Abstract
Suicide and nonfatal suicidal behaviors are major causes of mortality and morbidity worldwide. Variability in rates of suicide and suicidal behaviors within and between countries has been attributed to population and individual risk factors, including economic status and cultural differences, both of which can have suicide risk effects mediated through a variety of factors, of which perhaps the least understood is the role of diet. We therefore review the scientific literature concerning two major dietary lipid classes, cholesterol and polyunsaturated fatty acids (PUFAs), that have been associated with higher risk of suicide attempts and suicide. We consider potential mechanistic intermediates including serotonin transporters and receptors, toll-like receptors (TLRs), nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), and peroxisome proliferator activated receptors (PPARs). Based on this review, we describe a theoretical model linking cholesterol and PUFA status to suicide risk, taking into account the effects of cholesterol-lowering interventions on PUFA balance, membrane lipid microdomains (rafts) as a nexus of interaction between cholesterol and omega-3 PUFAs, and downstream effects on serotonergic neurotransmission and specific inflammatory pathways.
1. Introduction
1.1. Lipids and suicide
Suicide and suicidal behaviors are among the leading causes of death and injuries worldwide. Approximately 800,000 people die from suicide each year, and suicide is the second leading cause of death in the 15-29 year-old cohort. Ten to twenty times more individuals attempt suicide, indicating that both suicide and non-fatal suicidal behaviors are prevalent and need to be addressed. (World Health Organization, 2014)
To understand the causes of suicide, prevalent explanatory models have focused on psychological factors such as feelings of thwarted belongingness, perceived burdensomeness, and hopelessness (Van Orden et al., 2010); neurobiological factors such as genetic risk, serotonergic functioning, and altered stress responses (Mann et al., 1999, Oquendo et al., 2014); and cultural factors (Chu et al., 2018).
Rates of suicide and suicidal behaviors vary geographically, with higher rates of suicide occurring in lower per capita-income regions (World Health Organization, 2017). Some portion of this variability may be attributable to economic and cultural differences that influence nutrition and in this way can impact the diathesis or predisposition to suicide behavior. One nutritional factor proposed to impact suicide and suicidal behavior is dietary lipid intake, presumably through lipid effects on brain. Two major lipid classes have been implicated in suicide risk, cholesterol and polyunsaturated fatty acids (PUFAs). We here review the evidence associating low cholesterol and low n-3 relative to n-6 PUFAs with suicide and suicidal behaviors. Finally, we present a neurobiological model proposing that the actions and interactions of cholesterol and PUFA status may influence suicide risk through effects on decreased serotonergic neurotransmission and/or increased inflammation (see Figure 1).
Figure 1. Theoretical schematic of pathways through which an elevated n-6 to n-3 PUFA ratio could affect suicide risk.
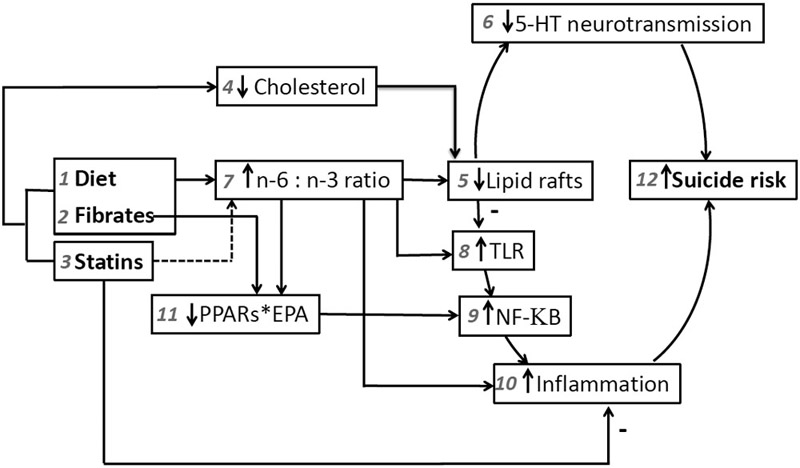
Treatment with (1) diet, (2) fibrates and (3) statins that lower (4) cholesterol can cause (5) disruption of lipid rafts with functional consequences, due to lipid raft regulation of serotonin transporters and receptors, resulting in (6) decreased serotonergic neurotransmission, which has been shown to increase (12) suicide risk. (1) Diets replacing saturated fats with polyunsaturated oils high in n-6 PUFAs and (2) fibrates also can cause an increase in (7) the ratio of n-6 to n-3 PUFAs. This is, effectively, a lowering of n-3 that also is expected to contribute to (5) destabilization of lipid rafts, although directional effects of PUFAs on lipid rafts are complex and incompletely understood. More clearly, a higher n-6 to n-3 PUFA ratio directly promotes (10) inflammation, which is associated with (12) suicide risk. Also, lower n-3 PUFAs can indirectly result in increased inflammation by lowering DHA-mediated inhibition of (8) TLR dimerization and activation, resulting in downstream increased activation of (9) NF-KB, a (10) pro-inflammatory molecule. Either (1) decreased n-3 intake or (2) fibrate competition with EPA can reduce EPA binding to PPARs. Since (11) the EPA*PPARs complex acts as a brake on (9) NF-KB, interference with the EPA*PPARs complex via both mechanisms also contributes to activation (disinhibition) of (9) NF-KB. Counter to these pro-inflammatory forces, (5) decreased lipid raft functioning could decrease (8) TLR recruitment into lipid rafts and activation; and (3) statins may have lesser effects on (7) the n-6 to n-3 ratio and they also exert pleiotropic (10) anti-inflammatory effects that may mitigate (12) suicide risk. See text for all references substantiating these relationships.
1.2. Low cholesterol and suicide risk
Cholesterol and cholesterol metabolites are abundant in the brain. Accounting for 2% of body weight, the brain has 25% of total body cholesterol (Dietschy and Turley, 2001). Cholesterol is essential for cell membrane stability and neurotransmission (Ghaemi et al., 2000). An association between cholesterol and suicide was first reported in a 1990 meta-analysis of primary intervention trials in cardiovascular illness, which found that cholesterol lowering treatments led to an excess in non-illness mortality, mostly suicide and injury (Muldoon et al., 1990). A second meta-analysis was carried out by the same group 11 years later, after hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, or statins, became the most commonly used cholesterol-lowering drug class. The authors concluded that overall, cholesterol-lowering treatments were not related to non-illness mortality, and that statins showed a tendency to reduce non-illness mortality (Muldoon, 2001). Non-statin treatments, however, including diet, did exhibit a trend (p=0.06) toward increased mortality from suicide, accidents and trauma (Muldoon et al., 2001).
In parallel, observational studies of cholesterol status in psychiatric populations have been summarized recently in a meta-analysis of 65 epidemiological studies, involving 510,392 participants, studying associations between serum lipid levels and ‘suicidality’ (Wu et al., 2016). Included were studies that assessed total serum cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and/or triacylglycerols (TAG). The outcome measure of ‘suicidality’ was defined as including suicidal ideation, suicide attempt, having threatened suicide, or death by suicide. The main results were that TC and LDL-C levels were lower in suicidal patients than in non-suicidal patients and healthy controls; HDL-C levels were lower in suicidal patients than in healthy controls; and TG levels were lower in suicidal than in non-suicidal patients. When all three groups were pooled, lower serum TC was associated with higher risk of suicidality, suicide attempts, and suicide.
1.3. Low dietary intake of polyunsaturated fatty acids and suicide risk
Another lipid class implicated in suicide risk is polyunsaturated fatty acids (PUFAs), and it has been suggested that PUFA status may be an important factor in cholesterol associations with suicide risk (Hibbeln and Salem, 1996), as has also has been postulated for cardiovascular risk (de Lorgeril et al., 2005). Comprised of long carbon chains with two or more double bonds and categorized as n-3 or n-6 based on the number of carbon atoms from the terminal methyl (omega) end to the first double bond of the carbon chain, PUFAs are found in every cell of the human body and present in multiple lipid classes: esterified to triacylglycerol, cholesterol (as cholesteryl esters) and phospholipids, as well as existing as non-esterified (‘free’) fatty acids (reviewed in (Jump, 2002)). Both n-3 and n-6 PUFAs are defined as essential because humans and most other mammals cannot synthesize these compounds de novo (Spector, 1999), although ingested shorter-chain fatty acids, alpha linolenic acid (ALA, 18:3n-3) and linoleic acid (LA, 18:2n-6) can be converted to long-chain PUFAs in the liver through a series of elongation and desaturation reactions. In the modern diet, whereas n-6 PUFAs are abundant in many plant-based oils and in meat from animals fed corn-based diets, the major source of n-3 PUFAs is seafood (Meyer et al., 2003, Simopoulos, 2011).
Several studies have linked PUFAs with suicide risk. A case-control study of emergency room patients showed that red blood cell levels of eicosapentaenoic acid (EPA, 20:5n-3) were lower in suicide attempters in comparison with controls (Huan et al., 2004). In a pilot study, low docosahexaenoic acid (DHA, 22:6n-3) percentages of total phospholipid fatty acids and elevated n-6 to n-3 ratios predicted suicidal behavior in patients with major depression (Sublette et al., 2006). Finally, a large (n=1600) retrospective case-control study of active duty US military personnel determined that low n-3 PUFA levels were associated with increased risk of suicide compared with other causes of death (Lewis et al., 2011). Higher blood levels of n-6 PUFAs also have been reported in association with higher suicide risk and depression in a study of 234 pregnant women (Vaz et al., 2014).
Lower n-3 PUFA levels are also observed in depressed patients compared with healthy controls, in plasma (Dinan et al., 2009, Féart et al., 2008, Frasure-Smith et al., 2004, Rees et al., 2009, Tiemeier et al., 2003) and serum (Conklin et al., 2007, Maes et al., 1999, Riemer et al., 2010, Schins et al., 2007) phospholipids, red blood cell membranes (Adams et al., 1996, Amin et al., 2008, Edwards et al., 1998, McNamara et al., 2010b, Peet et al., 1998), and adipose tissue (Mamalakis, 2002, Mamalakis et al., 2006a, Mamalakis et al., 2006b, Papandreou et al., 2011, Sarri et al., 2008), and confirmed by meta-analytic findings (Lin et al., 2010). These relationships are relevant since depression is one of the main risk factors associated with suicidal behavior (Teti et al., 2014).
Another suicide risk factor, the presence of impulsive/aggressive traits (van Heeringen and Mann, 2014), also has been observed to associate with lower n-3 PUFAs. In patients with deliberate self-harm, correlations were seen between low plasma levels of n-3 PUFAs and higher impulsivity scores (Garland et al., 2007). In context of substance use disorders, another risk factor for suicide (Tondo et al., 1999), low plasma EPA, was associated with aggression and impulsivity in adults with MDD and comorbid substance use disorders (Beier et al., 2014); and lower plasma levels of docosapentaenoic acid (DPA, 22:5n-6), DHA, and total n-3 PUFAs were found in aggressive cocaine addicts (Buydens-Branchey et al., 2003). Of note, a low cholesterol diet in nonhuman primates also is associated with serotonin neurotransmitter system deficits and greater aggressive behavior (Kaplan et al., 1994).
Meta-analyses provide variable conclusions concerning the therapeutic benefits of n-3 PUFAs in depression (Appleton et al., 2006, Appleton et al., 2010, Appleton et al., 2015, Bloch and Hannestad, 2012, Grosso et al., 2014, Martins, 2009, Martins et al., 2012, Mocking et al., 2016, Sublette et al., 2011, Yang et al., 2015); disparities appear to stem from differences with regard to depression severity, selection of outcome measures, composition of n-3 PUFA supplements, and estimates of negative publication bias. There is considerable support for the finding that n-3 PUFA supplements have greatest efficacy in patients who have a diagnosis of major depression and when the n-3 supplement contains a greater proportion of EPA compared to DHA (Appleton, 2010, Grosso, 2014, Martins, 2009, Martins, 2012, Sublette, 2011, Yang, 2015).
Given that both cholesterol and PUFAs have been implicated in suicide risk, Hibbeln & Salem (Hibbeln and Salem, 1995, 1996) have suggested that PUFA status might be a confounder in the putative relationship between plasma cholesterol and suicide, citing effects of cholesterol-lowering medications on the n-3 to n-6 PUFA balance. We here expand on this idea, describing biochemical and pathophysiologic mechanisms in support of a mediation hypothesis that lowering cholesterol may increase risk of suicidal behavior at least in part through effects on PUFAs.
2. Proposed model connecting cholesterol reduction with PUFA status and suicidal behavior
To postulate a pathogenic and/or causal relationship between cholesterol, PUFAs and suicidal behavior, biological plausibility is needed. As described below and modeled in Figure 1, putative mechanisms include alteration of membrane lipid raft structure by the proportions of cholesterol and n-3 PUFAs, affecting the functioning of membrane-bound proteins including serotonin receptors and transporters, and toll-like receptors. Cholesterol lowering also can increase the n-6:n-3 PUFA ratio, thereby promoting inflammation, since n-3 PUFAs tend to be anti-inflammatory and n-6 PUFAs tend to be pro-inflammatory (reviewed in (Liu et al., 2014)). More indirectly, low n-3 PUFAs disinhibit two inflammatory intermediates, nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) and peroxisome proliferator activated receptors (PPARs). Abnormal monoaminergic neurotransmission and the presence of neuroinflammation are two leading theories of biological pathways to suicide.
2.1. Effects of PUFAs and cholesterol on lipid rafts
The plasma membrane was conceptualized by Singer & Nicolson (Singer and Nicolson, 1972) as a fluid mosaic mixture of lipids and proteins. Alternative paradigms have since been developed, chief among them the lipid raft concept: a glycerophospholipid bilayer containing discrete, spatially and temporally dynamic microdomains (rafts), tightly packed, nano-scale membrane assemblies enriched in cholesterol, sphingolipids, and glycosylphosphatidylinositol (GPI)-anchored proteins (Hancock, 2006). Lipid rafts interact to bring together the components of signaling machinery and influence the trafficking of cellular constituents (Ikonen, 2001, Rajamoorthi et al., 2005).
Due to multiple double bonds, the acyl chains of long-chain PUFAs are extremely flexible and can rapidly change conformational states (Feller and Gawrisch, 2005), resulting in poor packing and higher fluidity, aversive to the tightly-packed, highly ordered cholesterol/sphingolipid raft domains. In vitro, disruption of lipid rafts is accomplished by reducing cholesterol through techniques such as depletion (Sjogren et al., 2006), sequestration (Schnitzer et al., 1994), or replacement with sterols that do not serve to form ordered domains (Vainio et al., 2006). Studies with in vivo mouse models (Fan et al., 2004, Fan et al., 2003), cell cultures (Grimm et al., 2011, Kim et al., 2008), and model membranes (Kinnun et al., 2018, Williams et al., 2012) also agree that n-3 PUFAs alter lipid raft composition. However, contradictory findings have been reported, as to whether n-3 PUFAs promote the formation of lipid rafts and cause increased membrane order, by herding the cholesterol and sphingolipids into larger domains (Kim, 2008, Kinnun, 2018); or cause decreased membrane order by lowering the levels of cholesterol (Ma et al., 2004) and sphingolipids (Fan, 2004, Fan, 2003) within lipid rafts, shifting the cholesterol to non-raft domains (Grimm, 2011). One study reports that, in contrast to a more global lowering of membrane cholesterol due to pharmacologic depletion, n-3 PUFAs reduce cholesterol only in caveolae (Ma, 2004), and notes that studies in T-cells, which lack caveolae, do not exhibit decreased cholesterol (Fan, 2004, Fan, 2003). Counterintuitively, given the aversion between cholesterol and unsaturated fatty acid chains, several studies in T-cells report that n-3 PUFAs incorporate into the lipid rafts (Fan, 2004, Fan, 2003, Stulnig et al., 2001). Thus prediction about in vivo clinical effects of cholesterol and n-3 PUFAs on lipid raft functioning is necessarily somewhat speculative.
2.1.1. Effects of PUFAs and cholesterol on lipid rafts: serotonergic neurotransmission.
Membrane lipid rafts are one arena where PUFA balance and cholesterol reduction intersect and may influence suicide risk by affecting functioning of membrane proteins such as monoaminergic transporters and receptors. Among monoaminergic transporters and receptors that are regulated by lipid rafts, we hypothesize that lipid raft alterations would have the greatest impact on suicide risk via effects on serotonin (5-hydroxytryptamine, 5-HT) transporters (SERT) and receptors, given the associations of the serotonergic pathway with suicidal behavior (reviewed in (Mann, 2003, Oquendo, 2014, van Heeringen and Mann, 2014)). Both SERT (Magnani et al., 2004, Samuvel et al., 2005), which regulates synaptic 5-HT concentrations and has effects on the 5-HT receptors, and the 5-HT receptors themselves, most notably the 5-HT1A (Kalipatnapu and Chattopadhyay, 2005, Kobe et al., 2008, Nothdurfter et al., 2011, Renner et al., 2007, Sjogren et al., 2008), 5-HT2A (Dreja et al., 2002, Mialet-Perez et al., 2012, Sommer et al., 2009), 5-HT3A (Eisensamer et al., 2005, Ilegems et al., 2005, Nothdurfter et al., 2010), and 5-HT7A (Sjogren, 2006, Sjogren and Svenningsson, 2007a, b), localize to lipid rafts.
In vitro studies find that disruption of lipid rafts by cholesterol-interfering agents produces, on average, a 50% decrease in the transport rate of the SERT and a concurrent reduction in SERT affinity for 5-HT, suggesting that lipid rafts may promote a high-affinity state of SERT (Magnani, 2004, Scanlon et al., 2001). Likewise, reduction of cholesterol reduces agonist and antagonist binding at the 5-HT1A (Kalipatnapu and Chattopadhyay, 2005, Sjogren, 2008) and 5-HT7A (Sjogren, 2006) receptors. Interestingly, co-accumulation of certain psychotropic drugs and 5-HT3A receptors in lipid raft fractions is associated with effects on serotonin-induced cation currents (Eisensamer, 2005), suggesting possible lipid raft-mediated effects of antidepressants and antipsychotics on therapeutic efficacy, including reduction of suicide risk.
Direct evidence of a relationship between PUFAs and serotonergic neurotransmission is limited to rat models, in which n-3 deficiency induces higher basal and lower stimulated levels of serotonin (Kodas et al., 2004) and increased central 5-HT turnover (McNamara et al., 2010a), while high n-6 diets cause alterations in 5-HT2A and 5-HT2C receptors and in 5-HT transporter binding (Dubois et al., 2006).
2.1.2. Effects of PUFAs and cholesterol on lipid rafts: toll-like receptors.
Another potential factor in suicide risk involving lipid rafts is the balance of opposing effects of cholesterol and DHA on the dimerization and recruitment of Toll-like receptors (TLR) into lipid rafts. TLRs are pattern recognition receptors that play a key role in recognizing pathogens and triggering immune responses by inducing microglial activation and cytokine production. TLR also activate nuclear factor kappa light chain enhancer of activated B cells (NF-κB), a heterodimeric transcription factor that when activated rapidly migrates into the nucleus of the cell and promotes inflammation through effects on target genes controlling the expression of multiple cytokines, chemokines, adhesion molecules and vascular cell adhesion molecules, and inducible enzymes (Hayden and Ghosh, 2012). Cholesterol and lipid rafts are necessary for TLR activation (Sadikot, 2012). DHA, on the other hand, as one of its anti-inflammatory actions, inhibits the dimerization of TLR, particularly TLR4, and its recruitment into lipid rafts (Wong et al., 2009), and thereby also prevents NF-κB translocation into the nucleus (Chen et al., 2017). Lowering of cholesterol could theoretically disrupt lipid rafts and reduce TLR function and inflammation, but cholesterol-lowering effects of reducing DHA can have the opposite effect, reversing the DHA inhibition of TLR and thus increasing inflammation. Thus a complex balancing of effects remains to be parsed out.
2.2. PUFA interactions with PPARs
NF-κB is also modulated by the peroxisome proliferator-activated receptors (PPARs) family of nuclear transcription factors, for which EPA, DHA and eicosanoids are natural ligands. Upon activation by EPA or other ligands, PPARs form heterodimers with retinoid X that bind to PPAR-responsive elements in the regulatory region of target genes, reducing the expression of proinflammatory molecules. Thus, in addition to inhibition of NF-κB via effects on TLR4, n-3 PUFAs downregulate NF-κB through binding to PPARγ (Rao and Lokesh, 2017). As cholesterol-lowering reduces both DHA effects on TLR and EPA effects on PPARγ, increased inflammation could ensue.
2.3. PUFA balance and inflammation
It has long been known that hormones and neurotransmitters which bind to membrane receptors that activate phospholipase A2 release PUFAs from the sn-2 position of membrane phospholipids to become substrates for eicosanoid biosynthesis, producing a variety of compounds with pro or anti-inflammatory effects. Most of the mediators formed from n-3 PUFAs have anti-inflammatory effects, while those synthesized n-6 PUFAs are mainly pro-inflammatory. Very recently, attention also has been focused on anti-inflammatory consequences of n-3 PUFA via effects on macrophages and microglia (Fourrier et al., 2017, Hopperton et al., 2016, Rombaldova et al., 2017, Shen et al., 2017). Elevation of the n-6:n-3 PUFA ratio thus causes a shift toward a pro-inflammatory state, which has emerged as a potentially important factor in the pathophysiology of suicidal behavior. Pro-inflammatory cytokines, particularly IL-6, are associated with suicidal ideation and both nonfatal suicide attempts and suicides (reviewed in (Gananca et al., 2016)).
2.4. Effect of cholesterol lowering interventions on PUFA levels
The initial observation of the association between lowering cholesterol serum levels and suicide came from intervention studies (Muldoon, 1990). However, those studies did not measure effects of the interventions on PUFA serum levels. As described below, there is some evidence that cholesterol-lowering interventions, besides modifying cholesterol, affect PUFA serum levels, which thus could perhaps be a mediator of the cholesterol-suicide connection.
2.4.1. Effect of fibrates on PUFAs
Fibrates are synthetic ligands for PPARα receptors, and it is through binding to these nuclear receptors that they act to alter lipid levels. Fibrates primarily reduce triglycerides, have a modest effect on HDL-C levels, and, depending upon the baseline triglyceride levels, may decrease LDL-C levels (in patients without baseline elevation in triglyceride levels) or may substantially increase LDL-C levels (in patients with very high baseline triglyceride levels). (Goldenberg et al., 2008)
Information regarding the effect of fibrates on PUFAs is conflicting, and it seems that the action varies with the type of fibrate. In animal models, bezafibrate, and to a lesser extent gemfibrozil, increase monounsaturated fatty acids (palmitoleic and oleic acids) and decrease PUFAs (mainly linoleic acid has been studied) in microsomal phospholipids (Vazquez et al., 1995). In humans, gemfibrozil produces major modifications in fatty acid composition, decreasing saturated fatty acids and increasing n-6 PUFA (Nyalala et al., 2008). A conflicting finding in an animal model (rat heart) is that clofibrate treatment reduces n-6 PUFAs (linoleic acid and arachidonic acid) and increases n-3 PUFA (DHA) and increases the unsaturation extent of myocardial fatty acids (Tian et al., 2006). Taken together, these results suggest that fibrates alter PUFAs serum levels, apparently diminishing n-3 PUFAs. However, this effect is not equivalent for all drugs.
2.4.2. Effect of statins on PUFAs
Statins were originally believed to be effective in treating cardiovascular illness by inhibiting the synthesis of mevalonate, acting on HMG-CoA reductase, and thereby reducing the serum levels of LDL and increasing HDL (Stancu and Sima, 2001). However, statins have pleiotropic biochemical effects beyond the inhibition of cholesterol synthesis and some of these effects modify PUFA levels, altering the balance of n-3 to n-6 PUFAs, apparently by affecting desaturation and elongation (Rise et al., 2001).
In cultured monocytic cells, simvastatin activates the formation of AA from LA, mainly acting at the delta 5 desaturation steps, and increases the mRNA levels of delta 5 desaturase (Rise et al., 2002). In patients with dyslipidemia, rosuvastatin and pitavastatin decrease serum DHA and increase the AA/DHA ratio (Nozue and Michishita, 2015); similarly, pravastatin and simvastatin treatment increase the AA/EPA ratio (Harris et al., 2004, Nakamura et al., 1998) and tend to increase the AA/DHA ratio (Harris, 2004).
Taken together, this information suggests that statins affect PUFA synthesis, particularly increasing serum concentration of AA or the concentration of AA relative to n-3 PUFAs (DHA and EPA), although as for fibrates, individual statin drugs may have differing effects.
2.4.3. Comparisons of fibrate and statin effects on PUFAs
Several studies have simultaneously assessed the effects of fibrates and statins. In one study of plasma lipids of hyperlipidemic patients, elevation of AA occurred in those treated with statins but not those treated with fibrates; AA elevation was associated with concomitant selective escalation of product/precursor ratios for delta 5 desaturation in hypercholesterolemic patients (Rise, 2001). The opposite result was seen in another study which compared the magnitude of effects of atorvastatin, simvastatin and gemfibrozil (Nyalala, 2008), on fatty acid composition in human plasma and red blood cell membranes; they found that n-6 PUFA levels were increased with both treatments, but the effects of gemfibrozil were significant while the effects of statins were not. Finally, a study comparing patients treated with fenofibrate vs. simvastatin for 3 months found significant increases in both groups for n-6 PUFAs, including AA and other intermediate n-6 species, and a concomitant decrease in the n-6 precursor, LA, but observed a decrease in two major n-3 PUFAs (ALA and DHA) in fibrates only (de Lorgeril, 2005). At this time, there is no resolution of these contradictory findings. Differentially stronger effects of fibrates on PUFA balance could, however, pertain to why the most recent meta-analysis (Muldoon, 2001) found a trend (p=0.06) in increased risk of suicide/violent death/accidents with respect to fibrates but no increase with respect to statins.
2.4.4. Effect of proprotein convertase subtilisin-kexin type 9 serine protease (PSCK9) inhibitors
New, highly effective cholesterol lowering drugs, PSCK9 inhibitors, have not been associated with suicidal behavior, according to a meta-analysis of 13,083 patients in 17 clinical trials (Lipinski et al., 2016); however, neurocognitive side effects (amnesia, alterations in memory and confusional state) were reported. Taking into account that there are still few studies published and with short-term follow-ups, potential effects of PSCK9 inhibitors on suicidal behavior should not be ruled out. In a cardiovascular context, effects of dietary n-3 PUFAs may be mediated by direct effects of DHA on PCSK9. (Graversen et al., 2016, Rodriguez-Perez et al., 2016, Yu et al., 2017)
2.4.5. Effect of cholesterol-lowering diets on PUFA levels
In the meta-analyses of Muldoon et al. (Muldoon, 1990, Muldoon, 2001), subjects undergoing dietary interventions (Z-score=1.77, p=0.08 (Muldoon, 1990)) or dietary interventions lumped with non-statin treatment (OR=1.32; CI=1.32-0.98; p=0.06 (Muldoon, 2001)) showed a trend toward increased death from non-illness mortality. Details of the dietary interventions were not available.
3. Discussion
We have expanded upon the previous work of Hibbeln and Salem, who postulated that, “fatty acids may be a critical variable which links cholesterol lowering therapies to suicide or depression,” (Hibbeln and Salem, 1996). Our theoretical model explicates several possible pathways whereby cholesterol-lowering treatments can affect PUFAs and lipid rafts, leading to alterations in serotonergic neurotransmission and/or inflammation and thereby increase suicide risk.
Complexities in the proposed model center around how effects of cholesterol lowering and changes in PUFA balance affect lipid rafts, which have some of the qualities of a “black box”. We know that aversive relationships between highly unsaturated fatty acids and large cholesterol and sphingolipid molecules have biophysical effects on lipid raft structure with functional consequences. However, lipid rafts pose extreme technical challenges for clinical study, as rafts are dynamic systems that are nanoscalar, both temporally and molecularly. Thus to date, current knowledge relies on studies in model membranes, cell culture, and some in vivo rodent studies.
Another complexity is that although statins have been shown to decrease cholesterol and increase n-6 PUFA blood levels with respect to n-3 levels, a meta-analysis performed in 2001 found that in contrast to fibrates and diet, statins showed not only no significant increase in deaths by accidents, violence, and suicide, but rather a tendency to reduce non-illness mortality (Muldoon, 2001). In concordance with our explanatory model, this may be due to a greater impact of fibrates compared with statins on PUFA status (de Lorgeril, 2005, Nyalala, 2008). Additionally, statin mechanisms of action other than lipid reduction have been recognized (Tousoulis et al., 2014), including anti-inflammatory properties. Meta-analyses suggest that statins’ benefits for atherosclerosis are primarily associated with their anti-inflammatory actions (An et al., 2017, Li et al., 2018), particularly in the case of lipophilic statins (Bonsu et al., 2015), which also comports with the premise in our model that lipid effects on suicide risk may relate to their effects on inflammatory state. Thus, statins’ anti-inflammatory properties may actually mitigate suicide risk.
Another relevant question is whether statins have any effects on aggression, which may be a psychiatric mediator or moderator of suicidal behavior. One randomized clinical trial (Golomb et al., 2015) has addressed this question, finding age and sex effects of statin treatment: aggression was decreased in men, particularly men with low baseline aggression, after outliers were removed; these outliers included men in whom statins induced markedly increased aggression, and this was found to be associated with statin-induced side effect of sleep problems. Moreover, aggression was increased in postmenopausal women. The same research group also reported on a case series of 12 patients who took statins and self-referred for assessment of subsequent mood or behavior changes including irritability, depressed mood, and suicidal ideation, suicide attempts and suicide completion (Cham et al., 2016). From a precision medicine standpoint, these findings suggest that although in the aggregate statins tend to decrease suicide risk, certain individuals may have a biological or behavioral phenotype that would confer vulnerability to statin-induced aggression, depression and suicide risk.
We have described mechanisms through which cholesterol-lowering drugs may increase the proportion of n-6 PUFAs. Dietary interventions also showed a trend toward increased suicide mortality (Muldoon, 1990, Muldoon, 2001). How does this relate to the hypothesis that cholesterol relationships to suicide risk are confounded by the underlying PUFA balance? The specifics of the dietary interventions included in these meta-analyses are not readily available for examination. However, U.S. dietary guidelines (Office of Disease Prevention and Healthy Promotion, 2017) on lipid consumption for 1990 stated, “Choose liquid vegetable oils most often because they are lower in saturated fat” and similarly for 2000, “Choose vegetable oils rather than solid fats (meat and dairy fats, shortening).” Although the Mediterranean diet had been proposed previously (Keys and Keys, 1975), possible benefits of using olive oil and n-3-rich oils were not widely recognized during that time period. Assuming that dietary interventions for improved heart health adhered to the nutritional wisdom of the time, then it is likely that study participants were instructed to choose vegetable oils over animal fats. Analysis of n-3 and n-6 PUFA concentrations in a sampling of 14 vegetable oils (olive oil and canola oil were not included) found the average amount of n-6 PUFAs to be 43.4 ± 24.7% of total fatty acid methyl esters while n-3 PUFAs made up only 0.47 ± 0.53% (Orsavova et al., 2015). Thus, comporting with our model, increasing intake of vegetable oils would be expected to have reduced saturated fats but increased intake of n-6 PUFAs.
Like any theoretical schema, our proposed model is an oversimplification. As yet understudied is the extent to which the two pathways we have identified, serotonergic neurotransmission and inflammation, may be related and create a final common pathway to suicide risk. Along these lines, there are reports that serotonin reuptake inhibitors inhibit activation of microglia (Su et al., 2015) and that an abnormal astrocyte-microglia balance is associated with impaired serotonergic functioning (Müller and Schwarz, 2007).
To better understand treatment effects of PUFAs, future studies should assess diet, taking into account not just concentrations of PUFAs but also percentage composition and the relative effects across the lipidome on different lipid classes (phospholipids, cholesteryl esters, unesterified fraction). Additional important factors to be studied include lipid-associated genetic variants and epigenetic marks that modulate the effects of lipid status on suicide risk.
4.1. Limitations
In reviewing the literature pertinent to cholesterol, PUFAs and suicide risk, we are hampered by the lack of a) clinical trials with suicidal behavior as outcome measures and b) studies that fully characterize the lipidome. Therefore, although evidence supports each step in our hypothesized model (Fig 1), and the model possesses face validity, in order to establish mechanisms of action as postulated in our model, mediation analyses would need to be undertaken, which require large sample sizes.
4.2. Conclusions
Based on a survey of relevant scientific literature, we propose links between low cholesterol, elevated n-6 to n-3 PUFAs, lower 5-HT neurotransmission, inflammation, and suicide risk. Effects of cholesterol-PUFA balance on lipid rafts are a mechanistic linchpin deserving of additional study in this regard. If our model is correct, the use of cholesterol-lowering treatments has implications for personalized medicine as well as an impact on public health. There could be preventative value, with respect to suicide, in administering n-3 PUFA supplements to cardiac patients for whom lower cholesterol is medically important and who have or develop psychiatric vulnerabilities.
Abbreviations:
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- PPARs
peroxisome proliferator-activated receptors
- TLR
Toll-like receptor
- NF-KB
nuclear factor kappa-light-chain-enhancer of activated B cells
- 5-HT
5-hydroxytryptamine (serotonin)
- PUFAs
polyunsaturated fatty acids.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Dr. Mann receives royalties from Research Foundation for Mental Hygiene for commercial use of the C-SSRS. Drs. Daray and Sublette have no conflicts to report.
References
- Adams P, Lawson S, Sanigorski A, Sinclair A, 1996. Arachidonic acid to eicosapentaenoic acid ratio in blood correlates positively with clinical symptoms of depression. Lipids. 31, S157–61. [DOI] [PubMed] [Google Scholar]
- Amin AA, Menon RA, Reid KJ, Harris WS, Spertus JA, 2008. Acute coronary syndrome patients with depression have low blood cell membrane omega-3 fatty acid levels. Psychosom. Med. 70, 856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J, Shi F, Liu S, Ma J, Ma Q, 2017. Preoperative statins as modifiers of cardiac and inflammatory outcomes following coronary artery bypass graft surgery: a meta-analysis. Interactive cardiovascular and thoracic surgery. 25, 958–65. [DOI] [PubMed] [Google Scholar]
- Appleton KM, Hayward RC, Gunnell D, Peters TJ, Rogers PJ, Kessler D, et al. , 2006. Effects of n-3 long-chain polyunsaturated fatty acids on depressed mood: systematic review of published trials. Am. J. Clin. Nutr 84, 1308–16. [DOI] [PubMed] [Google Scholar]
- Appleton KM, Rogers PJ, Ness AR, 2010. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. Am. J. Clin. Nutr 91, 757–70. [DOI] [PubMed] [Google Scholar]
- Appleton KM, Sallis HM, Perry R, Ness AR, Churchill R, 2015. Omega-3 fatty acids for depression in adults. Cochrane Database Syst Rev. 11, CD004692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier AM, Lauritzen L, Galfalvy HC, Cooper TB, Oquendo MA, Grunebaum MF, et al. , 2014. Low plasma eicosapentaenoic acid levels are associated with elevated trait aggression and impulsivity in major depressive disorder with a history of comorbid substance use disorder. J. Psychiatr. Res 57, 133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch MH, Hannestad J, 2012. Omega-3 fatty acids for the treatment of depression: systematic review and meta-analysis. Mol. Psychiatry 17, 1272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsu KO, Reidpath DD, Kadirvelu A, 2015. Effects of Statin Treatment on Inflammation and Cardiac Function in Heart Failure: An Adjusted Indirect Comparison Meta-Analysis of Randomized Trials. Cardiovascular therapeutics. 33, 338–46. [DOI] [PubMed] [Google Scholar]
- Buydens-Branchey L, Branchey M, McMakin DL, Hibbeln JR, 2003. Polyunsaturated fatty acid status and aggression in cocaine addicts. Drug Alcohol Depend. 71, 319–23. [DOI] [PubMed] [Google Scholar]
- Cham S, Koslik HJ, Golomb BA, 2016. Mood, Personality, and Behavior Changes During Treatment with Statins: A Case Series. Drug safety - case reports. 3, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wu S, Chen C, Xie B, Fang Z, Hu W, et al. , 2017. Omega-3 polyunsaturated fatty acid supplementation attenuates microglial-induced inflammation by inhibiting the HMGB1/TLR4/NF-kappaB pathway following experimental traumatic brain injury. J Neuroinflammation. 14, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J, Robinett EN, Ma JKL, Shadish KY, Goldblum P, Bongar B, 2018. Cultural versus classic risk and protective factors for suicide. Death Stud. [DOI] [PubMed] [Google Scholar]
- Conklin SM, Manuck SB, Yao JK, Flory JD, Hibbeln JR, Muldoon MF, 2007. High omega-6 and low omega-3 fatty acids are associated with depressive symptoms and neuroticism. Psychosom. Med 69, 932–4. [DOI] [PubMed] [Google Scholar]
- de Lorgeril M, Salen P, Guiraud A, Zeghichi S, Boucher F, de Leiris J, 2005. Lipid-lowering drugs and essential omega-6 and omega-3 fatty acids in patients with coronary heart disease. Nutr Metab Cardiovasc Dis. 15, 36–41. [DOI] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD, 2001. Cholesterol metabolism in the brain. Curr. Opin. Lipidol 12, 105–12. [DOI] [PubMed] [Google Scholar]
- Dinan T, Siggins L, Scully P, O'Brien S, Ross P, Stanton C, 2009. Investigating the inflammatory phenotype of major depression: focus on cytokines and polyunsaturated fatty acids. J. Psychiatr. Res 43, 471–6. [DOI] [PubMed] [Google Scholar]
- Dreja K, Voldstedlund M, Vinten J, Tranum-Jensen J, Hellstrand P, Sward K, 2002. Cholesterol depletion disrupts caveolae and differentially impairs agonist-induced arterial contraction. Arterioscler. Thromb. Vasc. Biol 22, 1267–72. [DOI] [PubMed] [Google Scholar]
- Dubois T, Deng C, Bell W, Huang X, 2006. Fatty acids differentially affect serotonin receptor and transporter binding in the rat brain. Neuroscience. 139, 1397–403. [DOI] [PubMed] [Google Scholar]
- Edwards R, Peet M, Shay J, Horrobin D, 1998. Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. J. Affect. Disord 48, 149–55. [DOI] [PubMed] [Google Scholar]
- Eisensamer B, Uhr M, Meyr S, Gimpl G, Deiml T, Rammes G, et al. , 2005. Antidepressants and antipsychotic drugs colocalize with 5-HT3 receptors in raft-like domains. The Journal of neuroscience : the official journal of the Society for Neuroscience. 25, 10198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan YY, Ly LH, Barhoumi R, McMurray DN, Chapkin RS, 2004. Dietary docosahexaenoic acid suppresses T cell protein kinase C theta lipid raft recruitment and IL-2 production. J. Immunol 173, 6151–60. [DOI] [PubMed] [Google Scholar]
- Fan YY, McMurray DN, Ly LH, Chapkin RS, 2003. Dietary (n-3) polyunsaturated fatty acids remodel mouse T-cell lipid rafts. J. Nutr 133, 1913–20. [DOI] [PubMed] [Google Scholar]
- Féart C, Peuchant E, Letenneur L, Samieri C, Montagnier D, Fourrier-Reglat A, et al. , 2008. Plasma eicosapentaenoic acid is inversely associated with severity of depressive symptomatology in the elderly: data from the Bordeaux sample of the Three-City Study. Am. J. Clin. Nutr 87, 1156–62. [DOI] [PubMed] [Google Scholar]
- Feller SE, Gawrisch K, 2005. Properties of docosahexaenoic-acid-containing lipids and their influence on the function of rhodopsin. Curr. Opin. Struct. Biol 15, 416–22. [DOI] [PubMed] [Google Scholar]
- Fourrier C, Remus-Borel J, Greenhalgh AD, Guichardant M, Bernoud-Hubac N, Lagarde M, et al. , 2017. Docosahexaenoic acid-containing choline phospholipid modulates LPS-induced neuroinflammation in vivo and in microglia in vitro. J Neuroinflammation. 14, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasure-Smith N, Lesperance F, Julien P, 2004. Major depression is associated with lower omega-3 fatty acid levels in patients with recent acute coronary syndromes. Biol. Psychiatry 55, 891–6. [DOI] [PubMed] [Google Scholar]
- Gananca L, Oquendo MA, Tyrka AR, Cisneros-Trujillo S, Mann JJ, Sublette ME, 2016. The role of cytokines in the pathophysiology of suicidal behavior. Psychoneuroendocrinology. 63, 296–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland MR, Hallahan B, McNamara M, Carney PA, Grimes H, Hibbeln JR, et al. , 2007. Lipids and essential fatty acids in patients presenting with self-harm. Br. J. Psychiatry 190, 112–7. [DOI] [PubMed] [Google Scholar]
- Ghaemi SN, Shields GS, Hegarty JD, Goodwin FK, 2000. Cholesterol levels in mood disorders: high or low? Bipolar Disord. 2, 60–4. [DOI] [PubMed] [Google Scholar]
- Goldenberg I, Benderly M, Goldbourt U, 2008. Update on the use of fibrates: focus on bezafibrate. Vascular health and risk management. 4, 131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb BA, Dimsdale JE, Koslik HJ, Evans MA, Lu X, Rossi S, et al. , 2015. Statin Effects on Aggression: Results from the UCSD Statin Study, a Randomized Control Trial. PLoS One. 10, e0124451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graversen CB, Lundbye-Christensen S, Thomsen B, Christensen JH, Schmidt EB, 2016. Marine n-3 polyunsaturated fatty acids lower plasma proprotein convertase subtilisin kexin type 9 levels in pre- and postmenopausal women: A randomised study. Vascular pharmacology. 76, 37–41. [DOI] [PubMed] [Google Scholar]
- Grimm MO, Kuchenbecker J, Grosgen S, Burg VK, Hundsdörfer B, Rothhaar TL, et al. , 2011. Docosahexaenoic acid reduces amyloid beta production via multiple pleiotropic mechanisms. J. Biol. Chem 286, 14028–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosso G, Pajak A, Marventano S, Castellano S, Galvano F, Bucolo C, et al. , 2014. Role of omega-3 fatty acids in the treatment of depressive disorders: a comprehensive meta-analysis of randomized clinical trials. PLoS One. 9, e96905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock JF, 2006. Lipid rafts: contentious only from simplistic standpoints. Nature reviews. Molecular cell biology. 7, 456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JI, Hibbeln JR, Mackey RH, Muldoon MF, 2004. Statin treatment alters serum n-3 and n-6 fatty acids in hypercholesterolemic patients. PLEFA. 71, 263–9. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S, 2012. NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 26, 203–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbeln JR, Salem N Jr., 1995. Dietary polyunsaturated fatty acids and depression: when cholesterol does not satisfy. Am. J. Clin. Nutr 62, 1–9. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR, Salem N Jr., 1996. Risks of cholesterol-lowering therapies. Biol. Psychiatry 40, 686–7. [DOI] [PubMed] [Google Scholar]
- Hopperton KE, Trepanier MO, Giuliano V, Bazinet RP, 2016. Brain omega-3 polyunsaturated fatty acids modulate microglia cell number and morphology in response to intracerebroventricular amyloid-beta 1–40 in mice. J Neuroinflammation. 13, 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huan M, Hamazaki K, Sun Y, Itomura M, Liu H, Kang W, et al. , 2004. Suicide attempt and n-3 fatty acid levels in red blood cells: a case control study in China. Biol. Psychiatry 56, 490–6. [DOI] [PubMed] [Google Scholar]
- Ikonen E, 2001. Roles of lipid rafts in membrane transport. Curr. Opin. Cell Biols 13, 470–7. [DOI] [PubMed] [Google Scholar]
- Ilegems E, Pick H, Deluz C, Kellenberger S, Vogel H, 2005. Ligand binding transmits conformational changes across the membrane-spanning region to the intracellular side of the 5-HT3 serotonin receptor. Chembiochem. 6, 2180–5. [DOI] [PubMed] [Google Scholar]
- Jump DB, 2002. The biochemistry of n-3 polyunsaturated fatty acids. J. Biol. Chem 277, 8755–8. [DOI] [PubMed] [Google Scholar]
- Kalipatnapu S, Chattopadhyay A, 2005. Membrane organization of the human serotonin(1A) receptor monitored by detergent insolubility using GFP fluorescence. Mol. Membr. Biol 22, 539–47. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Shively CA, Fontenot MB, Morgan TM, Howell SM, Manuck SB, et al. , 1994. Demonstration of an association among dietary cholesterol, central serotonergic activity, and social behavior in monkeys. Psychosom. Med 56, 479–84. [DOI] [PubMed] [Google Scholar]
- Keys AB, Keys M How to eat well and stay well the Mediterranean way. 1st ed Garden City, N.Y.,: Doubleday; 1975. [Google Scholar]
- Kim W, Fan YY, Barhoumi R, Smith R, McMurray DN, Chapkin RS, 2008. n-3 polyunsaturated fatty acids suppress the localization and activation of signaling proteins at the immunological synapse in murine CD4+ T cells by affecting lipid raft formation. J. Immunol 181, 6236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnun JJ, Bittman R, Shaikh SR, Wassall SR, 2018. DHA Modifies the Size and Composition of Raftlike Domains: A Solid-State (2)H NMR Study. Biophys. J 114, 380–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobe F, Renner U, Woehler A, Wlodarczyk J, Papusheva E, Bao G, et al. , 2008. Stimulation- and palmitoylation-dependent changes in oligomeric conformation of serotonin 5-HT1A receptors. Biochim. Biophys. Acta 1783, 1503–16. [DOI] [PubMed] [Google Scholar]
- Kodas E, Galineau L, Bodard S, Vancassel S, Guilloteau D, Besnard J, et al. , 2004. Serotoninergic neurotransmission is affected by n-3 polyunsaturated fatty acids in the rat. J. Neurochem 89, 695–702. [DOI] [PubMed] [Google Scholar]
- Lewis MD, Hibbeln JR, Johnson JE, Lin YH, Hyun DY, Loewke JD, 2011. Suicide deaths of active-duty US military and omega-3 fatty-acid status: a case-control comparison. J. Clin. Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GM, Zhao J, Li B, Zhang XF, Ma JX, Ma XL, et al. 2018. The anti-inflammatory effects of statins on patients with rheumatoid arthritis: A systemic review and meta-analysis of 15 randomized controlled trials. Autoimmunity reviews. [DOI] [PubMed] [Google Scholar]
- Lin PY, Huang SY, Su KP, 2010. A Meta-Analytic Review of Polyunsaturated Fatty Acid Compositions in Patients with Depression. Biol. Psychiatry 68, 140–7. [DOI] [PubMed] [Google Scholar]
- Lipinski MJ, Benedetto U, Escarcega RO, Biondi-Zoccai G, Lhermusier T, Baker NC, et al. , 2016. The impact of proprotein convertase subtilisin-kexin type 9 serine protease inhibitors on lipid levels and outcomes in patients with primary hypercholesterolaemia: a network meta-analysis. Eur. Heart J 37, 536–45. [DOI] [PubMed] [Google Scholar]
- Liu JJ, Green P, John Mann J, Rapoport SI, Sublette ME, 2014. Pathways of polyunsaturated fatty acid utilization: Implications for brain function in neuropsychiatric health and disease. Brain Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DW, Seo J, Davidson LA, Callaway ES, Fan YY, Lupton JR, et al. , 2004. n-3 PUFA alter caveolae lipid composition and resident protein localization in mouse colon. FASEB J. 18, 1040–2. [DOI] [PubMed] [Google Scholar]
- Maes M, Christophe A, Delanghe J, Altamura C, Neels H, Meltzer HY, 1999. Lowered omega3 polyunsaturated fatty acids in serum phospholipids and cholesteryl esters of depressed patients. Psychiatry Res. 85, 275–91. [DOI] [PubMed] [Google Scholar]
- Magnani F, Tate CG, Wynne S, Williams C, Haase J, 2004. Partitioning of the serotonin transporter into lipid microdomains modulates transport of serotonin. J. Biol. Chem. 279, 38770–8. [DOI] [PubMed] [Google Scholar]
- Mamalakis G, 2002. Depression and adipose essential polyunsaturated fatty acids. PLEFA. 67, 311–8. [DOI] [PubMed] [Google Scholar]
- Mamalakis G, Kalogeropoulos N, Andrikopoulos N, Hatzis C, Kromhout D, Moschandreas J, et al. , 2006a. Depression and long chain n-3 fatty acids in adipose tissue in adults from Crete. Eur. J. Clin. Nutr 60, 882–8. [DOI] [PubMed] [Google Scholar]
- Mamalakis G, Kiriakakis M, Tsibinos G, Hatzis C, Flouri S, Mantzoros C, et al. , 2006b. Depression and serum adiponectin and adipose omega-3 and omega-6 fatty acids in adolescents. Pharmacol. Biochem. Behav 85, 474–9. [DOI] [PubMed] [Google Scholar]
- Mann JJ, 2003. Neurobiology of suicidal behaviour. Nat Rev Neurosci. 4, 819–28. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Waternaux C, Haas GL, Malone KM, 1999. Toward a clinical model of suicidal behavior in psychiatric patients. Am. J. Psychiatry 156, 181–9. [DOI] [PubMed] [Google Scholar]
- Martins JG, 2009. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. J. Am. Coll. Nutr 28, 525–42. [DOI] [PubMed] [Google Scholar]
- Martins JG, Bentsen H, Puri BK, 2012. Eicosapentaenoic acid appears to be the key omega-3 fatty acid component associated with efficacy in major depressive disorder: a critique of Bloch and Hannestad and updated meta-analysis. Mol. Psychiatry 17, 1144–9. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Rider T, Tso P, Cole-Strauss A, Lipton JW, 2010a. Omega-3 fatty acid deficiency increases constitutive pro-inflammatory cytokine production in rats: relationship with central serotonin turnover. PLEFA. 83, 185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Rider T, Tso P, Dwivedi Y, Pandey GN, 2010b. Selective deficits in erythrocyte docosahexaenoic acid composition in adult patients with bipolar disorder and major depressive disorder. J. Affect. Disord 126, 303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer BJ, Mann NJ, Lewis JL, Milligan GC, Sinclair AJ, Howe PR, 2003. Dietary intakes and food sources of omega-6 and omega-3 polyunsaturated fatty acids. Lipids. 38, 391–8. [DOI] [PubMed] [Google Scholar]
- Mialet-Perez J, D'Angelo R, Villeneuve C, Ordener C, Negre-Salvayre A, Parini A, et al. , 2012. Serotonin 5-HT2A receptor-mediated hypertrophy is negatively regulated by caveolin-3 in cardiomyoblasts and neonatal cardiomyocytes. J. Mol. Cell. Cardiol 52, 502–10. [DOI] [PubMed] [Google Scholar]
- Mocking RJ, Harmsen I, Assies J, Koeter MW, Ruhe HG, Schene AH, 2016. Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Translational psychiatry. 6, e756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldoon MF, Manuck SB, Matthews KA, 1990. Lowering cholesterol concentrations and mortality: a quantitative review of primary prevention trials. BMJ. 301, 309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldoon MF, Manuck SB, Mendelsohn AB, Kaplan JR, Belle SH, 2001. Cholesterol reduction and non-illness mortality: meta-analysis of randomised clinical trials. BMJ. 322, 11–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller N, Schwarz MJ, 2007. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol. Psychiatry 12, 988–1000. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Hamazaki T, Jokaji H, Minami S, Kobayashi M, 1998. Effect of HMG-CoA reductase inhibitors on plasma polyunsaturated fatty acid concentrations in patients with hyperlipidemia. Int. J. Clin. Lab. Res 28, 192–5. [DOI] [PubMed] [Google Scholar]
- Nothdurfter C, Tanasic S, Di Benedetto B, Rammes G, Wagner EM, Kirmeier T, et al. , 2010. Impact of lipid raft integrity on 5-HT3 receptor function and its modulation by antidepressants. Neuropsychopharmacology. 35, 1510–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothdurfter C, Tanasic S, Rammes G, Rupprecht R, 2011. Modulation of ligand-gated ion channels as a novel pharmacological principle. Pharmacopsychiatry. 44 Suppl 1, S27–34. [DOI] [PubMed] [Google Scholar]
- Nozue T, Michishita I, 2015. Statin treatment alters serum n-3 to n-6 polyunsaturated fatty acids ratio in patients with dyslipidemia. Lipids Health Dis. 14, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyalala JO, Wang J, Dang A, Faas FH, Smith WG, 2008. Hypertriglyceridemia and hypercholesterolemia: effects of drug treatment on fatty acid composition of plasma lipids and membranes. PLEFA. 78, 271–80. [DOI] [PubMed] [Google Scholar]
- Office of Disease Prevention and Healthy Promotion. Dietary Guidelines. 2017. [Google Scholar]
- Oquendo MA, Sullivan GM, Sudol K, Baca-Garcia E, Stanley BH, Sublette ME, et al. , 2014. Toward a biosignature for suicide. Am. J. Psychiatry. 171, 1259–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsavova J, Misurcova L, Ambrozova JV, Vicha R, Mlcek J, 2015. Fatty Acids Composition of Vegetable Oils and Its Contribution to Dietary Energy Intake and Dependence of Cardiovascular Mortality on Dietary Intake of Fatty Acids. International journal of molecular sciences. 16, 12871–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papandreou C, Schiza SE, Tsibinos G, Mermigkis C, Hatzis CM, Kafatos AG, et al. , 2011. Gluteal adipose-tissue polyunsaturated fatty-acids profiles and depressive symptoms in obese adults with obstructive sleep apnea hypopnea syndrome: a cross-sectional study. Pharmacol. Biochem. Behav 98, 316–9. [DOI] [PubMed] [Google Scholar]
- Peet M, Murphy B, Shay J, Horrobin D, 1998. Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol. Psychiatry 43, 315–9. [DOI] [PubMed] [Google Scholar]
- Rajamoorthi K, Petrache HI, McIntosh TJ, Brown MF, 2005. Packing and viscoelasticity of polyunsaturated omega-3 and omega-6 lipid bilayers as seen by (2)H NMR and X-ray diffraction. J. Am. Chem. Soc 127, 1576–88. [DOI] [PubMed] [Google Scholar]
- Rao YP, Lokesh BR, 2017. Down-regulation of NF-kappaB expression by n-3 fatty acid-rich linseed oil is modulated by PPARgamma activation, eicosanoid cascade and secretion of cytokines by macrophages in rats fed partially hydrogenated vegetable fat. Eur. J. Nutr 56, 1135–47. [DOI] [PubMed] [Google Scholar]
- Rees AM, Austin MP, Owen C, Parker G, 2009. Omega-3 deficiency associated with perinatal depression: case control study. Psychiatry Res. 166, 254–9. [DOI] [PubMed] [Google Scholar]
- Renner U, Glebov K, Lang T, Papusheva E, Balakrishnan S, Keller B, et al. , 2007. Localization of the mouse 5-hydroxytryptamine(1A) receptor in lipid microdomains depends on its palmitoylation and is involved in receptor-mediated signaling. Mol. Pharmacol 72, 502–13. [DOI] [PubMed] [Google Scholar]
- Riemer S, Maes M, Christophe A, Rief W, 2010. Lowered omega-3 PUFAs are related to major depression, but not to somatization syndrome. J. Affect. Disord 123, 173–80. [DOI] [PubMed] [Google Scholar]
- Rise P, Marangoni F, Galli C, 2002. Regulation of PUFA metabolism: pharmacological and toxicological aspects. PLEFA. 67, 85–9. [DOI] [PubMed] [Google Scholar]
- Rise P, Pazzucconi F, Sirtori CR, Galli C, 2001. Statins enhance arachidonic acid synthesis in hypercholesterolemic patients. Nutr Metab Cardiovasc Dis. 11, 88–94. [PubMed] [Google Scholar]
- Rodriguez-Perez C, Ramprasath VR, Pu S, Sabra A, Quirantes-Pine R, Segura-Carretero A, et al. , 2016. Docosahexaenoic Acid Attenuates Cardiovascular Risk Factors via a Decline in Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Plasma Levels. Lipids. 51, 75–83. [DOI] [PubMed] [Google Scholar]
- Rombaldova M, Janovska P, Kopecky J, Kuda O, 2017. Omega-3 fatty acids promote fatty acid utilization and production of pro-resolving lipid mediators in alternatively activated adipose tissue macrophages. Biochem. Biophys. Res. Commun 490, 1080–5. [DOI] [PubMed] [Google Scholar]
- Sadikot R The Role of Cholesterol and Lipid Rafts in Regulation of TLR Receptors In: Levitan I, Barrantes FJ, editors. Cholesterol Regulation of Ion Channels and Receptors. Hoboken, NJ: John Wiley & Sons; 2012. [Google Scholar]
- Samuvel DJ, Jayanthi LD, Bhat NR, Ramamoorthy S, 2005. A role for p38 mitogen-activated protein kinase in the regulation of the serotonin transporter: evidence for distinct cellular mechanisms involved in transporter surface expression. J. Neurosci 25, 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarri KO, Linardakis M, Tzanakis N, Kafatos AG, 2008. Adipose DHA inversely associated with depression as measured by the Beck Depression Inventory. PLEFA. 78, 117–22. [DOI] [PubMed] [Google Scholar]
- Scanlon SM, Williams DC, Schloss P, 2001. Membrane cholesterol modulates serotonin transporter activity. Biochemistry (Mosc). 40, 10507–13. [DOI] [PubMed] [Google Scholar]
- Schins A, Crijns HJ, Brummer RJ, Wichers M, Lousberg R, Celis S, et al. , 2007. Altered omega-3 polyunsaturated fatty acid status in depressed post-myocardial infarction patients. Acta Psychiatr. Scand 115, 35–40. [DOI] [PubMed] [Google Scholar]
- Schnitzer JE, Oh P, Pinney E, Allard J, 1994. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J. Cell Biol. 127, 1217–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Yang Y, Ou T, Key CC, Tong SH, Sequeira RC, et al. , 2017. Dietary PUFAs attenuate NLRP3 inflammasome activation via enhancing macrophage autophagy. J. Lipid Res 58, 1808–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simopoulos AP, 2011. Evolutionary aspects of diet: the omega-6/omega-3 ratio and the brain. Mol. Neurobiol 44, 203–15. [DOI] [PubMed] [Google Scholar]
- Singer SJ, Nicolson GL, 1972. The fluid mosaic model of the structure of cell membranes. Science. 175, 720–31. [DOI] [PubMed] [Google Scholar]
- Sjogren B, Csoregh L, Svenningsson P, 2008. Cholesterol reduction attenuates 5-HT1A receptor-mediated signaling in human primary neuronal cultures. Naunyn. Schmiedebergs Arch. Pharmacol 378, 441–6. [DOI] [PubMed] [Google Scholar]
- Sjogren B, Hamblin MW, Svenningsson P, 2006. Cholesterol depletion reduces serotonin binding and signaling via human 5-HT(7(a)) receptors. Eur. J. Pharmacol 552, 1–10. [DOI] [PubMed] [Google Scholar]
- Sjogren B, Svenningsson P, 2007a. Caveolin-1 affects serotonin binding and cell surface levels of human 5-HT7(a) receptors. FEBS Lett. 581, 5115–21. [DOI] [PubMed] [Google Scholar]
- Sjogren B, Svenningsson P, 2007b. Depletion of the lipid raft constituents, sphingomyelin and ganglioside, decreases serotonin binding at human 5-HT7(a) receptors in HeLa cells. Acta Physiol (Oxf). 190, 47–53. [DOI] [PubMed] [Google Scholar]
- Sommer B, Montano LM, Carbajal V, Flores-Soto E, Ortega A, Ramirez-Oseguera R, et al. , 2009. Extraction of membrane cholesterol disrupts caveolae and impairs serotonergic (5-HT2A) and histaminergic (H1) responses in bovine airway smooth muscle: role of Rho-kinase. Can. J. Physiol. Pharmacol 87, 180–95. [DOI] [PubMed] [Google Scholar]
- Spector AA, 1999. Essentiality of fatty acids. Lipids. 34 Suppl, S1–3. [DOI] [PubMed] [Google Scholar]
- Stancu C, Sima A, 2001. Statins: mechanism of action and effects. Journal of cellular and molecular medicine. 5, 378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stulnig TM, Huber J, Leitinger N, Imre EM, Angelisova P, Nowotny P, et al. , 2001. Polyunsaturated eicosapentaenoic acid displaces proteins from membrane rafts by altering raft lipid composition. J. Biol. Chem 276, 37335–40. [DOI] [PubMed] [Google Scholar]
- Su F, Yi H, Xu L, Zhang Z, 2015. Fluoxetine and S-citalopram inhibit M1 activation and promote M2 activation of microglia in vitro. Neuroscience. 294, 60–8. [DOI] [PubMed] [Google Scholar]
- Sublette ME, Ellis SP, Geant AL, Mann JJ, 2011. Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. J. Clin. Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sublette ME, Hibbeln JR, Galfalvy H, Oquendo MA, Mann JJ, 2006. Omega-3 polyunsaturated essential fatty acid status as a predictor of future suicide risk. Am. J. Psychiatry 163, 1100–2. [DOI] [PubMed] [Google Scholar]
- Teti GL, Rebok F, Rojas SM, Grendas L, Daray FM, 2014. Systematic review of risk factors for suicide and suicide attempt among psychiatric patients in Latin America and Caribbean. Rev. Panam. Salud Publica 36, 124–33. [PubMed] [Google Scholar]
- Tian Q, Grzemski FA, Panagiotopoulos S, Ahokas JT, 2006. Peroxisome proliferator-activated receptor alpha agonist, clofibrate, has profound influence on myocardial fatty acid composition. Chem. Biol. Interact 160, 241–51. [DOI] [PubMed] [Google Scholar]
- Tiemeier H, van Tuijl HR, Hofman A, Kiliaan AJ, Breteler MM, 2003. Plasma fatty acid composition and depression are associated in the elderly: the Rotterdam Study. Am. J. Clin. Nutr 78, 40–6. [DOI] [PubMed] [Google Scholar]
- Tondo L, Baldessarini RJ, Hennen J, Minnai GP, Salis P, Scamonatti L, et al. , 1999. Suicide attempts in major affective disorder patients with comorbid substance use disorders. J. Clin. Psychiatry 60 Suppl 2, 63–9; discussion 75–6, 113–6. [PubMed] [Google Scholar]
- Tousoulis D, Psarros C, Demosthenous M, Patel R, Antoniades C, Stefanadis C, 2014. Innate and adaptive inflammation as a therapeutic target in vascular disease: the emerging role of statins. J. Am. Coll. Cardiol 63, 2491–502. [DOI] [PubMed] [Google Scholar]
- Vainio S, Jansen M, Koivusalo M, Rog T, Karttunen M, Vattulainen I, et al. , 2006. Significance of sterol structural specificity. Desmosterol cannot replace cholesterol in lipid rafts. J. Biol. Chem 281, 348–55. [DOI] [PubMed] [Google Scholar]
- van Heeringen K, Mann JJ, 2014. The neurobiology of suicide. The lancet. Psychiatry 1, 63–72. [DOI] [PubMed] [Google Scholar]
- Van Orden KA, Witte TK, Cukrowicz KC, Braithwaite SR, Selby EA, Joiner TE Jr., 2010. The interpersonal theory of suicide. Psychol. Rev 117, 575–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz JS, Kac G, Nardi AE, Hibbeln JR, 2014. Omega-6 fatty acids and greater likelihood of suicide risk and major depression in early pregnancy. J. Affect. Disord 152–154, 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez M, Alegret M, Lopez M, Rodriguez C, Adzet T, Merlos M, et al. , 1995. Different effects of fibrates on the microsomal fatty acid chain elongation and the acyl composition of phospholipids in guinea-pigs. Br. J. Pharmacol 116, 3337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JA, Batten SE, Harris M, Rockett BD, Shaikh SR, Stillwell W, et al. , 2012. Docosahexaenoic and eicosapentaenoic acids segregate differently between raft and nonraft domains. Biophys. J 103, 228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SW, Kwon MJ, Choi AM, Kim HP, Nakahira K, Hwang DH, 2009. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J. Biol. Chem 284, 27384–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Preventing suicide: A global imperative. Geneva: 2014. [Google Scholar]
- World Health Organization. Global Burden of Disease (GBD). 2017. [Google Scholar]
- Wu S, Ding Y, Wu F, Xie G, Hou J, Mao P, 2016. Serum lipid levels and suicidality: a meta-analysis of 65 epidemiological studies. J. Psychiatry Neurosci 41, 56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JR, Han D, Qiao ZX, Tian X, Qi D, Qiu XH, 2015. Combined application of eicosapentaenoic acid and docosahexaenoic acid on depression in women: a meta-analysis of double-blind randomized controlled trials. Neuropsychiatr Dis Treat. 11, 2055–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Huang T, Zheng Y, Wang T, Heianza Y, Sun D, et al. , 2017. PCSK9 variant, long-chain n-3 PUFAs, and risk of nonfatal myocardial infarction in Costa Rican Hispanics. Am. J. Clin. Nutr 105, 1198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]


