. doi: 10.1126/science.250.4985.1251. [DOI] [PubMed] [Google Scholar]
- Wasternack C, Ortel B, Miersch O, Kramell R, Beale MH, Greulich F, Feussner I, Hause B, Krumm T, Boland W, Parthier B. Diversity in octadecanoid-induced gene expression of tomato. J Plant Physiol. 1998;152:345–352. [Google Scholar]
- Weiler EW. Octadecanoid-mediated signal transduction in higher plants. Naturwissenschaften. 1997;84:340–349. [Google Scholar]
- Weiler EW, Albrecht T, Groth B, Xia ZQ, Luxem M, Liss, Andert L, Spengler P. Evidence for the involvement of jasmonates and their octadecanoid precursors in the tendril coiling response of Bryonia dioica. Phytochemistry. 1993;32:591–600. [Google Scholar]
- Weiler EW, Kutchan TM, Gorba T, Brodschelm W, Niesel U, Bublitz F. The Pseudomonas phytotoxin coronatine mimics octadecanoid signaling molecules of higher plants. FEBS Lett. 1994;345:9–13. doi: 10.1016/0014-5793(94)00411-0. [DOI] [PubMed] [Google Scholar]