Abstract
It is a major concern in neuroscience how different types of neurons work in neural circuits. Recent advances in optogenetics have enabled the identification of the neuronal type in in vivo electrophysiological experiments in broad brain regions. In optogenetics experiments, it is critical to deliver the light to the recording site. However, it is often hard to deliver the stimulation light to the deep brain regions from the brain's surface. Especially, it is difficult for the stimulation light to reach the deep brain regions when the optical transparency of the brain surface is low, as is often the case with recordings from awake animals. Here, we describe a method to record spike responses to the light from an awake mouse using a custom-made glass optrode. In this method, the light is delivered through the recording glass electrode so that it is possible to reliably stimulate the recorded neuron with light in the deep brain regions. This custom-made optrode system consists of accessible and inexpensive materials and is easy to assemble.
Keywords: Neuroscience, Issue 136, Neuroscience, electrophysiology, extracellular recording, auditory pathway, optogenetics, head fixed awake preparation
Introduction
The central nervous system consists of various types of neurons, which have different functions. How these different types of neurons work within the neural circuit is one of the major concerns in neuroscience. However, in many brain regions, it has been impossible to distinguish the neuronal types in in vivo recordings of electrical activities because there is no clear difference in the electrical spike signal itself, with some exceptions. Recent advances in optogenetics have made a breakthrough1,2. Using transgenic animals in which light-sensitive opsin (e.g., channelrhodopsin-2) is expressed in specific neuronal types, it became possible to distinguish the neuronal types efficiently in in vivo recordings3,4,5,6. In these animals, the neurons with light-sensitive opsin are excited by giving light stimuli during the electrical recordings, but other neurons are not. The opsin-positive neurons, therefore, are easily distinguished from other neuron types by their responses to light.
In optogenetics experiments, it is critical to deliver the light to the recording site. As a non-invasive method, the light is often directed from the brain's surface. However, because the light's strength reduces as it goes through brain tissue, it is hard to stimulate the deep brain regions from the brain's surface. Especially, it is difficult for the stimulation light to reach the deep brain regions when the optical transparency of the brain surface is low, as is often the case with recordings from awake animals. Electrophysiological experiments have often been performed on anesthetized animals because the body movement causes noise in the recordings. As is well documented, however, anesthesia is known to change the neural responses7,8,9,10. Thus, it is necessary to use awake animals in order to study neural responses without the artificial effects of anesthesia. Unlike the experiments with anesthetized animals, the electrophysiological recordings are performed after the recovery from surgery in the experiments with awake animals. During the interval between the surgery and the recordings, the tissue exudate often accumulates on the brain surface and makes the optical transparency of the brain surface low.
Here, we describe a method to record single-unit recordings from an awake mouse using a custom-made glass optrode. In this method, the light is delivered through the recording glass electrode so that it is possible to reliably stimulate the recorded neuron with light in deep brain regions. This custom-made optrode system consists of accessible and inexpensive materials and is easy to assemble.
Protocol
All procedures are done in accordance with the guiding principles of the Physiological Society of Japan and with the approval of the Animal Care Committee of Kanazawa Medical University.
1. Construction of the Glass Optrode Holder
NOTE: To build a glass optrode holder, a commercial electrode holder is used (Figure 1A).
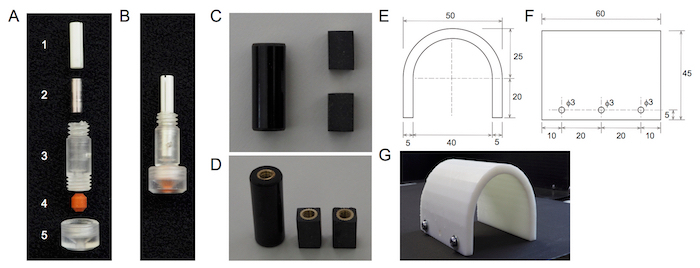
Gently pull out the steel tube for pressure control from the barrel of the holder.
By drilling, broaden the hole of the pin seat side in the barrel of the holder. Make the hole 3 mm in diameter and 12 mm in depth.
Put a stainless-steel pipe (3 mm in diameter, 0.5 mm in thickness, and 8 mm in length; Figure 1A2) in the hole.
Insert a ceramic split mating sleeve for ⌀2.5 mm ferrules (Figure 1A1) into the hole.
Fix the barrel of the electrode holder to an L-shaped rabbet with epoxy adhesive.
Make the holes (3 mm in diameter) in the rabbet and attach it to a manipulator with screws.
2. Head Post Installation
Prepare a custom-built head post made of a circuit board spacer. Make the columnar circuit board spacer into a cuboidal shape with a milling cutter and cut it into 2 head posts with a saw (Figure 1B). Flatten the bottom of the head post with a milling cutter.
Prepare the transgenic animal in which a light-sensitive opsin is expressed in a specific neuron type for the study. In this work, the transgenic mouse (VGAT-ChR2 mice) in which the inhibitory neurons express channelrhodopsin-2 (ChR2)11 is used.
Anesthetize the animal with a mixture of 0.3 mg/kg of medetomidine, 4.0 mg/kg of midazolam, and 5.0 mg/kg of butorphanol by intraperitoneal administration. To confirm that the animal is fully anesthetized, test its reactions to tail pinching.
Place the mouse in a stereotaxic frame on a heating pad. Clean the stereotaxic frame and the heating pad with 70% alcohol in advance. Place the teeth in the hole of the bite bar, and lightly tighten the nose clamp. Fix both sides of the head using ear bars. When the head is correctly fixed by the ear bars, tighten the nose clamp. Apply an ophthalmic ointment to the eyes.
Shave the head skin with small scissors and clean the scalp with a cotton swab of chlorhexidine gluconate. Before the surgery, sterilize all the surgical instruments and a head post with an autoclave (121 °C, 15 min).
Cut the scalp along the midline with scissors and push it aside. Make the incision from the back of the head to the eyes (approximately 20 mm). Remove the periosteum with a cotton swab dipped in 70% alcohol and dry the skull.
Attach the head post to the manipulator. Position the head post flat on the skull around the bregma using the manipulator.
Mix the monomer (4 drops), catalyst (1 drop), and polymer (1 small spoon) of dental cement on a small dish that is chilled in the refrigerator in advance. Put the dental cement (below 1 g) on the frontal and parietal bone of the skull to fix the head post to the skull.
After the dental cement becomes hard, remove the mouse from the stereotaxic frame. Put an antibiotic ointment on the exposed skin. Inject the medetomidine antagonist (0.3 mg/kg) and the antibiotics (oxytetracycline; 20 mg/kg). Place and monitor the mouse on the heat pad in a cage until it awakes, and return it to a housing cage.
House the animal in an individual housing cage with some enrichment device. Change the animal paper bedding daily to keep the cage clean and prevent infection. Carefully monitor whether the animal shows any sign of discomfort. Apply lidocaine to the wound if any pain is suspected. Administer meloxicam or carprofen once every 24 h for 48 h after both the head post installation and craniotomy surgeries. The local lidocaine can be given in addition if the NSAID is not adequate.
3. Acclimation
Two days (minimum) after the recovery from the anesthesia, acclimate the animals to the head-fixed state in the recording chamber. Perform the acclimation sessions for at least 5 days.
During the acclimation sessions, place the animal in the recording chamber with the head post fixed to a custom-built clamp. During the session, in order to limit movement, cover the body with a custom-made resin half tube (Figure 1C), made using a 3D printer. NOTE: On day 1, the acclimation session should be 5 min long. It is then increased daily to 10, 20, 40, and 60 min for days 2, 3, 4, and 5, respectively.
At the end of each session, give the animal a food reward. If the animal struggles continuously during the acclimation, stop the session.
4. Craniotomy
NOTE: After the acclimation, a craniotomy is made over the brain region for recording. The craniotomy is performed in the stereotaxic frame under anesthesia, as with the head post installation. The post-operation procedure is the same as the head post installation.
Before the craniotomy, clean the skull with a cotton swab with chlorhexidine gluconate and 70% alcohol.
Carefully scrape the skull over the recording site with a dental drill. When the bone becomes thin enough, cut it with the tip of the scalpel and remove it.
Put dental cement around the exposed region in order to strengthen the skull.
Cover the exposed brain area with silicone adhesive after the dental cement becomes hard. The post-surgery procedure is as described in step 2.8.
5. Electrophysiological Recording
Allow the animal to recover for at least 1 day after the craniotomy before performing the electrophysiological recording. Place the animal in the recording chamber in the same way it was done during the acclimation sessions when the recording starts.
Make glass pipettes from borosilicate glass capillaries (OD = 1.5 mm, ID = 0.9 mm, 90.0 mm long) pulled on an electrode puller. Their tip diameter is 2–3 µm, and their resistance is 4–6 MΩ when they are filled with 10 mM PBS. The length of the electrode is 40–50 mm. In some recordings, 2% neurobiotin is added to the 10 mM PBS.
Attach the filled glass pipette to the custom-made optrode holder.
Put the Ag-Cl coated silver wire (0.2 mm in diameter) into the glass pipette. Connect the silver wire to a pin via a thin coated electric wire. Connect the pin to the headstage of the recording amplifier.
Connect the fiber-optic patch cord (core = 960 µm, cladding = 1,000 µm, NA = 0.63) with a zirconia ferrule (OD = 2.5 mm) to the split mating sleeve on the optrode holder. Push the patch cord into the sleeve until the tip of the ferrule contacts the back of the glass pipette. The patch cord delivers the light (wavelength = 465 nm) from LED. The LED light is controlled by a single-channel LED driver.
Remove the silicone adhesive over the craniotomy. Put warmed saline (38 °C) on the brain surface periodically to prevent the brain surface from drying during the recording session. If necessary, remove the dura with sharp tweezers.
Insert the glass electrode into the brain tissue with a manipulator. Monitor the electrode resistance when it is inserted. Replace the electrode when the resistance is lower than expected because the tip of the electrode may be damaged. At the depth of interest, search and isolate the single unit spikes by adjusting the depth in 2 to 5 µm steps.
After the single unit is isolated, deliver the LED light and observe the response to the light stimuli.
Record the neural activities after the identification of the cell types. Perform the recordings for 2–3 h. If the recording is made on consecutive days (maximum of 2 days), cover the craniotomy with silicone adhesive. When the recording is finished, and the animal is sacrificed, retrieve the head post and put it in acetone to wash and reuse it.
Representative Results
In Figure 2, we examined the effects of the tip size and the length of the glass pipettes on the light power at the tip of the pipettes (Figure 2A-B). The light power was measured by an optical power meter placed 1 mm away from the tip. The full length was set to 50 ± 2 mm when the tip size varied, while the tip size was set to 2.5 µm when the full length varied. The shank of the glass pipette was set to 8 mm. The light power at the tip ranged from 3–5 mW (mean ± SD; Figure 2A: 3.77 ± 0.29 mW, n = 20; Figure 2B: 4.24 ± 0.31 mW, n = 21). The tip size (Figure 2A) of the pipettes was hardly correlated with the light power (R = 0.1555). The length of the pipette was only slightly correlated with the light power in a negative manner (R = -0.2054). These results suggest that the tip and length of the pipettes rarely affect the light power at the tip. We also checked if the amount of volume load of the solution (10 mM PBS) in the glass pipettes affects the light power (Figure 2C). The volume of the solution was assessed by the length of the solution in the pipette. The data were recorded from 4 pipettes (tip size: 0.25 µm, the full length: 50 ± 2 mm, the length of the shank: 8 mm). We found that varying the volume of the solution in the pipette did not affect the light power (Figure 2C).

In Figure 3, we show the spike responses recorded in the inferior colliculus (IC) of awake VGAT-ChR2 mice (2–4 months old). The IC is an auditory center in the midbrain and consists of glutamatergic and GABAergic neurons12,13, both of which respond to sound3,4. In the IC of VGAT-ChR2 mice, the GABAergic neurons specifically express ChR24. In previous work, it was shown that the GABAergic and glutamatergic neurons were excited or suppressed, respectively, when they were given light stimuli3,4. In the awake mice, we found both types of neurons (Figure 3A-B). When the light stimulus is delivered through the glass electrode, it evoked spike responses in some neurons (Figure 3A). In contrast, in other neurons, the light stimuli suppressed the spike responses evoked by sound stimuli (Figure 3B). These results show that a glass optrode can record the well-isolated single unit activity, and reliably stimulate the recorded neurons by light.

Figure 1: Optrode holder and recording accessories. Panels (A) and (B) show the optrode holder. Panel (A) shows the part structure of the optrode holder. (1) This is a ceramic split mating sleeve. (2) This is a stainless-steel tube. (3) This is a barrel of the holder. (4) This is a cone washer. (5) This is a polycarbonate cap. Panel (B) shows the assembled optrode holder. Panels (C) and (D) show the custom-built head post. Panel (C) shows a side view of the head post. Panel (D) shows an oblique view of the head post. The two head posts (right) are made from a circuit board spacer (left). Panels (E), (F), and (G) show the custom-made resin half tube. Panels (E) and (F) show dimensional drawings of the resin half tube. Panel (E) shows a front view of the resin half tube. Panel (F) shows a side view of the resin half tube. Panel (G) shows a photo image of the half tube. The numbers in panels (E) and (F) indicate the drawing dimensions in mm. Please click here to view a larger version of this figure.
Figure 2: The effect of the configuration of the glass pipettes on the light power at the tip. (A) The tip's size was plotted against the light power. The full length of the pipette was set to 50 ± 2 mm. The length of the shank was set to 8 mm. (B) The length of the pipette was plotted against the light power. The tip size was set to 0.25 µm. The length of the shank was set to 8 mm. (C) This panel shows the effect of the volume load of the solution (10 mM PBS) on the light power. The data from 4 pipettes are indicated by different symbols and lines. Please click here to view a larger version of this figure.
Figure 3: The light-evoked responses in the IC of awake VGAT-ChR2 mice. (A) The light stimuli (blue box, 0.03 s) evoked spike responses. (B) The light stimuli (blue box, 0.03 s) suppressed the spike responses evoked by sound (gray box). The upper 3 traces are the response to sound, and the lower 3 traces are the response when both sound and light were given. Please click here to view a larger version of this figure.
Discussion
Optogenetics has become a powerful tool in neuroscience. It has been utilized for identifying specific neuron types in vivo as well as manipulating the activities of specific neuronal pathways. The clarification of the neural activities of different neuronal types promotes the understanding of the mechanism of the neural circuits. Here, we demonstrated a method to deliver the light to the recording site through a glass electrode in the IC of awake VGAT-ChR2 mice.
There are several critical steps in the method described. First, it is a requirement to use animals with a sufficient and specific expression of opsins in the target neurons. To identify the responses to light with the extracellular recordings, it is necessary that the activation of the opsin evokes the spikes in the target neurons. In some transgenic strains, it is possible that the transgene expression level in neurons is low in young animals14, and there might be a minimum age for the experiment. Further, it may be necessary to perform some additional experiments to confirm if the responses to the light stimuli are specific to the target neuron types (e.g., immunohistochemistry). When the target neuron is excitatory, it would be necessary to distinguish the directly evoked responses from the synaptically evoked responses, which could be done by thresholding the latency of the response. Second, it is critical to ensure the brain tissue is as clean as possible. A maintenance of cleanliness is especially required in order to prevent any bleeding during the craniotomy and the recordings. Also, the surface of the brain should be prevented from drying after the craniotomy by covering the surface tightly with silicone adhesive.
To deliver the light efficiently from the electrode tip, it is critical to reduce any loss in the optical path. In our configuration of the optrode, the light power drops tenfold through the electrode holder: the light power is 45 mW at the end of the fiber-optic patch cord, while it is 3–5 mW at the tip of the glass pipette. As shown in the results, the tip size and the full length did not affect the light power at the tip (Figure 2). These results indicated that the light attenuation was fairly stable regardless of the configuration of the glass pipette. This light attenuation did not matter in the recording of IC neurons in VGAT-ChR2 mice because we have confirmed that it was possible to record light activated spikes in the ventral edge (2 mm depth) of the IC, although it may matter in other recording configurations. It would be hard to reduce the light attenuation dramatically, but there may be possibilities for improvement. First, it may be effective to use quartz instead of a borosilicate glass as an electrode pipette. It is known that quartz has better light conductivity, so that it may reduce the light loss through the electrode15. Also, it was reported that the polish of the end of the pipette reduced light loss at the contact surface between the pipette and patch cord15. If the improvement in the light attenuation is not enough, it would be effective to use a laser instead of LED, although laser costs more than LED.
One of the advantages of the glass pipette electrode is that it is possible to perform histological procedures by adding tracers or vectors to the filling solution. The histological procedures will add a great deal of useful information (the location of the recording, the morphology of the recorded neuron, etc.) to the electrophysiological data. Also, it was reported that it is possible to perform pharmacological surveys using the glass electrode with blockers in the filling solution15. In contrast to these advantages, the limitation of the glass pipette electrode is that it cannot record from multiple isolated units. To attain multiple recordings, it may be possible to put a tetrode wire into the glass pipette instead of a single Ag-Cl coated wire. In this case, it would be necessary to use a pipette with a larger tip size (30–60 µm, the typical tip size of a tetrode) and let the end of the tetrode out from the tip of the pipette.
While previously reported optrodes often required complex fabrication16,17, Magee and his colleagues have developed an optrode using standard glass pipettes as with the optrode we designed18. In their method, they insert a thin optical fiber into the pipette to deliver the light. To make the fiber thin enough to insert the shank of the pipette, they etched 125 µm cladding fiber with a 9 µm core to a diameter of 8–10 µm at the tip. The advantage of this method is that it would apply even for the bent pipette that is often used for iontophoresis. However, it might be hard to use LED as a light source because standard commercial LEDs would not achieve sufficient light power via a 9 µm core fiber.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors were supported by the Japan Society for the Promotion of Science KAKENHI Grant JP16K11200 and 17H02223, and the Grant for Research from Kanazawa Medical University S2016-8 and C2017-3. We thank Yuhichi Kuda for his support in taking the photos.
References
- Rajasethupathy P, Ferenczi E, Deisseroth K. Targeting neural circuits. Cell. 2016;165(3):524–534. doi: 10.1016/j.cell.2016.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore F, Schwartz EC, Salzman CD. Manipulating neural activity in physiologically classified neurons: triumphs and challenges. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2015;370(1677):20140216. doi: 10.1098/rstb.2014.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Bishop DC, Oliver DL. Identified GABAergic and glutamatergic neurons in the mouse inferior colliculus share similar response properties. Journal of Neuroscience. 2017;37(37):8952–8964. doi: 10.1523/JNEUROSCI.0745-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Bishop DC, Oliver DL. Long-lasting sound-evoked afterdischarge in the auditory midbrain. Scientific Reports. 2016;6:20757. doi: 10.1038/srep20757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz W, Tremblay R, Rudy B. Channelrhodopsin-assisted patching: in vivo recording of genetically and morphologically identified neurons throughout the brain. Cell Reports. 2014;9(6):2304–2316. doi: 10.1016/j.celrep.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima SQ, Hromadka T, Znamenskiy P, Zador AM. PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording. PLoS One. 2009;4(7):e6099. doi: 10.1371/journal.pone.0006099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwada S, Batra R, Stanford TR. Monaural and binaural response properties of neurons in the inferior colliculus of the rabbit: effects of sodium pentobarbital. Journal of Neurophysiology. 1989;61(2):269–282. doi: 10.1152/jn.1989.61.2.269. [DOI] [PubMed] [Google Scholar]
- Populin LC. Anesthetics change the excitation/inhibition balance that governs sensory processing in the cat superior colliculus. Journal of Neuroscience. 2005;25(25):5903–5914. doi: 10.1523/JNEUROSCI.1147-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque D, Malmierca MS. Stimulus-specific adaptation in the inferior colliculus of the mouse: anesthesia and spontaneous activity effects. Brain Structure and Function. 2015;220(6):3385–3398. doi: 10.1007/s00429-014-0862-1. [DOI] [PubMed] [Google Scholar]
- Cai R, Richardson BD, Caspary DM. Responses to predictable versus random temporally complex stimuli from single units in auditory thalamus: impact of aging and anesthesia. Journal of Neuroscience. 2016;36(41):10696–10706. doi: 10.1523/JNEUROSCI.1454-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, et al. Cell type-specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nature Methods. 2011;8(9):745–752. doi: 10.1038/nmeth.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Bishop DC, Oliver DL. Two classes of GABAergic neurons in the inferior colliculus. Journal of Neuroscience. 2009;29(44):13860–13869. doi: 10.1523/JNEUROSCI.3454-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Yanagawa Y, Koyano K. GABAergic neurons in inferior colliculus of the GAD67-GFP knock-in mouse: electrophysiological and morphological properties. Neuroscience Research. 2005;51(4):475–492. doi: 10.1016/j.neures.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Chen Q, et al. Imaging neural activity using Thy1-GCaMP transgenic mice. Neuron. 2012;76(2):297–308. doi: 10.1016/j.neuron.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai Y, Nishino E, Ohmori H. Simultaneous recording of fluorescence and electrical signals by photometric patch electrode in deep brain regions in vivo. Journal of Neurophysiology. 2015;113(10):3930–3942. doi: 10.1152/jn.00005.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeChasseur Y, et al. A microprobe for parallel optical and electrical recordings from single neurons in vivo. Nature Methods. 2011;8(4):319–325. doi: 10.1038/nmeth.1572. [DOI] [PubMed] [Google Scholar]
- Abaya TV, Blair S, Tathireddy P, Rieth L, Solzbacher F. A 3D glass optrode array for optical neural stimulation. Biomedical Optics Express. 2012;3(12):3087–3104. doi: 10.1364/BOE.3.003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner KC, et al. Conjunctive input processing drives feature selectivity in hippocampal CA1 neurons. Nature Neuroscience. 2015;18(8):1133–1142. doi: 10.1038/nn.4062. [DOI] [PMC free article] [PubMed] [Google Scholar]


