Abstract
It is well established that tobacco smoking is associated with vascular endothelial dysfunction in a causative and dose dependent manner primarily related to the tobacco smoke (TS) content of reactive oxygen species (ROS), nicotine, and oxidative stress (OS)–driven inflammation. Preclinical studies have also shown that nicotine (the principal e-liquid’s ingredient used in e-cigarettes (e-Cigs) can also cause OS, exacerbation of cerebral ischemia and secondary brain injury. Likewise, chronic e-Cig vaping could be prodromal to vascular endothelial dysfunctions. Herein, we provide direct evidence that similarly to TS, e-Cig promotes mitochondrial depolarization in primary brain vascular endothelial cells as well as the vascular endothelial cell line bEnd3. In addition, both TS and e-Cig exposure upregulated the transmembrane iron exporter Slc40a1 (crucial to maintain cellular iron and redox homeostasis) and that of porphyrin importer Abcb6 (linked to accelerated atherosclerosis). We then investigated in vivo whether gender plays a role in how chronic TS affect vascular endothelial functions. Our results clearly show chronic TS exposure differentially impacts the expression levels of Phase-II enzymes as well as the iron transporters previously investigated in vitro. Although the physiological implications of the genderspecific differential responses to TS are not fully clear, they do demonstrate that gender is a risk factor that needs to be investigated when assessing the potential impact of chronic smoking and perhaps e-Cig vaping.
Keywords: MitoChip, Blood brain barrier, Nrf2, Ferroportinl, Abe transporter, Redox, Gender
1. Introduction
TS is accountable for 434,000 deaths each year in United States (US) [1] and has been reported to enhance the risk of stroke [2] and other cerebrovascular/neurological disorders like Alzheimer’s [3] and vascular dementia [4]. OS, inflammation and the resulting brain micro-vascular impairment [5,6] are often the major prodromal factors linking TS to cerebrovascular toxicity. Additionally, in the past decade several alternative vaping products have hit the market, rapidly gaining consumers among adults and adolescents [7]. Electronic cigarettes have become the sought-after product partly due to the belief that they are safe. However, recently published data [8] from side by side experiments investigating the impact of e-Cig (Blu™; 24mg/mL nicotine) vs. TS (3R4F research cigarettes containing 9.4 mg tar and 0.726 mg nicotine/cigarette and equivalent to full flavor brands; University of Kentucky) on mouse primary brain microvascular endothelial cells (mBMEC) clearly show that OS promoted by 24 h exposure to e-Cigs extract was not dissimilar from that induced by cigarette smoke extract from 3R4F. Therefore, we decide to briefly investigate whether both TS and e-Cig can also impact mitochondrial functions as well as iron transporters such as Slc40al (also known as Ferroportin 1) which is heavily implicated in the regulation of cellular iron and redox hemostasis as well as AD brain pathology, infection, and oxidative stress [9,10]. In addition to Slc40al, Abcb6 (a mitochondrial ATP-binding cassette porphyrin importer we also investigated) is implicated in the onset of atherosclerosis [11,12].
Previous studies [13,14] suggest that females may respond to oxidative stress stimuli (such as those induced by chronic TS exposure) differently than male. Therefore we also investigated the role of gender in determining the impact of TS on the cerebrovascular cellular responses including the iron transporters previously examined in vitro and 2 major phase2 detoxification enzymes also involved in redox functions (glutathione S-transferase PI - GSTP1 and glutathione S-transferase A4 (GSTA4). While the extent of the physiological implications are not fully clear, our study strongly suggests that chronic TS exposure has a gender-dependent differential impact on the cerebrovascular system in respect to regulation of phase 2 detoxification enzymes and iron transporters. Thus this study provides a strong baseline work supporting further and more extended studies on to assess gender-specific differential response to TS and other pathological stimuli (possibly including e-cigarettes) as well gender-specific treatments.
2. Methods
2.1. Materials and reagents
Mitoprobe JC-1 kit was purchased from Life Technologies Corporation (Carlsbad, CA); rabbit anti-Slc40al (#NBP1–21502), rabbit anti-Abcb6 (#NBP2–58327), mouse anti-Gstpl (#NB110–60512) and rabbit anti Gsta4 (#H00002941-D01P) were purchased from Novus Biologicals (Littleton, CO). Mini-Protean® TGXTM gels for western blotting were purchased from Bio-rad (Hercules, CA, USA). Donkey anti-rabbit (#NA934) and sheep anti-mouse (#NA931) secondary antibodies were from GE Healthcare (Piscataway, NJ); Alexa Fluor” 488 or 555 conjugated goat anti-rabbit or anti-mouse antibodies were obtained from Invitrogen (Carlsbad, CA). RNeasy Plus Universal Mini Kit (#73404) were purchased from RNAeasy plus mini kit (Qiagen Inc, Germantown, MD). Sterile culture wares were purchased from Fisher Scientific (Pittsburgh, PA, USA) while molecular biology grade reagents and chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) or Bio-rad laboratories (Hercules, CA, USA). RNA Stabilization Reagent (RNA later, cat #76104).
2.2. Cell culture
Mouse bEnd.3 cell line [15,16], was obtained from American Type Culture Collection and cultured in complete DMEM with 10% FBS. Mouse primary brain microvascular endothelial cells (mBMECs, #C57– 6023) from Cell Biologics (Chicago, IL, USA) were seeded (p3–6) on gelatin coated cell culture flasks or glass chamber slides, cultured in recommended medium (Ml168) and maintained at 37 °C with 5% CO2 exposure. The culture medium was changed every other day until the cells reached confluency. Phase contrast microscopy and the expression of characteristic phenotypic markers confirmed the monolayer integrity of both mBMEC at confluency. Established cultures were exposed to 5% TS for 72 h.
2.3. Soluble tobacco smoke (TS) and e-cigarette (e-Cig) extract preparation
Soluble TS and e-Cig extracts were prepared according to the FTC standard smoking protocol (35 mL puff volume, 2 s puff duration, 58 s interval, 8 puffs per cigarette) using a Single Cigarette Smoking Machine (SCSM, CH Technologies Inc., Westwood, NJ, USA) according to previously published methods [17,18]. Extracts were prepared fresh for each cycle and used in culture at a 5% dilution [18,19].
2.4. In vivo experimental design and tissue preparation
The animal protocol for this work was approved by the Institutional Animal Care and Use Committee, TTUHSC, Lubbock, Texas. C57BL/6J male mice (age range 8–10 weeks old) were purchased from Jackson Laboratories. Mice of both gender (50:50) were divided into 2 groups including control and TS exposed. Mice were chronically exposed (via direct inhalation) to TS mixed with oxygenated air or oxygenated air alone, 6 times/day; 2 cigarettes/hour, 7 days/week for 4 weeks following previously established exposure method simulating realistic human smoking pattern20. Mice were sacrificed within an hour of their last TS exposure cycle at the end of the day. Mice were then decapitated under anesthesia to collect blood and brain for subsequent biochemical and molecular preparations.
2.5. Mitochondrial membrane potential
Following treatment, mitochondrial membrane potential was assessed by using the cationic dye, JC-1 (5′,5′,5′tetrachloro-11′,3,3′-tet- raethylbenzimidazolylcarbocyanine iodide), and a mitochondrial membrane potential disrupter, CCCP (carbonyl cyanide 3-chlor- ophenylhydrazone), as a positive control. Cells were washed with PBS and incubated with 50 μΜ (final concentration) of CCCP for minutes. 2 μΜ JC-1 dye (in PBS) was added to both control and treated groups along with the CCCP exposed group and incubated for 20 min. Afterwards, images were captured with EVOS inverted fluorescence microscope at 40X or fluorescence was measured in plate reader (excitation wavelength 488 nm, emission wavelength green (~529nm) and red (~ 590 nm).
2.6. Immunofluorescence and western blotting
Following treatment, cells were rinsed with PBS and fixed with ice-cold 4% buffered formalin for 10–15 min. Later, cells were permeabilized and subsequently blocked with 10% goat serum in PBS followed by overnight cold incubation with primary antibodies in blocking buffer (1:100–150) reactive against human or mouse proteins (Glutl, Nrf2, Nqol and Slc40al). Next day, cells were washed with blocking buffer and incubated for 1 h with Alexa Fluor 488 or 555-conjugated antirabbit or anti-mouse antibodies (1:1000). Cells were washed and cover- slipped with DAPI in Prolonged Gold Anti-fade reagent (Invitrogen, Carlsbad, CA). Images were captured with EVOS inverted fluorescence microscope at 40X.
Total protein was collected by cell lysis in RIPA buffer with protease inhibitors and quantitated by BCA assay [21]. Equal amounts of protein across samples were subjected to SDS-PAGE separation (4–20% graded gels) as described earlier [21,22]. Following gel to PVDF membrane electrotransfer of the protein bands, membranes were blocked with blocking buffer (0.1% tween-20 in TBS) containing 5% non-fat dry milk and 1% BSA. Subsequently, membranes were incubated overnight at 4 °C with primary antibodies in blocking buffer (in range of 1:200– 1:500), washed repeatedly and probed with HRP-conjugated secondary antibodies (1:5000). Bands were visualized by chemiluminescence reagent using X-ray film-based autoradiography and densities were analyzed by Li-Cor Image Studio software with β-actin as a loading control.
2.7. Quantitative RT-PCR
Total RNA (1 μg) extracted as mentioned above was reverse transcribed to cDNA by Superscript III first strand synthesis kit (Life Technologies, Carlsbad, CA). The cDNA strands were mixed with gene-specific forward and reverse primer pairs (Integrative DNA technologies, Coralville, IA; Table 1) and SYBR select master mix (Life Technologies). Template-free and RT-negative samples served as internal controls [21]. Amplification was performed in triplicates/sample on Bio-Rad CFX96 Touch Real-Time PCR system (95 °C for 5 min followed by 40 cycles of 95 °C-30 s, 58 °C-1 min and 72 °C-1 min and a terminal reaction at 72 °C-2 min). The threshold values (counts, Ct) for target genes and reference genes (Rpl21) were determined for each sample and relative expression of each target gene was calculated by ΔΔCt method [21].
Table 1.
Forward and reverse (5′−3′) primer sequences used for qRT-PCR experiments
| Mouse gene | Forward | Reverse |
|---|---|---|
| Slc40al | TGTCAGCCTGCTGTTTGCAGGA | TCTTGCAGCAACTGTGTCACCG |
| Abcb6 | GCCGTGATATGAACACACAG | GCCAAAAAGCACAAAGTCCC |
| Gstpl | TGTAATCGGCAAAGGAGATCTG | TCACCCTCATCTACACCAACr |
| Rpl21 | CCATAAGTGCTACCACGGCA | GCCCTTCTCTTTGGCTTCCT |
2.8. UHPLC-MS/MS
Plasma and brain levels of nicotine and its main metabolite cotinine were determined via HCPLC-MS/MS (Column: Kinetex-EVO-1.7 μΜ, 2.1*100 mm; Mobile Phase-A: 5mM Amm Bicarbonate; Mobile Phase- B: Methanol; Flow rate: 0.3mL/min) at the end of the 4 weeks of chronic TS exposure as previously described [20]. In brief, continine-d3 was used as internal standard (IS) as per FDA guidelines. Sample protein precipitation of 20 μL mouse plasma or brain homogenate was performed using acetonitrile at 1:8 ratio. Mass Spectrometer was operated in positive polarity under the multiple reaction-monitoring mode (MRM) using electro spray ionization technique and the transitions of m/z 163.2 → 132.1, m/z 177.2 → 98.0 and 180.2 → 101.2 were used to measure the NT, CN and IS, respectively. The elution of NT, CN and IS are at 1.89, 1.77and 1.76 min, respectively. This was achieved with a gradient mobile phase consisting of 5 mM ammonium bicarbonate, acetonitrile and methanol (3:1, v/v) at a flow rate of 0.3 mL/min on a Kinetex EVO C18 column.
2.9. Data analysis
Results are reported as mean ± SEM and analyzed for statistical significance by one-way ANOVA followed by Dunnett’s or Tukey’s multiple comparison tests using GraphPad Prism Software Inc. (La Jolla, CA, USA). Student’s t-test (two-tailed, unpaired) was used to compare control vs. treatment effects. P value < 0.05 was considered for statistical significance.
3. Results
3.1. Tobacco smoke and e-cigarette vapor similarly disrupt mitochondrial membrane potential in brain vascular endothelial cells
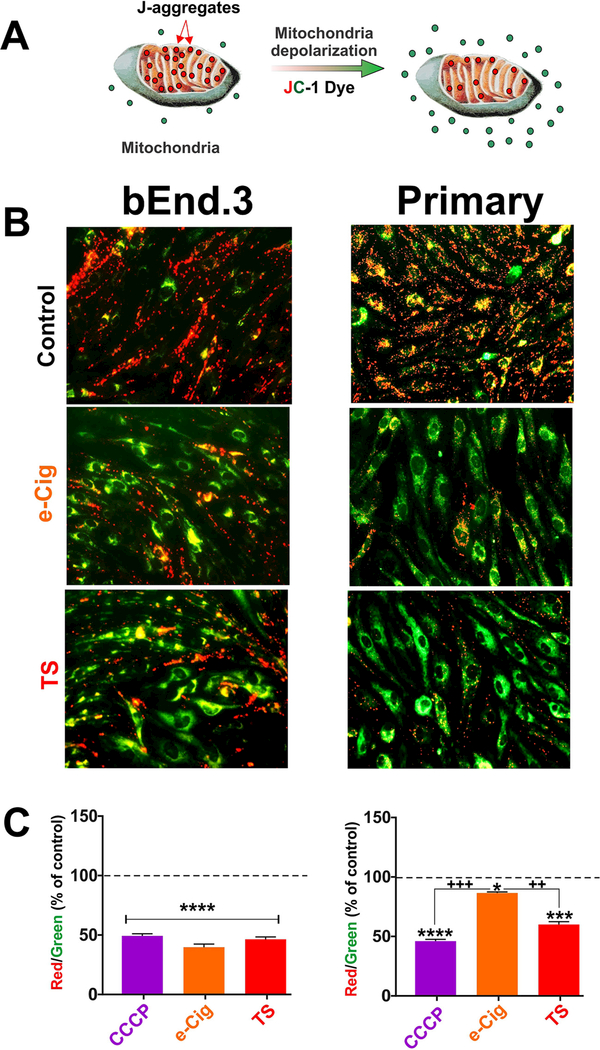
The major role of mitochondrial membrane potential (Δψ|m) is to drive ATP synthesis by oxidative phosphorylation. The balance between the generation of ATP and its consumption determines the magnitude of Δψ|m which keeps the electron travelling across electron transport chain to maintain an optimum electrochemical gradient. Enhanced ATP production or mitochondrial dysfunction can cause a reduction in mitochondrial membrane potential [23,24]. JC-1 is a cationic carbocya- nine dye exhibits potential-dependent accumulation in mitochondria, forming J aggregates (red fluorescence, emission wavelength 590 nm); upon depolarization it diffuses across mitochondria to form monomeric state showing green fluorescence with emission wavelength 529 nm (Fig. 1A). Acute exposure to TS and e-Cig reduced the red (~590nm)/ green (~ 529 nm) fluorescence intensity ratio suggesting depolarization/disruption of mitochondrial membrane potential (Fig. 1B) using CCCP as a potent mitochondrial membrane disruptor (positive control). The results observed in immunofluorescence image were further verified by assessing the fluorescence intensity in plate reader (Fig. 1C). Both TS and e-Cig produced strong mitochondrial membrane depolarization of comparable magnitude. However, while TS demonstrated a similar effect on mBMEC, the impact of e-Cig (although statistically significant), was not as substantial (Fig. 2).
Fig. 1. Mitochondrial membrane potential by JC-1 dye.
JC- 1, a cationic carbocyanine dye exhibits potential-dependent accumulation in mitochondria, forming J aggregates (red); upon depolarization it remains as monomer showing green fluorescence (A). A decrease in the red (∼590 nm)/green (∼529 nm) fluorescence intensity ratio by acute exposure to CCCP, e-Cig and TS is indicative of depolarization/disruption of mitochondrial membrane (B and C). Although both TS and e-Cig demonstrated mitochondrial membrane depolarizing effects in bEnd.3 cell line the effect of e-Cig was not observed in mBMEC. CCCP = Carbonyl cyanide 3-chlorophenylhy- drazone, mitochondrial membrane potential disruptor.”*”= P < 0.05; “**” P < 0.01;”***”P < 0.01 compared to control. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
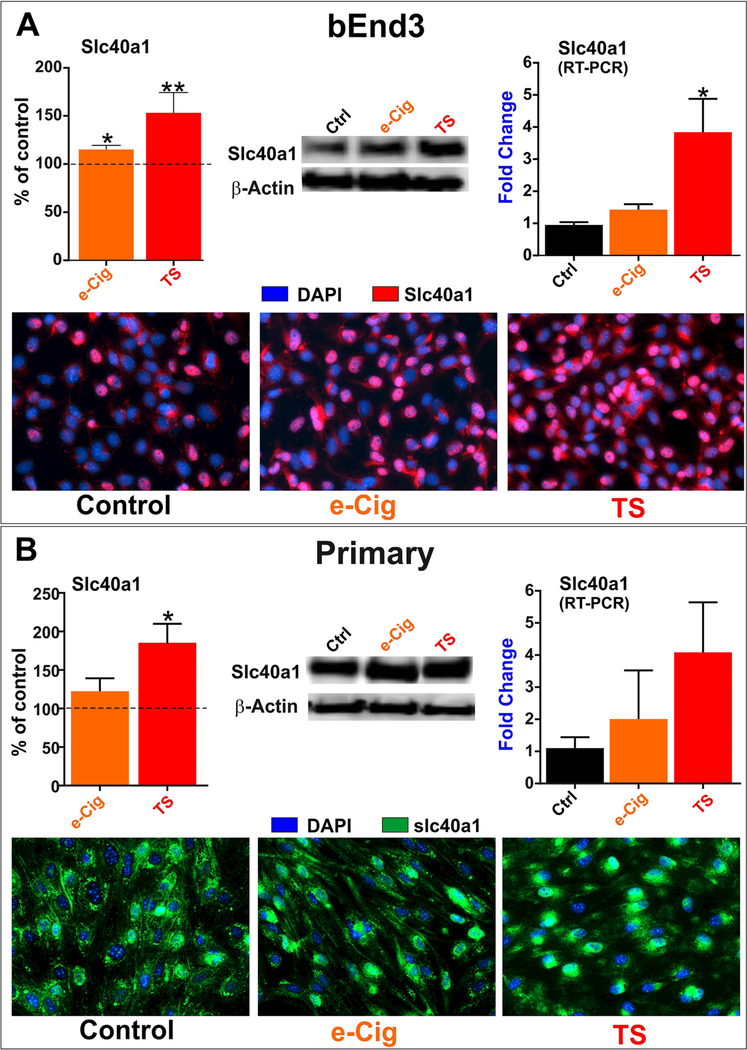
Fig. 2. Slc40al expression (in vitro).
Acute exposure to TS and e-Cig upregulated Slc40al expression in bEnd.3 (A) cell lines and mBMEC (B) demonstrated by western blotting, immunocytochemistry and RT-PCR. This effect was significantly higher following TS exposure compared to e-Cig in both cell type and all assays. “*” = P < 0.05; “**” p < 0.01 compared to control.
3.2. Acute TS/e-Cig exposure upregulates Slc40al expression at the brain microvascular endothelium
Acute TS and e-Cig exposure promoted (to a different extent) up- regulation of the transmembrane iron exporter Slc40al which is crucial to maintain cellular iron and redox homeostasis. The effect was observed in both bEnd.3 (2A) and mBMEC cells (2B) as demonstrated by western blotting, immunocytochemistry and RT-PCR.
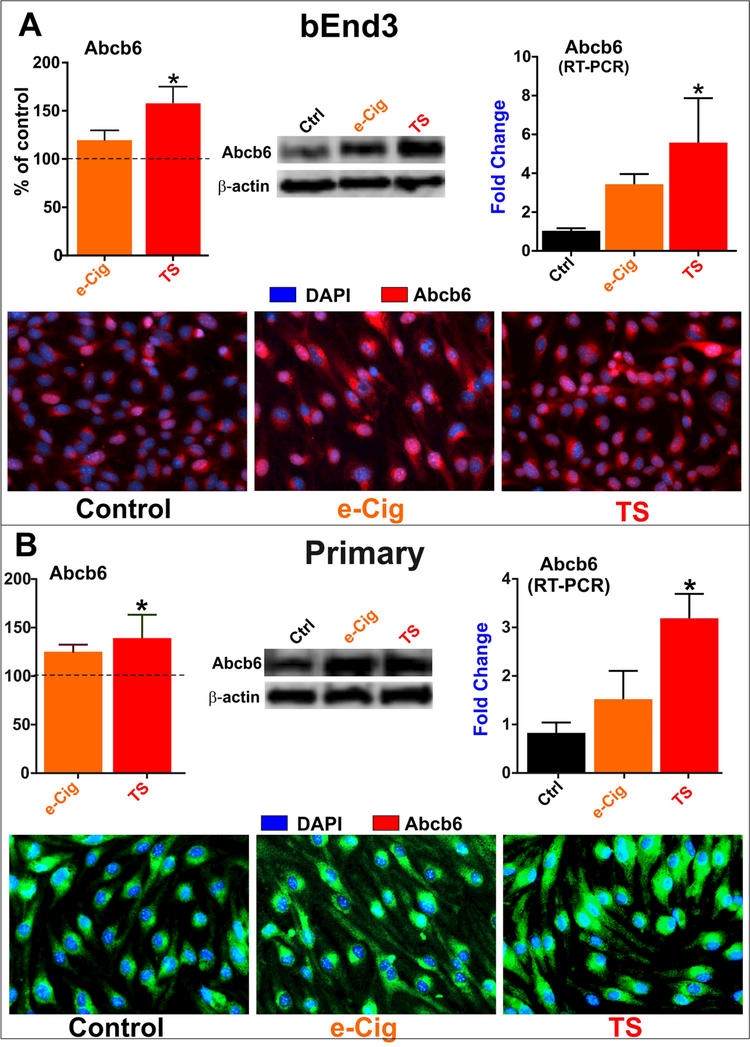
3.3. TS/e-Cig upregulates Abcb6 expression at brain microvasculature
The movement of anionic porphyrins across intracellular membranes and organelle such as mitochondria is crucial to many biological processes including ATP production. Herein we investigated the impact of acute TS and e-Cig exposure on the mitochondrial ATP-binding cassette porphyrin importer Abcb6. Acute exposure to TS and e-Cig upregulated Abcb6 expression in bEnd.3 (3A) and mBMEC cells (3B) as demonstrated by western blotting, immunocytochemistry and RT-PCR.
3.4. Gender specific effects of chronic TS exposure on Slc40al and Abcb6
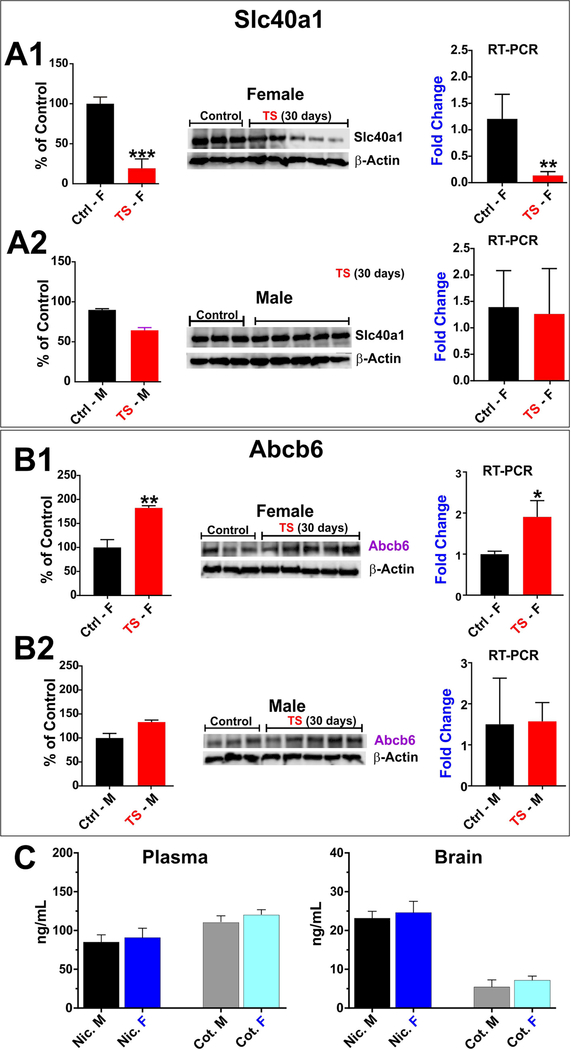
Following our in vitro observations, we extended our investigation in vivo for chronic exposure and also to look for evidences of gender specific responses. At this time we decided to focus primarily of TS exposure for which we have accumulated a more considerable bulk of previous data and reserve the study of e-Cig to a later time if warranted by this results.
Interestingly enough we observed a gender specific response to TS which resulted in a differential expression changes in females vs. males in response to identical TS exposure parameters. In fact, as shown in Fig. 4A1, while the expression levels of Slc40al resulted largely unchanged in male mice (as evidenced by western blotting and RT-PCR analyses), identical TS exposure resulted in a substantial down- regulation of this iron exporter in female mice (≈ 25% of the control levels; Fig. 4A2). By contrast, the TS effect on the porphyrin importer Abcb6 in female mice was opposite to that observed for Slc40al. As shown in Fig. 4B2, TS caused a significant upregulation of the expression level of this transporter in female mice ( ≈ 180% of the control levels) while bearing no significant effect on male mice (Fig. 4B1). Results were obtained by western blotting and RT-PCR. Also note (Fig. 4C) there was no statistically significant differences between the nicotine and cotinine levels (both plasma and brain tissues) measured at the end of the 4 weeks of chronic TS exposure between male and female mice. Specifically plasma nicotine levels were ≈ 83 ng/mL ± 9 (SEM) in male vs. 90 ng/mL ±12 (SEM) in female mice. Corresponding plasma levels of cotinine were ≈114 ng/mL ± 8 (SEM) and 119 ng/ mL ± 6 (SEM) in male and female mice respectively. This clearly indicates that levels of chronic TS exposure were uniform between the 2 groups. The brain concentrations (from whole brain homogenates) of both nicotine and cotinine were significantly lower compared to plasma. Specifically, the corresponding brain levels of nicotine and cotinine were ≈22 ng/mL ± 3 (SEM) and 5 ng/mL ± 3 (SEM) in male mice vs. 25 ng/mL ± 4 (SEM) and 7 ng/mL ± 2 (SEM) respectively in female mice. Note that BBB permeability of cotinine is significantly lower than nicotine. Nicotine and cotinine levels were assessed by UHPLC-MS/MS as previously described by our group [20].
Fig. 4. Gender specific dysregulation of Slc40al and Abcb6 (in vivo).
Chronic (4 weeks) smoking drastically minimized the expression of Slc40al in female mice (A1). The expression was also down- regulated in male mice to a lesser extent (A2). The effect of chronic TS exposure was opposite in Abcb6 expression. It remarkably elevated Abcb6 expression in female mice (Bl) but in case of male mice it was slightly upregulated (B2). Plasma and brain levels of nicotine (Nic.) and cotinine (Cot.) in male (M) and female (F) mice (n = 3–5) for each group following 4 weeks of chronic TS exposure (C). The concentrations of both nicotine and cotinine were significantly lower in brain than plasma for both male and female mice. No significant differences in terms of TS exposure (based on nicotine and cotitine plasma and brain levels) were found between male and female mice. “*”= P < 0.05; “**” P < 0.01; “***” P < 0.001 compared to control.
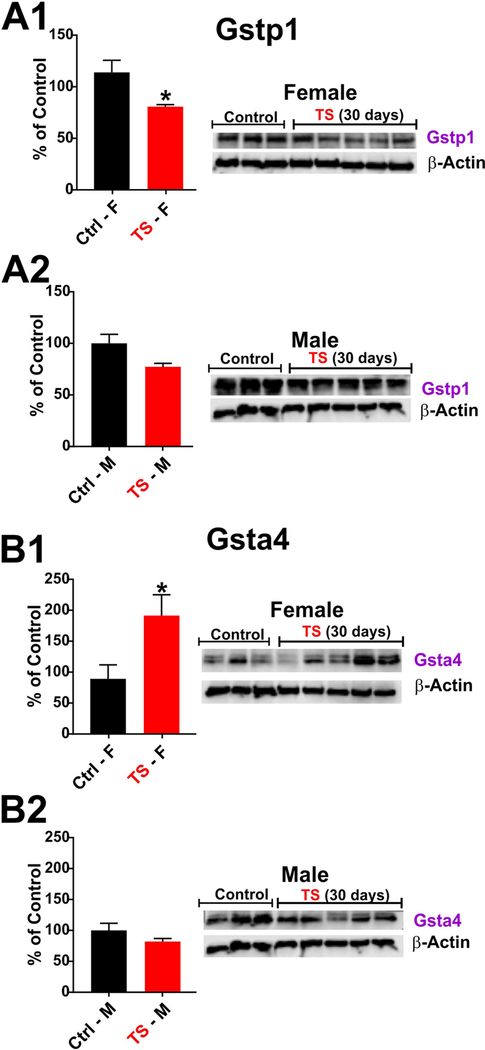
3.5. Gender specific effects of chronic TS exposure on Phase-II enzymes
Following our in vivo observation on the impact of chronic TS exposure on the above mentioned iron transporters, we decided to take a look at the potential impact of TS on Glutathione S-transferases (GSTs); a phase 2 detoxification enzymes which have a crucial protective role against xenobiotic and oxidative stress-induced toxicity. GSTs are a class of phase-II metabolic isozymes which catalyze the conjugation of xenobiotic substrates with the reduced form of glutathione (GSH) for the purpose of detoxification. GSTs are categorized into four main classes including alpha, mu, pi, and theta. Herein we investigated the impact of chronic TS exposure on glutathione S-transferase P1 (GSTP1) which represents the most prevalent mammalian isoenzyme of glutathione S-transferase superfamily and has important role in detoxification and antioxidant defense including maintenance of the redox homeostasis [25,26]. As shown in Fig. 5A1, Gstpl remained relatively unchanged in male mice following chronic (30 days) TS exposure as evidenced by western blotting analysis. However, in female mice (Fig. 5A2) was instead significantly downregulated ( ≈75% of control levels). By contrast, analysis of the expression level of glutathione S-transferase A4 (GSTA4), which is also involved in cellular defense against pharmacologically active electrophilic compounds, was instead significantly upregulated by TS in females mice ( ≈ 190% of control levels) while remained substantially unchanged in males.
Fig. 5. Gender specific differential effects of chronic smoking on phase-II enzymes.
Chronic (4 weeks) smoking significantly downregulated the expression of Gstpl in female mice (Al). The expression was also lowered in male mice as well but to a lesser extent (A2). The effect of chronic TS exposure was opposite in Gsta4 expression. It significantly elevated Gsta4 expression in female mice (Bl) but in case of male mice no change was observed (B2). (F = Female; M = Male); * = p < 0.05 vs control; + = p < 0.05 vs. test males. = P < 0.05; compared to control.
4. Discussion
Current scientific opinion considers ROS/OS-mediated pathways to play a major role in the pathogenesis of cerebrovascular and neurological disorders like stroke, and Alzheimer’s via ROS generation, inflammation, and blood-brain barrier (BBB) impairment. It is well established that active and passive tobacco smoking (TS) has been associated with vascular endothelial dysfunction in a causative and dose dependent manner primarily related to the release of reactive oxygen species (ROS). In addition, recent preclinical studies have also shown that nicotine (the principal e-liquid’s ingredient used in e-cigarettes (e-Cig) can also cause OS, exacerbation of cerebral ischemia and secondary brain injury. Herein we show that acute exposure to TS and e-Cig promotes mitochondrial depolarization in both primary brain vascular endothelial cells and bEnd3 cells although the impact of e-Cig (while statistically significant) was dampened in primary cells when compared to bEnd3 (see Fig. 1). What also our results revealed is that Iron transporters plays a major role in the regulation of mitochondrial membrane potential and redox activity. Therefore, we investigated the impact on TS and e-Cig on the expression level of 2 iron transporters. Specifically, Slc40al (better known as ferroportin 1) and Abcb6 (a mitochondrial ATP-binding cassette porphyrin importer). Deregulation (downregulation) of Slc40al has been implicated with several neurological disorders including AD brain pathology, abnormal forebrain patterning and neural tube closure and inflammation [10,27,28]. Noteworthy, deficiency of the Abcb6 transporter has been linked to accelerated atherosclerosis in mice [11]. Our in vitro results shows that acute TS exposure (and to a lesser extent e-Cig) promotes the upregu- lation of these transporters (Figs. 3 and 4). Based on previous work by our group, this is not unexpected since we have previously observed that acute TS exposure promotes upregulation/activation of a wide host of antioxidative and defensive response systems including the above mentioned transporters as well as phase 1 and 2 detoxification enzymes. The effect seems to be largely mediated by ROS through the activation of nuclear factor erythroid 2-related factor (Nrf2); a redox- sensitive transcriptional factor we previously reported to be activated (along with up regulation of its downstream targets including antioxidant and detoxifying enzymes) by acute TS/e-Cig exposure [8,19].
Fig. 3. Abcb6 expression (in vitro).
Acute exposure to TS and e-Cig upregulated Abcb6 expression in bEnd.3 (A) cell lines and mBMEC (B) demonstrated by western blotting, immunocytochemistry and RT-PCR. This effect was significantly higher following TS exposure compared to e-Cig in both cell type and all testing methods.“*” = P < 0.05; “**” P < 0.01 compared to control.
Preceding studies by our group have also shown that chronic TS and e-Cig exposure can impair the antioxidative Nrf2-ARE system [11,18] depressing these physiological defense responses and resulting into increased susceptibility to oxidative stress and associated inflammatory damages. At this stage, the differential response to e-Cig between bEnd.3 and primary cells cannot be mechanistically explained (which is also beyond the scope of this work) but the differential physiological responses to treatment we observed will need to be taken into consideration when selecting the most appropriate cell type in future studies involving either TS and/or e-cigarettes.
In relation to the in vivo study, previous work by others [13,14] suggests that females may respond to oxidative stress stimuli (such as those induced by chronic TS exposure) differently than male. What we observed here seems to strongly confirm these initial circumscriptive evidences. In fact, as shown in Fig. 4, Slc40al (ferroportinl) is significantly more downregulated by chronic TS exposure in female than male mice when compared to their corresponding controls. As mentioned above, downregulation of Slc40al has been implicated with the onset and progression of several neurological disorders including AD brain pathology, abnormal forebrain patterning and neural tube closure and inflammation [10,27,28]. By contrast, Abcb6 was instead significantly upregulated by chronic TS in females while remained relatively unchanged in males. Since deficiency of this transporter has been linked to accelerated atherosclerosis in mice11, our data also suggests that female smokers could be on the other hand, less susceptible to atherosclerosis than males. As a matter of fact, TS is one of the most relevant risk factors for the development of atherosclerosis [29] which is one of the most common causes of BBB lesions and is linked to the onset of AD [30]. Clinical studies have indeed shown that women are less likely to develop atherosclerosis than men during pre-meno- pausal age [31]. This also strongly suggests that hormonal differences may be likely responsible for the observed effects. The differential response observed between acute (in vitro) vs. chronic (in vivo) TS exposure builds on previous research from our group demonstrating that cells and tissue can effectively overcome acute oxidative stress stimuli (add pooja paper) but “long term” chronic exposures to these stimuli negatively impact cellular antioxidative response capabilities [19]. We have in fact previously shown that prolonged TS exposure increases oxidative stress compromising the Nrf2-ARE anti-oxidant pathway and causing cerebrovascular impairment in terms of loss of BBB integrity and impaired redox metabolism [19,21]. Alterations of gene transcription/translation related to the Nrf2-ARE pathways were among the most predominant in human BBB microvascular endothelial cells exposed to TS [18]. These data well fit with a hormonal-based regulation of the antioxidative response system since estrogen signaling has been shown (both in humans and rodents) to affect Nrf2 activity in a cell/ tissue-dependent manner. For example (and specific to our case), estrogen stimulates Nrf2 activation in cerebral nerve tissue and endothelial cells [32,33]. This well fit with our in vivo results. By contrast, estrogen exercises the opposite effect in the uterus [34].
Looking at detoxification enzymes of the glutathione family, GSTP1 is a phase 2 enzyme involved in xenobiotic metabolism, antioxidant defense and maintenance of the cellular redox hemostasis [25,26]. GSTP1 catalyzes the conjugation of hydrophobic and electrophilic compounds with reduced glutathione [26] and loss of this enzyme has been associated with nuclear oxidative stress damage and potentiation of cell injury by ROS such as those released by TS [35,36].
Herein (see Fig. 5) we observed that Gstpl was significantly downregulated by chronic TS exposure in female mice (only marginally in males). However, the impact of TS exposure on Gsta4, the other glutathione S-transferase enzyme we analyzed in vivo, was markedly different. In fact, chronic TS exposure induced a marked upregulation of Gsta4 in female mice but not in males. GSTA4 is a glutathione S-Transferase but of the alpha type and similarly to GSTP1, is also involved in the detoxification of active electrophilic compounds (by conjugation with glutathione). GSTA4 has been recently associated with protection against oxidative stress and atherogenesis through positive modulation of inducible nitric oxide synthase (iNOS) [37]. Thus, upregulation of GSTA4 could be a compensatory mechanism to counteract the downregulation of GSTP1 in female mice. However, the overall physiological impact of GSTA4 upregulation against GSTP1 downregulation needs to be specifically determined in more detailed studies.
In conclusion, although the physiological implications are not fully clear, what our work suggests is that in mice chronic TS exposure differentially impact redox and detoxification functions based on gender, thus gender could be considered one of the risk factors to be taken into account when assessing the possible health impact of chronic smoking. Our in vitro study also outlined the fact that similar to TS, e-cigarette vapor is also likely to negatively impact the brain vascular endothelium by promoting mitochondrial depolarization and affecting cellular iron transporters involved in the regulation of redox metabolism. Inasmuch our results suggest that e-cigarette may represent a potential prodromal risk factor for the onset of cerebrovascular impairments. Our in vivo study have shown strong evidences supporting a gender-specific response to TS toxicity which likely correlate with hormonal differences (estrogen) and are in line with current clinical observations suggesting that women are less susceptible to oxidative stress-related damage [13]. Therefore, future toxicological studies aimed at further determining the toxicological impact of either TS or e-cigarette vaping on public health will need to address potential gender differences.
Acknowledgements
Funding: This work was supported by the National Institutes of Health/National Institute on Drug Abuse2R01-DA029121–01Al to Dr. Luca Cucullo.
Abbreviations:
- BBB
blood-brain barrier
- CNS
central nervous system
- OS
oxidative stress
- Nrf2
nuclear factor erythroid 2-related factor 2
- Nqol
NAD(P)H: quinone oxidoreductase 1
- SFN
sulforaphane
- RT-PCR
reverse transcription polymerase chain reaction
- mBMEC
mouse primary brain microvascular endothelial cells
- Sic
solute carrier
- Abe
ATP-binding cassette
- VDAC
voltage-dependent anion channels
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the FDA.
Competing interests
The authors declare no competing interests.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.Org/10.1016/j.neulet.2018.05.045.
References
- [1].In The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General Publications and Reports of the Surgeon General, (2006). [PubMed]
- [2].Howard G, et al. , Cigarette smoking and progression of atherosclerosis: the atherosclerosis risk in communities (ARIC) study, JAMA 279 (1998) 119–124. [DOI] [PubMed] [Google Scholar]
- [3].Cataldo JK, Prochaska JJ, Glantz SA, Cigarette smoking is a risk factor for Alzheimer’s Disease: an analysis controlling for tobacco industry affiliation, J. Alzheimers Dis 19 (2010) 465–480, 10.3233/JAD-2010-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Anstey KJ, von Sanden C, Salim A, O’Kearney R, Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies, Am. J. Epidemiol 166 (2007) 367–378, 10.1093/aje/kwmll6. [DOI] [PubMed] [Google Scholar]
- [5].Uttara B, Singh AV, Zamboni P, Mahajan RT, Oxidative stress and neurode- generative diseases: a review of upstream and downstream antioxidant therapeutic options, Curr. Neuropharmacol 7 (2009) 65–74, 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Arnson Y, Shoenfeld Y, Amital H, Effects of tobacco smoke on immunity, inflammation and autoimmunity, J. Autoimmun 34 (2010) J258–265, 10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- [7].Hildick-Smith GJ, et al. , A practitioner’s guide to electronic cigarettes in the adolescent population, J. Adolesc. Health 57 (2015) 574–579, 10.1016/j.jadohealth.2015.07.020. [DOI] [PubMed] [Google Scholar]
- [8].Kaisar MA, et al. , Offsetting the impact of smoking and e-cigarette vaping on the cerebrovascular system and stroke injury: is Metformin a viable countermeasure? Redox Biol. 13 (2017) 353–362, http://dx.doi.Org/10.1016/j.redox.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kasvosve I, Effect of ferroportin polymorphism on iron homeostasis and infection, Clin. Chim. Acta 416 (2013) 20–25, http://dx.doi.Org/10.1016/j.cca.2012.ll.013. [DOI] [PubMed] [Google Scholar]
- [10].Raha AA, Vaishnav RA, Friedland RP, Bomford A, Raha-Chowdhury R, The systemic iron-regulatory proteins hepcidin and ferroportin are reduced in the brain in Alzheimer’s disease, Acta Neuropathol. Commun 1 (2013) 55, 10.1186/2051-5960-1-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Murphy AJ, et al. , Deficiency of ATP-binding cassette transporter B6 in megakaryocyte progenitors accelerates atherosclerosis in mice, Arterioscler. Thromb. Vasc. Biol 34 (2014) 751–758, 10.1161/ATVBAHA.113.302613. [DOI] [PubMed] [Google Scholar]
- [12].Schumacher T, Benndorf RA, ABC transport proteins in cardiovascular disease-a brief summary, Molecules 22 (2017), 10.3390/molecules22040589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Borras C, et al. , Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males, Free Radic. Biol. Med 34 (2003) 546–552. [DOI] [PubMed] [Google Scholar]
- [14].Vina J, et al. , Females live longer than males: role of oxidative stress, Curr. Pharm. Des 17 (2011) 3959–3965. [DOI] [PubMed] [Google Scholar]
- [15].Omidi Y, et al. , Evaluation of the immortalised mouse brain capillary endothelial cell line, b.End3, as an in vitro blood-brain barrier model for drug uptake and transport studies. Brain Res. 990 (2003) 95–112. [DOI] [PubMed] [Google Scholar]
- [16].Scott GS, Bowman SR, Smith T, Flower RJ, Bolton C, Glutamate-stimulated peroxynitrite production in a brain-derived endothelial cell line is dependent on N- methyl-D-aspartate (NMDA) receptor activation, Biochem. Pharmacol 73 (2007) 228–236, http://dx.doi.Org/10.1016/j.bcp.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Naik P, et al. , Oxidative and pro-inflammatory impact of regular and denicotinized cigarettes on blood brain barrier endothelial cells: is smoking reduced or nicotine-free products really safe? BMC Neurosci. 15 (2014) 51, 10.1186/1471-2202-15-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Naik P, Sajja RK, Prasad S, Cucullo L, Effect of full flavor and denicotinized cigarettes exposure on the brain microvascular endothelium: a microarray-based gene expression study using a human immortalized BBB endothelial cell line, BMC Neurosci. 16 (2015) 38, 10.1186/sl2868-015-0173-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Prasad S, et al. , Role of Nrf2 and protective effects of Metformin against tobacco smoke-induced cerebrovascular toxicity, Redox Biol. 12 (2017) 58–69, 10.1016/j.redox.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kaisar MA, Kallem RR, Sajja RK, Sifat AE, Cucullo L, A convenient UHPLC-MS/MS method for routine monitoring of plasma and brain levels of nicotine and cotinine as a tool to validate newly developed preclinical smoking model in mouse, BMC Neurosci. 18 (2017) 71, 10.1186/sl2868-017-0389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sajja RK, Green KN, Cucullo L, Altered Nrf2 signaling mediates hypoglycemia- induced blood-brain barrier endothelial dysfunction in vitro, PLoS One 10 (2015) e0122358, 10.1371/journal.pone.0122358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Prasad S, et al. , Impact of cigarette smoke extract and hyperglycemic conditions on blood-brain barrier endothelial cells, Fluids Barriers CNS 12 (2015) 18, 10.1186/sl2987-015-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Logan A, et al. , Assessing the mitochondrial membrane potential in cells and In vivo using targeted click chemistry and mass spectrometry, Cell Metab. 23 (2016) 379–385, http://dx.doi.Org/10.1016/j.cmet.2015.ll.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Suski JM, et al. , Relation between mitochondrial membrane potential and ROS formation, Methods Mol. Biol 810 (2012) 183–205, 10.1007/978-l-61779-382-0_12. [DOI] [PubMed] [Google Scholar]
- [25].Hayes JD, Flanagan JU, Jowsey IR, Glutathione transferases, Annu. Rev. Pharmacol. Toxicol 45 (2005) 51–88, 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- [26].Sanchez-Gomez FJ, et al. , Detoxifying enzymes at the cross-roads of inflammation, oxidative stress, and drug hypersensitivity: role of glutathione transferase P 1–1 and aldose reductase, Front. Pharmacol 7 (2016) 237, 10.3389/fphar.2016.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Urrutia P, et al. , Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells, J. Neurochem 126 (2013) 541–549, 10.llll/jnc.12244. [DOI] [PubMed] [Google Scholar]
- [28].Mao J, et al. , The iron exporter ferroportin 1 is essential for development of the mouse embryo, forebrain patterning and neural tube closure, Development 137 (2010) 3079–3088, 10.1242/dev.048744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Messner B, Bernhard D, Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis, Arterioscler. Thromb. Vasc. Biol 34 (2014) 509–515, 10.1161/ATVBAHA.113.300156. [DOI] [PubMed] [Google Scholar]
- [30].Gupta A, Iadecola C, Impaired Abeta clearance: a potential link between atherosclerosis and Alzheimer’s disease, Front. Aging Neurosci 7 (2015) 115, 10.3389/fnagi.2015.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fairweather D, Sex differences in inflammation during atherosclerosis, Clin. Med. Insights Cardiol 8 (2014) 49–59, 10.4137/CMC.S17068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang T, et al. , Estrogen receptor and PI3 K/Akt signaling pathway involvement in S-(−)equol-induced activation of Nrf2/ARE in endothelial cells, PLoS One 8 (2013) e79075, 10.1371/journal.pone.0079075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Meng X, et al. , Suppression of NADPH oxidase- and mitochondrion-derived superoxide by Notoginsenoside R1 protects against cerebral ischemia-reperfusion injury through estrogen receptor-dependent activation of Akt/Nrf2 pathways, Free Radic. Res 48 (2014) 823–838, 10.3109/10715762.2014.911853. [DOI] [PubMed] [Google Scholar]
- [34].Ansell PJ, et al. , In vitro and in vivo regulation of antioxidant response element- dependent gene expression by estrogens, Endocrinology 145 (2004) 311–317, 10.1210/en.2003-0817. [DOI] [PubMed] [Google Scholar]
- [35].Fletcher ME, et al. , Influence of glutathione-S-transferase (GST) inhibition on lung epithelial cell injury: role of oxidative stress and metabolism, Am. J. Physiol. Lung Cell. Mol. Physiol 308 (2015) L1274–1285, 10.1152/ajplung.00220.2014. [DOI] [PubMed] [Google Scholar]
- [36].Mian OY, et al. , GSTP1 Loss results in accumulation of oxidative DNA base damage and promotes prostate cancer cell survival following exposure to protracted oxidative stress, Prostate 76 (2016) 199–206, 10.1002/pros.23111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yang Y, et al. , Endothelial glutathione-S-transferase A4–4 protects against oxidative stress and modulates iNOS expression through NF-kappaB translocation, Toxicol. Appl. Pharmacol 230 (2008) 187–196, http://dx.doi.Org/10.1016/j.taap.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]