Abstract
Failed back surgery syndrome (FBSS) refers to persistent, chronic pain following spinal surgery. Spinal cord stimulation with dorsal epidural leads can be used to treat back and leg pain in FBSS patients. This paper presents a detailed protocol for using spinal cord stimulation with surgical leads in FBSS patients. In our department, with the patient under general anesthesia, we place the lead in the epidural space by means of a small laminectomy at the 10th thoracic level. Placement of the lead is followed by a 1 month trial period with an externalized lead. If pain relief is greater than 50% at the end of this 1 month stimulation trial (required by Belgian reimbursement criteria), an internal pulse generator is then placed under the skin and connected to the lead in a second surgical procedure. We have demonstrated that using this technique in rigorously selected FBSS patients can significantly improve back pain, leg pain, patient activity, and quality of life for a sustained period of time.
Keywords: Behavior, Issue 136, Low back pain, failed back surgery syndrome, spinal cord stimulation, multicolumn lead, neuropathic pain, leg pain
Introduction
Failed back surgery syndrome (FBSS) refers to persistent, chronic pain following spinal surgery. Before FBSS can be established, other conditions, such as persistent nerve root compression, permanent nerve root injury as a consequence of the original compression or surgery, arachnoiditis, incorrect initial diagnoses, and documented mechanical causes for low back pain (such as spinal instability, posterior joint osteoarthritis, spondylolysthesis, and discogenic pain), should be excluded1,2,3. FBSS patients may have severe or disabling neuropathic pain, and the syndrome is worrisome since it is chronic and resistant to conventional treatment. Its incidence and prevalence are similar to those of rheumatoid arthritis4. It is thus a great burden for industrialized societies5.
Spinal cord stimulation (SCS) with dorsal epidural leads can be used to treat back pain (BP) and leg pain (LP) in FBSS patients, although the SCS pain relief mechanism has not been fully investigated. According to the gate control theory, which was first proposed by Ronald Melzack and Patrick Wall in 1965, the brain monitors the activity of a tiny neural network distributed along the dorsal horn of the spinal cord that is regulated by nociceptive but also non-nociceptive afferents6. These complex circuits in the superficial dorsal horn of the spinal cord are responsible for relieving pain in a specific location in the body when an intense tactile stimulus is applied to the same place7,8. However, several studies have shown that SCS is not only dependent on this theory. Several neurotransmitters (acetylcholine, gamma-aminobutyric acid, and serotonin) have been reported to have a role in the SCS mechanism9,10,11,12, as well as numerous brain areas13,14 and different spinal fibers15.
An SCS device includes an epidural lead, internal pulse generator (IPG), and an extension cable to connect the lead to the IPG. The stimulation settings are adjusted by an external remote control. Two types of electrode can be used, namely, percutaneous and surgical leads.
The efficacy of SCS in improving the radicular LP component of FBSS has already been demonstrated in previously published studies, including the EVIDENCE study16, and two new technologies have recently been shown to be effective for treating the BP component, which is known to be recalcitrant to SCS. The first one is high-frequency stimulation17. The second one is the use of surgical multicolumn lead3,18. The main disadvantages of the former technique are that their batteries need more frequent charging and that the programming optimization is challenged by longer "wash-in" times19. The latter technique is more invasive than the percutaneous high-frequency stimulation technique because it requires a small laminectomy, making peroperative paresthesia mapping under local anesthesia challenging, although possible3. Indeed, this procedure can be performed under local anesthesia supplemented by conscious sedation or spinal anesthesia3. In our institution, when targeting back pain in FBSS patients, we place such surgical leads through a small laminectomy at the 10th thoracic level with the patient under general anesthesia. The lasting efficacy of this method with such multicolumn leads for treating LP and BP in FBSS patients has been demonstrated by 3 year follow-up18. A detailed protocol of our method is given below.
Protocol
1. Patient Selection
Make a diagnosis of FBSS20.
- Be sure that the FBSS patient meets all the inclusion criteria before being accepted for an SCS trial:
- Chronic low back pain and/or radicular leg pain that were refractory to optimal medical therapy for a minimum of 6 months.
- Persistent pain despite 3 types of pharmacological treatments (paracetamol, non-steroidal anti-inflammatory drugs, opioids, antidepressant medications, anticonvulsant medications, etc.).
- Lemniscal pathways must remain at least partially intact (for paresthesia coverage of the painful area).
Rule out exclusion criteria for SCS in FBSS patients such as active infection, drug addiction or ongoing withdrawal, psychological contraindications, documented mechanical causes of low BP (discogenic pain, spondylolisthesis, spinal instability, and posterior joint osteoarthritis), and coagulopathy (due to risk of bleeding and epidural hematoma). Make sure that no coagulopathy is present by asking relevant questions, examining the patient and, if necessary, checking relevant blood tests for coagulopathy.
2. Neuromodulation Specialist
If the FBSS patient meets the criteria for SCS, refer him to the neuromodulation specialist (NMS). NOTE: The neuromodulation specialist in our team is a nurse. He is fully dedicated to pain and neuromodulation and is the coordinator of the pluridisciplinary team, which includes a psychologist, a physiotherapist, and a specialist pain nurse.
Describe the anatomy of the pain pathways to the patient and explain the difference between nociceptive and neuropathic pain. Explain the sites of action of the different analgesic and co-analgesic medications. NOTE: It is important that the patient understands the nature of neuropathic pain and can differentiate it from nociceptive pain. Once the patient has understood the physiology of their neuropathic pain, they are ready to learn about SCS.
Explain to the patient the theoretical concept of SCS. The technique is believed to inhibit chronic pain by stimulating the large-diameter afferent nerve fibers in the spinal cord, in accordance with the gate control theory of pain.
Measure the Visual Analog Scales (VAS) score21 for leg and back pain.
- Explain the objectives of the therapy:
- Paresthesia coverage of the painful area of 50 to 70%.
- Reduction of pain (VAS score).
- Reduction in analgesic and co-analgesic medications.
- Improvement in the quality of sleep.
- Improvement in the quality of life.
- Improvement in the distance walked.
Explain the different components of an SCS device (epidural lead, internal pulse generator, and the extension cable connecting the lead to the IPG) and let the patient manipulate them to understand the system better.
3. Creation of the Health Insurance File by the NMS
Describe in this file the characteristics of the patient's pain, quality of life, and quality of sleep, but also the patient's activities, walked distance, and analgesic and co-analgesic consumption. NOTE: Under Belgian law, psychiatric evaluation is mandatory before any surgery to implant a foreign body. The psychiatrist has to rule out any psychiatric disorder and evaluate the patient's functional capacities in order to be able to accept and live with a foreign body.
Attach the patient's medical record and the psychiatric evaluation to the Health Insurance File.
4. Implantation of the Multicolumn Lead
Obtain the patient's informed consent after explaining the details of the surgical procedure, including the complications (infection 3–6%; serious neurological complications 0.54–1.71%; epidural hematoma 0.19–0.63%; spinal cord injury 0.022–0.067%; and cerebrospinal fluid leakage 0.05–0.001%)22. NOTE: The long testing period required in Belgium is presumed to be the main reason for the higher infection rate (11%) observed in our institution, and we are currently trying to address this issue. This must be explained to the patient.
In addition to the above risks, which are similar to those associated with other spinal procedures, explain the other risks normally associated with the use of an implant, such as allergic or immune system responses to the implanted materials; lead, extension, or neurostimulator erosion through the skin or migration; and formation of reactive tissue around the lead in the epidural space that can result in delayed spinal cord compression and paralysis, requiring surgical intervention. Time to onset can range from weeks to many years after implant. NOTE: The neurological complication rates for percutaneous dorsal column stimulation leads are slightly higher (infection 3–6%; serious neurological complications 0–2.35%; epidural hematoma 0.75%; spinal cord injury 0.03–2.35%; and cerebrospinal fluid leakage 0.3%)22.
If the patient doesn't have any contraindication, conduct an MRI to rule out any anomaly of the conus medullaris projection such as spinal dysraphism or other congenital spinal cord anomalies23.
Perform antibiotic prophylaxis 30 min before incision using a single intravenous (IV) dose of Cefazolin, 50 mg/kg, with a maximum of 2 gm, through a 16 G needle placed in a peripheral route.
With the patient under general anesthesia (use 0.1 µg/kg Sunfentanil, 2 mg/kg Propolipid 1%, 0.3 mg/kg Ketamine Hydrochloride (see Table of Materials), and 0.3 mg/kg Rocuronium) and in the prone position, define the incision site between the spinous processes of T10 and T11 under fluoroscopic assistance.
Infiltrate the incision site with 5 mL of a 20 mL solution of 0.5% Bupivacaine and 1:200,000 adrenaline (see Table of Materials).
Incise until the thoracic aponeuroses are revealed.
Dissect the paravertebral musculature on both sides of T10's supraspinous process and place the retractor.
Resect the supraspinous and interspinous ligaments and dissect the lamina of T10 (5 mm on each side and 5 mm in the craniocaudal direction).
Before inserting the lead, perform a partial laminectomy of T10 to create enough space to allow the insertion of the lead (no need to remove the entire lamina).
Insert the phantom lead into the epidural space, while keeping the insertion of the flavum ligament on the upper part of the T11 lamina intact. NOTE: This T11 lamina is used as a guide for the insertion of the lead into the epidural space.
Place the lead as medially as possible under fluoroscopic guidance. The final lead's position is reached when placed midline in the projection of the bodies of T8–T9 under fluoroscopic control.
Fix the lead to the interspinous ligament of T11 by doing a single interrupted stitch.
Tunnel the extensions from your operative field to the lateral side of the back (often the right one) by using the tunneling tool provided by the company, and then connect them to the lead.
Check fluoroscopically to be sure the lead has not moved and is still in the epidural space, midline, in the projection of T8–T9.
Remove the retractor and make sure there is no bleeding.
Suture the musculo-aponeurotic layer by means of simple interrupted stitches of braided synthetic absorbable suture material (see Table of Materials).
Connect the two intrinsic external extensions to the two extrinsic external extensions. Tunnel them in the subcutaneous fat in such a way that they exit through the skin 15 cm laterally to the incision site. NOTE: The choice of the side is discussed preoperatively with the patient and depends on the implantation location of the internal pulse generator.
Wrap the intrinsic and extrinsic extensions and suture the subcutaneous layer by means of simple interrupted stitches of braided synthetic absorbable suture material (see Table of Materials).
Make a dermic subcuticular suture using finer absorbable 3/0 suture material (see Table of Materials) and apply antiseptic and bandages. During this step, administer a single IV dose of 1g Paracetamol and 100 mg Tradonal.
5. Postoperative Trial
When the lead is placed, connect it to an external stimulator for a period of four weeks. NOTE: This long test period is required by the Belgian law as a condition to reimburse the implantation of the pulse generator during a second operation.
Begin the programming sessions a few hours after surgery. The objective of the therapy is to cover the painful area by pleasant paresthesia.
Connect the external neurostimulator to the extrinsic external lead extensions.
Place the clinician programmer on the external neurostimulator. Turn the clinician programmer on and then turn the neurostimulation on. Start by setting the pulse width (PW) to 260 µs, at a rate of 60 Hz.
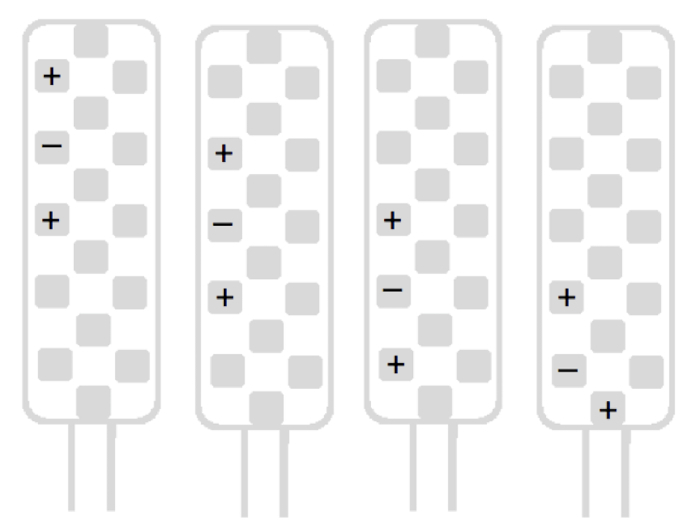
If LP was bilateral, perform a first screening using a guarded cathode (+-+), starting with the top-left tripolar longitudinal stimulation configuration (Figure 1), in order to identify the laterality of the lead (right/left).
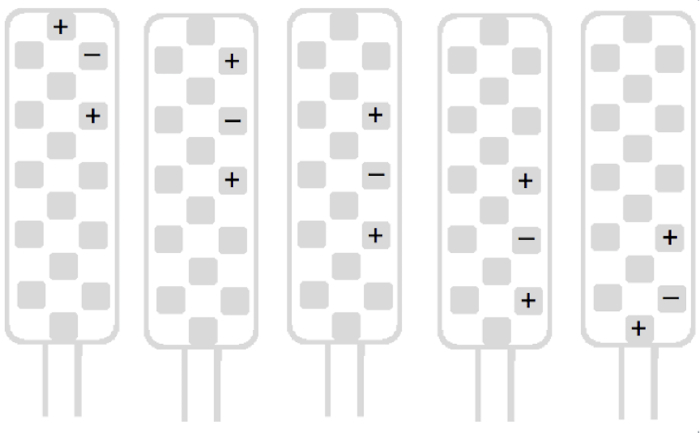
Perform a rapid scan of the left side with the four remaining left-side tripolar longitudinal stimulation configurations (Figure 2). An idea of the painful metameric level is determined during this unilateral screening. Thereafter, scan the right side following the same scheme (Figure 3).
Finally, activate the stimulation configuration(s) allowing better paresthesia coverage of the painful leg area(s). NOTE: If there is bilateral LP, two different stimulation configurations must be programmed to obtain optimal paresthesia coverage of the two painful areas.
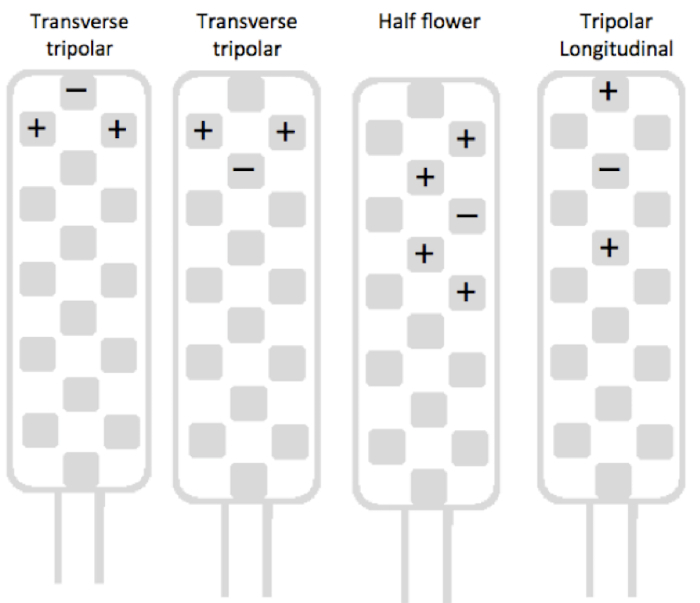
For back pain, test a transverse tripolar configuration at the top of the lead first, using the guarded cathode configuration (Figure 4). If this configuration gives insufficient paresthesia coverage, test a "half flower-like pattern" configuration of four anodes around a cathode (Figure 4). If this configuration does not provide sufficient paresthesia coverage and/or pain suppression again, use a tripolar longitudinal configuration in order to verify possible bilateral stimulation by one channel (Figure 4).
Fine-tune back pain area coverage by increasing PW to 450 µs. This makes it possible to enlarge the area of stimulation. Adjust the pulse rate between 40 and 60 Hz to obtain the most satisfying paresthesia coverage of the painful area. NOTE: These settings are based on experience with this multicolumn surgical lead and may of course vary with a different device or stimulation strategy (high-intensity stimulation, for example). For more detailed instructions, please consult the Programmer Quick Guide24.
Ensure close follow-up (at least once a week) of the patient during the trial phase (1 month in Belgium) to check appropriate wound healing and optimize paresthesia coverage by repeating Steps 5.7, 5.8 and 5.9. Use sterile technique bandage changes22. Educate the patient about the signs and symptoms of an emerging surgical site infection.
At the end of the trial period, use sharp scissors to cut the external extension flush with the skin in order to reduce the risk of infection until the next surgical procedure.

6. Implantation of the Internal Pulse Generator
If pain relief is higher than 50% at the end of the one-month trial period (Belgian reimbursement criteria), obtain the patient's informed consent and set a date for the IPG placement. Pain relief is assessed by using the VAS scale22. NOTE: The patient decides on the site of the IPG placement after the advantages and drawbacks of the different locations are discussed with the NMS.
Perform antibiotic prophylaxis 30 minutes before incision using a single intravenous dose of Cefazolin 50 mg/kg, with a maximum of 2 g, with a 16 G needle.
With the patient under general anesthesia (same protocol as mentioned in Step 4.3), make a 7 cm-long and 2 cm-deep incision and create a pocket for the IPG in the subcutaneous fat of the abdominal wall or the external quadrant of a buttock, according to patients' wishes (as discussed before surgery - Step 6.1).
Excise the dorsal scar made for the lead implantation (Step 4.3), dissect the lead extensions from the surrounding tissues, and disconnect the lead from its external extrinsic extensions.
Tunnel the intrinsic extension of the lead towards the implantation site of the IPG.
Connect the lead's intrinsic extensions to its IPG.
Suture the subcutaneous layer by simple interrupted stitches of 2/0 and 1/0 braided synthetic absorbable suture material.
Make a dermal subcuticular suture using finer absorbable 3/0 suture material and apply antiseptic and bandages. During this step, administer a single IV dose of Paracetamol 1 gm and Tradonal 100 mg.
7. Postoperative Care and Follow-up Visits
With the clinician programmer, set the programming parameters according to the ones that worked the best during the trial period (Step 5.7).
Teach the patient how to use the programmer25. Explain again the different components of the neurostimulation system. Educate the patient to avoid activities requiring excessive twisting or stretching.
Instruct the patient always to inform any healthcare personnel that they have an implanted neurostimulation system before any procedure is begun. Provide instructions about the warnings (CT-scan, defibrillation and cardioversion, electrocautery, lithotripsy, MRI, microwave ablation, and security screening devices) and precautions (bone growth stimulators, dental drills and ultrasonic probes, electrolysis, electromagnetic field devices, laser procedures, psychotherapeutic procedures, radiation therapy, and household items) for a neurostimulation system.
Ensure close follow-up of the patient after the surgery to check for surgical site infection and appropriate paresthesia coverage.
Representative Results
A prospective, non-randomized, controlled study of 62 consecutive FBSS patients who were implanted with a multicolumn lead using this method in our center, CHR Citadelle, in Liège, Belgium, was recently published in Neuromodulation18 . All patients had had previous spinal surgery, had been screened for possible secondary causes of their pain, and were refractory to optimal medical therapy. Multicolumn surgical leads were placed in all 62 patients in the projection of T8-T9 in the midline position under general anesthesia with fluoroscopic control. Fifty-four patients (87%) had >50% pain reduction at the end of the trial period and were implanted with an IPG.
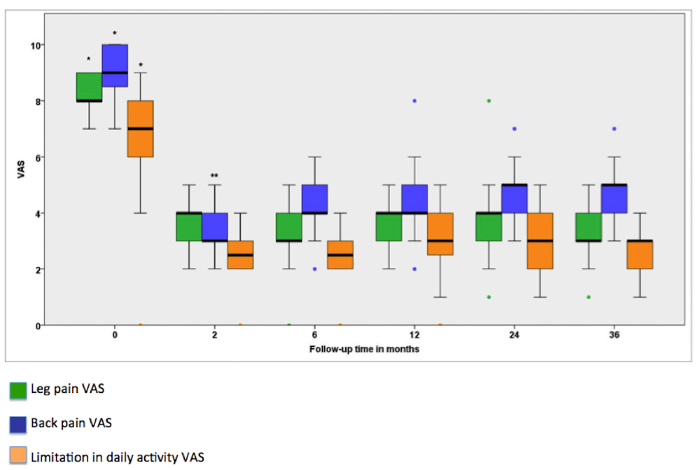
This study showed that BP, LP, and limitation of daily activity were significantly improved during the entire follow-up, as shown in Figure 5. The quality of sleep improved and the percentages of patients taking any kind of analgesic and co-analgesic medication on a regular basis decreased. We observed an overall complication rate of 19%, with infection of the material being the most frequent complication (7 cases).
For more details on this study, please read our previous article18.
Figure 1:Top left tripolar stimulation configuration. This is the first one tested in order to identify the laterality of the lead. Please click here to view a larger version of this figure.
Figure 2 : Four left tripolar longitudinal stimulation configurations used in unilateral screening to obtain an idea of the painful metameric level. Please click here to view a larger version of this figure.
Figure 3 : Main types of stimulation configurations for right side screening. Please click here to view a larger version of this figure.
Figure 4:Main types of stimulation configuration groups used for back pain screening. The transverse tripolar group includes six possible transverse tripolar configurations, all with a transverse guarded cathode located at the six rostrocaudal levels of the lead. The half flower-like pattern group includes sixteen configurations, all with a cathode surrounded by four anodes. These twenty-two configurations are possible only with multicolumn leads. The tripolar longitudinal group includes all longitudinal guarded cathode configurations. Please click here to view a larger version of this figure.
Figure 5: Evolution of VAS for leg pain (green), back pain (blue), and limitation in daily activity (orange) across the 36 month follow-up period. Thick horizontal lines are the medians, boxes indicate the 25th and 75th percentiles, and error bars are the maximum and minimum. Closed circles correspond to outliers that are above or below 2 times the 75th or 25th percentile. The Friedman test was statistically significant for leg pain (χ2(5) = 65.35; P <0.0001), back pain (χ2(5) = 91.64; P <0.0001), and limitation in daily activity (χ2(5) = 77.38; P <0.0001). Post hoc comparisons using Wilcoxon signed-rank test and a two-tailed statistically significant P threshold of 0.0033 indicated that VAS scores for leg pain, back pain, and limitation in daily activity were significantly higher at baseline than at 2, 6, 12, 24, and 36 months(*), and that VAS scores for back pain were significantly lower at 2 months than at 6, 12, 24, and 36 months(**). This figure has been modified from a previous article18. Please click here to view a larger version of this figure.
Discussion
Recent studies have demonstrated that SCS can reliably improve the different pain components of FBSS patients, improve their quality of life (e.g., quality of sleep and physical activity), and these benefits are sustained during a long-term follow-up3,17,18,19.
Depending on the localization of the pain, we use a different approach. When the patient has only unilateral LP, a percutaneous 8-contact lead is placed in the posterior spinal epidural space under fluoroscopic imaging and the final position of the lead is based on intraoperative paresthesia mapping involving patient feedback. When the patient has bilateral LP or a BP component, a surgical 16-contact lead is used. Unfortunately, correct placement of this paddle electrode requires a small laminectomy, which would clearly be uncomfortable for patients under local anesthesia. That explains why this multicolumn lead is not positioned according to the topography of the pain, but strictly anatomically, in the projection of the T8–T9 vertebral bodies. Considering the geometry of that surgical electrode and the variety of programming possibilities, a standard positioning of the device allows optimal paresthesia coverage of BP and LP areas. Other teams use two 8-contact percutaneous leads for treating both leg and back pain17. This technique allows them to avoid a general anesthesia, but in a subset of patients with extensive spinal fusions percutaneous access cannot be obtained and the surgical electrode is the only option. Besides, to target the BP area, very high frequency (10 kHz or burst) stimulations are applied through those percutaneous leads. That depletes the battery much more rapidly, leading to more frequent recharging or surgical replacement19.
When the lead is placed, it is connected to an external stimulator for four weeks. This long test period, which is required by Belgian law as a condition to reimburse the implantation of the pulse generator in a second operation, might explain why infection of the material is the most frequent complication of this surgery. The prolonged transcutaneous access could favor bacterial contamination. Several authors have indeed recommended a shorter test period26.
SCS is a field in constant evolution and new methods to improve FBSS patients' outcomes are constantly being studied. Recently, the observational SCS-LUMINA study found that a new device called "Anatomically Guided Neural Targeted" provides even better LP and BP relief than conventional SCS. This new device is compatible with percutaneous and paddle leads and could be an important tool in the treatment of LP and BP in FBSS patients19. It is important constantly to reset the place of each tool in the therapeutic arsenal for the treatment of FBSS patients in light of medical and technological advances.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors would like to thank Gabrielle Leyden for revising the final draft of this paper.
References
- Schofferman J, Reynolds J, Herzog R, Covington E, Dreyfuss P, O'Neill C. Failed back surgery: Etiology and diagnostic evaluation. Spine Journal. 2003;3(5):400–403. doi: 10.1016/s1529-9430(03)00122-0. [DOI] [PubMed] [Google Scholar]
- Hazard RG. Failed back surgery syndrome: surgical and nonsurgical approaches. Clinical Orthopaedics and Related Research. 2006;443(443):228–232. doi: 10.1097/01.blo.0000200230.46071.3d. [DOI] [PubMed] [Google Scholar]
- Rigoard P, et al. An Algorithmic Programming Approach for Back Pain Symptoms in Failed Back Surgery Syndrome Using Spinal Cord Stimulation with a Multicolumn Surgically Implanted Epidural Lead: A Multicenter International Prospective Study. Pain Practice. 2015;15(3):195–207. doi: 10.1111/papr.12172. [DOI] [PubMed] [Google Scholar]
- Thomson S. Failed back surgery syndrome - definition, epidemiology and demographics. British Journal of Pain. 2013;7(1):56–59. doi: 10.1177/2049463713479096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balagué F, Mannion AF, Pellisé F, Cedraschi C. Non-specific low back pain. Lancet. 2012;379(9814):482–491. doi: 10.1016/S0140-6736(11)60610-7. [DOI] [PubMed] [Google Scholar]
- Melzack R, Wall PD. Pain Mechanisms: A New Theory. Science. 1965;150(3699):971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- González JCA, Melzack R, Wall P. The gate theory: Beyond the scientific concept two worlds scientists dedicated to the understanding of pain. Revista de la Sociedad Espanola del Dolor. 2013;20(4):191–202. [Google Scholar]
- Loeser D, John MR. Concepts of pain. The Lancet. 1992;353(May 8):1607–1609. [Google Scholar]
- Cui JG, Linderoth B, Meyerson BA. Effects of spinal cord stimulation on touch-evoked allodynia involve GABAergic mechanisms. An experimental study in the mononeuropathic rat. Pain. 1996;66(2-3):287–295. doi: 10.1016/0304-3959(96)03069-2. [DOI] [PubMed] [Google Scholar]
- Song Z, Meyerson BA, Linderoth B. Muscarinic receptor activation potentiates the effect of spinal cord stimulation on pain-related behavior in rats with mononeuropathy. Neuroscience Letters. 2008;436(1):7–12. doi: 10.1016/j.neulet.2008.02.044. [DOI] [PubMed] [Google Scholar]
- Song Z, Meyerson BA, Linderoth B. Spinal 5-HT receptors that contribute to the pain-relieving effects of spinal cord stimulation in a rat model of neuropathy. Pain. 2011;152(7):1666–1673. doi: 10.1016/j.pain.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Waszak PM, et al. Spinal Cord Stimulation in Failed Back Surgery Syndrome: Review of Clinical Use, Quality of Life and Cost-effectiveness. Asian Spine Journal. 2016;10(6):1195–1204. doi: 10.4184/asj.2016.10.6.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishima H, et al. Modulation of neuronal activity after spinal cord stimulation for neuropathic pain; H(2)15O PET study. Neuroimage. 2010;49(2):2564–2569. doi: 10.1016/j.neuroimage.2009.10.054. [DOI] [PubMed] [Google Scholar]
- Stancák A, et al. Functional magnetic resonance imaging of cerebral activation during spinal cord stimulation in failed back surgery syndrome patients. European Journal of Pain (London, England) 2008;12(2):137–148. doi: 10.1016/j.ejpain.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Yang F, et al. Bipolar spinal cord stimulation attenuates mechanical hypersensitivity at an intensity that activates a small portion of A-fiber afferents in spinal nerve-injured rats. Neuroscience. 2011;199:470–480. doi: 10.1016/j.neuroscience.2011.09.049. [DOI] [PubMed] [Google Scholar]
- North RB, et al. Spinal cord stimulation versus re-operation in patients with failed back surgery syndrome: an international multicenter randomized controlled trial (EVIDENCE study) Neuromodulation: Journal of the International Neuromodulation Society. 2011;14:330–335. doi: 10.1111/j.1525-1403.2011.00371.x. [DOI] [PubMed] [Google Scholar]
- Kapural L, et al. Comparison of 10-kHz High-Frequency and Traditional Low-Frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain: 24-Month Results From a Multicenter, Randomized, Controlled Pivotal Trial. Neurosurgery. 2016;79(5):667–677. doi: 10.1227/NEU.0000000000001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remacle TY, Bonhomme VL, Renwart HJP, Remacle JM. Effect of Multicolumn Lead Spinal Cord Stimulation on Low Back Pain in Failed Back Surgery Patients: A Three-Year Follow-Up. Neuromodulation: Technology at the Neural Interface. 2017;20(7):668–674. doi: 10.1111/ner.12603. [DOI] [PubMed] [Google Scholar]
- Veizi E, et al. Spinal Cord Stimulation (SCS) with Anatomically Guided (3D) Neural Targeting Shows Superior Chronic Axial Low Back Pain Relief Compared to Traditional SCS-LUMINA Study. Pain Medicine (Malden, Mass) 2017;18(8):1534–1548. doi: 10.1093/pm/pnw286. [DOI] [PubMed] [Google Scholar]
- Chan C, Peng P. Failed back surgery syndrome. Pain Medicine. 2011;12(4):577–606. doi: 10.1111/j.1526-4637.2011.01089.x. [DOI] [PubMed] [Google Scholar]
- Hawker G, Mian S, Kendzerska T, French M. Measures of adult pain. Arthritis Care and Research. 2011;63(11):240–252. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- Deer TR, et al. The Neurostimulation Appropriateness Consensus Committee (NACC) Safety Guidelines for the Reduction of Severe Neurological Injury. Neuromodulation. 2017;20(1):15–30. doi: 10.1111/ner.12564. [DOI] [PubMed] [Google Scholar]
- Gupta P, Kumar A, Kumar A, Goel S. Congenital Spinal Cord Anomalies: A Pictorial Review. Current Problems in Diagnostic Radiology. 2013;42(2):57–66. doi: 10.1067/j.cpradiol.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Medtronic N'VISION® Programmer Quick Guide. 2009.
- Medtronic MyStim® Programmer Quick Guide. 2014.
- Frey ME, Manchikanti L, Benyamin RM, Schultz DM, Smith HS, Cohen SP. Spinal cord stimulation for patients with failed back surgery syndrome: a systematic review. Pain Physician. 2009;12(2):379–397. [PubMed] [Google Scholar]


