Abstract
The palps of locust mouthparts are considered to be conventional gustatory organs that play an important role in a locust's food selection, especially for the detection of non-volatile chemical cues through sensilla chaetica (previously named terminal sensilla or crested sensilla). There is now increasing evidence that these palps also have an olfactory function. An odorant receptor (LmigOR2) and an odorant-binding protein (LmigOBP1) have been localized in the neurons and accessory cells, respectively, in the sensilla basiconica of the palps. Single sensillum recording (SSR) is used for recording the responses of odorant receptor neurons, which is an effective method for screening active ligands on specific odorant receptors. SSR is used in functional studies of odorant receptors in palp sensilla. The structure of the sensilla basiconica located on the dome of the palps differs somewhat from the structure of those on the antennae. Therefore, when performing an SSR elicited by odorants, some specific advice may be helpful for obtaining optimum results. In this paper, a detailed and highly effective protocol for an SSR from insect palp sensilla basiconica is introduced.
Keywords: Neuroscience, Issue 136, Single sensillum recordings, palp, olfaction, sensilla basiconica, chemosensory neuron, locust
Introduction
Animals have evolved a range of chemosensory organs that sense exogenous chemical cues. In insects, the most important chemosensory organs are the antennae and the palps. On these organs, several types of chemosensory hairs, called chemosensory sensilla, are innervated by chemosensory neurons (CSNs) within the hairs. CSNs in chemosensory sensilla recognize specific chemical cues through signal transduction from chemical stimuli to electrical potentials that are subsequently transferred up to the central nervous systems1,2,3.
CSNs express various chemosensory receptors [e.g., odorant receptors (ORs)], ionotropic receptors (IRs), and gustatory receptors (GRs) on their membranes, which encode exogenous chemical cues associated with different types of chemosensation4,5,6. The characterization of CSNs is key to the elucidation of cellular and molecular mechanisms of insect chemoreception. Now single sensillum recording (SSR) is a widely-used technique for the characterization of insect CSNs in the antennal sensilla of many insects, including flies7, moths8, beetles9, aphids10, locusts11, and ants12. However, few studies have applied an SSR to insect palps13,14,15,16,17, because the particular structures of their sensilla make an electrophysiological recording difficult18.
Swarms of locusts (Orthoptera) often cause serious crop damage and economic loss19. The palps are believed to play an important role in the food selection of locusts20,21,22,23,24. Two types of chemosensory sensilla are investigated by a scanning electron microscope (SEM). Usually, 350 sensilla chaetica and 7 - 8 sensilla basiconica are observed on each dome of the locust palps18. Sensilla chaetica are gustatory sensilla that sense non-volatile chemical cues, whereas sensilla basiconica have an olfactory function, sensing volatile chemical cues.
On locust palps, the diameters of the hair sockets of the sensilla basiconica (ca. 12 µm), are much greater than those of sensilla chaetica (ca. 8 µm)18,25. The cuticular wall of the sensilla basiconica on the palps is much thicker than that of antennal sensilla18. In addition, the dome of the palp has fluid contents within a highly flexible cuticle. These characteristics mean that a penetration with a microelectrode and an acquisition of good electrophysiological signals is more difficult than for antennal sensilla. In this paper, a detailed and highly effective SSR protocol for locust palp sensilla basiconica is presented with a video.
Protocol
1. Preparation of Instruments and Insect
- Preparing tungsten electrodes and stimuli solutions
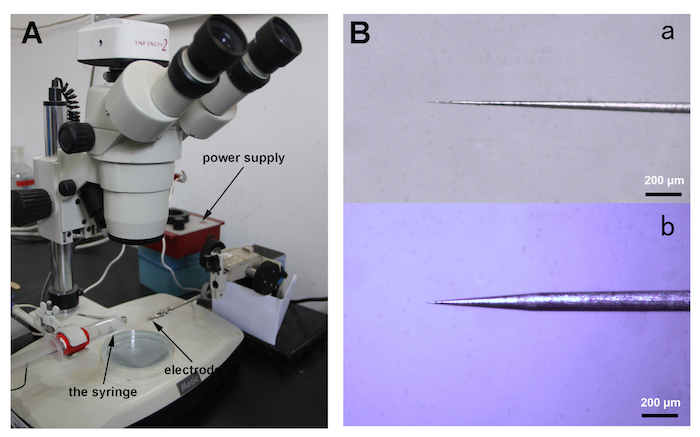
- Fix a new tungsten wire (diameter of 0.125 mm, length of 75 mm) into a micromanipulator and sharpen it in a 10% (w/v) sodium nitrite (NaNO2) solution in a syringe at 10 V provided by a power supply for about 1 min under a stereomicroscope (40X magnification).
- Dip the sharpened tungsten wire repeatedly into the 10% NaNO2 solution, about 4 mm at 5 V in < 1 min (Figure 1A).
- Examine the diameter of the sharpened tungsten tip frequently under the stereomicroscope until it is fine enough to penetrate the cuticle of a locust palp olfactory sensillum (Figure 1B).
- Prepare the stimulus solutions. Dilute each of the chemical stimulus substance in mineral oil. Dilute 1-nonanol and nonanoic acid at 10% dilutions. Dilute E-2-hexenal and hexanal at 10-2, 10-3, 10-4, and 10-5.
- Prepare Pasteur tubes carrying the stimuli: insert filter paper strips (length of 2 cm, width of 0.5 cm) into the Pasteur tubes, add the diluted stimulus solutions (each 10 µl) to the filter paper strips, and then plug the Pasteur tubes with pipette tips (1 ml).
- Prepare the insect
- Rear locusts (Locusta migratoria) with fresh wheat seedlings under crowded conditions at a relative humidity of 60%, a temperature of 28 - 30 °C, and a photoperiod of 18:6 h (light:dark). Choose 1- to 3-day-old 5th instar locust nymphs and remove the antennae with fine scissors to avoid any interference when recording.
- Preparing the locust maxillary palp holder
- Use a glass slide (25 mm x 75 mm) as the base of the maxillary palp holder (MPH). Attach a plastic piece (1 mm in height, 10 mm in width, 35 mm in length) to a corner of the glass slide with double-sided adhesive tape, and finally fix a cover glass (18 mm x 18 mm) on top of the plastic piece with double-sided adhesive tape. Place a small piece of red rubber tape onto the cover glass as a non-slip layer. The plastic piece and the cover glass constitute the platform for the locust palp. The height of the platform is approximately 1.5 mm.
- Install a tungsten wire (diameter of 0.125 mm, length of 36 mm) at a distance of 1.5 mm parallel to the inside edge of the platform. Fix the two ends of the wire onto the platform with double-sided adhesive tape.
2. Preparation of Locust Maxillary Palps
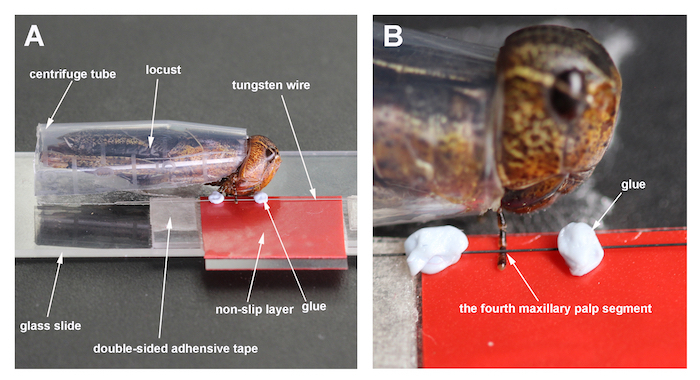
Cut a centrifuge tube (1.5 ml) vertically in half and cut off the bottom. Place the locust into the prepared tube. Leave the ventral region and the head of the locust exposed. Fix the assembly to the glass slide with double-sided adhesive tape (Figure 2A).
Pull the right maxillary palp onto the platform.
Put the tungsten wire at the fourth segment of the palp. Place adhesive putty on each side of the tungsten wire, about 2 mm from the maxillary palp (Figure 2A and 2B).
3. Single Sensillum Recordings
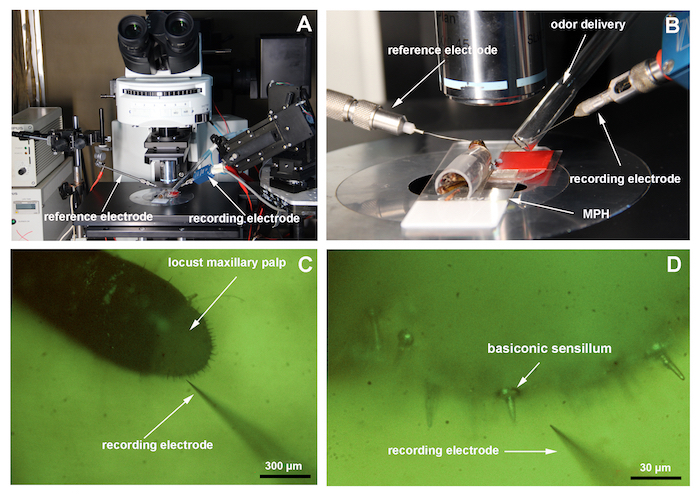
Place the locust maxillary palp preparation under a microscope at a low magnification (100X). Adjust the position of the preparation until the palp is perpendicular to the recording electrode (Figure 3A).
Insert the reference electrode (tungsten electrode) into the locust eye using a micromanipulator. Move the recording electrode (tungsten electrode) close to the maxillary palp with the micromanipulator (Figure 3B and 3C).
Adjust the odor delivery device to about 1 cm from the maxillary palp (Figure 3B).
Open the recording software Auto Spike 32. Set the recording parameters as follows: the recording scale on 500 µV; the high cutoff of the filter on 300 Hz, the low cutoff on 200 Hz; and the pretrigger on 10 s.
Connect the recording electrode to a 10x universal AC/DC amplifier.
Switch the microscope to a high magnification (500X). Insert the recording electrode into the base of a basiconic sensillum on the maxillary palp and delicately adjust the recording electrode to obtain good spontaneous spikes (Figure 3D).
Open the stimulus controller to deliver a continuous air stream at 20 ml/s. Set the stimulation time to 1 s. Record signals for 10 s, starting 10 s before the onset of the stimulus pulse.
Use a 10x universal AC/DC amplifier to amplify the signals. Feed the signals into the IDAC 4. Analyze the signals with the Auto Spike 32 software. AC signals are band-pass filtered between 200 to 300 Hz. Use Auto Spike 32 to distinguish peak-to-trough amplitudes from noises. Calculate the responses of the neurons as the increases in action potential frequencies (spikes per second) over the spontaneous frequencies. Perform a statistical analysis using GraphPad Prism 7.
Representative Results
Two sensilla subtypes (pb1 and pb2) on the locust maxillary palp are identified based on different response dynamics to chemical odorants (10% 1-nonanol and 10% nonanoic acid). The neurons in pb1 produce significantly more spikes to 1-nonanol than to nonanoic acid while the neurons in pb2 are significantly less activated by 1-nonanol compared with nonanoic acid (Figure 4). Hexanal and E-2-Hexenal can evoke a locust palp opening response (POR)26. Hexanal is an abundant host plant green leaf volatile which may contribute to a further confirmation to the food source26. The spikes elicited in the pb1 neurons last longer than those of pb2 when they are stimulated by E-2-hexenal (Figure 4). The neurons in pb1 and pb2 exhibit similarly robust responses to hexanal (Figure 4). Comparing the mean changes of all spikes between the periods 5 s before and 5 s after the stimulation indicates that the response to 1-nonanol is significantly higher than to nonanoic acid in pb1, but contrarily in pb2 (Figure 5). The neurons in these two subtypes of sensilla respond dose-dependently to E-2-hexenal and hexanal, and their response patterns to these two aldehydes differ (Figure 6A and 6B).
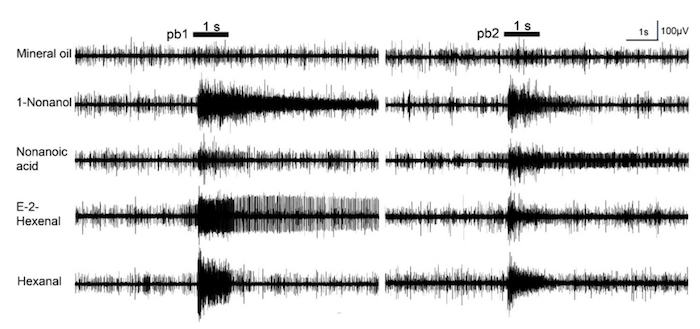
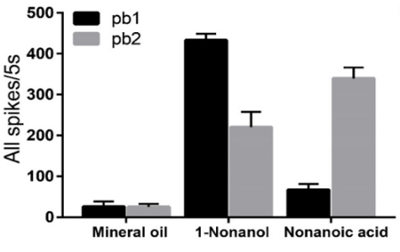
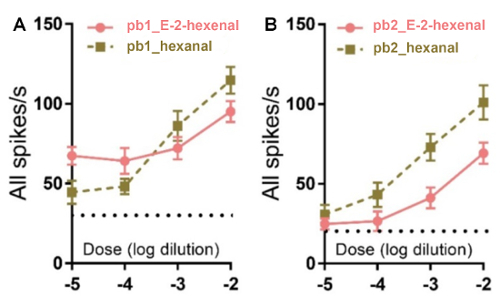
Figure 1. Electrode preparation. (A) This panel shows a general view of the electrode sharpening apparatus. The syringe containing 10% NaNO2 (left) is used to sharpen the electrode (right). (B) This panel shows a close view of the electrode tip (a: suitable; b: unsuitable). Please click here to view a larger version of this figure.
Figure 2. Locust maxillary palp holder (MPH). (A) The MPH and a locust are mounted on the glass slide before positioning it under the microscope. (B) This panel shows a close-up of the locust maxillary palp, fixed by tungsten wire on the platform. Please click here to view a larger version of this figure.
Figure 3. Single sensillum recordings. (A) This panel shows a view of the electrophysiology setup. (B) This panel shows a close view of the locust preparation mounted on the microscope. (C) This image shows the locust maxillary palp at 100X magnification. (D) This image shows the palp at 500X magnification. The arrow indicates a basiconic sensillum. Please click here to view a larger version of this figure.
Figure 4. Response traces of single sensillum recordings of the locust maxillary palp. In this panel, pb1 stands for subtype 1 of the palp sensilla basiconica; pb2 stands for subtype 2 of the palp sensilla basiconica. The bars above the traces indicate the stimulus duration (1 s). For these recordings, all odors are used at 10% dilutions except for E-2-hexenal and hexanal, which are diluted to 1%. This figure has been modified from Zhang et al.26. Please click here to view a larger version of this figure.
Figure 5. Comparison of mean numbers of spikes in neurons in pb1 and pb2 stimulated by nonanoic acid and 1-nonanol. The mean numbers of the spikes are calculated in the periods 5 s before and after stimulation. In pb1, the mean numbers of the spikes in the neurons responding to 1-nonanol increase significantly higher than those of the spikes in neurons responding to nonanoic acid (n = 11 palps; ANOVA with post hoc t-tests; p < 0.0001), in contrast to pb2 (n = 10 palps; ANOVA with post hoc t-tests; p = 0.0110). The error bar represents SEM. This figure has been modified from Zhang et al.26. Please click here to view a larger version of this figure.
Figure 6. The patterns of neurons in pb1 and pb2 responding dose-dependently to E-2-hexenal and hexanal. (A) This panel shows the patterns of the neurons in pb1 (± SEM; n = 12 palps). (B) This panel shows the patterns of the neurons in pb2 (± SEM; n = 10 palps). This figure has been modified from Zhang et al.26. Please click here to view a larger version of this figure.
Discussion
Insects rely on palps to detect food odors, and their palps are believed to play an important role in speciation13,27. The palps are simple olfactory organs and are receiving increasing attention as an attractive model for the exploration of the neuromolecular networks underlying chemosensation28.
Insect labellar and palp SSRs have been successfully performed on Drosophila melanogaster, Anopheles gambiae, and Culex quinquefasciatus13,14,15,16,17 but have rarely been reported in the form of a video presentation16,29. In contrast, video data on antennal SSRs are available for Drosophila, the navel orangeworm moth (Amyeloistransitella), Schistocerca Americana, and the bed bug (Cimex lectularius)16,30,31,32,33.
Locust palp sensilla basiconica have a particular structure that differs from that of locust antennal sensilla and many other insect sensilla. Using the method described here, action potentials generated by locust palp sensilla basiconica subtypes pb1 and pb2 could be recorded and discriminated (Figure 4 and Figure 5).
The critical step is the penetration of the recording electrode. The recording electrode should be inserted into the base of the sensillum and advanced until good signals are acquired. In addition, it is important to prevent the dome of the palp from collapsing when the recording electrode is inserted into the base of the sensillum. To achieve this, we set up a platform including a special locust maxillary palp holder (MPH) and used a tungsten wire to compress the fourth segment of the palp. Many repetitions of this procedure demonstrate that this is effective. Based on the response patterns of the neurons in the sensilla to several odorants, we have, for the first time, identified two subtypes of sensilla basiconica on the locust maxillary palp, namely pb1 and pb2.
The limitation of the technique outlined in this publication is that it could be used to record big insects (e.g., moths, beetles, and locusts) while not to record small insects (e.g., flies and mosquitoes), which have their own platforms and techniques13,14,15,16,17. This technique is complementary to existing methods.
In conclusion, a highly effective protocol of an SSR from insect palp sensilla basiconica is described in detail. This protocol could provide researchers with a useful technique in the study of molecular and cellular mechanisms of insect olfaction on the mouthpart. This method linked with gas chromatography could be used to identify natural electrophysiologically-active ligands in extracts of favorable food resources.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work is supported by a grant from the National Natural Science Foundation of China (No.31472037). Any mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply a recommendation.
References
- Carey AF, Carlson JR. Insect olfaction from model systems to disease control. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(32):12987–12995. doi: 10.1073/pnas.1103472108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal WS. Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Annual Review of Entomology. 2013;58:373–391. doi: 10.1146/annurev-ento-120811-153635. [DOI] [PubMed] [Google Scholar]
- Zhang J, Walker WB, Wang G. Pheromone reception in moths: from molecules to behaviors. Progress in Molecular Biology and Translational Science. 2015;130:109–128. doi: 10.1016/bs.pmbts.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Amrein H, Morozov PS, Rzhetsky A, Axel R. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell. 1999;96(5):725–736. doi: 10.1016/s0092-8674(00)80582-6. [DOI] [PubMed] [Google Scholar]
- Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136(1):149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annual Review of Neuroscience. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- de Bruyne M, Foster K, Carlson JR. Odor coding in the Drosophila antenna. Neuron. 2001;30:537–552. doi: 10.1016/s0896-6273(01)00289-6. [DOI] [PubMed] [Google Scholar]
- Roelofs W, et al. Sex pheromone production and perception in European corn borer moths is determined by both autosomal and sex-linked genes. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(21):7585–7589. doi: 10.1073/pnas.84.21.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson MC, Leal WS, Hansson BS. Olfactory receptor neurons detecting plant odours and male volatiles in Anomala cuprea beetles (Coleoptera: Scarabaeidae) Journal of Insect Physiology. 2001;47(9):1065–1076. doi: 10.1016/s0022-1910(01)00087-7. [DOI] [PubMed] [Google Scholar]
- Zhang R, et al. Molecular basis of alarm pheromone detection in aphids. Current Biology. 2017;27(1):55–61. doi: 10.1016/j.cub.2016.10.013. [DOI] [PubMed] [Google Scholar]
- Cui X, Wu C, Zhang L. Electrophysiological response patterns of 16 olfactory neurons from the trichoid sensilla to odorant from fecal volatiles in the locust, Locusta migratoria manilensis. Archives of Insect Biochemistry and Physiology. 2011;77(2):45–57. doi: 10.1002/arch.20420. [DOI] [PubMed] [Google Scholar]
- Sharma KR, et al. Cuticular hydrocarbon pheromones for social behavior and their coding in the ant antenna. Cell Reports. 2015;12(8):1261–1271. doi: 10.1016/j.celrep.2015.07.031. [DOI] [PubMed] [Google Scholar]
- de Bruyne M, Clyne PJ, Carlson JR. Odor coding in a model olfactory organ: the Drosophila maxillary palp. Journal of Neuroscience. 1999;19(11):4520–4532. doi: 10.1523/JNEUROSCI.19-11-04520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed Z, Leal WS. Maxillary palps are broad spectrum odorant detectors in Culex quinquefasciatus. Chemical Senses. 2007;32(8):727–738. doi: 10.1093/chemse/bjm040. [DOI] [PubMed] [Google Scholar]
- Lu T, et al. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Current Biology. 2007;17(18):1533–1544. doi: 10.1016/j.cub.2007.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino M, Nakagawa T, Vosshall LB. Single sensillum recordings in the insects Drosophila melanogaster and Anopheles gambiae. Journal of Visualized Experiments. 2010;36:e1725. doi: 10.3791/1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant AJ, Wigton BE, Aghajanian JG, O'Connell RJ. Electrophysiological responses of receptor neurons in mosquito maxillary palp sensilla to carbon dioxide. Journal of Comparative Physiology A. 1995;177(4):389–396. doi: 10.1007/BF00187475. [DOI] [PubMed] [Google Scholar]
- Blaney W. The ultrastructure of an olfactory sensillum on the maxillary palps of Locusta migratoria (L.) Cell and Tissue Research. 1977;184(3):397–409. doi: 10.1007/BF00219899. [DOI] [PubMed] [Google Scholar]
- Hassanali A, Njagi PGN, Bashir MO. Chemical ecology of locusts and related acridids. Annual Review of Entomology. 2005;50:223–245. doi: 10.1146/annurev.ento.50.071803.130345. [DOI] [PubMed] [Google Scholar]
- Chapman RF. Contact chemoreception in feeding by phytophagous insects. Annual Review of Entomology. 2003;48:455–484. doi: 10.1146/annurev.ento.48.091801.112629. [DOI] [PubMed] [Google Scholar]
- Chapman RF, Sword G. The importance of palpation in food selection by a polyphagous grasshopper (Orthoptera: Acrididae) Journal of Insect Behavior. 1993;6:79–91. [Google Scholar]
- Winstanley C, Blaney W. Chemosensory mechanisms of locusts in relation to feeding. Entomologia Experimentalis et Applicata. 1978;24:750–758. [Google Scholar]
- Blaney W, Duckett A. The significance of palpation by the maxillary palps of Locusta migratoria (L.): an electrophysiological and behavioural study. Journal of Experimental Biology. 1975;63:701–712. doi: 10.1242/jeb.63.3.701. [DOI] [PubMed] [Google Scholar]
- Blaney W. Electrophysiological responses of the terminal sensilla on the maxillary palps of Locusta migratoria (L.) to some electrolytes and non-electrolytes. Journal of Experimental Biology. 1974;60:275–293. doi: 10.1242/jeb.60.2.275. [DOI] [PubMed] [Google Scholar]
- Jin X, Zhang S, Zhang L. Expression of odorant-binding and chemosensory proteins and spatial map of chemosensilla on labial palps of Locusta migratoria (Orthoptera: Acrididae) Anthropod Structure & Development. 2006;35(1):47–56. doi: 10.1016/j.asd.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li H, Zhang L. Two olfactory pathways to detect aldehydes on locust mouthpart. International Journal of Biological Sciences. 2017;13(6):759–771. doi: 10.7150/ijbs.19820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dweck HKM, et al. Olfactory channels associated with the Drosophila maxillary palp mediate short- and long-range attraction. eLife. 2016;5:e14925. doi: 10.7554/eLife.14925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohbot JD, Sparks JT, Dickens JC. The maxillary palp of Aedes aegypti, a model of multisensory Integration. Insect Biochemistry and Molecular Biology. 2014;48:29–39. doi: 10.1016/j.ibmb.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Delventhal R, Kiely A, Carlson JR. Electrophysiological recording from Drosophila labellar taste sensilla. Journal of Visualized Experiments. 2014;84:e51355. doi: 10.3791/51355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng R, Lin HH, Wang JW, Su CY. Electrophysiological recording from Drosophila trichoid sensilla in response to odorants of low volatility. Journal of Visualized Experiments. 2017;125:e56147. doi: 10.3791/56147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed Z, Leal WS. Electrophysiological measurements from a moth olfactory system. Journal of Visualized Experiments. 2011;49:e2489. doi: 10.3791/2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha D, Leong K, Katta N, Raman B. Multi-unit recording methods to characterize neural activity in the locust (Schistocerca Americana) olfactory circuits. Journal of Visualized Experiments. 2013;71:e50139. doi: 10.3791/50139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Liu N. Using single sensillum recording to detect olfactory neuron responses of bed bugs to semiochemicals. Journal of Visualized Experiments. 2016;107:e53337. doi: 10.3791/53337. [DOI] [PMC free article] [PubMed] [Google Scholar]


