Abstract
Streptococcus suis is not only a major bacterial pathogen of pigs worldwide but also an emerging zoonotic agent. In humans and pigs, meningitis is a major manifestation of S. suis infections. A suitable infection model is an essential tool to understand the mechanisms of diseases caused by pathogens. Several routes of infection in mice have been developed to study the pathogenesis of S. suis infection. However, the intraperitoneal, intranasal, and intravenous routes of infection are not suitable for studying the roles of S. suis surface components in meningitis directly in the brain, such as the extracellular matrix from biofilms. Although intracisternal inoculation has been used for S. suis infection, the precise injection site has not been described. Here, the intracranial subarachnoidal route of infection was described in a mouse model to investigate the roles of biofilms in S. suis meningitis. S. suis planktonic cells or biofilm state cells were directly injected into the subarachnoid space of mice through the injection site located 3.5 mm rostral from the bregma. Histopathological analysis and increased mRNA expression of TLR2 and cytokines of the brain tissue from mice injected with biofilm state cells clearly indicated that S. suis biofilm plays definitive roles in S. suis meningitis. This route of infection has obvious advantages over other routes of infection, allowing the study of the host-bacterium interaction. Furthermore, it permits the effect of bacterial components on host immune responses directly in the brain to be assessed, and mimics bacterial entrance into the central nervous system. This route of infection can be extended for investigating the mechanisms of meningitis caused by other bacteria. In addition, it can also be used to test the efficacy of drugs against bacterial meningitis.
Keywords: Immunology and Infection, Issue 137, Streptococcus suis, intracranial subarachnoidal, biofilm, meningitis, mouse infection model, bacterial virulence
Introduction
Streptococcus suis (S. suis) is a major bacterial pathogen of pigs worldwide, causing severe diseases including meningitis, pneumonia, septicaemia, endocarditis, and arthritis1. It is also an emerging zoonotic agent. So far, it has been reported that nine serotypes can cause infection in humans, including serotypes 2, 4, 5, 9, 14, 16, 21, 24, and 312,3,4. In humans and pigs, meningitis is one of the major clinical signs of S. suis infections. In Vietnam and Thailand, S. suis is the major cause of meningitis in adults5. Microbial biofilms are microorganisms that adhere to each other and are concentrated at an interface; they are essential for bacterial virulence, survival in diverse environments, and antibiotic resistance5. Biofilms are typically surrounded by an extracellular matrix that generally contains polysaccharides, proteins, and DNA6. The latter is able to elicit host inflammatory responses and cytokine production7. Biofilm formation has been reported to be involved in streptococcal meningitis in previous studies. Biofilms contribute to Streptococcus agalactiae meningitis in a tilapia fish model and biofilm formation has been revealed within brain tissues and around meningeal surfaces in vivo through intra-abdominal inoculation8. During meningitis, Streptococcus pneumoniae is in a biofilm-like state and bacteria in such a biofilm state were more effective in inducing meningitis in a mouse infection model9. In addition, in our previous study, the biofilm state associated with S. suis in mouse brain contributes to bacterial virulence by survival analysis10. However, direct evidence for biofilm involvement in S. suis meningitis requires further investigation.
Animal models of S. suis infection have been developed in mice using the intraperitoneal (i.p.)11, intranasal (i.n.)12, intravenous (i.v.)13, and the intracisternal (i.c.) routes of infection14,15,16. However, the i.p., i.n., and i.v. routes of infection are not suitable for studying the roles of S. suis surface components in meningitis directly in the brain. These include extracellular matrix from biofilms. Although the i.c. inoculation was used for S. suis infection, the precise injection site has not been described in those papers. In contrast, the stereotaxic coordinates of the injection site for intracranial subarachnoidal inoculation has clearly been described in a previous study17. This allowed easy recognition of the inoculation point and more simplistic experimental protocol. In addition, the intracranial subarachnoidal route of infection mimics bacterial entrance into the central nervous system from the sinuses or the middle ear17, and the relationship between the middle ear and meningitis caused by S. suis has been demonstrated by Madsen et al18. Moreover, by applying the intracranial subarachnoidal route of infection in mice, we have demonstrated that S. suis small RNA rss04 contributes to meningitis in our previous study10.
In the present study, the intracranial subarachnoidal route of infection was used in mice to investigate the roles of biofilms in S. suis meningitis. Mice were infected with planktonic cells or biofilm state cells of S. suis by this route of infection. Histopathological analysis and increased mRNA expression of TLR2 and cytokines from brain tissue of mice injected with biofilm state cells clearly indicated that S. suis biofilm contributes to meningitis.
Protocol
The mouse infection experiments were approved by the Laboratory Animal Monitoring Committee of Jiangsu Province, China and performed in the Laboratory Animal Center of Nanjing Agricultural University (Permit number: SYXK (Su) 2017-0007).
1. Preparation of Bacteria
Note: S. suis serotype 2 virulent strain P1/7 was isolated from a diseased pig with meningitis19. Strain P1/7 was grown in Todd-Hewitt broth (THB, formula per liter of THB: Heart Infusion, 3.1 g; neopeptone, 20.0 g; dextrose, 2.0 g; sodium chloride, 2.0 g; disodium phosphate, 0.4 g; sodium carbonate, 2.5 g) and plated on Todd-Hewitt agar (THA, formula per liter of THA: Heart Infusion, 3.1 g; neopeptone, 20.0 g; dextrose, 2.0 g; sodium chloride, 2.0 g; disodium phosphate, 0.4 g; sodium carbonate, 2.5 g; agar, 15.0 g) at 37 °C and 5% CO2.
- Collection of strain P1/7 planktonic cells
- Collect 5 mL of planktonic cells from mid-log phase culture (OD600=0.6), centrifuge for 3 min at 8000 × g, and then wash 3 times with PBS.
- Resuspend the cells with 5 mL of 25% glycerol in THB, aliquot into 5 tubes, and then store at -80 °C.
- Collection of strain P1/7 biofilm state cells
- Take 20 mL of an overnight culture and add to 180 mL of fresh THB; divide the diluted culture into 10 round culture plates equally, and then put the plates in an incubator at 37 °C in 5% CO2 for 24 h.
- After incubation, shake the plate gently to resuspend the bacteria that have not adhered to the plate, and then discard the supernatant by aspiration. NOTE: Shake the plate gently and avoid resuspending the sediment.
- Add 5 mL of PBS to harvest biofilm state cells, resuspend the sediment completely, and then transfer the sample to a new tube.
- Sonicate the biofilm state cells from the last step with the following parameters: 60 W, 4 cycles, 5 s on and 10 s off.
- Centrifuge for 3 min at 8000 × g and store the supernatant at -80 °C. NOTE: The supernatant may contain biofilm components and it can be used to resuspend the biofilm bacteria before infection as described in the animal experiments (Step 3).
- Resuspend the sediment with 10 mL 25% glycerol in THB and then store the biofilm state cells at -80 °C.
2. Scanning Electron Microscopy (SEM) Analysis
Fix the collected biofilm state cells and planktonic cells using 4% paraformaldehyde for 12 - 24 h at 4 °C.
Rinse the samples quickly with distilled water and dehydrate the samples sequentially with an increasing concentration of ethanol (25%, 50%, 70%, 90%, and 100%) for 30 min in each solution.
Adhere the dried samples to metal holders with double-sided tape and finally coat them in an evaporator with gold and palladium.
Observe the samples by a scanning electron microscope using the following parameters: EHT (Extra High Tension) = 10 kV; WD (Working Distance) = 7.0 or 8.0 mm; Magnification = 5000 ×; Signal A= SE1.
3. Animal Experiments
- Use SPF 6-week-old female BALB/c mice in this study. Infect all mice through the intracranial subarachnoidal route of infection whose injection site is located 3.5 mm rostral from the bregma. The stereotaxic coordinates of the injection site were clearly described by Chiavolini et al17.
- For histopathological analysis, infect two groups of mice (5 mice per group) using planktonic cells or biofilm state cells at a dose of 3 × 107 colony-forming units (CFU), and another 5 mice were injected with PBS as a control.
- For detection of mRNA expression of TLR2 and cytokines in brain, infect two additional groups of mice (6 mice per group) using planktonic cells or biofilm state cells at a dose of 3 × 107 CFU.
- Determine the number of viable bacteria by plating serial dilutions on to THA.
- Euthanize all mice at 12 h post-infection for further research. NOTE: Glycerol in the stock medium must be removed before infection. Both planktonic cells and biofilm state cells recovered from -80 °C are washed 3 times with PBS. Then, planktonic cells are resuspended with PBS and diluted to the appropriate dose for infection; biofilm state cells are resuspended with the stored supernatant (from step 1.2.5) and diluted to the appropriate dose for infection. The volume for infection should not be more than 50 µL.
- Detecting the mRNA expression of TLR2 and cytokines in brain tissue
- Extract the total RNA from brain tissue using a RNA extraction kit.
- For each mouse, use whole brain tissue for extracting RNA. Divide whole brain tissue into 5 tubes. Take up to 100 mg brain tissue and add 1 mL of lysis solution to each tube containing the lysing matrix provided by the kit.
- Process the tube in a homogenizer for 40 s at a setting of 6.0, and then centrifuge the tube at 12000 × g and 4 °C for 5 min.
- After centrifugation, transfer the upper phase to a new microcentrifuge tube.
- Incubate the transferred sample for 5 min at room temperature; add 300 µL of chloroform, vortex for 10 s, and then incubate for 5 min at room temperature.
- Centrifuge the tube at 12000 ×g and 4 °C for 5 min. Transfer the upper phase to a new tube.
- Add 500 µL of cold absolute ethanol to the tube, before inverting it for 5 times, and then store the sample at -20 °C for at least 1 h.
- Centrifuge the tube at 12000 ×g and 4 °C for 15 min and remove the supernatant. Wash the pellet with 500 µL of cold 75% ethanol in RNase-free H2O.
- Remove the ethanol, air dry the pellet for 5 min at room temperature, and resuspend the RNA in 100 µL of RNase-free H2O.
- Incubate the RNA for 5 min at room temperature and determine the RNA concentration using a RNA bioanalyzer.
- Perform cDNA synthesis, including elimination of genomic DNA (gDNA), using a thermocycler with a reverse transcription (RT) reagent kit.
- Add 1 µg of RNA to a tube containing 2 µL of 5x gDNA elimination buffer, 1 µL of gDNA elimination enzyme and RNase-free H2O until the volume reaches 10 µL. Incubate for 2 min at 42 °C.
- Add 10 µL of master mix (1 µL of RT enzyme Mix I, 1 µL of RT Primer Mix, 4 µL of 5x Buffer 2, and 4 µL of RNase-free H2O) to the reaction solution from the last step and then mix gently. Proceed immediately with the RT reaction: 37 °C for 15 min and then 85 °C for 5 s.
- Perform the quantitative real-time PCR (RT-qPCR) analysis using a Real-Time PCR machine with a SYBR RT-qPCR kit. Add 10 µL of 2x enzyme, 0.8 µL of forward primer, 0.8 µL of reverse primer, 0.4 µL of 50x ROX Reference Dye II, 2 µL of cDNA template, and RNase-free H2O until a total volume of 20 µL.
- Run each sample in triplicate using the following thermal parameters: 30 s at 94 °C, followed by 40 cycles of 5 s at 95 °C, and 34 s at 60 °C, with a final stage of 15 s at 95 °C, 1 min at 60 °C, and 15 s at 95 °C. Primers for RT-qPCR are listed in Table 1. Housekeeping genes B2m and 18s rRNA were used as the internal controls.
- Calculate the relative fold change based on the 2-ΔΔCt method.
- Observation of histological sections
- After 24 h fixation with 10% formalin, reconstruct the fixed brain tissue into appropriate sizes of tissue pieces at 2 - 3 mm.
- Put the tissue pieces in an embedding cassette and immerse in a new fixative solution for dehydration with an automatic vacuum dehydrator.
- Take out the fixed tissue and dehydrate it sequentially in increasing concentrations of ethanol (70%, 80%, 95%, 95%, and 100%) for 30 min in each solution.
- Transparentize the sample with xylene for 15 min, dip it in liquid paraffin at 60 °C for 90 min, and then embed using an embedding machine.
- Cut the paraffin block embedded tissue into 3 µm slices, tile the slice on water at 45 °C, and then dry it in the air.
- Incubate the section for 3 h at 60 °C, stain it using the haematoxylin and eosin (HE) method, and seal it with the neutral gum.
- Observe the histopathological changes of the brain with an optical microscope. A four-point grading system was used to evaluate histological changes. Use an unpaired t test for statistical analysis. Congestion/haemorrhage: absent, score 0; small area of focal congestion/haemorrhage, score 1; large area of focal congestion/haemorrhage, or multiple sites of small area of focal congestion/haemorrhage, score 2; up to three large focal congestion/haemorrhage sites, score 3; diffuse congestion/haemorrhage, score 4. Necrosis: absent, score 0; focal necrosis, score 1; diffuse occurrence of 1 - 10 necrotic cells, score 2; foci of confluent necrosis, score 3; extensive confluent necrosis, score 4. Inflammatory cell infiltration: absent, score 0; few and focal infiltrations, score 1; multifocal infiltration or infiltrations with formation of aggregates, score 2; up to three aggregates, score 3; and more than four aggregates, score 4.
Representative Results
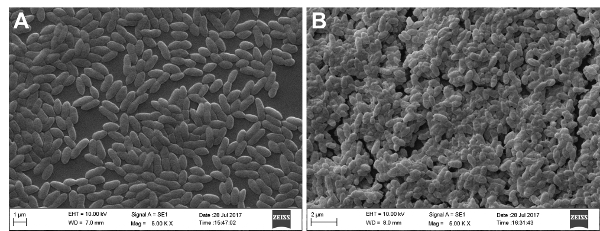
SEM analysis was performed to examine biofilm formation under the experimental conditions. As shown in Figure 1, there is a significant difference in biofilm formation between planktonic cells (Figure 1A) and biofilm state cells (Figure 1B). SEM analysis showed that biofilm bacteria were in clumps and multiple layers and they were encased in the extracellular matrix, while planktonic bacteria were much less dense and mainly dispersed individually.
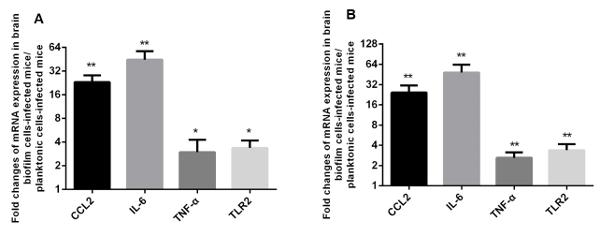
The components of the extracellular matrix of biofilms, such as DNA, are able to elicit host inflammatory responses and cytokine production. Since TLR2 and cytokines CCL2, IL-6, and TNF-α are involved in cerebral inflammatory responses related to meningitis according to previous studies10,11,20, the mRNA expression of these genes was compared in vivo for mice infected with planktonic cells and those infected with biofilm state cells. This was done to explore the effect of biofilms on inflammatory response in murine brain tissue. As shown in Figure 2, at 12 h post-infection, the expression of TLR2, CCL2, IL-6, and TNF-α from brains of infected mice was significantly higher for biofilm state cells compared with planktonic cells.
Mice infected with biofilm bacteria showed much more severe signs of meningitis (rigid posture, ataxia, or convulsions) than those infected with planktonic bacteria. Histological examination of the brain tissue from mice infected with S. suis strain P1/7 showed congestion/haemorrhage, necrosis, and inflammatory cell infiltration in the meninges, cerebral cortex, ventricles, or diencephalon (Figure 3). Compared with mice infected with planktonic bacteria (Figure 3E-H), at 12 h post-infection, far more severe pathological changes were observed in brain tissue from mice infected with biofilm bacteria (Figure 3A-D), including large areas of congestion/haemorrhage, necrosis, or intense inflammatory cell infiltration. The gross pathological changes in the brain from mice infected with planktonic and biofilm bacteria was recorded in Table 2. Neither pathological changes nor neurological symptoms were observed from mice injected with PBS. Taken together, these data clearly show that S. suis biofilms contribute to induction of meningitis.
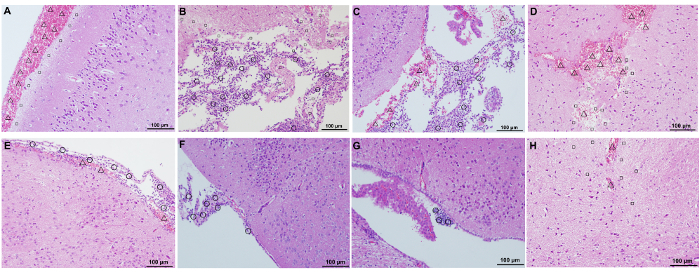
Figure 1: SEM images of strain P1/7 planktonic cells and biofilm state cells. Bacteria were cultured in THB medium. SEM analysis showed that planktonic cells (A) were much less dense and mainly dispersed individually, while biofilm bacteria (B) were clustered in clumps and multiple layers and they were encased in the extracellular matrix. Please click here to view a larger version of this figure.
Figure 2: Biofilms activate the mRNA expression of CCL2, IL-6, TNF-α, and TLR2 in mouse brain tissue in vivo. Six BALB/c mice per group were each injected through the intracranial subarachnoidal route of infection with 3 × 107 CFU of planktonic cells or biofilm state cells. Mice were euthanized at 12 h post-infection. The results are presented as the fold change of mRNA expression in biofilm state cell-infected mice compared with planktonic cell-infected mice. (A) The gene B2m was used as the reference gene; (B) the gene 18s rRNA was used as the reference gene. An unpaired t-test was used for statistical analysis. **p ≤0.01, and *p ≤0.05. Please click here to view a larger version of this figure.
Figure 3: Histological analysis of the brain tissue of mice infected with strain P1/7 planktonic bacteria and biofilm state bacteria. A-D, brain samples from mice infected with biofilm state bacteria; E-H, brain samples from mice infected with planktonic bacteria. A, B, E, and F: meninges and cerebral cortex; C and G: ventricles; D and H: diencephalon. Δ, congestion/haemorrhage; □, necrosis; ○: inflammatory cell infiltration. Magnification = 200X; scale bar = 100 µm. Please click here to view a larger version of this figure.
| Primer Name | Sequence (5’-3’) | Gene symbol |
| IL-6 forward | CTTCCATCCAGTTGCCTTCT | Il6 |
| IL-6 reverse | CTCCGACTTGTGAAGTGGTATAG | Il6 |
| TLR2 forward | CACTATCCGGAGGTTGCATATC | Tlr2 |
| TLR2 reverse | GGAAGACCTTGCTGTTCTCTAC | Tlr2 |
| CCL2 forward | CTCACCTGCTGCTACTCATTC | Ccl2 |
| CCL2 reverse | ACTACAGCTTCTTTGGGACAC | Ccl2 |
| TNF-α forward | TTGTCTACTCCCAGGTTCTCT | Tnf |
| TNF-α reverse | GAGGTTGACTTTCTCCTGGTATG | Tnf |
| β2m forward | GGTCTTTCTGGTGCTTGTCT | B2m |
| β2m reverse | TATGTTCGGCTTCCCATTCTC | B2m |
| 18s forward | GTAACCCGTTGAACCCCATT | 18s rRNA |
| 18s reverse | CCATCCAATCGGTAGTAGCG | 18s rRNA |
Table 1: Primers for RT-qPCR.
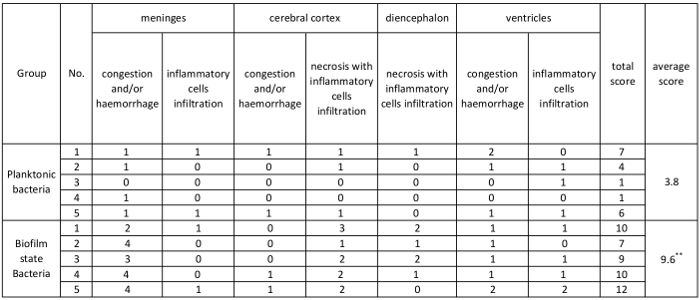 Table 2: The gross histopathological changes in the brain from mice infected with S. suis strain P1/7. A four-point grading system was used to evaluate histological changes, as described in Protocol section 3.3.7. The total score was calculated as the sum of scores from different histopathological changes. The average was calculated as the total score/number of mice. An unpaired t-test was used for statistical analysis. **p ≤0.01.
Table 2: The gross histopathological changes in the brain from mice infected with S. suis strain P1/7. A four-point grading system was used to evaluate histological changes, as described in Protocol section 3.3.7. The total score was calculated as the sum of scores from different histopathological changes. The average was calculated as the total score/number of mice. An unpaired t-test was used for statistical analysis. **p ≤0.01.
Discussion
The intracranial subarachnoidal route of infection described here has obvious advantages over other routes of infection. It allows investigators to study the host-bacterium interaction and the effect of bacterial components on host immune responses directly in the brain, which mimic bacterial entrance into the central nervous system. Thus, this route of infection can be extended for investigating the mechanisms of meningitis caused by other bacteria. In addition, it can also be used to test the efficacy of drugs against bacterial meningitis.
In order to obtain good results using this model, the following critical steps are explicit. Biofilm formation needs to be examined under experimental conditions. In the present study, it was examined using scanning electron microscope analysis. Other methods can also be used to examine the biofilm formation, such as tissue culture plate method and tube method21. One day before infection, aliquots of both planktonic cells and biofilm state cells need to be thawed from -80 °C to determine the CFU. The next day, bacteria should be diluted to the appropriate dose according to the CFU for infection. The mock-infected control group injected with PBS needs to be included and the mice from the mock-infected control group should exhibit no manifestations during the duration of the infection experiment. Different strains may have different infectious doses, so a preliminary experiment is strongly recommended to determine the appropriate infectious dose. In the present study, a dose of 3 × 107 CFU for infection was used based on our preliminary experiment. This dose of biofilm bacteria was able to induce severe signs of meningitis and subsequent death in all 5 mice 48 h after infection in the preliminary experiment.
The disruption of the blood-brain or blood-cerebrospinal fluid barriers by S. suis are an important step in causing meningitis22,23,24. The intracranial subarachnoidal route of infection is not suitable for evaluating the ability of S. suis to break through these barriers. Other routes of infection, such as i.p., i.v., or i.n., can be used to achieve this goal.
Here, we first demonstrate that S. suis biofilm contributes to the induction of meningitis using the intracranial subarachnoidal route of infection. This infection model not only helps to further understand the mechanisms of S. suis meningitis but also is suitable for studying the pathogenesis of meningitis caused by other bacteria and the efficacy of new drugs against bacterial meningitis.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by grants from the National Key Research and Development Program of China [2017YFD0500102]; the National Natural Science Foundation of China [31572544]; the State Key Laboratory of Veterinary Etiological Biology [SKLVEB2016KFKT005]; the Shanghai Agriculture Applied Technology Development Program, China [G2016060201].
References
- Gottschalk M, Xu J, Calzas C, Segura M. Streptococcus suis: a new emerging or an old neglected zoonotic pathogen? Future Microbiology. 2010;5:371–391. doi: 10.2217/fmb.10.2. [DOI] [PubMed] [Google Scholar]
- Goyette-Desjardins G, Auger JP, Xu J, Segura M, Gottschalk M. Streptococcus suis, an important pig pathogen and emerging zoonotic agent-an update on the worldwide distribution based on serotyping and sequence typing. Emerging Microbes & Infections. 2014;3(6):e45. doi: 10.1038/emi.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerdsin A, et al. Emergence of Streptococcus suis serotype 9 infection in humans. Journal of Microbiology, Immunology and Infection. 2017;50(4):545–546. doi: 10.1016/j.jmii.2015.06.011. [DOI] [PubMed] [Google Scholar]
- Hatrongjit R, et al. First human case report of sepsis due to infection with Streptococcus suis serotype 31 in Thailand. BMC Infect Diseases. 2015;15:392. doi: 10.1186/s12879-015-1136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fittipaldi N, Segura M, Grenier D, Gottschalk M. Virulence factors involved in the pathogenesis of the infection caused by the swine pathogen and zoonotic agent Streptococcus suis. Future Microbiology. 2012;7(2):259–279. doi: 10.2217/fmb.11.149. [DOI] [PubMed] [Google Scholar]
- Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nature Reviews Microbiology. 2004;2(2):95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- Fuxman Bass JI, et al. Extracellular DNA: a major proinflammatory component of Pseudomonas aeruginosa biofilms. Journal of Immunology. 2010;184(11):6386–6395. doi: 10.4049/jimmunol.0901640. [DOI] [PubMed] [Google Scholar]
- Isiaku AI, et al. Biofilm is associated with chronic streptococcal meningoencephalitis in fish. Microbial Pathogenesis. 2017;102:59–68. doi: 10.1016/j.micpath.2016.10.029. [DOI] [PubMed] [Google Scholar]
- Oggioni MR, et al. Switch from planktonic to sessile life: a major event in pneumococcal pathogenesis. Molecular Microbiology. 2006;61(5):1196–1210. doi: 10.1111/j.1365-2958.2006.05310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G, et al. Streptococcus suis small RNA rss04 contributes to the induction of meningitis by regulating capsule synthesis and by inducing biofilm formation in a mouse infection model. Veterinary Microbiology. 2017;199:111–119. doi: 10.1016/j.vetmic.2016.12.034. [DOI] [PubMed] [Google Scholar]
- Dominguez-Punaro MC, et al. Streptococcus suis serotype 2, an important swine and human pathogen, induces strong systemic and cerebral inflammatory responses in a mouse model of infection. Journal of Immunology. 2007;179(3):1842–1854. doi: 10.4049/jimmunol.179.3.1842. [DOI] [PubMed] [Google Scholar]
- Seitz M, et al. A novel intranasal mouse model for mucosal colonization by Streptococcus suis serotype 2. Journal of Medical Microbiology. 2012;61(Pt 9):1311–1318. doi: 10.1099/jmm.0.043885-0. [DOI] [PubMed] [Google Scholar]
- Busque P, Higgins R, Caya F, Quessy S. Immunization of pigs against Streptococcus suis serotype 2 infection using a live avirulent strain. Canadian Journal of Veterinary Research. 1997;61(4):275–279. [PMC free article] [PubMed] [Google Scholar]
- Williams AE, Blakemore WF. Pathology of Streptococcal meningitis following intravenous intracisternal and natural routes of infection. Neuropathology and Applied Neurobiology. 1990;4(4):345–356. doi: 10.1111/j.1365-2990.1990.tb01268.x. [DOI] [PubMed] [Google Scholar]
- Dominguez-Punaro MC, et al. Severe cochlear inflammation and vestibular syndrome in an experimental model of Streptococcus suis infection in mice. European Journal of Clinical Microbiology & Infectious Diseases. 2012;31(9):2391–2400. doi: 10.1007/s10096-012-1581-2. [DOI] [PubMed] [Google Scholar]
- Auger JP, Fittipaldi N, Benoit-Biancamano MO, Segura M, Gottschalk M. Virulence Studies of Different Sequence Types and Geographical Origins of Streptococcus suis Serotype 2 in a Mouse Model of Infection. Pathogens. 2016;5(3) doi: 10.3390/pathogens5030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiavolini D, et al. Method for inducing experimental pneumococcal meningitis in outbred mice. BMC Microbiology. 2004;4:36. doi: 10.1186/1471-2180-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen LW, Svensmark B, Elvestad K, Jensen HE. Otitis interna is a frequent sequela to Streptococcus suis meningitis in pigs. Veterinary Pathology. 2001;38(2):190–195. doi: 10.1354/vp.38-2-190. [DOI] [PubMed] [Google Scholar]
- Holden MT, et al. Rapid evolution of virulence and drug resistance in the emerging zoonotic pathogen Streptococcus suis. PLoS One. 2009;4(7):e6072. doi: 10.1371/journal.pone.0006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Punaro Mde L, et al. In vitro characterization of the microglial inflammatory response to Streptococcus suis, an important emerging zoonotic agent of meningitis. Infection and Immunity. 2010;78(12):5074–5085. doi: 10.1128/IAI.00698-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan A, et al. Evaluation of different detection methods of biofilm formation in the clinical isolates. Brazilian Journal of Infectious Diseases. 2011;15(4):305–311. [PubMed] [Google Scholar]
- Vanier G, et al. New putative virulence factors of Streptococcus suis involved in invasion of porcine brain microvascular endothelial cells. Microbial Pathogenesis. 2009;46(1):13–20. doi: 10.1016/j.micpath.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Takeuchi D, et al. The contribution of suilysin to the pathogenesis of Streptococcus suis meningitis. Journal of Infectious Diseases. 2014;209(10):1509–1519. doi: 10.1093/infdis/jit661. [DOI] [PubMed] [Google Scholar]
- Tenenbaum T, et al. Polar bacterial invasion and translocation of Streptococcus suis across the blood-cerebrospinal fluid barrier in vitro. Cellular Microbiology. 2009;11(2):323–336. doi: 10.1111/j.1462-5822.2008.01255.x. [DOI] [PubMed] [Google Scholar]


