Abstract
Lung cancer is a deadly treatment refractory disease that is biologically heterogeneous. To understand and effectively treat the full clinical spectrum of thoracic malignancies, additional animal models that can recapitulate diverse human lung cancer subtypes and stages are needed. Allograft or xenograft models are versatile and enable the quantification of tumorigenic capacity in vivo, using malignant cells of either murine or human origin. However, previously described methods of lung cancer cell engraftment have been performed in non-physiological sites, such as the flank of mice, due to the inefficiency of orthotopic transplantation of cells into the lungs. In this study, we describe a method to enhance orthotopic lung cancer cell engraftment by pre-conditioning the airways of mice with the fibrosis inducing agent bleomycin. As a proof-of-concept experiment, we applied this approach to engraft tumor cells of the lung adenocarcinoma subtype, obtained from either mouse or human sources, into various strains of mice. We demonstrate that injuring the airways with bleomycin prior to tumor cell injection increases the engraftment of tumor cells from 0-17% to 71-100%. Significantly, this method enhanced lung tumor incidence and subsequent outgrowth using different models and mouse strains. In addition, engrafted lung cancer cells disseminate from the lungs into relevant distant organs. Thus, we provide a protocol that can be used to establish and maintain new orthotopic models of lung cancer with limiting amounts of cells or biospecimen and to quantitatively assess the tumorigenic capacity of lung cancer cells in physiologically relevant settings.
Keywords: This Month in JoVE, Issue 136, Lung adenocarcinoma, orthotopic mouse models, intratracheal injection, bleomycin, engraftment, metastasis
Introduction
Lung cancer is the leading cause of cancer related deaths worldwide1. Patients with lung cancer eventually succumb from metastasis to distant organs, notably to the central nervous system, liver, adrenal glands, and bones2,3,4. Thoracic malignancies have been traditionally classified as small cell lung cancer (SCLC) or non-small cell lung cancer (NSCLC)5. NSCLC is the most frequently diagnosed malignancy and can be subdivided into different histological subtypes, including lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC)6. Genomic analysis of resected human primary lung cancers has revealed that tumors within a given histotype can also express diverse molecular perturbations, further contributing to their divergent clinical progression and confounding patient prognosis. The remarkable heterogeneity of lung cancers represents a significant challenge to the rational design, pre-clinical testing, and implementation of effective therapeutic strategies. Consequently, there is a need to expand the repertoire of tractable experimental lung cancer models to study the diverse cellular origins, molecular subtypes, and stages of this disease.
Various approaches using animal models have been employed to study lung cancer in vivo, each with their own advantages and disadvantages depending on the biological question(s) of interest. Genetically engineered mouse models (GEMMs) can target specific genetic alterations in a given progenitor cell type, resulting in tumors that progress within an immunocompetent host7. While extremely powerful and clinically relevant, the latency, variability, and/or lung tumor morbidity associated with GEMMs can be prohibitive to certain quantitative measurements and the detection of late stage metastasis in distant organs8. A complementary approach is the use of allograft models, whereby lung cancer cells, obtained either directly from a mouse tumor or derived first as established cell lines in culture, are re-introduced into syngeneic hosts. Analogously, lung cancer xenografts are established from human cell lines or patient derived tumor samples. Human cell line xenografts or patient derived xenografts (PDXs) are generally maintained in immunocompromised mice and therefore preclude complete immune-surveillance9. Despite this drawback, they provide an avenue to propagate limiting amounts of human biospecimens and study fundamental in vivo properties of human cancer cells, which encode for more complex genomic aberrations than GEMM tumors.
One useful property of allografts and xenografts is that they are amenable to traditional limiting cell dilution assays, employed to quantify the frequency of tumor initiating cells (TICs) within a malignant cell population10. In these experiments, a defined number of cells are injected subcutaneously into the flank of animals and the frequency of TICs can be estimated based on tumor take rate. Subcutaneous tumors however can be more hypoxic11 and may not model key physiological constraints of the lung tumor microenvironment. Intratracheal delivery of epithelial stem or progenitor cells into the lungs of mice is a method to study pulmonary regeneration and airway stem cell biology12. However, the engraftment rate from this technique can be relatively low, unless the lungs are first subjected to physiological forms of injury, such as viral infection13,14. Support from inflammatory stromal cells and/or the disruption of the lung basement membrane may improve retention of transplanted cells into relevant stem cell niches in the distal airways15. Fibrosis inducing agents can also pre-condition the lungs to enhance engraftment of induced pluripotent cells16 and mesenchymal stem cells17. Whether similar forms of airway injury can affect the engraftment rate, tumor initiating capacity, and outgrowth of lung cancer cells has yet to be systematically assessed.
In this study, we describe a method to increase the efficiency of orthotopic lung cancer cell engraftment, by pre-conditioning the lungs of mice with injury. LUAD arises in the distal airways with a significant subset of these cancers developing a fibrotic stroma18 that often correlates with poor prognosis19. Bleomycin, a natural nonribosomal hybrid peptide-polyketide, has been extensively utilized to induce pulmonary fibrosis in mice20. Airway instillation of bleomycin first promotes epithelial attrition in the alveoli and recruitment of inflammatory cells, including macrophages, neutrophils and monocytes21. This is followed by tissue remodeling in the distal airways, basement membrane reorganization22,23 and extracellular matrix (ECM) deposition24. The effects of a single bleomycin injection are transient, with fibrosis resolving after 30 days in most studies25. Using both allograft and xenograft models, we tested if pre-conditioning the airways of mice with bleomycin could significantly increase the take rate of LUAD cells in the lungs.
Protocol
All experiments were carried out in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) at Yale University.
1. Set Up / Preparation of the Reagents.
- Bleomycin Caution: Based on the Globally Harmonized System (GHS) of Classification and Labelling of Chemicals, bleomycin is classified as a GHS08 health hazard.
- Prepare bleomycin in a chemical hood. Resuspend 15 U into 5 mL of sterile phosphate buffered saline (PBS).
- Aliquot 100-200 µL of the solution into glass vials and freeze them at -20 °C for future use. Properly label the tubes with the date of resuspension. Use within 6 months from this date. NOTE: Each mouse strain has a different sensitivity to bleomycin26. Testing different doses of bleomycin for each mouse strain is recommended. The mouse strains and the corresponding doses of bleomycin used in representative experiments are listed in Table 1.
- Mice
- Purchase mice needed for the experiment and allow mice 7 days to acclimate before injection. Perform infusions in a biosafety hood. Transfer animals housed in a non-BSL2 compliant room to a BSL2 room using standard institutional procedures prior to bleomycin infusion. Inject mice at 6-8 weeks of age. Caution: Inject mice following institutional guidelines for hazardous agents, biosafety level 2 (BSL2) and IACUC approval. NOTE: Mice purchased for the representative experiments are listed in the Table of Materials. Only male mice have been used.
- Lung Cancer Cell Lines
- Culture desired cell lines in their respective media. Before injections, perform short tandem repeat DNA profiling or genetic testing to ensure proper cell line identification. To facilitate in vivo and ex vivo imaging, infect cell lines with a lentivirus expressing the thymidine kinase, green fluorescent protein (GFP) and luciferase fusion reporter27, and sort reporter positive cells by fluorescence activated cell sorting (FACS) prior to engraftment. Test lines for mycoplasma every 6 months.
- Culture H2030 human cancer cell line as recommended by the manufacturer using Roswell Park Memorial Institute medium (RPMI) with 10% Fetal Bovine Serum (FBS), penicillin-streptomycin, and amphotericin B.
- Culture 368T1 murine cell line (derived from a KrasG12D;p53-/- mouse) using Dulbecco's Modified Eagle's medium (DMEM) with 10% FBS, penicillin-streptomycin, and amphotericin B.
- Culture PC9 human cancer cell line using RPMI with 10% FBS, penicillin-streptomycin, and amphotericin B.
2. Bleomycin Treatment
Plan to inject mice intratracheally with bleomycin 14 days prior to tumor cell engraftment.
Thaw bleomycin stock on ice 2 h prior to injection.
Once thawed, dilute bleomycin to the desired working concentration (0.02 U/50 µL or 0.005 U/50 µL), and keep it on ice. NOTE: Concentration of bleomycin depends on the mouse strain used for the experiments (Table 1). Titration of bleomycin in mice should be performed to determine optimal dose.
Prepare anesthesia by diluting ketamine and xylazine to a final concentration of 10 mg/mL and 1 mg/mL, respectively, Using a 1 mL syringe and 27 G needle, anesthetize each mouse by injecting intraperitoneally ketamine/xylazine solution at 100/10 mg/kg respectively.
Monitor the breathing of mice and employ a toe pinch. Confirm proper anesthetization when mouse does not respond to toe pinch. Apply vet ointment on eyes to prevent dryness while under anesthesia.
Place 1 mouse at a time on an intubation platform by hanging its front teeth with its back against the platform.
Illuminate the upper chest using a fiber-optic illuminator to help with visualization of the trachea. Open the mouth of the mouse and pull the tongue out gently with sterile flat forceps.
Use an intravenous catheter without the needle to avoid blocking breathing. Position the catheter over the white light emitted from the opening of the trachea.
Insert the catheter into the trachea until the top of the catheter reaches the front teeth. Confirm proper placement of the catheter in the trachea by visualizing the white light shining through the opening of the catheter in the mouth.
Using a pipette, dispense 50 µL of bleomycin or vehicle (PBS) directly into the catheter to ensure that the entire volume is inhaled. Perform this step under sterile conditions.
If the catheter is correctly inserted, the mouse will immediately inhale the contents of the catheter. Wait a few seconds until the entire volume travels down the catheter. Then remove the catheter from the trachea and dispose in 10% bleach solution. NOTE: The average time per mouse of steps 2.7-2.11 is around 5 min.
If the mouse is not inhaling the liquid, carefully monitor breathing and adjust the catheter position. If the mouse stops breathing, remove the catheter immediately and allow the mouse to resume breathing normally before re-inserting the catheter.
Place the injected mouse on an IACUC approved heating pad on their back on a flat surface for recovery. The whole procedure will take 10 min per mouse. Do not return an animal that has undergone injection to the company of other animals until fully recovered.
Place a "Hazardous Chemical in Use" Card on the cage for 24 h.
3. Monitoring Mice Post-Intubation
Monitor the mice for respiratory distress immediately after intubation and then every 15 min until mice wake up from anesthesia. Do not leave mice unattended until they have regained sufficient consciousness to maintain sternal recumbency.
Inject ketoprofen (5 mg/kg) intraperitoneally to alleviate pain.
Examine the mice 24 h post intubation and 3 times a week for any signs of morbidity.
Keep a record of the date of procedure, animal identification, type of procedure, type of anesthetic, and post-operative monitoring observations with times.
Monitor the mice for weight loss, respiratory distress, behavioral abnormalities, and a body condition score <2 (segmentation of vertebral column evident/dorsal pelvic bones are palpable) bi-weekly following injection of bleomycin.
Euthanize mice under respiratory distress or mice that have experienced 15% loss of body mass by CO2 or by ketamine/xylazine followed by cervical dislocation. Confirm euthanasia by conducting palpation for respiratory activity.
4. Engraftment of Lung Adenocarcinoma Cell Lines.
NOTE: Perform engraftment of cells 14 days after the injection of bleomycin (step 2.1).
Allow cells to grow in 75 cm2 flasks undisturbed for 3 days prior to the day of injection with corresponding media as stated in step 1.3. NOTE: They should be 80% confluent at the day of injection (which corresponds to 2-3 x 106 in each flask depending on the cell line).
Wash the cells growing on 75 cm2 flasks with 5 mL of PBS at room temperature.
Aspirate PBS and add 1.5 mL of trypsin to the cells and incubate the cells at 37 °C until the cells detach (typically 2-5 min). NOTE: We recommend checking every 2 min for cell detachment from the plastic by simple visualization of the bottom of the flask or under a light microscope. Do not exceed 5 min of incubation with trypsin.
Neutralize trypsin by adding 4 mL of media containing 10% FBS (same media as step 4.1). Collect the cells into a 15 mL tube and centrifuge it at 200 x g for 3 min at room temperature.
Aspirate the media and resuspend the cell pellet in 5 mL of PBS to wash cells. Centrifuge the cells at 200 x g for 3 min at room temperature to pellet cells. Repeat this 1 time.
Aspirate the PBS and resuspend the pellet in 1.5 mL of PBS per flask.
Mix 50 µL of cell mixture with 50 µL of trypan blue and count the cells using a Cell Counter/Hemocytometer chamber28.
Prepare cell suspension by diluting the cells in PBS to inject 1 x 105 cells (or other amount indicated) in 50 µL per mouse. Keep the cells on ice prior to injection. NOTE: Prepare at least two times more cells for injection to avoid running out of cell suspension.
Prepare anesthesia by diluting ketamine and xylazine to a final concentration of 10 mg/mL and 1 mg/mL, respectively, Using a 1 mL syringe and 27 G needle, anesthetize each mouse by injecting intraperitoneally ketamine/xylazine solution at 100/10 mg/kg respectively.
Monitor the breathing of mice and employ a toe pinch. Confirm proper anesthetization when mouse does not respond to toe pinch. Apply vet ointment on eyes to prevent dryness while under anesthesia.
Place 1 mouse at a time on an intubation platform by hanging its front teeth with its back against the platform.
Illuminate the upper chest using a fiber-optic illuminator to help with visualization of the trachea.
Resuspend the cells gently using a p200 pipette to make sure they do not form clumps. Open the mouth of the mouse and pull the tongue out gently with sterile flat forceps.
Use an intravenous catheter with the needle removed to avoid blocking breathing. Position the catheter over the white light emitted from the opening of the trachea.
Insert the catheter into the trachea until the top of the catheter reaches the front teeth. Confirm proper placement of the catheter in the trachea by visualizing the white light shining through the opening of the catheter in the mouth.
Using a pipette, dispense 50 µL of cells directly into the catheter to ensure that the entire volume is inhaled. Perform this step under sterile conditions. NOTE: If the catheter is correctly inserted, the mouse will immediately inhale the contents of the catheter.
Wait a few seconds until the entire volume travels down the catheter, and then remove the catheter from the trachea and dispose in 10% bleach solution.
If the mouse is not inhaling the liquid, carefully monitor breathing and adjust the catheter position. If the mouse stops breathing, remove the catheter immediately and allow the mouse to resume breathing normally before re-inserting the catheter. NOTE: The average time per mouse of steps 4.11-4.18 is around 5 min.
Place the injected mouse on an IACUC approved heating pad on their back on a flat surface for recovery. NOTE: The whole procedure will take 10 min per mouse. Do not return an animal that has undergone injection to the company of other animals until fully recovered.
Shave the entire rib area both ventrally and dorsally of B6129SF1/J and other mouse strains with dark hair using an electric shaver prior to imaging.
2-3 min after cell injection, inject 100 µL of luciferin retro-orbitally (15 mg/mL) using an insulin needle. Alternate the eye used for injection every imaging session.
After at least 2 min, place up to 5 mice in an animal bioluminescence imager in the dorsal recumbency position and acquire a ventral picture using luminescence settings29.
Under the control panel adjust the resolution and sensitivity settings to measure luminescence of a given cell line. For most applications, start with 3 min (or "Auto" if saturated), binning=Medium (4), F/Stop=1, Field of View=D, Subject Height=1.50. NOTE: If the injection is successful, luminescence signal is detected in the upper chest area, in one lung or both lungs.
Flip mice onto sternal recumbency position and acquire a dorsal picture as above. NOTE: If cell injection is not performed properly, cells may cluster at the entrance of the trachea or in the neck.
Inject ketoprofen 5 mg/kg intraperitoneally to alleviate pain.
Move mice back to their cage and place a "BSL-2 Agent in Use" Card on the cage.
Monitor mice for respiratory distress immediately after intubation, and then every 15 min until mice wake up from anesthesia. Do not leave mice unattended until they have regained sufficient consciousness to maintain sternal recumbency.
Examine the mice 24 h post intubation and three times a week for any signs of morbidity.
Keep record in the logbook of the date of procedure, animal identification, type of procedure, type of anesthetic and post-operative monitoring observations and times.
Monitor mice for weight loss, respiratory distress, behavioral abnormalities, and a body condition score <2 (segmentation of vertebral column evident/dorsal pelvic bones are palpable) bi-weekly following inoculation of bleomycin. NOTE: Mice with lung tumors may exhibit respiratory irritation, weight loss, cachexia, and/or death.
Euthanize mice under respiratory distress or mice that have experienced 15% loss of body mass by CO2 or by ketamine/xylazine followed by cervical dislocation if collecting tissues. Confirm euthanasia by conducting palpation for respiratory activity.
5. Monitoring of Tumor Growth by Bioluminescence Imaging
Repeat imaging of the mice at day 3 post-engraftment and weekly thereafter.
Anesthetize mice by injecting ketamine/xylazine (as described in steps 2.4-2.5) or by inhaled isoflurane (2%). Monitor breathing of mice and employ a toe pinch to confirm proper anesthetization. Apply vet ointment on eyes to prevent dryness while under anesthesia.
Shave the entire rib area both ventrally and dorsally of B6129SF1/J and other mouse strains with dark hair using an electric shaver prior to imaging.
Once the mice are under anesthesia, inject 100 µL of luciferin retro-orbitally (15 mg/mL) using an insulin needle. Wait 2 min. Alternate the eye used for injection every imaging session.
Place mice in the imager and acquire dorsal and ventral images as described in steps 4.22-4.24.
Place mice back into the cage after imaging, on their back on a flat surface for recovery. NOTE: The whole procedure will take 5 min per group of 5 mice. Do not leave mice unattended until they have regained sufficient consciousness to maintain sternal recumbency.
- Analyze the images using the imager/bioluminescence analysis software.
- Select the appropriate images and load them as a group in the bioluminescence analysis software.
- Click ROI tool | square to add a square region of interest (ROI). Place the ROI over the upper chest area, covering the entire lung image. Repeat this for each mouse image.
- Right click and select Copy all ROI function to apply the same ROI to all images in the group and position them in the upper chest of the mice, both ventral and dorsal views.
- In the upper left corner of the image window, select the Photons function. Click the Measure control panel function to measure the ROIs. Copy and paste the output table containing total flux (photons/s) into a software of choice to analyze the data further.
- Normalize the daily ROI value of each mouse to its ROI value measured on day 0.
6. Tissue Isolation and Processing.
Anesthetize mice by injecting ketamine/xylazine (as described in steps 2.4-2.5 or by inhaled isoflurane (2%). Monitor breathing of mice and employ a toe pinch. Confirm proper anesthetization when mouse does not respond to toe pinch. Apply vet ointment on eyes to prevent dryness while under anesthesia.
Inject 100 µL of luciferin retro-orbitally (15 mg/mL) using an insulin needle. Wait 2 min.
Image mice following steps 4.22-4.24 from this protocol.
Inject into the other eye from step 6.2 another 100 µL of luciferin retro-orbitally using an insulin needle prior to euthanasia.
Euthanize the mice by intraperitoneal injection of ketamine/xylazine (see step 2.4 for details) followed by cervical dislocation if mice are under deep anesthesia in accordance with guidelines provided by IACUC. Confirm euthanasia by conducting palpation for respiratory activity.
Using sterile surgical scissors make a skin incision below the sternum. Open the muscle layer along the diaphragm using forceps and surgical scissors and expose the thoracic cavity by cutting through the rib cage on either of the lateral sides avoiding the internal thoracic arteries.
Perfuse the mouse by making a small incision in the right atrium of the heart and inject 5 mL of PBS through the left ventricle. The color of lung tissue will go from red to pale pink-white if the blood perfusion is done properly.
Excise the lungs from the thoracic cavity carefully by grabbing the esophagus or heart with forceps. Gently pull the tissue and use surgical scissors to carefully cut connecting tissue avoiding puncture of the lung tissue. Preserve the trachea as much as possible for inflation of the lung airways at a later step.
Wash the lung by submerging and swirling the tissue 3 times in a 6 well plate filled with 3 mL of PBS to remove excess blood.
Place the lung onto the lid of a 6 well plate, and place the lid containing the tissue in the bioluminescent imager and acquire a luminescent image30. NOTE: We recommend selecting the following settings from the "Acquisition control panel" "Field of view=A" and "Subject Height=0.75 cm" and then "Auto exposure" to avoid getting saturated images.
Excise and image other organs, as done in step 6.9-6.10, where metastasis have been identified by luminescence, such as brain, liver, adrenal, bone, lymph nodes, kidney or spleen31.
Perfuse the lung with 1 mL of 4% paraformaldehyde by inserting the needle into the trachea of the mouse. The lungs will inflate if well-perfused. Caution: 4% paraformaldehyde is a toxic, a health hazard GHS07 and GHS08.
Transfer the lungs into a 15 mL conical with 5 mL of 4% paraformaldehyde.
Fix the lungs or other tissues by incubating the tissue with 4% paraformaldehyde overnight at 4 °C in a shaking device at 30-50 rpm.
Embed lungs (or other tissues) in paraffin or optimum cutting temperature compound depending on the application32. NOTE: Paraffin is the preferred method to preserve the lung structure and for Masson Trichrome and Hematoxylin-Eosin staining.
Representative Results
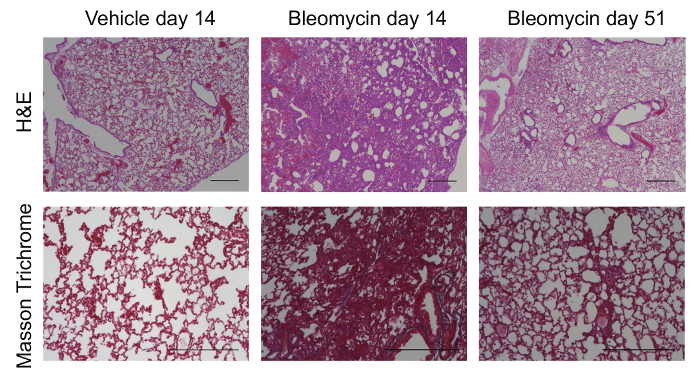
To increase the efficiency of LUAD cancer cell engraftment into the lungs of mice, we developed a protocol that first pre-conditions the airways using bleomycin followed by orthotopic tumor cell injection (Figure 1). We confirmed that even when administered into immunocompromised athymic mice, bleomycin induced transient fibrosis by day 14 as evidenced by loss of airway architecture and increased collagen deposition (Figure 2). Gross fibrosis in these mice resolved 50 days post bleomycin injection (Figure 2; right panels) consistent with prior studies24.
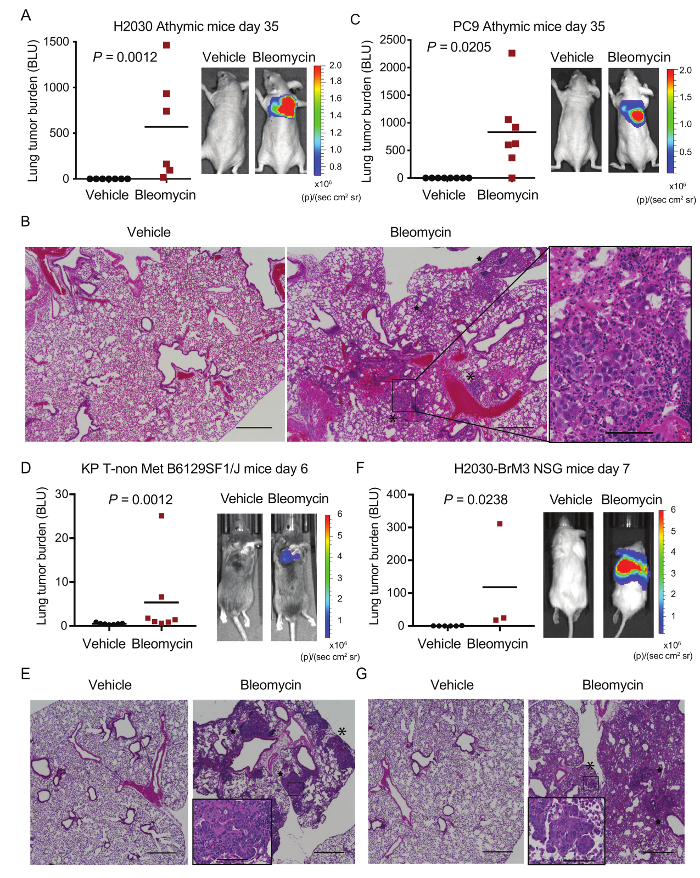
Based on these observations, we engrafted LUAD cells of different origins into the lungs of various mouse strains that had been pre-conditioned with a single dose of vehicle or bleomycin 14 days prior. For example, a suspension of LUAD cells from the established human LUAD cell line, H2030, was delivered intratracheally into the lungs of athymic mice. After 35 days, mice pretreated with bleomycin had high lung tumor burden as detected by bioluminescence (Figure 3A). Conversely, in vehicle-treated animals, no tumors were detected over the same time frame (Figure 3A). Lung tumor burden was confirmed by histology following necropsy. Lungs pretreated with vehicle had no evidence of tumor nodules whereas bleomycin pre-treated lungs had large tumor nodules (Figure 3B). Using this protocol, we also increased the engraftment of another human LUAD cell line into athymic mice (PC9; Figure 3C) and a murine LUAD cell line that was injected into syngeneic immunocompetent B6129SF1/J mice (Figure 3D). The early lesions observed in bleomycin pre-treated syngeneic mice were mainly grade 1 and 2 and were well differentiated, resembling tumors arising in situ from KrasG12D/+;p53-/- GEMMs33. All engrafted LUADs grew both in the proximal and distal lung (Figure 3B, Figure 3E, Figure 3G; asterisk (distal), star (proximal)). The establishment of PDXs from lung cancer biospecimens (from biopsies or surgical resections) is relatively inefficient, even when human cells are subcutaneously transplanted into severely immunodeficient animals such as the NOD scid gamma (NSG) mouse strain34. To evaluate the potential application of our method to PDXs, we also injected a highly metastatic cell sub-population of the H2030 cell line, termed H2030-BrM3 cells35, intratracheally in NSG mice that had been pre-conditioned with vehicle or bleomycin. We note that NSG mice may be more susceptible to the toxic effects of bleomycin (data not shown) and that a dose lower than 0.02 units per mouse is advisable when working with this strain. Nevertheless, our protocol also significantly increased the engraftment and tumor burden in the lungs of NSG mice (Figure 3F, Figure 3G).
We next used this protocol to test the engraftment rates of limiting amounts of cells using the H2030 cell line in athymic mice. Mice pre-treated with bleomycin were injected with 5 x 105, 5 x 104, or 5 x 103 H2030 cells. 85.7-100% of mice developed lung tumors between 7 and 10 weeks at all dilutions tested (Table 2). We conclude that engraftment of a relatively low number of tumor cells in the lung is possible if mice are pre-conditioned with bleomycin. Overall, our protocol could be tailored to several strains of mice and various LUAD cell line models to increase the engraftment rate of cancer cells into the lungs from 0-16.7% to 71.4-100% (Table 2).
Finally, to evaluate the putative kinetics of LUAD cell outgrowth and metastatic dissemination from bleomycin pre-conditioned lungs, we characterized the behavior of highly metastatic H2030-BrM3 cells using our protocol. Following orthotopic engraftment in vehicle-treated mice, tumor cell attrition was observed by bioluminescence at day 3 post-injection and lung tumor growth could not be detected thereafter or at endpoint (day 35) (Figure 4A, Figure 4B). Conversely, we detected lung tumor outgrowth in mice pre-treated with bleomycin as early as day 7 post-injection (Figure 4A). After 39-57 days of tumor expansion, the morbidity and bioluminescent intensity associated with high lung tumor burden limits the analysis of disseminated disease (data not shown). Therefore, we imaged metastasis in distant organs ex vivo at necropsy. In animals that had lung tumor burden for 57 days, small lesions could also be detected in tissues such as the brain, liver, kidney, adrenal glands, and bone hind limbs at variable frequencies (Figure 4C). Therefore, we conclude that spontaneous disseminated disease in clinically relevant sites can be generated from this orthotopic method.
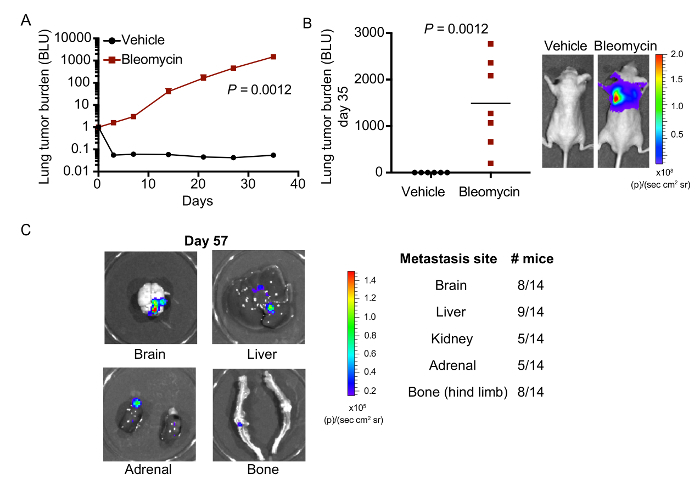
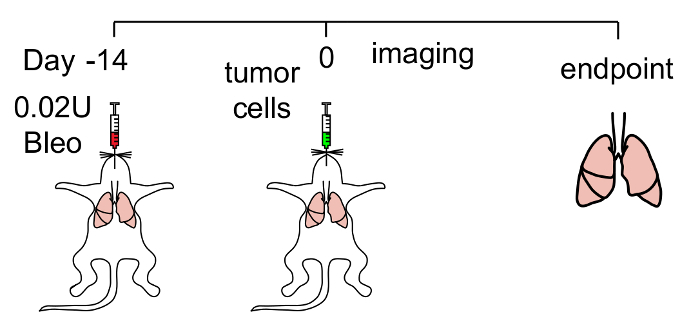
Figure 1: Method to pre-condition the lungs for tumor cell engraftment. Schematic of the protocol used. Lung injury is performed by injecting bleomycin intratracheally. LUAD cells are then injected into the trachea of mice 14 days after. Mice are subsequently imaged weekly for luminescence to monitor tumor engraftment, growth and distant metastasis. Please click here to view a larger version of this figure.
Figure 2: Bleomycin causes transient lung fibrosis and ECM remodeling. Representative images of Hematoxylin-Eosin (H&E) and Masson Trichrome staining of lungs from vehicle and bleomycin treated mice 14 days post-injection (peak of fibrosis) and at 51 days post-injection (fibrosis resolution) in the absence of tumor cell injection. Scale bar = 500 µm. Please click here to view a larger version of this figure.
Figure 3: Lung engraftment of LUAD cell lines in multiple mouse models is increased in mice pre-treated with bleomycin.A) Athymic mice pre-treated with vehicle or bleomycin were injected with 5x105 H2030 cells. Relative lung tumor burden at endpoint (day 35 normalized to day 0 post-injection) as measured by bioluminescence is plotted. For each group, a mouse with median tumor burden is shown. n=6-7 mice per group. B) Representative H&E images of lungs pre-treated with vehicle or bleomycin from A at day 35. Asterisk = distal; Star = proximal. C) Athymic mice treated as in A were injected with 1x105 PC9 cells. Tumor burden and representative images are measured and shown as in A. n=7 mice per group. D-E) B6129SF1/J mice pre-treated with vehicle or bleomycin were injected with 1x105 368T1 KrasG12D/+ p53-/- (KP T-non Met) cells. Tumor burden, representative images and H&Es are measured and depicted as in A and B. n= 7 mice per group. F-G) NSG mice pre-treated with vehicle or bleomycin were injected with 1 x 105 H2030-BrM3 cells. Tumor burden, representative images and H&Es (day 9 and day 10 post-injection) are measured and shown as in A and B. n=3-6 mice per group. Scale bar = 500 µm. Inset = high magnification of tumor cell engrafted areas. Scale bar of insets = 100 µm. p values calculated by the Mann-Whitney test. Please click here to view a larger version of this figure.
Figure 4: LUAD cells engrafted in bleomycin-treated mice metastasize to multiple distant organs.A) Athymic mice pre-treated with vehicle or bleomycin were injected with 5x105 H2030-BrM3 cells. Lung tumor burden was measured weekly using bioluminescence. Dorsal total photon flux normalized to day 0 is shown. B) Tumor burden by bioluminescence of the lungs from A at endpoint (day 35 post-injection). For each group, a mouse with median tumor burden is shown. n=6-7 mice per group. C) Representative images of organs with detectable metastasis from the same mouse at necropsy (left). Table with indicated frequencies of mice with metastasis in distant organs at endpoint (day 39 to 57 post-engraftment) (right). Ex-vivo organs with visual bioluminescent signal above 1.5 x 104 photons/(sec cm2 steradian) were counted as positive metastasis. Please click here to view a larger version of this figure.
| Mouse Strain | Units/mouse |
| Athymic | 0.02 |
| NSG | 0.02 |
| B6129SF1/J | 0.005 |
Table 1: List of recommended doses of bleomycin for mouse strains tested.
| Mouse Strain | Cell Line | Number of cells injected | % engraftment | p value | |
| Vehicle | Bleomycin | ||||
| Athymic | H2030 | 5x105 | 0% (0/7) | 100% (6/6) | 0.0006 |
| Athymic | H2030 | 5x104 | n.d. | 85.7% (6/7) | n.a. |
| Athymic | H2030 | 5x103 | n.d. | 85.7% (6/7) | n.a. |
| Athymic | PC-9 | 1x105 | 0% (0/7) | 85.7% (6/7) | 0.0023 |
| NSG | H2030-BrM3 | 1x105 | 16.7% (1/6) | 100% (3/3)* | 0.0476 |
| B6129SF1/J | 368T1 | 1x105 | 0% (0/7) | 71.4% (5/7)* | 0.0105 |
Table 2: Summary table of the engraftment frequency (%) using the indicated mouse strains, cell lines and cell numbers. Engraftment was determined by in vivo luminescence imaging and confirmed by ex vivo imaging with histology of the tissues between 37 and 50 days post-injection. * Positive engraftment was confirmed by in vivo luminescence at day 10 post-injection.p values calculated by one-sided Fischer's exact test. n.a. = not applicable; n.d. = not determined.
Discussion
Striking clinical parallels have been documented between lung cancer and other chronic diseases of the lung36. In particular, patients with idiopathic pulmonary fibrosis (IPF) have an increased predilection for developing lung cancer, and this association is independent of smoking history37,38. IPF is characterized by progressive destruction of lung architecture and impaired respiratory function through deposition of ECM39. Also, following surgical resection, early stage NSCLC patients with concurrent IPF have a poor outcome40. Most NSCLC tumors contain a fibrotic component at the time of diagnosis, and the extent of this stromal reaction correlates with poor prognosis18. Acute or sustained tissue damage from fibrosis may increase the incidence of tumorigenesis in multiple ways. For instance, after injury, the process of epithelial regeneration may expand the pool of progenitor cells susceptible to transformation. Alternatively, the influx and function of various immune cells may help establish an immunosuppressive microenvironment, while the activation of myofibroblasts may secrete growth factors or deposit tumor-promoting ECM. Accordingly, our method of pre-conditioning the lungs with a fibrosis inducing agent may prove to be particularly apt at studying the effects of lung cancer co-morbidities (e.g. fibrosis) and modeling NSCLC subtypes, which are rich in extracellular matrix and an inflammatory stroma41.
Overall, our method significantly enhanced the engraftment of tumor cells injected into the airways via the trachea. This observation holds true for mouse strains with varying degrees of immunocompetence. Other methods of tumor cell injection into the lungs include tail vein injection or direct injection into the lung parenchyma via the chest wall. For the specific purpose of studying lung cancer cell tumorigenicity, these methods are sub-optimal, as the former requires tumor cells to survive in circulation and extravasate before outgrowth (a process more akin to metastasis into the lungs), while the latter can cause cells to leak out into the pleural space soon after injection (data not shown). Both methods may confound loco-regional quantification of lung cancer cell outgrowth. Alternatively, our protocol ensures that seeded tumor cells are first retained in the airways surrounded by the lung stroma. Critical to the success of this protocol, are the proper intubation of the mice, tolerable dose of bleomycin, and timing of airway pre-conditioning relative to tumor cell injection. We also demonstrate that this protocol allows the cells to subsequently disseminate to other tissues, including the brain. Although the morbidity associated with lung tumor burden may still limit comprehensive characterization of distant macrometastasis, this approach may be useful to quantify and study circulating tumor cells originating from the lungs.
Despite the advantages of the method described herein, several technical variables may influence the ultimate efficiency of engraftment and monitoring of tumor progression. First, it is well documented that the fibrotic response to bleomycin in mice is strain-dependent. For instance, C57Bl/6 mice are more prone to fibrosis when compared to Balb/c mice42. Second, sensitivity to bleomycin is gender and age dependent. In our experience, females and younger mice are more prone to adverse effects over the entire protocol. This may limit tumorigenesis studies requiring direct comparisons between male and female, or young and old animals. Moreover, intratracheal instillation of mice younger than 7 weeks is technically challenging and adjustments to the catheter size may be required. Third, bleomycin is toxic and may be mutagenic due to its ability to induce DNA breaks. It is important to test the tolerable dose of bleomycin for each mouse strain prior to performing the experiments. In this proof of principle study, we provide suggested doses for male mice across various strains. Additionally, mice with dark fur are less suitable for bioluminescent imaging due to low tissue penetration and masking of the bioluminescent signal by the fur. Depilating the mice may improve image measurements, but alternative methods can also be employed such as Magnetic Resonance Imaging and fluorescent imaging using probes of long wavelength such as TdTomato or Katushka43. Finally, the potential immunogenicity of a given reporter protein should be considered when selecting a tumor detection modality for a given mouse model.
Bleomycin remodels the distal lungs by first damaging alveolar type 2 (AT2) cells which then regenerate in an attempt to repair the alveoli44. Since AT2 cells are one of the major cell types to give rise to NSCLC, pre-conditioning the lungs with bleomycin may also modify the initiation and progression of tumors arising in situ from GEMMs. Other types of injury, including influenza infection13 or naphthalene instillation14, have been shown to increase the engraftment of human and murine lung stem/progenitor cells. Notably, naphthalene damages stem/progenitor cells in the bronchio-alveolar junctions and potentially increases tumor initiation in GEMMs45. Fine-tuning the type of injury and airway niche targeted to the engrafted cell types may further optimize our method to study lung cancer allografts or xenografts of different origins. Finally, we propose that this method might be implemented for the standard generation of lung cancer PDXs. The success rate of establishing lung cancer PDXs from human biospecimens by subcutaneous transplantation in NSG mice is relatively low (30-40%)46,47. Orthotopic growth of PDXs may not only increase this success rate with limiting amounts of specimen (from human lungs), but also generate more physiologically relevant conditions to test the pharmacokinetics and pharmacodynamics of lung cancer therapeutics.
Disclosures
The authors declare no competing financial interests.
Acknowledgments
This study was funded by grants from the National Cancer Institute (R01CA166376 and R01CA191489 to D.X. Nguyen) and the Department of Defense (W81XWH-16-1-0227 to D.X. Nguyen).
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA-Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- Gaspar LE. Brain metastases in lung cancer. Expert Rev Anticanc. 2004;4:259–270. doi: 10.1586/14737140.4.2.259. [DOI] [PubMed] [Google Scholar]
- Hess KR, et al. Metastatic patterns in adenocarcinoma. Cancer. 2006;106:1624–1633. doi: 10.1002/cncr.21778. [DOI] [PubMed] [Google Scholar]
- Hoffman PC, Mauer AM, Vokes EE. Lung cancer. Lancet. 2000;355:479–485. doi: 10.1016/S0140-6736(00)82038-3. [DOI] [PubMed] [Google Scholar]
- Travis WD. Pathology of lung cancer. Clin Chest Med. 2002;23(1):65–81. doi: 10.1016/s0272-5231(03)00061-3. [DOI] [PubMed] [Google Scholar]
- Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer. 2014;14:535–546. doi: 10.1038/nrc3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CF, et al. Mouse models of human non-small-cell lung cancer: raising the bar. Cold Spring Harb Sym. 2005;70:241–250. doi: 10.1101/sqb.2005.70.037. [DOI] [PubMed] [Google Scholar]
- Meuwissen R, Berns A. Mouse models for human lung cancer. Gene Dev. 2005;19:643–664. doi: 10.1101/gad.1284505. [DOI] [PubMed] [Google Scholar]
- Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501:346–354. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12:133–143. doi: 10.1038/nrc3184. [DOI] [PubMed] [Google Scholar]
- Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- Leblond AL, et al. Developing cell therapy techniques for respiratory disease: intratracheal delivery of genetically engineered stem cells in a murine model of airway injury. Hum Gene Ther. 2009;20:1329–1343. doi: 10.1089/hum.2009.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan AE, et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature. 2015;517:621–625. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo W, et al. p63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature. 2015;517:616–620. doi: 10.1038/nature13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney JE, Kim CF. Tracing the potential of lung progenitors. Nat Biotechnol. 2015;33:152–154. doi: 10.1038/nbt.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Morales JE, Calame DG, Alcorn JL, Wetsel RA. Transplantation of human embryonic stem cell-derived alveolar epithelial type II cells abrogates acute lung injury in mice. Mol Ther. 2010;18:625–634. doi: 10.1038/mt.2009.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz LA, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, et al. Prognostic significance of the size of central fibrosis in peripheral adenocarcinoma of the lung. Ann Thorac Surg. 2000;69:893–897. doi: 10.1016/s0003-4975(99)01331-4. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research N. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–550. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotton CJ, Chambers RC. Bleomycin revisited: towards a more representative model of IPF? Am J Physiol-Lung C. 2010;299:L439–L441. doi: 10.1152/ajplung.00258.2010. [DOI] [PubMed] [Google Scholar]
- Hay J, Shahzeidi S, Laurent G. Mechanisms of bleomycin-induced lung damage. Arch Toxicol. 1991;65:81–94. doi: 10.1007/BF02034932. [DOI] [PubMed] [Google Scholar]
- Vaccaro CA, Brody JS, Snider GL. Alveolar wall basement membranes in bleomycin-induced pulmonary fibrosis. Am Rev Respir Dis. 1985;132:905–912. doi: 10.1164/arrd.1985.132.4.905. [DOI] [PubMed] [Google Scholar]
- Venkatesan N, Ebihara T, Roughley PJ, Ludwig MS. Alterations in large and small proteoglycans in bleomycin-induced pulmonary fibrosis in rats. Am J Resp Crit Care. 2000;161:2066–2073. doi: 10.1164/ajrccm.161.6.9909098. [DOI] [PubMed] [Google Scholar]
- Moore BB, Hogaboam CM. Murine models of pulmonary fibrosis. Am J Physiol-Lung C. 2008;294:L152–L160. doi: 10.1152/ajplung.00313.2007. [DOI] [PubMed] [Google Scholar]
- Izbicki G, Segel MJ, Christensen TG, Conner MW, Breuer R. Time course of bleomycin-induced lung fibrosis. Int J Exp Pathol. 2002;83:111–119. doi: 10.1046/j.1365-2613.2002.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier DJ, Phan SH, McGarry BM. The effects of the nude (nu/nu) mutation on bleomycin-induced pulmonary fibrosis. A biochemical evaluation. Am Rev Respir Dis. 1983;127:614–617. doi: 10.1164/arrd.1983.127.5.614. [DOI] [PubMed] [Google Scholar]
- Ponomarev V, et al. A novel triple-modality reporter gene for whole-body fluorescent, bioluminescent, and nuclear noninvasive imaging. Eur J Nucl Med Mol I. 2004;31:740–751. doi: 10.1007/s00259-003-1441-5. [DOI] [PubMed] [Google Scholar]
- Morten BC, Scott RJ, Avery-Kiejda KA. Comparison of Three Different Methods for Determining Cell Proliferation in Breast Cancer Cell. J. Vis. Exp. 2016. [DOI] [PMC free article] [PubMed]
- Tseng JC, Kung AL. Quantitative bioluminescence imaging of mouse tumor models. Cold Spring Harbor protocols. 2015. pdb prot078261. [DOI] [PubMed]
- Byrne FL, McCarroll JA, Kavallaris M. Analyses of Tumor Burden In Vivo and Metastasis Ex Vivo Using Luciferase-Expressing Cancer Cells in an Orthotopic Mouse Model of Neuroblastoma. Methods Mol Biol. 2016;1372:61–77. doi: 10.1007/978-1-4939-3148-4_5. [DOI] [PubMed] [Google Scholar]
- Parkinson CM, et al. Diagnostic necropsy and selected tissue and sample collection in rats and mice. J. Vis. Exp. 2011. [DOI] [PMC free article] [PubMed]
- Tammela T, et al. A Wnt-producing niche drives proliferative potential and progression in lung adenocarcinoma. Nature. 2017;545:355–359. doi: 10.1038/nature22334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPage M, Dooley AL, Jacks T. Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat Protoc. 2009;4:1064–1072. doi: 10.1038/nprot.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne AT, et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat Rev Cancer. 2017;17:254–268. doi: 10.1038/nrc.2016.140. [DOI] [PubMed] [Google Scholar]
- Nguyen DX, et al. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell. 2009;138:51–62. doi: 10.1016/j.cell.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu G, Nyberg F, Morgan G. The epidemiology of interstitial lung disease and its association with lung cancer. Brit J Cancer. 2004;91(Suppl 2):S3–S10. doi: 10.1038/sj.bjc.6602061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard R, Venn A, Lewis S, Britton J. Lung cancer and cryptogenic fibrosing alveolitis. A population-based cohort study. Am J Resp Crit Care. 2000;161:5–8. doi: 10.1164/ajrccm.161.1.9906062. [DOI] [PubMed] [Google Scholar]
- Nagai A, Chiyotani A, Nakadate T, Konno K. Lung cancer in patients with idiopathic pulmonary fibrosis. Tohoku J Exp Med. 1992;167:231–237. doi: 10.1620/tjem.167.231. [DOI] [PubMed] [Google Scholar]
- Rock JR, et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci USA. 2011;108:E1475–E1483. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, et al. Survival after surgery for pathologic stage IA non-small cell lung cancer associated with idiopathic pulmonary fibrosis. Ann Thorac Surg. 2011;92:1812–1817. doi: 10.1016/j.athoracsur.2011.06.055. [DOI] [PubMed] [Google Scholar]
- Stevens LE, et al. Extracellular Matrix Receptor Expression in Subtypes of Lung Adenocarcinoma Potentiates Outgrowth of Micrometastases. Cancer Res. 2017;77:1905–1917. doi: 10.1158/0008-5472.CAN-16-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JH, Lazo JS. High dose continuous infusion of bleomycin in mice: a new model for drug-induced pulmonary fibrosis. J Pharmacol Exp Ther. 1987;243:1185–1194. [PubMed] [Google Scholar]
- Shcherbo D, et al. Bright far-red fluorescent protein for whole-body imaging. Nature Methods. 2007;4:741–746. doi: 10.1038/nmeth1083. [DOI] [PubMed] [Google Scholar]
- Aso Y, Yoneda K, Kikkawa Y. Morphologic and biochemical study of pulmonary changes induced by bleomycin in mice. Lab Invest. 1976;35:558–568. [PubMed] [Google Scholar]
- Kim CF, et al. Identification of bronchioalveolar stem cells in normal lung and lung. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Fichtner I, et al. Establishment of patient-derived non-small cell lung cancer xenografts as models for the identification of predictive biomarkers. Clin Cancer Res. 2008;14:6456–6468. doi: 10.1158/1078-0432.CCR-08-0138. [DOI] [PubMed] [Google Scholar]
- Zhang XC, et al. Establishment of patient-derived non-small cell lung cancer xenograft models with genetic aberrations within EGFR, KRAS and FGFR1: useful tools for preclinical studies of targeted therapies. J Transl Med. 2013;11:168. doi: 10.1186/1479-5876-11-168. [DOI] [PMC free article] [PubMed] [Google Scholar]


