Abstract
Metamemory involves the cognitive ability to assess the strength of one's memories. To explore the possibility of metamemory in non-human animals, numerous behavioral tasks have been created, many of which utilize an option to decline memory tests. To assess metamemory in rats, we utilized this decline-test option paradigm by adapting previous visual delayed-match-to-sample tests (DMTS)1,2 developed for primate species to an odor-based test suitable for rodents. First, rats are given a sample to remember by digging in a cup of scented sand. After a delay, the rat is presented with four distinctly scented cups, one of which contains the identical scent experienced during the sample; if this matching cup is selected, then the rat obtains a preferred, larger reward. Selection of any of the other three non-matching sand-filled scented cups results in no reward. Retention intervals are individually titrated such that subjects perform between 40 and 70% correct, therefore ensuring rats sometimes remember and sometimes forget the sample. Here, the operational definition of metamemory is the ability to distinguish between the presence and absence of memory through behavioral responding. Towards this end, on two-thirds of trials, a decline option is presented in addition to the four choice cups (choice trials). If the decline-test option- an unscented colored sand cup, is selected, the subject receives a smaller less-preferred reward and avoids the memory test. On the remaining third of trials, the decline-test option is not available (forced trials), causing subjects to guess the correct cup when the sample is forgotten. On choice tests, subjects that know when they remember should select the decline option when memory is weak rather than take the test and choose incorrectly. Therefore, significantly higher performance on chosen tests as compared to forced memory tests is indicative of the adaptive use of the decline-test response and metacognitive responding.
Keywords: Behavior, Issue 136, Metamemory, episodic memory, cognitive control, memory monitoring, comparative cognition, memory awareness, odor memory, working memory
Introduction
Metamemory is the ability to assess the strength of one's memories2,3,4. While humans have consistently demonstrated this cognitive ability, there is debate over the existence of metamemory in non-human animals e.g.5,6. One common method for testing for the possibility of metamemory is to present nonhuman animals with a decline-test option, which, if selected results in a guaranteed, but smaller reward than the reward for a correct response to the test. If subjects can monitor their memory states, then they should decline tests when their memories are weak (for reviews see:7,8,9,10).
Over the past few decades, procedures developed for human and nonhuman primates11 and pigeons12 have been adapted in several ways to further test for the presence of metamemory in monkeys1,2,13. Perhaps the most seminal study of metamemory in monkeys was Hampton's1 delayed match-to-sample test (DMTS). After training in the MTS task, delays between the sample and test were titrated so that the difficulty level was intermediate such that monkeys had a chance of remembering and forgetting the sample. Following the delay, monkeys were presented with the option to decline the test. Results showed that subjects performed significantly more accurately on trials in which they chose to take the test than on forced trials, suggesting that monkeys monitored their memory strength of the sample1.
Most metamemory paradigms tested thus far have utilized visual or auditory stimuli, which is not surprising given that the most widely tested species have been highly visual nonhuman primates e.g.11,14. Of the previous limited research that exists on metacognition in rats, the sensory modality used has also been visual or auditory15,16,17,18. Given the recent robust evidence for metacognitive responding when an odor-based task was used19, it is possible that the previous equivocal results in rats could have been due to the lack of standardized paradigms used. We designed a paradigm19 which capitalized on rat's primary sense, olfaction, by adapting the DMTS paradigm similar to the one used in monkeys described above1. The task described here involves digging in sand-filled cups to obtain a food reward, taking advantage of rodent's natural digging behavior.
Metamemory can be assessed in rats using the odor-based DMTS task presented here. First, a hierarchy of food rewards is established so subjects demonstrate a clear preference for a more valued reward. Rats are then trained to dig in a cup of scented sand to obtain a reward. Following the presentation of a scented sample cup, subjects must choose one of four distinctly scented cups. A reward is buried at the bottom of the cup with the same scent as the sample. After rats are proficient on the basic MTS tests, retention intervals (RIs) are titrated for each rat so that performance falls between a 40% and 70% success rate. On 2/3 of the trials, rats are given the choice to decline the memory test for a less preferred reward. Rats' metacognitive ability is assessed by comparing performance on trials in which the decline-test option is presented (choice trials) to trials in which rats are forced to take the test (forced trials).
While only Long-Evans rats were tested in this paradigm, the task could be used to test other strains of laboratory rats and mice to further study metamemory in rodent species. It will also be beneficial to test aged rodents or transgenic mice with Alzheimer's disease pathologies to better understand how memory disorders affect metacognitive processes.
Protocol
All methods described here have been approved by the Institutional Animal Care and Use Committee (IACUC) of Providence College.
1. Creating the Testing Apparatus
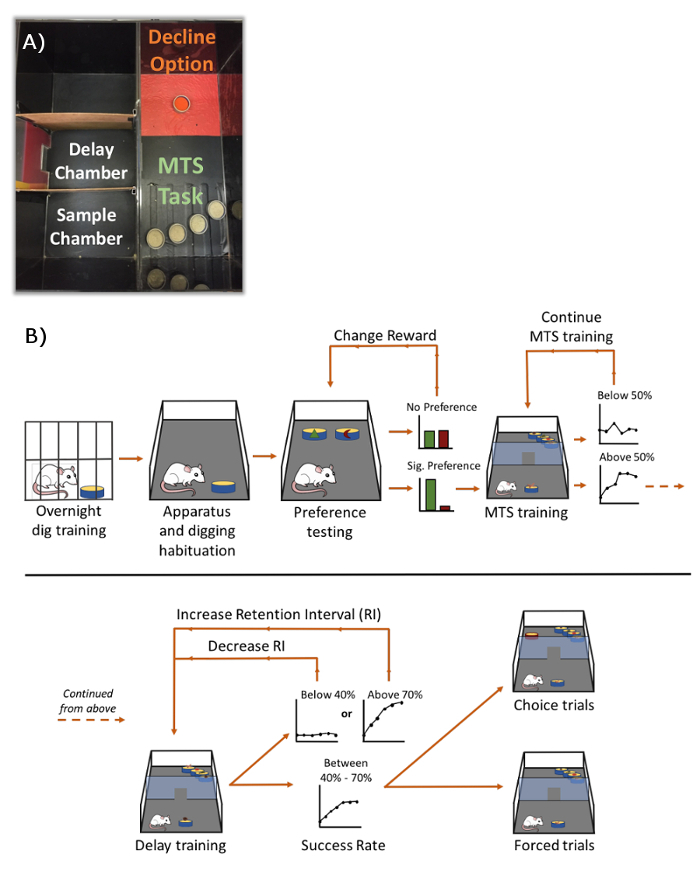
- Use a Social Interaction Chamber (76.2 x 114.3 cm.), which is divided into 3 separate rooms.
- Divide the center room into a sample and delay camber using a cardboard insert that can be lifted by sliding through metal tracks attached to the side walls.
Use opaque contact paper to cover the clear walls to prevent visual access to other rooms.
On one side of the room-on-the-right (testing room; see Figure 1), cover the floor with sand granules and red contact paper. This will serve as visual and tactile a discriminative cue for the decline option.
On the opposite side of the testing room, cover the floor with raised stripes using strips of masking tape to serve as a visual and tactile a discriminative cue for the choice test. Cover the stripes with black contact paper. Leave the remaining middle third of the chamber unaltered.
2. Developing Preferred Food Reward Hierarchy/Preference Testing
- Select possible food rewards for testing. Ensure one reward to be a larger reward and therefore "preferred" over the other.
- Use a whole piece of cereal as larger, "preferred" reward and a ¼ piece of cereal as smaller, "less preferred" reward. Note: Cereals can include sweetened cereal or dehydrated marshmallow. It is also possible to use two different rewards (e.g. cereal vs. standard rodent pellet (TestDiet, 45 mg)), but it was difficult to determine a reliable preference ranking within and across individuals when quality rather than quantity was the source of variation across rewards.
Bring rats (adult male Long Evans) into dark testing room 30 min prior to testing begins. Use red lighting to illuminate testing chamber, if rats are in the dark phase of their day's cycle. If not, test under normal lighting conditions as shown in video.
Place the two rewards, the whole piece of cereal and the ¼ piece of cereal, in opposite corners of the right room of the chamber (see Figure 1).
- Place the rat on the opposite side of the chamber, facing away from the rewards, and allow the rat to eat one of the rewards. Note: To pass preference testing and to ensure that the larger piece of cereal is the preferred reward, the subject must eat the larger reward on 4 out of 5 trials (5 trials per session) for 2 consecutive sessions. Run two sessions per day.
- If, after 10 sessions, the rat has not shown a preference for the larger piece of cereal, introduce a different reward.
- Throughout testing, to ensure that rats' preferences remain consistent, perform preference checks once a week.
- Run preference checks in the same manner as preference testing, with the whole and 1/4 piece of cereal on opposite corners of the chamber. Note: 2 trials comprise a preference check.
- If rats select the less preferred reward for both trials, test rat on a full preference test of 5 trials.
- If rat passes, it may continue testing.
- If rat fails, cease testing and introduce a different type or amount of reward.
3. General Methods
Use consistent experimenters throughout testing so rats are fully habituated to experimenter odors and handling. Note: It is assumed that prior to testing the rats have been handled for at least 1 week by humans, so that they are habituated to being picked up and carried by the experimenters.
Bring rats in their shoebox cages (home-cage) into the testing room 30 min before testing begins to acclimate them to the new room.
If testing is done during the dark phase of the rats’ cycle, keep the testing environment dark, with the exception of red lights to illuminate the apparatus.
Ensure that the scented choice cups are arranged in the proper order as determined by a randomization script that includes for counterbalancing. Bait the correct cup with the whole piece of cereal.
Only allow the rat to dig in one cup and immediately remove the rat from the apparatus once a cup has been selected as indicated by digging. Place the rat in home-cage where the rat can finish consuming the reward.
- The inter-trial-interval (ITI) is fixed to 4 min. Begin timing on a stopwatch once rat is in the home-cage.
- During the ITI, clean the apparatus by vacuuming any spilled sand and wiping down the floor with 70% isopropyl alcohol. Discard the reward if it was not eaten during the previous trial. Also, once a week, clean out ceramic cups by discarding old sand and wiping cups with isopropyl alcohol.
- Prepare the sand cups for the next trial by rearranging the order based on the randomization script, and baiting the correct cup.
4. Training to Dig in Sand Cups and Habituation to Testing Apparatus
Pour approximately ½ cup of play sand into ceramic cups (6.99 cm. in diameter and 8.81 cm tall).
Place the sand-filled cup with food rewards in each rat's home cage overnight. Note: Rats are required to successfully dig in the cup and eat the reward overnight for two consecutive nights.
- Habituate rat to testing apparatus by placing the subject in apparatus for 5 min.
- Spread food rewards throughout the apparatus to encourage apparatus exploration and traveling through opened doors. Ensure that rats do not urinate, defecate, or freeze inside the chamber for two days in a row.
Transfer odorless sand-filled cup with food reward to the testing apparatus. Ensure that rats successfully dig in the sand and retrieve the reward at least two days in a row with no freezing behavior, defecation, or urination. If necessary, remove the ceramic cups and place cereals in the chamber.
5. Match-to-Sample (MTS) Training
- Create 4 different scented piles of sand by mixing approximately 3 cups of play sand with ¼ teaspoon of scented spice (cinnamon, coffee, paprika, and thyme). To control for odor cues, crumble one piece of reward in incorrect choice cups.
- Remix scented sand once per week and store in glass containers so that scent strength is consistent across testing days.
- Pour ½ cup of each scented sand into 4 cups and arrange the cups in a semi-circle on the black contact paper (MTS Task area; Figure 1A). Ensure that all cups are equidistant from the entrance door.
- Organize the cups by placing them in the assigned position based on the output from the programming software "R" using the sampling without replacement function.
- Out of view of the subject, bury a full piece of reward in the correct scented cup. Bury ½ piece of reward in an identically-scented sample cup. Bury all rewards at least a ½ way down the height of the sand.
- Pseudo-randomly choose the correctly scented cup, the only constraint is that no one scent is the sample scent more than 2 times in a row.
Place the subject in the sample chamber and lower the sample cup using a bent spatula (handle-tray at a 90-degree angle). Allow subject to dig in the sample cup and retrieve the ½ cereal reward. Remove the cup as soon as the subject begins eating the reward.
Once the subject has finished eating the reward, lift the cardboard room divider and open the door to the testing chamber as the subject enters the delay room.
- Allow the subject to "select" one of the cups by digging. As soon as the subject makes a choice and retrieves a reward if present, remove the subject from the chamber and place in the transfer cage. Rats may sniff each cup, but they are only allowed one choice as indicated by digging.
- During the 4-min ITI, clean the chamber with 70% isopropyl alcohol. Vacuum any sand that may have spilled and replenish sand cups if needed.
- Obtain next sample cup and bait both the sample and testing cup. If the subject selects the incorrect cup in the previous trial, remove and discard the uneaten reward.
Ensure that subjects receive one 6-trial MTS session per day. In order to pass MTS training, subjects must choose the correct cup three of the six trials in two consecutive sessions. Note: This ensures that performance is above chance but below the ceiling on the 4-choice test.
6. Delay Titration in Delayed Match to Sample (DMTS)
Continue training subjects on MTS as before (starting with the delay of 0 s). Note: Performance must remain between 40 and 70% correct, to ensure that performance is well above chance (25%), but below the ceiling. Performance in this range allows subjects to experience instances when they when remember the same and instances when they forget the sample. This creates an opportunity for use of the decline-test response to be advantageous to the subject when chosen trials are introduced.
- If performance exceeds 70% correct for 2 consecutive 6-trial sessions, increase the delay from 0 to 30 s.
- In trials on which the delay is not 0 s, use a stopwatch to time each delay. After the subject has consumed the sample, raise the cardboard divider wall and allow subject to enter into the delay chamber. Close the cardboard divider and begin timing the delay on the stopwatch.
- After 30 s has passed, open the door to the testing room, and shut the door once the subject has entered the testing chamber.
- Carry on the rest of the trial as before.
If performance continues to remain above 70% correct for 2 consecutive 6-trial sessions, increase each subsequent delay by 1.5 times the previous delay.
If performance falls below 40% for 2 consecutive sessions, the delay is returned to the previous one used.
If performance remains between 40% and 70% for 2 consecutive sessions, set that delay as the subject-specific criterion delay.
7. Decline Use Training
After each session of DMTS training, place another cup of a different textured and colored sand, opposite the scented sand cups. Bait the cup with the less-preferred reward, ¼ piece of the cereal, which will be the decline cup. Remove the scented sand cups from the chamber.
Carry out the trial as during DMTS training, allowing the subject to sample a randomly scented cup and wait in the delay chamber for the subject-specific delay.
Allow subject to dig in the unscented sand cup and retrieve the reward. Then remove the subject from the chamber. This will be considered a "forced decline" trial.
Conduct two forced decline trials after each session of DMTS training.
8. Testing: Forced vs. Choice Trials
- Test subjects on DMTS using the criterion delay. Test each subject on one 12-trial session per day.
- Randomly select 8 of the trials to be "choice" trials and place the decline cup opposite the scented cups. Use randomization script to determine the order of trial-type with the constraint that no more than 3 choice or forced trials occur in a succession. Note: The remaining 4 trials will be "forced," with the decline cup removed from the chamber.
- Depending on the subject's criterion delay, it may be possible to test 2 or 3 subjects during one rat's ITI. During 1 subject's ITI, run the second subject's trial. Ensure to clean the chamber with isopropyl alcohol after each trial.
- Test subjects on ten sessions.
During each trial, once the rat enters the delay chamber ensure that he either turn left to decline the test or turn right to take the test (see Figure 1A; top-down photograph). Record if rat travels from one side of the room to the other (e.g. "decides to take the test" and then later traverses to the decline-test side of the room). Note: The rat, therefore, makes a prospective metamemory choice as he chooses to take or decline the test before encountering the four choice odors (see results paper19 for thorough discussion of concurrent vs. prospective metamemory tests).
- Calculate the accuracy on forced trials continuously after two sessions of testing and monitor decline-test use.
- If accuracy rises above 70% correct over the course of two sessions, cease testing and return to delay titration with a longer delay.
- If decline-test use approaches 0% or 100% consistently (over 2 sessions), pause testing and consider increasing or decreasing the value of the decline-test response ensuring the decline rates to be between 10% and 40%.
- If accuracy falls below 40% correct for 2 sessions, cease testing and return to delay titration with a shorter delay.
- Return to testing after accuracy on forced trials remains between 40% and 70% correct for two consecutive sessions.
- Throughout the testing, to ensure that rats' preferences remain the same, perform preference checks (2 trials a session) once a week. Preference checks are described above.
9. Suggested Generalization Tests
- Generalization tests, such as those described in the results paper19 can be used to determine if metacognitive responding transfers across conditions in which no single association with external stimuli is likely to control responding.
- Test rats on no-sample probe trials, in which no sample phase is presented but the RI and test phase of trials is identical to normal trials. Randomly intermix 4 probe trials with 8 normal sample trials to comprise a 12-trial session. Test subjects on 10 sessions. Compare the decline rates on no sample trials with the decline rates on normal trials.
- Test rats on double sample trials, in which subjects are presented with the original sample scent twice to increase memory strength. Randomly intermix probe trials with normal sample trials as explained above. Compare decline rates on double sample trials with the decline rates on normal trials.
- Test rats with three different RIs: the criterion delay, one delay which is shorter (e.g. 0 s) than the criterion delay, and one which is longer (e.g. 4 min). For each delay within a session present subjects with four choice trials and two forced trials, such that sessions are comprised of 18 trials. Randomly intermix all trials and test rats on ten sessions. Compare the decline rates of trials on three delays, where memory strength should be decreased and increased, respectively.
10. Data Analysis
- After testing is complete, calculate average proportion correct for forced and chosen trials for each subject.
- To find the proportion of correct forced trials, divide the number of correct forced trials by the total number of forced trials.
- To find the proportion of correct chosen trials, first subtract the trials declined from the total number of choice trials. Divide the number of correct choice trials by the total number of choice trials minus declined trials for each rat.
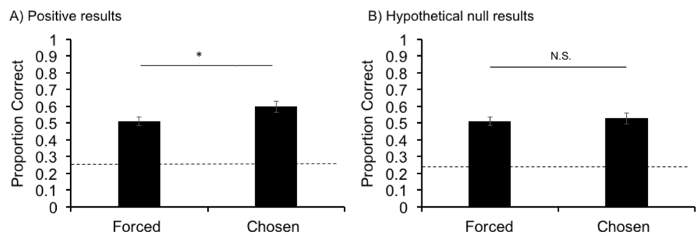
- Conduct a t-test on group data to compare the proportion of correct forced trials to the proportion of correct chosen trials (see Figure 2).
- If generalization tests are run, conduct t-tests on decline rates on normal trials vs. probe trials as described in 9. For mixed delay trials, as described in 9.1.3, compare forced and chosen tests with a repeated measures ANOVA with delay and trial-type as a within-subject factor. Also, conduct a one-way ANOVA on decline use across delays to determine if accuracy varied with RI.
Representative Results
In order to study the metacognitive responding of the subject, the proportion of correct forced trials (baseline memory performance) is compared with the proportion of correct chosen trials, or trials in which there is a decline option present. Positive results are indicated by significantly higher accuracy on chosen trials as compared to forced trials (Figure 2A). These results indicate that rats may be monitoring their memory states and declining the task when their memories are weak, leading to an increase in accuracy when a decline option is present on chosen trials.
If performance is not significantly more accurate on chosen trials as compared to forced trials, results are null. This hypothetical lack of evidence for metamemory in rats (Figure 2B) is indicated by failure to use the decline-test option when it would have been adaptive to do so: on trials in which the sample was forgotten.
If generalization tests are run, metacognitive performance would be indicated if subjects decline no-sample trials significantly more normal sample trials and decline double sample trials significantly more than normal sample trials. Decline use should show an inverse relationship with memory accuracy as indicated on accuracy on forced trials. If accuracy on forced tests does not vary with trial condition, change parameters (e.g. increase RI) as it is impossible to determine if subjects are or are not behaving metacognitively. On mixed delays tests, accuracy on chosen tests should be significantly higher than accuracy on forced tests and subjects should be more likely to decline tests with longer RIs and least likely to decline tests with shorter RIs. See results paper for more details on generalization tests and respective interpretations19.
Figure 1. Photograph of experimental apparatus and process. A) Photograph of apparatus adapted from19 with permission. B) Experimental process.The first step of habituation, teaching rats to dig in sand-filled cups, takes place over two nights. Next, the rats are habituated to the experimental apparatus and are trained to dig in sand-filled cups there. Subjects are then given the choice of two rewards, represented as a green triangle and red crescent (whole piece of cereal vs. a quarter piece of cereal). After rats show a significant preference for one reward, they transition to MTS training, in which cups are filled with scented sand, represented by images of cinnamon, paprika, thyme, and coffee. If the success rate is below 50% for two sessions, the subject continues training. If the success rate of the rats is at 50% or above, the subject progresses to delay titration. During delay titration, rats continue with MTS training, but a delay between the sample and test is introduced (starting with 0 s). If rats' memory accuracy is above 70%, then the RI is increased. If accuracy falls below 40%, the RI is decreased, and MTS training continues. This loop continues until rats' accuracy falls between 40% and 70% for two consecutive sessions. At this point, rats progress to the experimental test which consists of randomly intermixed forced and choice trials. Please click here to view a larger version of this figure.
Figure 2. Positive results indicating metacognitive responding and hypothetical null results indicating lack of metacognitive responding. A) Positive results adapted from 19 with permission. The average proportion correct of forced and chosen trials was derived from the performance of 9 rats on 120 trials each. *=p<.05 on paired two-tailed t-test. A significantly higher proportion of trials answered correctly on chosen trials as compared to forced trials indicates the appropriate use of the decline-test response. B) An equivalent proportion of trials answered correctly on forced and chosen trials indicates lack of declining tests on the basis of memory strength. Chance, 0.25, is indicated by the dotted line. Error bars represent standard error of the means. Please click here to view a larger version of this figure.
Discussion
Presented here is a novel method for assessing metacognitive responding in rats using an odor-based DMTS task. Due to olfaction as rodents' primary sense, the use of odor is preferable to visual cues in rats and mice 20,21. The use of digging in the sand is a natural foraging behavior of rats, making the task ecologically-relevant for rodent species.
A crucial step in the protocol is ensuring that there is a hierarchy of food rewards, one of which is clearly preferred over the other. During early phases of the study, rats were required to choose between the presumed to be preferred reward, a piece of sweetened cereal, and small standard food pellet. However, preference testing yielded indiscriminate choice behavior, causing the use of quantity as the source of reward variation. Rats chose between a whole piece of cereal and a ¼ piece of cereal, and reliably favored the larger quantity. Without a preference, whether food quality or quantity, there is no incentive to take the test over declining it, so this step is crucial.
Another important aspect of the protocol is establishing proper RIs and ITIs so that MTS accuracy remains within the range of 40 to 70% correct. If delays are too short, and interference is minimal- due to long ITIs, rats never experience "forgetting" the sample. Conversely, if RIs are too long and the ITIs are too short, the test becomes too difficult and rats never remember the sample. Such responses can be detected not only by accuracy on forced trials but by decline rates that are either too high or too low. Subjects should decline about 10-50% of choice trials: if a subject declines every trial or never declines, data cannot be interpreted as positive or negative for the presence of metamemory and the relative value of the decline-test response needs to be re-evaluated. Throughout the experiment, average performance on forced trials is consistently calculated at the end of two days of testing. As rats' progress through more trials of DMTS, their performance may improve, which would initiate a return delay titration (see Figure 1B) such that delays can be appropriately increased such that accuracy remains below 70% correct. If memory for the sample is consistently above 70% consider decreasing the ITI. If memory for the sample is consistently below 40% consider increasing the ITI and/or increasing the odor sample size from four to 10-20 odors to decrease memory interference.
Depending on the strain of rats used, housing environment, and light-dark cycle animals are housed in, memory may be weaker or stronger. Compared to other laboratory rat strains, such as Wistar or Sprague Dawleys, Long Evans are known to acquire cognitive tasks with relatively fewer trials22,23. It may, therefore, take more stages of habituation, and more training for other rodents to learn the task. Rats in the present study were housed in highly enriched environments with access to exercise so it is possible their memories were relatively strong24,25,26. Rats used here were also housed on a reversed light-dark cycle which may increase cognitive performance since they were tested during their dark cycle.
Adaptive use of the decline-test response, indicated by significantly higher performance on choice as compared to forced tests suggests metacognitive responding. However, metacognitive responding could result from reliance on internal memory cues or external cues8. To determine if in fact adaptive use of the decline-test response is the result of internal memory cues, experimenters should aim to eliminate as many external cues as possible8,14. External cues are considered any publicly available test-specific stimuli or cues that subjects could use to guide decline-test use8. Potential external cues in this task include relative salience of individual odors, sample duration, response latency, and duration of RIs. Generalization tests as described here and in more detail the results paper19 are useful in ruling out behavioral responding based on external cues like environmental cue associations due to cue inconsistency in task parameters across experiments.
In this paradigm, the rat is required to make the decision to take or decline the test before the memory test is presented (see 8.2), largely eliminating the potential for response competition to control use of the decline-test response. Response competition is the propensity to take the test based on the sight or smell of the correct test option when the metacognitive choice is presented concurrently with the primary memory test8. Requiring rats to choose to take or decline the test before encountering the memory test itself significantly reduces the possibility that response competition controls use of the decline-test response. However, one improvement to this design would be to have rats choose to take or decline the test by making a particular response in a room separate from the memory test itself. This would further increase the prospective nature of this paradigm, which may allow for the more successful elimination of external cues8,19. An additional improvement for future studies is to, if possible, record response latency at the point when the rat enters the testing room to the time it takes for the subject to turn left or turn right (decline or take the test, respectively). This would allow the researchers to determine if response latency served as an external cue subjects learned to associate with particular responses overtime (see8). It would also be enlightening to record if and when subjects change their response (see 8.2; e.g. travel from the MTS choice side of the room to the decline test area or vice versa). As discussed in the results paper, we observed this behavior only once, which adds to the conclusion that rats made a decision to take or decline the test as soon as they entered the testing room and before they encountered the memory test itself, which they did by systematically sampling each choice odor.
Because rats tested in this paradigm transferred metacognitive responding across multiple generalization tests, it was concluded that rats were capable of metamemory- they could monitor their memories for the sample19. It will be important for other research groups to test this paradigm or similar adaptations of it as corroborating evidence from different laboratories is needed before it can be concluded with certainty that rats are capable of metacognitive responding and that behavior is controlled by an internal rather than external cue. Testing for the presence of this important mnemonic ability, which degrades with age, may prove useful in translational approaches to studying memory dysfunction, such as with transgenic mice and models of Alzheimer's disease. It would be especially fruitful for researchers to employ this behavioral paradigm in conjunction with brain lesions, temporary brain inactivation's or activations (e.g. optogenetics, chemogenetics), and with electrophysiological recordings, as these investigations may elucidate the neural mechanisms underlying metacognitive processes.
Disclosures
There are no disclosures to report.
Acknowledgments
We thank Rebecca Burwell for the use of her laboratory during video recording and Robert Vera for help with a previous version of this manuscript.
References
- Hampton RR. Rhesus monkeys know when they remember. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5359–5362. doi: 10.1073/pnas.071600998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templer VL, Hampton RR. Rhesus monkeys (Macaca mulatta) show robust evidence for memory awareness across multiple generalization tests. Animal Cognition. 2012;15(3):409–419. doi: 10.1007/s10071-011-0468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton JE, Shettleworth SJ. Memory without awareness: Pigeons do not show metamemory in delayed matching to sample. Journal of Experimental Psychology-Animal Behavior Processes. 2008;34(2):266–282. doi: 10.1037/0097-7403.34.2.266. [DOI] [PubMed] [Google Scholar]
- Nelson TO, Narens L. Metamemory: A theoretical framework and new findings. Psychology of Learning and Motivation. 1990;26:125–322. [Google Scholar]
- Carruthers P. Meta-cognition in animals: A skeptical look. Mind & Language. 2008;23(1):58–89. [Google Scholar]
- Crystal JD. Where Is the Skepticism in Animal Metacognition? Journal of Comparative Psychology. 2014;128(2):152–154. doi: 10.1037/a0034427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornell N. Where Is the "Meta" in Animal Metacognition? Journal of Comparative Psychology. 2014;128(2):143–149. doi: 10.1037/a0033444. [DOI] [PubMed] [Google Scholar]
- Hampton RR. Multiple demonstrations of metacognition in nonhumans: Converging evidence or multiple mechanisms. Comparative cognition & behavior reviews. 2009;4:17–28. doi: 10.3819/ccbr.2009.40002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Couchman JJ, Beran MJ. Animal Metacognition: A Tale of Two Comparative Psychologies. Journal of Comparative Psychology. 2014;128(2):115–131. doi: 10.1037/a0033105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Zakrzewski AC, Church BA. Formal models in animal-metacognition research: the problem of interpreting animals' behavior. Psychonomic Bulletin & Review. 2016;23(5):1341–1353. doi: 10.3758/s13423-015-0985-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Shields WE, Washburn DA. Memory monitoring by animals and humans. Journal of Experimental Psychology-General. 1998;127(3):227–250. doi: 10.1037//0096-3445.127.3.227. [DOI] [PubMed] [Google Scholar]
- Inman A, Shettleworth SJ. Detecting metamemory in nonverbal subjects: A test with pigeons. Journal of Experimental Psychology-Animal Behavior Processes. 1999;25(3):389–395. [Google Scholar]
- Brown EK, Templer VL, Hampton RR. An assessment of domain-general metacognitive responding in rhesus monkeys. Behavioural Processes. 2017;135:132–144. doi: 10.1016/j.beproc.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile BM, Schroeder GR, Brown EK, Templer VL, Hampton RR. Evaluation of Seven Hypotheses for Metamemory Performance in Rhesus Monkeys. Journal of Experimental Psychology-General. 2015;144(1):85–102. doi: 10.1037/xge0000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote AL, Crystal JD. Metacognition in the rat. Current Biology. 2007;17(6):551–555. doi: 10.1016/j.cub.2007.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote AL, Crystal JD. "Play it Again": a new method for testing metacognition in animals. Animal Cognition. 2012;15(2):187–199. doi: 10.1007/s10071-011-0445-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki S, Okanoya K. Rats Show Adaptive Choice in a Metacognitive Task With High Uncertainty. Journal of Experimental Psychology-Animal Learning and Cognition. 2017;43(1):109–118. doi: 10.1037/xan0000130. [DOI] [PubMed] [Google Scholar]
- Kirk CR, McMillan N, Roberts WA. Rats Respond for Information: Metacognition in a Rodent? Journal of Experimental Psychology-Animal Behavior Processes. 2014;40(2):249–259. doi: 10.1037/xan0000018. [DOI] [PubMed] [Google Scholar]
- Templer VL, Lee KA, Preston AJ. Rats know when they remember: transfer of metacognitive responding across odor-based delayed match-to-sample tests. SpringerLink; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A, Vosshall LB. Better smelling through genetics: mammalian odor perception. Current Opinion in Neurobiology. 2008;18(4):364–369. doi: 10.1016/j.conb.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett SA. The rat: A study in behavior. Transaction Publishers; 2007. [Google Scholar]
- Andrews JS. Possible confounding influence of strain, age and gender on cognitive performance in rats. Cognitive Brain Research. 1996;3(3):251–267. doi: 10.1016/0926-6410(96)00011-0. [DOI] [PubMed] [Google Scholar]
- Kumar G, Talpos J, Steckler T. Strain-dependent effects on acquisition and reversal of visual and spatial tasks in a rat touchscreen battery of cognition. Physiology & Behavior. 2015;144(Supplement C):26–36. doi: 10.1016/j.physbeh.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. European Journal of Neuroscience. 2005;21(2):513–521. doi: 10.1111/j.1460-9568.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J. Neurobiol. 1999;39(4) doi: 10.1002/(sici)1097-4695(19990615)39:4<569::aid-neu10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nature reviews. Neuroscience. 2000;1(3):191. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]


