Abstract
Damaged cells at risk for neoplastic transformation can be neutralized by apoptosis or engagement of the senescence program, which induces a permanent cell-cycle arrest and a bioactive secretome implicated in tumor immunosurveillance. While from an evolutionary perspective senescence is beneficial in that it protects against malignancies, the accumulation of senescent cells in tissues and organs with aging and at sites of various pathologies is largely detrimental. With induction of senescence in cancer cells emerging as a therapeutic concept, it will be important to consider these detrimental effects, including tumor promoting properties that may drive the formation of secondary tumors or cancer relapse. In this review, we discuss the complex relationship between senescence and cancer, and highlight important considerations for therapeutics.
Keywords: Cellular senescence, Cancer therapy, SASP, Senotherapy
Senescent cells: modulators of aging and cancer
Advanced age is the leading risk factor for numerous chronic diseases including various types of cancer [1]. Although the causes and mechanisms of aging remain poorly understood, senescent cells have emerged as a central contributor to premature and natural aging [2], and age-related diseases [3–5]. Various studies in mice demonstrate that senescent cells represent a druggable target to extend healthy lifespan and ameliorate various chronic diseases [2–4, 6]. These findings have prompted collective interest in the fundamental biology of senescent cells, not only in cell culture, but also in tissues and organs across species, with the ultimate goal to identify molecular vulnerabilities for therapeutic purposes [7] (see Box 1).
Box 1. Key aspects of senescent cells.
Cellular senescence refers to a molecular program activated in response to environmental cues or stress including but not limited to, the end of replicative lifespan/telomere erosion, DNA damage, mechanical stress, or oncogenic stimuli. Senescent cells are commonly characterized by a durable cell-cycle arrest, apoptosis resistance, and a bioactive secretome referred to as the senescence-associated secretory phenotype (SASP). A combination of multiple senescence markers are widely used to identify senescent cells, such as p16INK4A, p14ARF (murine p19ARF) and p21CIP1/WAF1 (encoded by CDKN1A), DNA damage markers (e.g. γ-H2AX-foci, 53BP1-foci), senescence-associated β-Galactosidase activity, chromatin alterations such as Lamin B deficiency or heterochromatin foci, and expression of several SASP factors (pro-inflammatory cytokines, growth factors, extracellular enzymes, and matrix-metalloproteinases). The release of these bio-active molecules affect and potentially harm neighboring cells or signal to the immune system, which collectively may contribute to tissue deterioration or remodeling, chronic pathologies, and organismal aging. It should be noted that in the case of cancer, where the genomic region containing CDKN2A is frequently deleted, it is important to evaluate additional markers of senescence as p16INK4A-independent senescence may also occur in these cases.
Rational targeting of senescent cells, particularly in the context of cancer, requires a comprehensive understanding of the molecular and physiological properties of senescent cells, their different phenotypic variations, and their complex association to cancer, which can be both beneficial and detrimental. Acutely generated forms of senescent cells (see Glossary), that arise during wound healing or embryogenesis for example, are thought to enhance organismal fitness by inhibiting neoplastic transformation [8] or recruiting immune cells [9], However, chronically existing senescent cells during aging and chronic diseases can be deleterious for the organism, for instance by creating a microenvironment that promotes neoplastic growth [10], metastasis [11], or immunosuppression [12]. Below, we discuss the various forms of cancer-associated senescent cells in human and mouse tissues as well as their therapeutic implications. We propose that senescent cell removal, senotherapy, is not only a viable therapeutic option for aging and age-related diseases, but also for combination, two-stage cancer treatment - pro-senescence chemotherapy followed by senotherapy. This approach could maximize chemotherapeutic efficiency, preventing cancer relapse, and maintain an anti-tumor tissue microenvironment.
Senescent cell types implicated in cancer
Senescent neoplastic cells
Historically, cellular senescence has been described as a tumor-protective mechanism that inhibits uncontrolled proliferation of cancer-prone cells. Activation of particular oncogenes or the loss of certain tumor suppressor genes induces the senescence program to establish a durable cell-cycle arrest [8] (Figure 1A, Key Figure). This mechanism is described in a plethora of cellular systems with multiple oncogenes in vitro, as well as in murine tissues, including but not limited to: liver (RAS activation [9]), lymphocytes (RAS activation [13]), skin (BRAF activation [14]), thyroid gland (BRAF or RAS activation [11, 15]), mammary gland (RAS activation [16]), prostate (Pten or Skp2 loss [17, 18]), colon (Csnk1a1 loss [19]), and pituitary gland (Pttg1 loss [20]). Evidence for “oncogene-induced senescence” (OIS) in human primary tumors has also been reported. For instance, melanocytes with oncogenic BRAF mutations undergo senescence and remain benign in melanocyte nevi [21, 22]. Likewise, senescence markers have been identified in early-stage prostate tumors [17], including colon adenomas [10], astrocytomas [23], and neurofibromas [24].
Figure 1, Key Figure. Cancer-associated senescent cells affect tumors in multiple ways.
Acutely senescent cells that arise due to oncogene-activation (A, oncogenic RAS for example) or chemotherapy (B) show tumor suppressing properties, including cell cycle arrest and SASP production that may promote immunosurveillance. Prolonged presence of these cells, however, in addition to tumor-induced or paracrine senescence in the stroma (C, D), or age-related senescence (E) can promote several hallmarks of cancer. Stromal senescent cells may arise from paracrine signals originating from tumor cells (C, gray and white secreted factors) or other senescent cells (D, colored SASP factors). Age-related senescent cells are hypothesized to promote both, neoplastic transformation of adjacent cells and proliferation of tumor cells (E). Immunosenescence (F) is a complex process, but largely renders immune cells (especially T-cells) unresponsive to activating signals and also promotes a SASP with pro-tumorigenic capacities.
Inactivation of senescence pathways in mice, for instance through inactivation of the Cdkn2a encoded cell-cycle inhibitors p16INK4A and p19ARF (human p14ARF) leads to early death from tumors [16, 25], illustrating why natural selection favored the senescence program. Furthermore, alteration of CDKN2A in humans, either genetically or epigenetically, is one of the most frequent events in neoplastic lesions [26, 27], indicating that disruption of the senescence program is a major event during human tumor development. p16 can also be predictive of tumor subtype, as high p16 levels distinguish early stage small-cell lung cancer from lung adenocarcinoma [28] [29], or early stage papillary thyroid microcarcinoma from papillary thyroid carcinoma [30]. Tumor subtypes often show distinct therapeutic response profiles, suggesting that p16 levels could predict therapeutic efficacy [28]. In prostate oropharynx cancer, elevated p16 levels correlate with a superior response to radiation therapy [31]. On the other hand, it has to be taken into consideration that p16 levels may increase outside the context of senescence, for example due to Rb1 loss [32], another key cell cycle regulator with frequent loss-of-function mutations in human tumors [26]. Overall, senescent cells are found in both benign and pre-malignant tumors, suggesting that cellular senescence is an evolutionary cancer-protective mechanism designed to enhance organismal fitness.
Therapy-induced senescent cells
Albeit metabolically active, senescent cells are cell cycle arrested, and therefore, cellular senescence has been viewed as a desirable outcome during cancer treatment (Figure 1B). To this end, senescence-inducing compounds have been developed, including CDK4/6 inhibitors such as Abemaciclib, Palbociclib, and Ribociclib. Because this class of drugs has shown promise in treating several cancers in pre-clinical and clinical studies [33–35], high-throughput screens have been employed to find additional drug targets that trigger senescence in cancer cells [36]. Studies in mice, support the beneficial effects of senescence induction in tumor cells as it not only leads to tumor stalling, but also activates a SASP-mediated immune response (see Box 1) that can result in elimination of the senescent tumor cells, as well as neighboring neoplastic cells, and ultimately tumor regression [9, 37, 38].
Conversely, accumulating evidence indicates that senescent tumor cells promote tumor relapse, aggressiveness, and metastases (Figure 1B). It has been reported that p53-mediated senescence in mammary tumors can hinder chemotherapeutic efficiency and promotes rapid cancer relapse, compared to slowly relapsing tumors in p53-mutant mice that fail to arrest but undergo apoptosis due to mitotic catastrophe [39]. Similarly, p16-positive patient tumors are associated with cancer recurrence [40, 41]. Strikingly, a recent study shows that therapy-induced senescence is associated with stem cell and self-renewing features and can promote both, cancer initiation and aggressiveness, in several tumor mouse models including B-cell lymphoma and T-cell acute lymphoblastic leukemia [42]. Besides cancer recurrence, senescent cells within thyroid tumors have also been linked to invasion, suggesting that cancer metastasis is promoted by senescent cell non-autonomous features [11]. Importantly, while chronic senescent cells induced by radiation therapy or chemotherapeutic drugs contribute to local and systemic inflammation, targeted removal of these cells in transgenic mice ameliorates cancer recurrence and detrimental side effects including bone marrow suppression and cardiac dysfunction [6, 43]. Therefore, although senescence induction in cancer cells is a viable therapeutic option to reduce initial tumor growth, chronically persisting senescent cells need to be removed to minimize regression risk and avoid deleterious side effects.
Senescence-induction in tissue adjacent to tumors
The presence of senescent cells within tissues can promote proliferation of neighboring cells, including preneoplastic cells [10]. This property of senescent cells has been well-studied in vivo using xenograft models and co-injection of cancer cells and either senescent or non-senescent fibroblasts [12, 44–46]. In vitro studies show that senescent cell non-autonomous effects, via secretion of SASP factors (further detailed in a later section), induce growth, angiogenesis, and invasive properties in neighboring cells [10, 47, 48]. Established tumors or neoplastic cells can also induce cellular senescence in neighboring cells (Figure 1C). Indeed, senescent cells have been identified in the stroma of hepatocellular carcinoma [49] and ovarian cancer [50], and using a p16-luciferase mouse model, one group showed that injection of tumor cells induced senescence in the stroma surrounding tumors [51].
Stromal senescent cells drive tumor growth in several studies, and the gene expression profile of cancer-associated fibroblasts and senescent cells are similar suggesting that senescent cells drive neoplastic cell proliferation through similar paracrine mechanisms [52, 53]. In fact, increased p16 levels in the stroma surrounding human mammary ductal carcinoma in situ lesions predict disease recurrence, independent of other typical histological markers [54]. Recent studies have also shown that senescent cells can promote tumor growth by establishing an immunosuppressive microenvironment via secreting cytokines that recruit myeloid-derived suppressor cells, which inhibit T-lymphocyte-mediated targeting of tumor cells [55]. Overall, senescent cells are induced by neighboring neoplastic cells or tumors, and support a pro-tumorigenic microenvironment and increased risk of relapse.
In addition, senescent cells can also potentiate their own effects by inducing senescence in neighboring cells through paracrine mechanisms (bystander effect) via the SASP or gap junction-mediated cell-cell contact (Figure 1D) [56, 57]. Indeed, several studies have demonstrated this effect in vitro using senescent cell conditioned media, and have shown that numerous SASP factors or signaling pathways, including TGFB1 [58, 59], ROS-activated NFκB signaling [60], IL8 and CXCL1 [61], and cGAS-STING signaling [62] can mediate the induction of paracrine senescence. Further, another group showed that short-term exposure of normal cells to SASP from senescent cells induces expression of stem cell markers conferring regenerative capacity, however, prolonged exposure induces senescence [63], suggesting that only short-term exposure may be beneficial. Induction of senescence in neighboring cells has also been demonstrated in vivo, in pituitary stem cell clusters in mouse models of pediatric craniopharyngioma [64] and ischemic retinal cells in a mouse model of ischemic retinopathy [65]. Senescent cells clusters have also been identified in the thymus of aged mice [66], hepatocytes from mouse livers [56], and intervertebral discs of patients suffering from intervertebral disc degeneration [67]. Together, paracrine senescence induction by neighboring senescent cells represents a mechanism for senescent cells to potentiate their effects, and may amplify negative impacts on cancer (Figure 1D), aging, and other age-related diseases.
Aging-related senescent cells
Aging is a major risk factor for cancer, and most tumors are diagnosed in aged patients [68]. In addition, 5-year survival for many cancer types dramatically declines with age [68, 69]. Epidemiological studies show that familial factors correspond with both reduced cancer and longevity, and most genetic and dietary modifications in mice that impact aging, also impact cancer [69, 70]. Further, a number of progeroid syndromes (Hutchinson-Gilford progeria syndrome, Werner syndrome, Bloom syndrome, Xeroderma pigmentosum, Ataxia telangiectasia, and Mosaic variegated aneuploidy syndrome) are also associated with the development of cancer [71].
Although, historically cancer aggressiveness has been thought to decrease with age, several tumor types, including acute myeloid leukemia and ovarian cancer have a worse prognosis with increasing age [72, 73]. Experimental evidence for a relation between aging and cancer from animal models is variable, and appears to be tumor or cell-type dependent [69]. In prostate cancer and melanoma xenograft experiments, no change in growth or faster growth in young mice was observed, respectively [74, 75]. However, in these studies, 12-month-old mice were used as “aged” mice, but the severity of age-related tissue deterioration or presence of senescent cells at this age may be limited. Implantation of neoplastic liver epithelial cells into livers of young and old rats, however, resulted in reduced proliferation and more apoptosis in young rats [76]. This suggests that differences between tumor or cell-type and/or the site of implantation, may explain the variation in results.
In other genetic approaches, continued senescent cell removal in naturally aging mice (INK-ATTAC transgenic) throughout adulthood, extended lifespan and delayed tumor latency [2], suggesting a detrimental role for age-related senescent cells in tumor progression. This result is further supported by timed somatic p53 deletion in young and old mice, where reduced tumor latency was observed in aged mice [77]. Further, using an inducible conditional mouse model expressing the cell cycle inhibitor p27Kip1 to mimic skin aging, other researchers discovered the presence of stromal senescent cells and increased recruitment of suppressive myeloid cells, which inhibit tumor immune surveillance and promote tumor formation [12]. Collectively, these studies show that accumulation of senescent cells in tissues with aging promotes tumor formation and growth (Figure 1E), and highlights these cells as optimal therapeutic targets not only for the amelioration of age-related deterioration, but also for cancer prevention and treatment.
Cancer and the aging immune system
Both the adaptive and innate immune systems are capable of infiltrating and clearing tumor cells. While T-cells (CD4+ helper and CD8+ cytotoxic), tumor-associated macrophages, and natural killer (NK) cells prevent tumor growth by targeting antigenic tumor cells, regulatory T-cells that secrete immunosuppressive cytokines as well as myeloid and stromal cells suppress T-cell responses in lesions that have lost immunogenicity [9, 78, 79]. Interestingly, these same immune cell types are effective in eliminating senescent cells [9, 37, 38, 80, 81]. The immune system undergoes profound changes with aging as reflected by increased susceptibility to infection, autoimmunity, response to vaccination, and cancer development [82, 83]. With increasing age, the adaptive immune system’s ability to mount T-cell-mediated responses and regulation of the innate immune system decline, which may impact on both senescent and tumor cell clearance [84, 85].
Interestingly, accumulation of aged immune cells, referred to as immunosenescence, increases with age in both B- and T-cell populations (Figure 1F) [86]. We focus here on T-cell immunosenescence as T-cells function in immunosurveillance of tumors and senescent cells. T-cell immunosenescence can be induced by multiple mechanisms, including, but not limited to repeated or chronic T-cell stimulation (viruses, pathogens, tumor antigens, or immunogenic self-antigens) and a deregulated inflammatory environment [86, 87]. Senescent T-cells are nonresponsive to stimulation, but are metabolically active and produce cytokines, including IL6 and TNFα [86]. Senescent T-cells can be pro-tumorigenic through their ability to suppress proliferation of responder T-cells [88], but can also modulate macrophage cell fate and contribute to anti-tumoral functions [89].
One of the hallmarks of cancer is the ability of tumor cells to escape from immune surveillance [90]. Several recent studies have shown that immunosurveillance of tumor and senescent stromal cells are important tumor protection mechanisms. It has been shown that oncogene-induced senescent hepatocytes secret chemokines, which facilitate clearance by the adaptive immune system (CD4+ T-cell-mediated), whereas impaired immune surveillance resulted in the development of hepatocellular carcinomas [9]. This suggests that decreased immune surveillance, as observed with age, may drive tumor formation. Indeed, in a mouse model of squamous cell carcinoma, conditional induction of mutant HRAS in keratinocytes resulted in dysplastic changes and 50% tumor incidence in aged mice only, which showed increased cellular senescence in dermal immune cells [91]. Contrarily, two studies demonstrated that senescent cells within tumors facilitated NK cell recruitment and tumor elimination, suggesting that senescent cells may provide beneficial immune attraction properties [37, 80]. Together, these results suggest that presence of senescent cells may be a benefit or detriment to neoplastic cells/tumors, by averting or attracting immune cells.
In addition, the immunosuppressive nature of the tumor microenvironment limits the ability of immune cells to infiltrate and target tumor cells [92]. Senescent cells within tumor stroma, for example, may deter immune cell infiltration and drive tumorigenesis. One study showed that myeloid-derived suppressor cells (MDSCs) promoted an age-related increase in lung cancer growth in mice, and that these cells increase with age in the circulation of humans and the spleens of mice [93]. Further, in a model of skin aging, senescent stromal cells were sufficient to recruit and increase MDSCs, which inhibit T-cell responses and promote tumor growth [12]. Overall, the aging process increases the senescent cell burden and impairs immune function, which, in turn escalates senescent cell accumulation and inferior neoplastic surveillance, establishing a pro-tumorigenic environment (Figure 1F).
The SASP and cancer
Senescent cells restrict and contribute to cancer via both, cell autonomous (restriction of cell proliferation or transformation) and cell non-autonomous mechanisms (SASP) that can result in extracellular matrix remodeling, growth stimulation or suppression of adjacent cells, or signaling to the immune system. Senescence-associated paracrine signaling seems to be context-dependent, with the type of senescence stimuli and cell type having dramatic consequences on the SASP profile [47, 94].
SASP establishment and regulation can be orchestrated, at least in vitro, by multiple signaling pathways and transcription factor networks, including NFκB signaling [95], the p38 MAPK pathway [96], the cGAS-STING pathway [62, 97], inflammosome activation [57], TGF-β signaling [98], JAK-STAT signaling [55], PI3K-AKT-mTOR signaling [99], GATA4 activation [100], and C/EBP-β activation [10] (Figure 2). Which of these signaling pathways and networks are active seems dependent on senescent cell maturation [63, 98] and origin [42]. Extensive cross-talk among pathways and networks has been observed [99, 101]. Each SASP signaling pathway may drive expression, translation, or protein stability of numerous SASP factors. However, only a few of these factors have been mechanistically linked to physiological events in tissues or diseases, and mechanistic action of single components is still largely based on studies performed in cultured cells. Below, we describe select SASP factors to illustrate their context/potential in impacting cancer-associated processes (Figure 2).
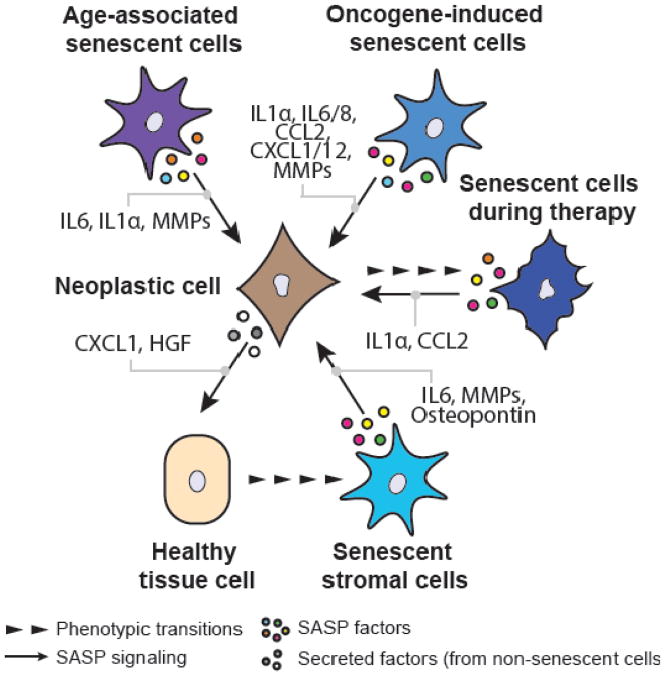
Figure 2. Secretory diversity of cancer-associated senescent cells.
Selected SASP components with tumor-modulating activities for each of the discussed senescence types are depicted. While age-associated senescent cells, oncogene-induced senescent cells and therapy-induced senescent cells often seem to secret cytokines and chemokines (including IL6, CCL2, etc.), the pro-tumorigenic activity of stromal senescent cells benefits mostly from secretion of growth factors (such as Osteopontin) and matrix-metalloproteinases (MMPs). Tumor cells themselves are also able to secret bioactive factors that, in some instances, are causally implicated in the development of stromal senescent cells. HGF (Hepatocyte growth factor).
IL1α is an important SASP initiator, and is activated in therapy-induced [99, 101], oncogene-induced [102], and age-related senescent cells [2]. IL1α drives autocrine pro-inflammatory signaling including NFκB activation, and expression of key cytokines such as IL6 and IL8 [99]. IL1α can act locally as a membrane-bound cytokine that may recruit hematopoietic cells or be cleaved by extracellular proteases and promote systemic inflammation. IL1α signaling may therefore not only contribute to senescent cell immunosurveillance, but also tissue ‘inflammaging’. Studies to determine the role of senescent cell-derived IL1α in tumor growth have yet to be conducted (see [103]).
IL6 and IL8 are two of the most investigated pro-inflammatory SASP factors, and have been linked to oncogene-induced senescent cells, senescent stroma [10–12, 15, 104], and murine senescent cells during natural aging, progeria and disease [2, 4, 105]. Besides promoting an inflammatory response and immunosurveillance to control liver tumor progression [80], CXCR2 receptor activation via IL6 and IL8 reinforces senescence and cell cycle arrest, through elevated ROS production and activation of the DNA damage response [61, 101]. In some instances, however, stromal cell-derived IL6 can act in immunosuppression [12]. Although functions ascribed to IL6 and IL8 such as pro-fibrotic signaling [106] or pro-proliferative signaling [107] are unexplored in the context of senescence, investigating these characteristics may be of integral importance in the context of cancer and cancer-associated senescence.
The chemokines such as CXCL1/GROα are broadly expressed in several senescence contexts. CXCL1 is not only highly expressed in oncogene-induced senescent cells in vitro and in mice [50, 104], but also in human ovarian cancer samples [50]. Secretion of CXCL12, by cancer-inherent, likely oncogene-induced senescent cells promotes thyroid tumor invasion and metastasis in mice [11], and the cytokine CCL2/MCP-1 has been linked to OIS in the liver and immune surveillance of pre-malignant hepatocytes [38] or senescent liver tumor cells [37]. However, in the context of established hepatocellular carcinoma, CCL2 among others restricts NK cell function through recruitment of immunosuppressive myeloid cells, and facilitates establishment of advanced disease [38]. On the other hand, CXCL1 can also be secreted by tumor cells and confers paracrine stromal senescence that, in turn, could promote tumor growth [50]. While these studies illustrate senescent cell-derived chemokines as integral SASP components, their physiological contributions to cancer are of a complex, context-dependent nature.
Growth factors or extracellular vesicles with growth-stimulatory properties are secreted by senescent cells and may contribute to tumor initiation, growth, and angiogenesis [47, 108]. Vascular endothelial cell proliferation may be mediated by senescent cells of various origins through secretion of pro-angiogenic VEGF resulting in tumor vascularization [45]. Osteopontin (OPN), a secreted glycoprotein, is highly produced by senescent stromal cells in murine skin papillomas, and co-injection of OPN-deficient senescent cells restricts tumor growth compared to OPN-expressing senescent cells [109]. Conversely, hepatocyte growth factor derived from tumor cells or ascitic fluid of an ovarian cancer-patient can also induce senescence in mesothelial cells, which can modulate ovarian cancer development and potentially metastasis [110, 111].
Matrix-metalloproteinases (MMPs), secreted enzymes that process and degrade extracellular matrix (ECM) components, contribute to tissue remodeling and are often released by senescent cells. This class of SASP factors is well described in multiple tissues with age [2, 112] and age-related diseases [3, 4], as well as in thyroid tumors associated with senescent cells [11]. While destruction of the ECM barrier per se may facilitate tumor growth and cell invasion, growth factors and cytokines that are sequestered by ECM components can also be liberated by MMP activity (see [113]). Indeed, senescent cell-derived MMPs were shown to support tumor growth [44] and promote VEGF-stimulated tumor vascularization of murine xenografts [114]. Further, stromal cell-derived MMP1 can cleave protease-activated receptors on tumor cells to enable migration and tumor cell invasion [115], however, whether this mechanism applies to senescent cell-derived MMPs remains to be explored. Therefore, while tumor-inherent senescent cells could render tumor tissue permissive to cancer growth, vascularization or cell invasion, age-associated senescent cells may render target tissue permissive to metastases.
Overall, the SASP of age-related senescent cells and of senescent cells in established tumors appears to be mostly detrimental, as it catalyzes several hallmarks of cancer and removal of senescent cells during natural aging delays tumor latency [2]. One key feature is the interplay between tumor cells, cancer-associated senescent cells, and the immune system orchestrating immune responses. Although only few studies address this relationship, it is apparent that senescent cell- and tumor-immune surveillance is complex and often context-dependent. Molecular-mechanistic insights into the implicated events, proteins, and kinetics are required to understand and predict therapeutic outcome. Although, dissecting the identity and origin of donor and recipient cell during paracrine signaling are technically challenging, recent advances in single-cell sequencing techniques and single-cell proteomics will aide these efforts and address the notion that targeting of senescent cells or their secretome in cancer patients may represent a viable therapeutic option that should be considered to supplement chemotherapy.
Senotherapy as an anti-cancer strategy
Although the central objective of chemo- and radiation therapies is to prevent the proliferation of cancer cells through induction of cellular senescence or cell death [116], the persistence of therapy-induced senescent cells after treatment is detrimental. The use of senotherapy in combination with currently used cancer therapies should be taken into consideration to control this problem [43]. There are a number of cancer types discussed here, which represent suitable candidates for consideration of combination cancer and senotherapies (see Table 1).
Table 1.
Senescence-associated Cancer Types and Therapeutic Potential
| Tissue/Tumor type | Model | Senescent cell type | Potential senotherapeutic outcome (aspect) | Refs. |
|---|---|---|---|---|
| Brain | ||||
| Adamantinomatous craniopharyngioma | Mouse, Human | Oncogene-induced, age-related | Beneficial (initiation) | 64 |
| Breast | ||||
| Mammary tumors | Mouse | Therapy-induced | Beneficial (recurrence) | 39 |
| Xenograft (breast cancer) | Mouse, co-injection (tumor and senescent cells) | Therapy-induced | Benefical (growth) | 44 |
| Xenograft (mammary epithelial cancer) | Mouse, co-injection (tumor and senescent cells) | Replicative, oncogene-induced, and p16 overexpression | Beneficial (vascularization) | 45 |
| Mammary ductal carcinoma | Human | Tumor-induced | Beneficial (recurrence) | 54 |
| Liver | ||||
| Hepatocellular carcinoma | Mouse | Oncogene-induced (and others?) | Detrimental, early stages (immunosurveillance) Benefical, late stages (immunosurveillance) |
9, 37, 38 |
| Hepatocellular carcinoma | Mouse | Genetic p53 re-activation | Detrimental (immunosurveillance) | 80 |
| Hepatocellular carcinoma | Human | Age-related or tumor-induced? | Unclear | 49 |
| Hepatic tumors | Mouse, tumor cell injection | Age-related | Beneficial (growth) | 76 |
| Lung | ||||
| Lung cancer | Mouse, tumor cell injection | Age-related | Beneficial (growth) | 93 |
| Lymphoma/Leukemia | ||||
| B-cell lymphoma | Mouse, tumor cell injection | Therapy-induced | Beneficial (initiation, growth) | 42 |
| T-cell acute lymphoblastic leukemia | Mouse, tumor cell injection | Therapy-induced | Beneficial (initiation, growth) | 42 |
| Multiple | ||||
| Lymphoma, Sarcoma, Carcinoma (age-related cancer in mice) | Mouse | Age-related | Beneficial (growth, initiation?) | 2, 77 |
| Xenograft (breast, pancreatic, endometrial, and lung cancer) | Mouse, tumor cell injection | Tumor-induced | Unclear | 51 |
| Xenograft (human epidermal keratinocytes, immortalized mouse mammary epithelial cells, human breast cancer) | Mouse, co-injection (tumor and senescent cells) | Oncogene-induced, replicative | Beneficial (initiation, growth) | 46 |
| Ovary | ||||
| Ovarian carcinoma | Human | Tumor-induced | Benefical (growth) | 50 |
| Prostate | ||||
| Prostate carcinoma | Human | Unclear | Beneficial (recurrence) | 40, 41 |
| Prostate cancer | Mouse | Pten deletion | Beneficial (immunosurveillance, growth, and chemo-resistance) | 55 |
| Skin | ||||
| Skin papillomas | Mouse | Tumor-induced | Beneficial (growth) | 109 |
| Skin (squamous cell carcinoma) | Mouse | Age-related | Beneficial (initiation, growth?) | 91 |
| Skin (squamous cell carcinoma) | Mouse, co-injection (tumor and senescent cells) | Genetic p27 overexpression | Beneficial (immunosurveillance, growth) | 12 |
| Thyroid | ||||
| Papillary thyroid carcinoma | Human | Unclear, potentially oncogene-induced | Beneficial (metastasis) | 11 |
Indeed, treatment with the CDK4/6 inhibitor Palbociclib, is initially effective in inhibiting melanoma tumor growth, however, prolonged treatment induced senescence and SASP production in stromal cells, which became tumor promoting [117]. In addition, inhibition of SHP2 prevented and arrested mammary tumor growth in mice through the induction of senescence, however, the activation of STAT3 and SASP secretion, suppressed immune surveillance [118]. Further, two mouse studies showed that removal of senescent cells after cancer therapy alleviated their detrimental effects, including reduced bone marrow suppression, cardiac dysfunction, cancer recurrence, and improved physical activity and strength [6, 43]. Together, these results underpin the relevance and potential benefit of senotherapy following cancer therapeutics.
Three principle categories can be considered for senotherapy: permanent removal of senescent cells (senolysis), immune-mediated senescent cell clearance, and SASP neutralization [7]. Although, senescent cells have been eliminated without negative consequences during aging and disease [2, 4, 105], acutely generated senescent cells in adults exhibit some beneficial effects in wound healing [2, 119], and tissue regeneration [63]. Senolytic drugs which target the anti-apoptotic response in senescent cells, such as signaling through BCL-2 family members (Navitoclax/ABT-263 or ABT-737), have proven effective in inducing cell death in senescent cells, however, these compounds are unlikely to meet required safety due to the risks of thrombocytopenia and neutropenia [7, 120, 121]. However, these risks can be minimized by short-term treatment, and potentially localized delivery, where applicable. Effective targeting of senescent tumor cells has been achieved using inhibition of lysosomal ATPases, thereby exploiting the high metabolic activity of cyclophosphamide- or Adriamycin-induced senescent lymphoma cells in mice [122].
Treatment to enhance immune activity could also be harnessed to improve the anti-tumor activity of senescent cell-recruited immune cells [123]. This could be achieved through the use of Ipilimumab, an antibody that enhances activation of cytotoxic CTLA-4 receptor, or with antibodies against the PD1 immune checkpoint, both of which are in clinical use for the treatment of melanoma [124]. SASP modulation may also be employed, and can be achieved by blocking pro-SASP signaling or inhibiting individual SASP components [7]. Blocking of pro-SASP signaling can be complicated as perturbation of these pathways are tumorigenic in some cases, for example IL6 is required to maintain the senescent cell state [101]. In addition, SASP reduction through NFκB inhibition in a lymphoma mouse model, disrupts immunosurveillance following therapy-induced senescence, and leads to treatment resistance and relapse [95]. Similarly, inhibition of mTORC1, a component of PI3K-AKT-mTOR pathway, with rapamycin diminishes p53 translation in Pten-deficient senescent cells and promotes murine prostate tumorigenesis [125]. On the other hand, STAT3 inhibition had beneficial effects in alleviating detrimental SASP effects, and resulted in reduced secretion of immunosuppressive cytokines triggering a strong CD8+ T-lymphocyte response and prostate tumor regression [55]. Together, this suggests that inhibition of pro-SASP signaling is pathway dependent, and further investigation into the efficiency and risk of these strategies is required. Inhibition of selected SASP components can also be beneficial because of reduced off-target effects. Perhaps the most prominent SASP factors, for which approved drugs are available, include IL1α (IL1 receptor drug Anakinra, currently used for treatment of rheumatoid arthritis), and IL6 (IL6 antibody Siltuximab, currently used for treatment of Castleman disease; IL6 receptor inhibitor Tocilizumab, currently used for treatment of rheumatoid arthritis) [126–128]. These strategies have not yet been tested in preclinical models of cancer or aging, but represent promising targets for future study. Together, several suitable approaches to targeting senescent cells in combination with chemotherapy or in the context of aging to promote effective therapy, minimize relapse, and delay or prevent cancer onset exist, however, further testing of these strategies in cancer is required.
In addition, careful consideration of the timeline for senotherapy in combination with cancer therapy should be taken into consideration, as described senescent cells have both beneficial and detrimental effects on tumor initiation, growth, and relapse, in a cell/tumor type dependent fashion. With the current knowledge, incorporation of senotherapy may be beneficial prior cancer therapy to increase therapeutic efficacy by removing existing senescent cells, following cycles of cancer therapy to improve therapeutic outcome, and after final treatment to reduce risk of recurrence and ameliorate negative impacts of indirect senescence induction during therapy. In all cases, senescent cell removal by senolysis or improving immune targeting would be most efficacious, however, if modulation of particular SASP factors can prove beneficial, with minimal off-target effects this may also be a viable option. In all instances, however, additional study using preclinical animal models is required to determine safety and efficiency of these strategies.
Concluding Remarks and Future Directions
Cellular senescence is a feature of cancer that can be induced by multiple mechanisms in and around tumors, and can have both beneficial and detrimental effects on tumor initiation, growth, therapeutic efficacy, and tumor recurrence. Features of these different senescent cell types, and the mechanisms for their phenotypic impact on neoplastic cells remain incompletely understood, however, and in depth in vivo analysis is currently lacking (see Outstanding Questions). Although, these studies are technically challenging, it is difficult to translate in vitro findings. Further, given the complex and important role of immune surveillance in tumorigenesis and cellular senescence, experimentation in immunocompetent animal models is required. In addition, the role of senescent cells in different tumor types appears quite variable, and furthering our understanding of these differences is an important consideration for both cancer and senotherapy. With the current knowledge, it seems that the detrimental effects of senescent cells in cancer appear to outweigh the beneficial effects that are observed in some instances. But, increasing our understanding of the differences between the SASP of senescent cells derived from multiple mechanisms, and how these components contribute to immune attraction and deterrence will be critical for consideration of combination cancer and senotherapy. Although, additional studies are required to determine the safety and efficiency of combination cancer and senotherapy, this concept shows great promise in improving current cancer therapeutics and overall of health and outcomes of cancer patients.
Outstanding Questions.
To what extent can combination cancer therapy and senotherapy be employed to improve therapeutic efficacy, lower risk of recurrence, and ultimately improve patient outcome?
Can removal of age-related senescent cells in humans reduce cancer risk?
Do different cell/tumor types have a different dependence on senescent cells, i.e. more or less beneficial or detrimental roles, within their niche?
What is the mechanism for senescent cell induction of regenerative capacity in neighboring cells with short-term exposure, and can this contribute to the protumorigenic properties of senescent cells?
Which properties of senescent cells determine their role in immune attraction or deterrence, and how can these be differentially mediated in senescent cells induced by similar mechanisms? Does immune efficiency underlie these differences?
Are beneficial, tumor-suppressing senescent cells modulating immunosurveillance differently compared to detrimental, cancer-promoting senescent cells?
How do senescent cell features and SASP from senescent cells induced by different mechanisms (oncogene-induced, therapy-induced, tumor-induced, age-related, and bystander-induced) differ in vivo, and how does this impact the tumor microenvironment and immune surveillance?
Which SASP components are involved in driving growth and bystander senescence in neighboring cells, immune attraction, and immune deterrence in vivo?
How do tumors/neoplastic cells induce senescence in neighboring cells/tumor stroma?
Highlights.
Senescent cells are a cell cycle arrested, but highly bioactive cell type. Although the proportion of senescent cells in tissues is relatively low, these cells are causally implicated in aging and an ever-expanding list of diseases including cancer.
Cancer-associated senescent cells can modulate all stages of tumor development, with their contributions being either detrimental or beneficial towards tumor initiation, growth, metastasis, or cancer relapse.
Although highly context-dependent, the senescence-associated secretory phenotype (SASP) serves many functions in the tumor-microenvironment including mitogenic induction, immune surveillance, or immune deterrence.
A two-step anti-cancer therapeutic concept, senescence-inducing chemotherapy followed by senotherapy, may represent a viable option to maximize therapeutic efficiency and patient outcome.
Glossary
- Acute senescent cells
describes senescent cells that are generated quickly after an environmental insult or stress (for example during wound healing) or during programmed senescence in embryogenesis. These cells are typically eliminated by the immune system in a fast and efficient manner. Since these cells are only temporarily present and are associated with physiological processes, acute senescent cells are hypothesized to be beneficial for the organism
- CDKN2A
Gene encoding the tumor suppressors p16INK4A and p19ARF (human p14ARF), both of which regulate cell cycle
- Chronic senescent cells
a subset of senescent cells, that are not efficiently removed or evade immune cell clearance, and therefore accumulate relatively slowly in several tissues during aging or at sites of chronic pathologies. These senescent cells are viewed as detrimental for disease progression and aging
- Inflammaging
describes a hypothesis that tissue deterioration is associated with low-grade tissue inflammation, usually in the context of aging and age-related accumulation of senescent cells, which secret pro-inflammatory cytokines
- Immunosurveillance (immune surveillance)
is a complex process by which immune cells from the innate or adaptive immune system detect and remove pathogens or damaged cells, which can include senescent cells
- INK-ATTAC mouse model
a transgenic mouse model containing a FK506-binding-protein-caspase 8 (FKBP-Casp8) fusion protein and green fluorescent protein (GFP) under the control of a minimal Cdkn2aInk4a (p16) promoter fragment that is transcriptionally active in senescent cells, allowing for elimination of senescent cells in the presence of the dimerizer AP20187 (AP), which activates FKBP-Casp8
- PD1 immune checkpoint
PD1, programmed cell death protein 1, is a cell surface receptor and immune checkpoint that guards against autoimmunity by promoting apoptosis in antigen-specific T-cells while simultaneously suppressing apoptosis in regulatory T-cells.
- SHP2
SRC homology phosphatase 2, also known as PTPN11 (tyrosine-protein phosphatase non-receptor type 11), is an enzyme and signaling molecule that regulates cell growth, mitotic cell cycle, differentiation, and oncogenic transformation
- STAT3
signal transducer and activator of transcription 3 is a transcription factor that is phosphorylated by Janus kinases (JAKs) in response to cytokines and growth factors, triggering translocation to the nucleus where it acts as a transcriptional activator and mediates cell growth and apoptosis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kennedy BK, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker DJ, et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530:184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Childs BG, et al. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science. 2016;354:472–477. doi: 10.1126/science.aaf6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeon OH, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017;23:775–781. doi: 10.1038/nm.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sturmlechner I, et al. Cellular senescence in renal ageing and disease. Nat Rev Nephrol. 2017;13:77–89. doi: 10.1038/nrneph.2016.183. [DOI] [PubMed] [Google Scholar]
- 6.Chang J, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22:78–83. doi: 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Childs BG, et al. Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov. 2017;16:718–735. doi: 10.1038/nrd.2017.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serrano M, et al. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 9.Kang TW, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–551. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 10.Kuilman T, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 11.Kim YH, et al. Senescent tumor cells lead the collective invasion in thyroid cancer. Nat Commun. 2017;8:15208. doi: 10.1038/ncomms15208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruhland MK, et al. Stromal senescence establishes an immunosuppressive microenvironment that drives tumorigenesis. Nat Commun. 2016;7:11762. doi: 10.1038/ncomms11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braig M, et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–665. doi: 10.1038/nature03841. [DOI] [PubMed] [Google Scholar]
- 14.Dhomen N, et al. Oncogenic Braf induces melanocyte senescence and melanoma in mice. Cancer Cell. 2009;15:294–303. doi: 10.1016/j.ccr.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 15.Vizioli MG, et al. Oncogenic RAS-induced senescence in human primary thyrocytes: molecular effectors and inflammatory secretome involved. Oncotarget. 2014;5:8270–8283. doi: 10.18632/oncotarget.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarkisian CJ, et al. Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat Cell Biol. 2007;9:493–505. doi: 10.1038/ncb1567. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin HK, et al. Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature. 2010;464:374–379. doi: 10.1038/nature08815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elyada E, et al. CKIalpha ablation highlights a critical role for p53 in invasiveness control. Nature. 2011;470:409–413. doi: 10.1038/nature09673. [DOI] [PubMed] [Google Scholar]
- 20.Chesnokova V, et al. Senescence mediates pituitary hypoplasia and restrains pituitary tumor growth. Cancer Res. 2007;67:10564–10572. doi: 10.1158/0008-5472.CAN-07-0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollock PM, et al. High frequency of BRAF mutations in nevi. Nature genetics. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- 22.Bennett DC. Human melanocyte senescence and melanoma susceptibility genes. Oncogene. 2003;22:3063–3069. doi: 10.1038/sj.onc.1206446. [DOI] [PubMed] [Google Scholar]
- 23.Jacob K, et al. Genetic aberrations leading to MAPK pathway activation mediate oncogene-induced senescence in sporadic pilocytic astrocytomas. Clin Cancer Res. 2011;17:4650–4660. doi: 10.1158/1078-0432.CCR-11-0127. [DOI] [PubMed] [Google Scholar]
- 24.Courtois-Cox S, et al. A negative feedback signaling network underlies oncogene-induced senescence. Cancer Cell. 2006;10:459–472. doi: 10.1016/j.ccr.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharpless NE, et al. The differential impact of p16(INK4a) or p19(ARF) deficiency on cell growth and tumorigenesis. Oncogene. 2004;23:379–385. doi: 10.1038/sj.onc.1207074. [DOI] [PubMed] [Google Scholar]
- 26.Solimini NL, et al. Recurrent hemizygous deletions in cancers may optimize proliferative potential. Science. 2012;337:104–109. doi: 10.1126/science.1219580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalgo ML, et al. The role of DNA methylation in expression of the p19/p16 locus in human bladder cancer cell lines. Cancer Res. 1998;58:1245–1252. [PubMed] [Google Scholar]
- 28.LaPak KM, Burd CE. The molecular balancing act of p16(INK4a) in cancer and aging. Mol Cancer Res. 2014;12:167–183. doi: 10.1158/1541-7786.MCR-13-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Otterson GA, et al. Absence of p16INK4 protein is restricted to the subset of lung cancer lines that retains wildtype RB. Oncogene. 1994;9:3375–3378. [PubMed] [Google Scholar]
- 30.Vizioli MG, et al. Evidence of oncogene-induced senescence in thyroid carcinogenesis. Endocr Relat Cancer. 2011;18:743–757. doi: 10.1530/ERC-11-0240. [DOI] [PubMed] [Google Scholar]
- 31.Lassen P, et al. Impact of HPV-associated p16-expression on radiotherapy outcome in advanced oropharynx and non-oropharynx cancer. Radiother Oncol. 2014;113:310–316. doi: 10.1016/j.radonc.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 32.Parry D, et al. Lack of cyclin D-Cdk complexes in Rb-negative cells correlates with high levels of p16INK4/MTS1 tumour suppressor gene product. EMBO J. 1995;14:503–511. doi: 10.1002/j.1460-2075.1995.tb07026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geoerger B, et al. A Phase I Study of the CDK4/6 Inhibitor Ribociclib (LEE011) in Pediatric Patients with Malignant Rhabdoid Tumors, Neuroblastoma, and Other Solid Tumors. Clin Cancer Res. 2017;23:2433–2441. doi: 10.1158/1078-0432.CCR-16-2898. [DOI] [PubMed] [Google Scholar]
- 34.Goldman JW, et al. Treatment Rationale and Study Design for the JUNIPER Study: A Randomized Phase III Study of Abemaciclib With Best Supportive Care Versus Erlotinib With Best Supportive Care in Patients With Stage IV Non-Small-Cell Lung Cancer With a Detectable KRAS Mutation Whose Disease Has Progressed After Platinum-Based Chemotherapy. Clin Lung Cancer. 2016;17:80–84. doi: 10.1016/j.cllc.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Turner NC, et al. Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N Engl J Med. 2015;373:209–219. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 36.Wang L, et al. High-Throughput Functional Genetic and Compound Screens Identify Targets for Senescence Induction in Cancer. Cell reports. 2017;21:773–783. doi: 10.1016/j.celrep.2017.09.085. [DOI] [PubMed] [Google Scholar]
- 37.Iannello A, et al. p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J Exp Med. 2013;210:2057–2069. doi: 10.1084/jem.20130783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eggert T, et al. Distinct Functions of Senescence-Associated Immune Responses in Liver Tumor Surveillance and Tumor Progression. Cancer Cell. 2016;30:533–547. doi: 10.1016/j.ccell.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jackson JG, et al. p53-mediated senescence impairs the apoptotic response to chemotherapy and clinical outcome in breast cancer. Cancer Cell. 2012;21:793–806. doi: 10.1016/j.ccr.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee CT, et al. Overexpression of the cyclin-dependent kinase inhibitor p16 is associated with tumor recurrence in human prostate cancer. Clin Cancer Res. 1999;5:977–983. [PubMed] [Google Scholar]
- 41.Henshall SM, et al. Overexpression of the cell cycle inhibitor p16INK4A in high-grade prostatic intraepithelial neoplasia predicts early relapse in prostate cancer patients. Clin Cancer Res. 2001;7:544–550. [PubMed] [Google Scholar]
- 42.Milanovic M, et al. Senescence-associated reprogramming promotes cancer stemness. Nature. 2018;553:96–100. doi: 10.1038/nature25167. [DOI] [PubMed] [Google Scholar]
- 43.Demaria M, et al. Cellular Senescence Promotes Adverse Effects of Chemotherapy and Cancer Relapse. Cancer Discov. 2017;7:165–176. doi: 10.1158/2159-8290.CD-16-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu D, Hornsby PJ. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 2007;67:3117–3126. doi: 10.1158/0008-5472.CAN-06-3452. [DOI] [PubMed] [Google Scholar]
- 45.Coppe JP, et al. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J Biol Chem. 2006;281:29568–29574. doi: 10.1074/jbc.M603307200. [DOI] [PubMed] [Google Scholar]
- 46.Krtolica A, et al. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci U S A. 2001;98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coppe JP, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laberge RM, et al. Glucocorticoids suppress selected components of the senescence-associated secretory phenotype. Aging Cell. 2012;11:569–578. doi: 10.1111/j.1474-9726.2012.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paradis V, et al. Replicative senescence in normal liver, chronic hepatitis C, and hepatocellular carcinomas. Hum Pathol. 2001;32:327–332. doi: 10.1053/hupa.2001.22747. [DOI] [PubMed] [Google Scholar]
- 50.Yang G, et al. The chemokine growth-regulated oncogene 1 (Gro-1) links RAS signaling to the senescence of stromal fibroblasts and ovarian tumorigenesis. Proc Natl Acad Sci U S A. 2006;103:16472–16477. doi: 10.1073/pnas.0605752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burd CE, et al. Monitoring tumorigenesis and senescence in vivo with a p16(INK4a)-luciferase model. Cell. 2013;152:340–351. doi: 10.1016/j.cell.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 53.Bavik C, et al. The gene expression program of prostate fibroblast senescence modulates neoplastic epithelial cell proliferation through paracrine mechanisms. Cancer Res. 2006;66:794–802. doi: 10.1158/0008-5472.CAN-05-1716. [DOI] [PubMed] [Google Scholar]
- 54.Witkiewicz AK, et al. Association of RB/p16-pathway perturbations with DCIS recurrence: dependence on tumor versus tissue microenvironment. Am J Pathol. 2011;179:1171–1178. doi: 10.1016/j.ajpath.2011.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Toso A, et al. Enhancing chemotherapy efficacy in Pten-deficient prostate tumors by activating the senescence-associated antitumor immunity. Cell reports. 2014;9:75–89. doi: 10.1016/j.celrep.2014.08.044. [DOI] [PubMed] [Google Scholar]
- 56.Nelson G, et al. A senescent cell bystander effect: senescence-induced senescence. Aging Cell. 2012;11:345–349. doi: 10.1111/j.1474-9726.2012.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Acosta JC, et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol. 2013;15:978–990. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mikula-Pietrasik J, et al. Bystander senescence in human peritoneal mesothelium and fibroblasts is related to thrombospondin-1-dependent activation of transforming growth factor-beta1. Int J Biochem Cell Biol. 2013;45:2087–2096. doi: 10.1016/j.biocel.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 59.Hubackova S, et al. IL1- and TGFbeta-Nox4 signaling, oxidative stress and DNA damage response are shared features of replicative, oncogene-induced, and drug-induced paracrine ‘bystander senescence’. Aging (Albany NY) 2012;4:932–951. doi: 10.18632/aging.100520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nelson G, et al. The senescent bystander effect is caused by ROS-activated NF-kappaB signalling. Mech Ageing Dev. 2018;170:30–36. doi: 10.1016/j.mad.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Acosta JC, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 62.Gluck S, et al. Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat Cell Biol. 2017;19:1061–1070. doi: 10.1038/ncb3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ritschka B, et al. The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev. 2017;31:172–183. doi: 10.1101/gad.290635.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mario Gonzalez-Meljem J, et al. Stem cell senescence drives age-attenuated induction of pituitary tumours in mouse models of paediatric craniopharyngioma. Nat Commun. 2017;8:1819. doi: 10.1038/s41467-017-01992-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oubaha M, et al. Senescence-associated secretory phenotype contributes to pathological angiogenesis in retinopathy. Sci Transl Med. 2016;8:362ra144. doi: 10.1126/scitranslmed.aaf9440. [DOI] [PubMed] [Google Scholar]
- 66.Burnley P, et al. Role of the p63-FoxN1 regulatory axis in thymic epithelial cell homeostasis during aging. Cell Death Dis. 2013;4:e932. doi: 10.1038/cddis.2013.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang X, et al. Metabolic triggered inflammation in osteoarthritis. Osteoarthritis Cartilage. 2015;23:22–30. doi: 10.1016/j.joca.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 68.de Magalhaes JP. How ageing processes influence cancer. Nat Rev Cancer. 2013;13:357–365. doi: 10.1038/nrc3497. [DOI] [PubMed] [Google Scholar]
- 69.Anisimov VN, et al. Relationships between cancer and aging: a multilevel approach. Biogerontology. 2009;10:323–338. doi: 10.1007/s10522-008-9209-8. [DOI] [PubMed] [Google Scholar]
- 70.Christensen K, et al. Cancer and longevity--is there a trade-off? A study of cooccurrence in Danish twin pairs born 1900–1918. J Gerontol A Biol Sci Med Sci. 2012;67:489–494. doi: 10.1093/gerona/gls087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kubben N, Misteli T. Shared molecular and cellular mechanisms of premature ageing and ageing-associated diseases. Nat Rev Mol Cell Biol. 2017;18:595–609. doi: 10.1038/nrm.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maas HA, et al. The influence of age and co-morbidity on treatment and prognosis of ovarian cancer: a population-based study. Gynecol Oncol. 2005;97:104–109. doi: 10.1016/j.ygyno.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 73.Lancet JE, et al. Acute myelogenous leukemia and aging. Clinical interactions. Hematol Oncol Clin North Am. 2000;14:251–267. doi: 10.1016/s0889-8588(05)70287-2. [DOI] [PubMed] [Google Scholar]
- 74.Reed MJ, et al. The effects of aging on tumor growth and angiogenesis are tumor-cell dependent. Int J Cancer. 2007;120:753–760. doi: 10.1002/ijc.22351. [DOI] [PubMed] [Google Scholar]
- 75.Ershler WB, et al. B16 murine melanoma and aging: slower growth and longer survival in old mice. J Natl Cancer Inst. 1984;72:161–164. doi: 10.1093/jnci/72.1.161. [DOI] [PubMed] [Google Scholar]
- 76.McCullough KD, et al. Age-dependent induction of hepatic tumor regression by the tissue microenvironment after transplantation of neoplastically transformed rat liver epithelial cells into the liver. Cancer Res. 1997;57:1807–1813. [PubMed] [Google Scholar]
- 77.Hinkal G, et al. Timed somatic deletion of p53 in mice reveals age-associated differences in tumor progression. PLoS One. 2009;4:e6654. doi: 10.1371/journal.pone.0006654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Braumuller H, et al. T-helper-1-cell cytokines drive cancer into senescence. Nature. 2013;494:361–365. doi: 10.1038/nature11824. [DOI] [PubMed] [Google Scholar]
- 79.Galon J, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 80.Xue W, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Krizhanovsky V, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Panda A, et al. Human innate immunosenescence: causes and consequences for immunity in old age. Trends Immunol. 2009;30:325–333. doi: 10.1016/j.it.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gruver AL, et al. Immunosenescence of ageing. J Pathol. 2007;211:144–156. doi: 10.1002/path.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hewitt G, et al. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat Commun. 2012;3:708. doi: 10.1038/ncomms1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Le Garff-Tavernier M, et al. Human NK cells display major phenotypic and functional changes over the life span. Aging Cell. 2010;9:527–535. doi: 10.1111/j.1474-9726.2010.00584.x. [DOI] [PubMed] [Google Scholar]
- 86.Vicente R, et al. Cellular senescence impact on immune cell fate and function. Aging Cell. 2016;15:400–406. doi: 10.1111/acel.12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Effros RB, et al. In vitro senescence of immune cells. Exp Gerontol. 2003;38:1243–1249. doi: 10.1016/j.exger.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 88.Montes CL, et al. Tumor-induced senescent T cells with suppressor function: a potential form of tumor immune evasion. Cancer Res. 2008;68:870–879. doi: 10.1158/0008-5472.CAN-07-2282. [DOI] [PubMed] [Google Scholar]
- 89.Ramello MC, et al. Tumor-induced senescent T cells promote the secretion of pro-inflammatory cytokines and angiogenic factors by human monocytes/macrophages through a mechanism that involves Tim-3 and CD40L. Cell Death Dis. 2014;5:e1507. doi: 10.1038/cddis.2014.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 91.Golomb L, et al. Age-associated inflammation connects RAS-induced senescence to stem cell dysfunction and epidermal malignancy. Cell Death Differ. 2015;22:1764–1774. doi: 10.1038/cdd.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Feig C, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110:20212–20217. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen S, et al. Myeloid-derived suppressor cells promote age-related increase of lung cancer growth via B7-H1. Exp Gerontol. 2015;61:84–91. doi: 10.1016/j.exger.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 94.Coppe JP, et al. A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLoS One. 2010;5:e9188. doi: 10.1371/journal.pone.0009188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chien Y, et al. Control of the senescence-associated secretory phenotype by NF-kappaB promotes senescence and enhances chemosensitivity. Genes Dev. 2011;25:2125–2136. doi: 10.1101/gad.17276711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Freund A, et al. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30:1536–1548. doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dou Z, et al. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature. 2017;550:402–406. doi: 10.1038/nature24050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hoare M, et al. NOTCH1 mediates a switch between two distinct secretomes during senescence. Nat Cell Biol. 2016;18:979–992. doi: 10.1038/ncb3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Laberge RM, et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol. 2015;17:1049–1061. doi: 10.1038/ncb3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kang C, et al. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science. 2015;349:aaa5612. doi: 10.1126/science.aaa5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Orjalo AV, et al. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc Natl Acad Sci U S A. 2009;106:17031–17036. doi: 10.1073/pnas.0905299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tasdemir N, Celik S. Self-reported pain relief interventions of patients before emergency department arrival. Int Emerg Nurs. 2016;28:20–24. doi: 10.1016/j.ienj.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 103.Di Paolo NC, Shayakhmetov DM. Interleukin 1alpha and the inflammatory process. Nat Immunol. 2016;17:906–913. doi: 10.1038/ni.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yoshimoto S, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 105.Baker DJ, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fielding CA, et al. Interleukin-6 signaling drives fibrosis in unresolved inflammation. Immunity. 2014;40:40–50. doi: 10.1016/j.immuni.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ning Y, et al. Interleukin-8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int J Cancer. 2011;128:2038–2049. doi: 10.1002/ijc.25562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Takasugi M, et al. Small extracellular vesicles secreted from senescent cells promote cancer cell proliferation through EphA2. Nat Commun. 2017;8:15729. doi: 10.1038/ncomms15728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pazolli E, et al. Senescent stromal-derived osteopontin promotes preneoplastic cell growth. Cancer Res. 2009;69:1230–1239. doi: 10.1158/0008-5472.CAN-08-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mikula-Pietrasik J, et al. Oxidative stress contributes to hepatocyte growth factor-dependent pro-senescence activity of ovarian cancer cells. Free Radic Biol Med. 2017;110:270–279. doi: 10.1016/j.freeradbiomed.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 111.Mikula-Pietrasik J, et al. Ovarian cancer-derived ascitic fluids induce a senescence-dependent pro-cancerogenic phenotype in normal peritoneal mesothelial cells. Cell Oncol (Dordr) 2016;39:473–481. doi: 10.1007/s13402-016-0289-1. [DOI] [PubMed] [Google Scholar]
- 112.Malaquin N, et al. Senescent fibroblasts enhance early skin carcinogenic events via a paracrine MMP-PAR-1 axis. PLoS One. 2013;8:e63607. doi: 10.1371/journal.pone.0063607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bonnans C, et al. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Woenne EC, et al. MMP inhibition blocks fibroblast-dependent skin cancer invasion, reduces vascularization and alters VEGF-A and PDGF-BB expression. Anticancer Res. 2010;30:703–711. [PubMed] [Google Scholar]
- 115.Boire A, et al. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120:303–313. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 116.Nardella C, et al. Pro-senescence therapy for cancer treatment. Nat Rev Cancer. 2011;11:503–511. doi: 10.1038/nrc3057. [DOI] [PubMed] [Google Scholar]
- 117.Guan X, et al. Stromal Senescence By Prolonged CDK4/6 Inhibition Potentiates Tumor Growth. Mol Cancer Res. 2017;15:237–249. doi: 10.1158/1541-7786.MCR-16-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lan L, et al. Shp2 signaling suppresses senescence in PyMT-induced mammary gland cancer in mice. EMBO J. 2015;34:1493–1508. doi: 10.15252/embj.201489004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Demaria M, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31:722–733. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kaefer A, et al. Mechanism-based pharmacokinetic/pharmacodynamic meta-analysis of navitoclax (ABT-263) induced thrombocytopenia. Cancer Chemother Pharmacol. 2014;74:593–602. doi: 10.1007/s00280-014-2530-9. [DOI] [PubMed] [Google Scholar]
- 121.Tse C, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 122.Dorr JR, et al. Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature. 2013;501:421–425. doi: 10.1038/nature12437. [DOI] [PubMed] [Google Scholar]
- 123.Lasry A, Ben-Neriah Y. Senescence-associated inflammatory responses: aging and cancer perspectives. Trends Immunol. 2015;36:217–228. doi: 10.1016/j.it.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 124.Ott PA, et al. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19:5300–5309. doi: 10.1158/1078-0432.CCR-13-0143. [DOI] [PubMed] [Google Scholar]
- 125.Alimonti A, et al. A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis. The Journal of clinical investigation. 2010;120:681–693. doi: 10.1172/JCI40535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fleischmann R. Anakinra in the treatment of rheumatic disease. Expert Rev Clin Immunol. 2006;2:331–340. doi: 10.1586/1744666X.2.3.331. [DOI] [PubMed] [Google Scholar]
- 127.Deisseroth A, et al. FDA approval: siltuximab for the treatment of patients with multicentric Castleman disease. Clin Cancer Res. 2015;21:950–954. doi: 10.1158/1078-0432.CCR-14-1678. [DOI] [PubMed] [Google Scholar]
- 128.Karsdal MA, et al. IL-6 receptor inhibition positively modulates bone balance in rheumatoid arthritis patients with an inadequate response to anti-tumor necrosis factor therapy: biochemical marker analysis of bone metabolism in the tocilizumab RADIATE study ( NCT00106522) Semin Arthritis Rheum. 2012;42:131–139. doi: 10.1016/j.semarthrit.2012.01.004. [DOI] [PubMed] [Google Scholar]