Abstract
Since 1970, the Gynecologic Oncology Group (GOG) has been at the forefront of evaluating and helping to implement ground breaking and paradigm changing research in the management of cervical cancer. While the most dramatic example of this impact was a series of clinical trials published in 1999 that evaluated chemoradiation therapy versus radiation therapy alone for patients with various clinical scenarios, including both locally advanced as well as post radical hysterectomy patients, investigation has continued to further refine and improve therapy. In 2014, based on the results of GOG protocol 240, bevacizumab became the first approved targeted therapy in a gynecologic cancer in the United States. Most recently, clinical trial work from the GOG is changing the standard of care for all clinical scenarios. Finally, an emphasis on survivorship and special populations are now top priorities.
Keywords: Cervical cancer, Chemotherapy, Chemoradiation, Clinical trials, Historical perspective, Survivorship
Introduction
While screening has dramatically decreased the incidence of cervical cancer in developed countries, cervical cancer remains a significant public health challenge. Most recent estimates suggest that 13,240 women will be diagnosed with cervical cancer in the United States in 2018, while 4,170 are expected to die in the same time period (1). Although there is continued excitement about HPV vaccination and its potential ability to further decrease cancer precursors and ultimately the incidence of cervical cancer (2), higher vaccination rates as well as time to realize the full benefits of vaccination in countries like the United States are needed. Accordingly, investigations into the ideal management of cervical cancer will remain a critical part of gynecologic cancer care for several more decades at least.
The Gynecologic Oncology Group (GOG), and now NRG Oncology, have been among if not the, principal participants among the cooperative groups focused on improving cervical cancer treatment and outcomes in the United States. Arguably, the most important contribution from GOG/NRG Oncology was a series of clinical trials that lead to the National Cancer Institute’s landmark announcement in 1999 regarding the use of chemoradiation in patients receiving radiation as a therapeutic modality for different cervical cancer clinical scenarios (3–6). Previously, reviews and meta-analyses have highlighted not only the use of chemoradiation (7– 9), but both the development and use of various chemotherapeutic agents, primarily in a series of GOG sponsored clinical trials, in treating women with recurrent cervical cancer (10, 11).
Since this seminal work was published and incorporated into clinical practice, much of the subsequent evaluation in cervical cancer patient management has focused on ways to tailor surgical therapy in lower stage tumors, including attention to fertility preservation, evaluate the impact of therapy on overall quality of life in an attempt to limit future treatment related morbidities, evaluate the use of pre-therapy imaging, and to improve the effectiveness of both primary chemoradiation therapy and therapy for recurrent disease. This review will concentrate on both completed and ongoing clinical trials from the GOG/NRG Oncology from the last 2 decades including a review of common scenarios encountered in clinical practice.
Review
1. Preinvasive Disease
Although pre-invasive disease is often out of the purview of cooperative group mandates, the GOG did perform a randomized placebo-controlled phase 2 trial (GOG 207) that utilized daily celecoxib in patients with high-grade cervical dysplasia that underwent both serial colposcopic examinations at pre-specified time points, as well as an end of therapy excisional procedure (12). In this trial of 130 women with biopsy proven cervical intraepithelial neoplasia (CIN) 3, the use of 400mg celecoxib daily, as compared to matching placebo for between 14–18 weeks, did not appear to increase HPV infection clearance, although serum vascular endothelial growth factor (VEGF) levels may predict a better likelihood of response to therapy.
2. Early Stage Tumors - Stage IA1-IB1
Patients with early stage cervical cancer are most commonly treated with one of several different surgical procedures, depending on both their fertility desires and the stage of disease (13). For patients with Stage IA1 tumors, multiple fertility preserving and non-radical surgical options to include Loop Electrocautery Excisional Procedure (LEEP), Cold Knife Conization (CKC), and various approaches to simple hysterectomy are utilized. More recently, it has been recognized that fertility preservation may be an acceptable alternative in patients with Stage IA2 and IB1 tumors, that previously were managed with radical hysterectomy and lymphadenectomy. Following observations of the low rate of parametrial involvement, the increased use of minimally invasive surgical techniques and the observation of the relative effectiveness and safety of radical trachelectomy with lymphadenectomy, approaches to surgical management have focused on tailoring the procedure to the patients’ tumor with a general approach of a less radical and fertility preserving surgery.
To further evaluate this approach in low stage tumors, GOG 278 (NCT 01649089) was developed to evaluate both physical function as well as quality of life in women with stage I squamous cell, adenocarcinoma or adenosquamous cervical cancer. Specifically, women with stage IA1 cervical cancer with lymphovascular space invasion (LVSI), Stage IA2 or Stage IB1 less than 2 centimeters in greatest dimension, that do not desire radical therapy and are undergoing either a simple non-radical hysterectomy or a CKC, both with pelvic lymphadenectomy are eligible. Patients are assessed for the impact of these procedures not only on the potential impact on bladder, bowel and sexual functioning, but in terms of the incidence and severity of lymphedema, recurrence and survival. At present, accrual is nearly 80% complete and patients undergo dedicated surveillance for at least 3 years after therapy.
3. Early Stage Tumors – Intermediate Recurrence Risk following Radical Hysterectomy
Although radical hysterectomy with lymphadenectomy is definitive therapy and associated with an excellent prognosis for most patients undergoing surgery, the GOG performed a prospective evaluation of surgical pathology specimens and outcomes, GOG 49, in order to determine which pathologic findings were associated with a higher likelihood of nodal spread (14). When specifically evaluating Stage IB patients that underwent a radical hysterectomy with pelvic and para-aortic lymphadenectomy, investigators identified depth of invasion, parametrial involvement, LVSI, tumor grade and gross versus occult primary tumor involvement as being associated with a higher risk of pelvic nodal involvement (15). This pathologic data allowed the development of clinical trials in order to determine potential superior therapeutic options for patients with higher risk of cervical cancer recurrence.
To evaluate the clinical impact of pathologic risk factors in node negative and cervix confined disease, the GOG performed a trial, GOG 92, to determine the ideal treatment of patients deemed to have an intermediate risk of recurrence following a radical hysterectomy and lymphadenectomy. This trial evaluated women with a combination of LVSI, large tumor diameters and varying degrees of cervical stromal invasion with categories depicted in Table 1 (16). Among 277 patients enrolled on the study, 137 received 46 – 50.4 Gy via external beam irradiation without vaginal brachytherapy. Preliminary results noted two-year superior recurrence free survival for patients receiving radiation, 88% versus 79%, at the expense of a nearly three-fold increase in grade 3/4 adverse events, 6% versus 2.1%. Subsequent analysis of longer term surveillance demonstrated a continued marked decrease in the risk of recurrence, HR 0.54 (90% CI 0.35–0.81, p =0.007) without an improvement in overall survival (OS), HR 0.70 (90% CI 0.45–1.05, p =0.074) (17). Moreover, the protection against recurrence with radiation seemed more pronounced in those with adenocarcinomas or adenosquamous carcinomas, HR 0.23, (90% CI 0.07–0.74, p =0.019).
Table 1.
Characteristics of Intermediate Risk Factors for Cervical Cancer Recurrence in Patients undergoing Radical Hysterectomy with Lymphadenectomy (16, 17)
| Tumor Size | Stromal Invasion | LVSI |
|---|---|---|
| Any Tumor Size | Deep 1/3 | Present |
| ≥ 2cm | Middle 1/3 | Present |
| ≥ 5cm | Superficial 1/3 | Present |
| ≥ 4cm | Deep or Middle 1/3 | Absent |
While GOG 92 did not include chemotherapy, based on the cumulative data in both locally advanced disease as well as for high-risk early stage disease, many providers have recommended the addition of cisplatin chemotherapy to radiation in these patients even in the absence of clinical trial data. Accordingly, GOG 263 (NCT 01101451) was developed as a replacement or follow-up trial to GOG 92 to answer this exact question in a randomized study in women with Stage I-IIA cervical cancer. GOG 263 compares the use of external radiation therapy alone without brachytherapy or in combination with 6 cycles of weekly Cisplatin 40mg/m2 in women with intermediate risk pathologic findings following a radical hysterectomy with pelvic lymphadenectomy. Importantly, this trial will answer if the potential benefit in improved survival for patients with intermediate risk cervical cancer, is warranted or is outweighed by the potential added toxicity of cisplatin.
4. Early Stage Tumors - High Recurrence Risk following Radical Hysterectomy
Similarly, GOG 109 which was performed in conjunction with both the Southwest Oncology Group and Radiation Therapy Oncology Group, evaluated the role of chemotherapy added to post-operative external beam radiation therapy to the pelvis (4). While GOG 49 was able to predict the impact of intermediate risk factors on recurrence and positive nodal status, much of the data available on higher risk features was retrospective (18, 19). Nonetheless, based on the relatively consistent information that noted not only a higher risk of recurrence but also inferior survival, a prospective trial was developed which randomly assigned patients to either 49.3 Gy alone or in combination with cisplatin 70mg/m2 and 5-fluorouracil (5FU) 1000mg/m2/d over 96 hours every 3 weeks. Of the 243 assessable patients on the two arms, the majority had positive pelvic nodes (207/243), with both positive parametrial involvement (83/243) and margins (12/243) much less common. At four years, patients on the combined chemoradiation arm had both superior PFS, 80% vs. 63%, and OS, 81% vs. 71%, with the hazard ratio point estimates for recurrence 2.01 (p=0.003) and death 1.96 (p=0.007) both inferior in the radiation only patients. Importantly based on study design, chemotherapy in this trial may have acted as a radiation sensitizer, in an adjunctive fashion after radiation of both with cycles 3 and 4 being administered following completion of external beam radiation therapy, or both.
Analogous to the treatment approach utilized where GOG 263 was developed as a replacement trial for GOG 92 in order to answer questions regarding chemoradiation for intermediate risk disease, the RTOG and GOG developed a trial, RTOG 0724/GOG0724 (NCT 00980954), which adds adjuvant chemotherapy to a standard post radical hysterectomy chemoradiation backbone. All patients in the trial will receive between 45–50.4 Gy of external beam radiation therapy administered either in a standard fashion or via Intensity Modulated Radiation Therapy (IMRT) with or without brachytherapy. Patients on the experimental arm with then commence adjuvant chemotherapy within four to six weeks from completion of chemoradiation which consists of four cycles of paclitaxel 135mg/m2 and carboplatin AUC 5 administered every three weeks. The primary objective of this trial is to determine if the experimental arm improves disease-free survival with OS being one of several secondary endpoints.
5. Quality of Life/Survivorship
While surgical excision is associated with generally favorable prognosis without the need for adjuvant therapy in many patients with Stage I cervical cancer, intermediate and high-risk patients notwithstanding, radical surgery can result in severe long-term sequelae. Consequently, the GOG developed GOG 244 (NCT 00956670) to prospectively evaluate the incidence of lower extremity lymphedema in a group of cervical, as well as endometrial and vulvar cancer patients, undergoing radical gynecologic surgery with concurrent lymphadenectomy. In addition to evaluating the incidence of lymphedema, various risk factors for the development of lymphedema as well as its potential impact on patient quality of life will be explored. Carlson recently presented the initial data for 138 cervical cancer patients with a median age of 44 (range 25–83) from the total study sample size of 1054 (20). Study endpoints of a limb volume change (LVC) of greater than 10% for any of the post-operatively visits between 6 weeks and 24 months were considered consistent with lymphedema, which was present in 48 or 35% of the cervical cancer patients. Moreover, LVC of a greater than 15% change was present in 35 or 25%, with an LCV of greater than 20% limited to 17 or 12% of these patients.
6. Pre-therapy imaging
Debate continues regarding the role of lymph node assessment in patients with locally advanced cervical cancer and the impact of the discovery and removal of occult nodal metastatic disease. In early locally advanced cervical cancer trials, patients generally underwent surgical assessment of their lymph nodes. However, with improving radiographic techniques and the development of Positron Emission Testing (PET) scanning capabilities, the role of pre-radiation surgical nodal assessment was questioned. Specifically, GOG 165 was the first chemoradiation trial that did not require a surgical assessment of the para-aortic lymph nodes (21). A subsequent ancillary analysis of data from both GOG 165 and GOG 120 noted differences in outcomes based on clinical versus surgical staging of the para aortic nodes (22). As noted in this analysis, although the radiation dose in GOG 165 was 5 Gy greater than that used in GOG 120 and administered over a shorter treatment course (10 versus 8 weeks), survival for patients with stage III or IV disease was worse in GOG 165, potentially suggesting a negative impact of imaging negative but pathologically positive para-aortic lymph nodes that were not surgically resected.
In order to determine if more modern imaging, such as PET imaging was sufficient to detect nodal metastatic disease in patients with locally advanced cervical cancer, the GOG partnered with the American College of Radiology Imaging Network (ACRIN) to answer this question (23). Specifically, in GOG 233, 153 patients with locally advanced cervical cancer underwent pre-operative PET combined with contrast-enhanced diagnostic CT imaging followed by pelvic and abdominal lymphadenectomy. Surgical approach included either laparoscopic or an extraperitoneal approach with a goal of removing lymph nodes from 4 regions bilaterally: obturator, external iliac, common and para-aortic lymph node basins. In this trial, whose primary objective was to determine the accuracy of PET/CT to detect abdominal retroperitoneal lymph node metastasis, 43 of 153 (28.1%) patients with an adequate PET/CT and some pathology had pathologically proven abdominal lymph node metastasis; however, secondary to exclusion criteria including inadequate lymph node dissection, failure to perform pre-operative PET/CT, poort quality PET/CT or lack of pathologic submission, only 109 patients meet all criteria both from an imaging and surgical assessment standpoint. When comparing the combination of PET/CT versus CT alone, sensitivity was 0.50 versus 0.42 (p=0.052) and specificity was 0.85 versus 0.89 (p=0.21) respectively. These results suggest perhaps a modest improvement for PET/CT compared to CT alone.
7. Locally Advanced Disease
While chemoradiation with single agent cisplatin 40mg/m2 administered weekly remains the standard of care for patients with locally advanced cervical cancer, continued investigations have evaluated potential modifications or additions to this chemoradiation backbone in order to improve patient survival. As noted previously, the landmark clinical trials from 1999 radically altered the management of women with cervical cancer that was not amenable to a surgical resection (3–6). Importantly, updated results have been published from several of these trials which note a continued and durable benefit for chemoradiation compared to radiation alone (24, 25). In order to evaluate the impact of anemia on survival, the GOG commenced GOG trial 191 which utilized standard cisplatin based chemoradiation with or without recombinant human erythropoietin (EPO) (26). Interestingly, in this trial where women were administered EPO to keep their hemoglobin greater than 12.0 g/dL, the observation of more thromboembolic events in the experimental arm caused the trial to be closed early which limits interpretation. More recently, the GOG has compared both a prolonged venous infusion of 5-fluorouracil (5FU) versus weekly cisplatin as well as the addition of tirapazamine, a hypoxic cell sensitizer, to standard chemoradiation in two Phase 3 randomized controlled trials (21, 27). Although 5FU had previously been evaluated in patients with locally advanced cervical cancer, this was in combination with other chemotherapy agents and had not been compared head-to-head with weekly cisplatin (6, 21). Accordingly, GOG 165 evaluated a prolonged venous infusion of 5FU 225mg/m2/d for five days versus cisplatin 40mg/m2 weekly for up to 6 doses, where 5FU was predicted to decrease the risk of recurrence by one-third. During a planned interim data analysis, not only was 5FU not superior to cisplatin, it was inferior and was associated with a higher risk of both treatment failure, RR 1.29 (95% CI 0.91–1.80) and death, RR 1.35 (95% CI 0.96–1.97), which appropriately lead to the premature closure of the study. Although the trial was considered negative, it was the first trial which did allow the use of high dose rate brachytherapy in locally advanced cervical cancer patients.
Tirapazamine, which is thought to increase the cytotoxicity of cisplatin and had been evaluated both in recurrent and primary cervical cancer previously, was utilized with the standard cisplatin based chemoradiation backbone and compared to the standard chemoradiation backbone alone (27). Similar to GOG 165, the experimental regimen was predicted to decrease the risk of recurrence by nearly one-third, specifically 30% for GOG 219, when compared to standard therapy. Based on an interim safety analysis, the dose of tirapazamine was decreased which resulted in better tolerance of therapy. Unfortunately, during the study, the drug became unavailable which resulted in premature closure of the trial. At the time of study closure, 402 of 750 (53.6%) planned patients were accrued, and in the 387 evaluable patients, both the three- year PFS, 63.0% vs. 64.4%, and three-year OS, 70.5% vs. 70.6%, were similar in the cisplatin/tirapazamine arm and cisplatin alone arms respectively (27). Unfortunately, these results add little to the current management of locally advanced cervical cancer. Pertinent and collated details from GOG chemoradiation trials for locally advanced cervical cancer are presented in Table 2.
Table 2.
Patient outcomes in GOG sponsored Phase 3 chemoradiation trials for locally advanced cervical cancer (3,5,6,21,26,27)
| Protocol | Stages | Chemotherapy | N | Proportion Disease Free |
Proportion Alive |
RR/HR Overall Survival |
|---|---|---|---|---|---|---|
| GOG 85 |
II, III, IVA |
HU 5FU+CP |
191 177 |
47% 57%* @8.7 yrs |
43% 55%* @8.7 yrs |
Referent 0.74 (90%CI 0.58–0.95) |
| GOG 120 |
IIB, III, IVA |
HU CP CP+5FU+HU |
177 176 173 |
26% 46%* 43%* @10 yrs |
34% 53%* 53%* @10 yrs |
Referent 0.57 (95%CI 0.43–0.75 0.51 (95%CI 0.38–0.67) |
| GOG 123 |
IB | None CP |
186 183 |
60% 71%* @6 yrs |
64% 78%* @6 yrs |
Referent 0.63 (95%CI 0.43–0.91) |
| GOG 165 |
IIB, IIIB, IVA |
CP 5FU |
159 157 |
57% 40% @4 yrs |
64% 55% @4 yrs |
Referent 1.37(95% CI 0.96–1.94) |
| GOG 191 |
IIB, IIIB, IVA |
CP CP+EPO |
52 57 |
65% 58% @3 yrs |
75% 61% @3 yrs |
NR |
| GOG 219 |
IIB, IIIB, IVA |
CP CP+TPZ |
194 185 |
63% 64.4% @3 yrs |
70.6% 70.5% @3 yrs |
Referent 1.174 (95% Cl 0.652– 2.112) |
N - number of evaluable patients per study arm; RR - relative risk; HR - hazard ratio; NR - not reported; GOG - Gynecologic Oncology Group; CP - cisplatin; HU - hydroxyurea; 5FU - 5-Flurouracil; EPO - recombinant human erythropoietin; TPZ - tirapazamine;
Statistically significant differences
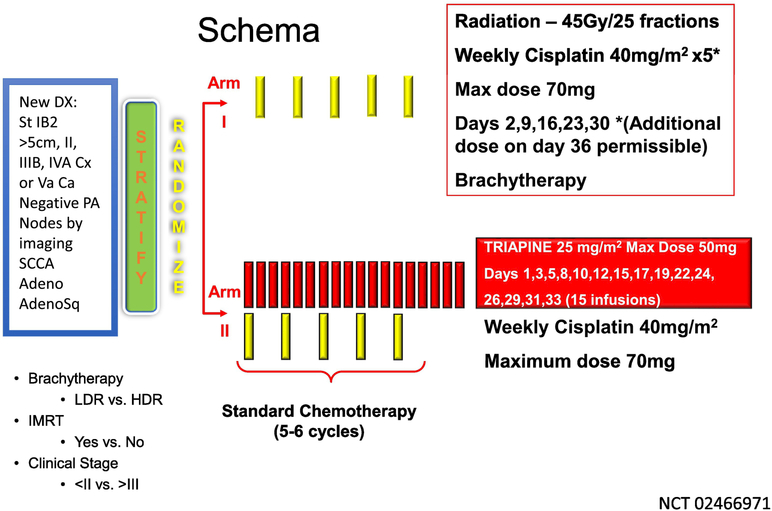
While both GOG 165 and GOG 219 are considered negative studies in that no regimen was found to be superior to the standard cisplatin chemoradiation backbone, two additional trials have evaluated other novel approaches in a similar group of patients. The first, is a combined cooperative group trial, referred to as the OUTBACK trial (GOG274) and evaluates the use of 4 cycles of paclitaxel 155mg/m2 and carboplatin AUC5 administered every three weeks following chemoradiation therapy (NCT 01414608). GOG 274 has completed enrollment with greater than 600 women enrolled by the GOG with results eagerly awaited. Triapine, a ribonucleotide reductase inhibitor, has been combined with standard chemoradiation therapy in patients with both cervical as well as vaginal cancer. Based on early encouraging preliminary results from Kunos and colleagues (28, 29), triapine was included in the replacement trial for GOG 274, GY006 (NCT02466971), a randomized phase 2 trial which will enroll nearly 200 women (Figure 1). Results from both GOG 274 as well as GY006 will help inform whether modifications to the chemoradiation backbone warrant further evaluation.
Figure 1.
Schema for GY006 evaluating the addition of the ribonucleotide reductase inhibitor, triapine (NSC#663249; IND#68338), to standard cisplatin based chemoradiation in locally advanced cervical and vaginal cancer.
8. Recurrent / Metastatic Disease
Bonomi and colleagues in an early GOG phase 3 clinical trial, presented milestone data when they demonstrated the optimal dose of cisplatin in the recurrent setting to be 50mg/m2 every 21 days, as comparted to other options of either Cisplatin 100mg/m2 every 21 days or cisplatin 20mg/m2 days 1–5 (30). Building upon this historic finding, subsequent phase 3 trials evaluated various cisplatin-based combinations which unfortunately provided limited patient benefit, as although combination therapy was often associated with superior response rate (RR) and PFS, OS was not improved and this was at the expense of added toxicity (31, 32). However, Moore and colleagues reported exciting results from GOG 169 when paclitaxel 135mg/m2/24 hours was combined with cisplatin 50mg/m2, as compared to single agent cisplatin 50mg/m2 (33). In addition to an improved RR of 35% vs. 19% and improved PFS of 4.8 vs. 2.8 months, quality of life was assessed for the first time in a metastatic cervical cancer randomized trial and demonstrated no apparent decrement with combination therapy.
With the widespread adoption of chemoradiation into primary therapy for locally advanced cervical cancer, response rates to both single agent platinum and platinum-based combination therapies were noted to decline as compared to historical controls (33, 34). In GOG 179, the combination of 0.75mg/m2 topotecan on days 1–3 with cisplatin 50mg/m2 day 1 every 3 weeks as compared to single agent cisplatin 50mg/m2 lead to FDA approval, based on superior outcomes including an improvement in median OS of 9.4 months vs. 6.5 months (p=0.017) for the combination arm. However, GOG 204, a four-arm trial that compared platinum doublets was subsequently performed and closed early for futility when none of the four combination arms: paclitaxel and cisplatin, topotecan and cisplatin, gemcitabine and cisplatin or vinorelbine were judged to be more effective than the others (35).
Although not a prospective evaluation, Moore and colleagues retrospectively evaluated patient data from GOG protocols 110, 169 and 179 in an attempt to determine if prognostic factors exist which may predict response or lack of response to chemotherapy in the recurrent and metastatic setting (36). Factors identified among 428 patients in these three phase 3 trials, that appeared to be associated with response to chemotherapy included: performance status > 0, pelvic disease, receipt of prior radiosensitizer, time interval from diagnosis to first recurrence < 1 year and African American race. Patients where then divided into three categories based on the number of risk factors present from Low Risk (0–1 factors) to High Risk (4–5 factors) and those with 2 or 3 factors being considered Mid Risk. This classification schema, often referred to as the Moore Criteria, was then applied to patients in another Phase 3 trial, GOG 149, to determine if indeed it accurately predicted differential outcomes. When the criteria were applied to the GOG 149 patients, response rate and survival did vary and appeared to correlate with the number of risk factors present. Specifically, median OS ranged from 11.93 months for those with Low Risk factors, to 5.58 months for those with High Risk factors. Moreover, in patients with High Risk factors, the overall response rate to additional chemotherapy was only 14.3% with a median progression free survival of a mere 3.38 months.
While cytotoxic chemotherapy has been the mainstay of treatment for recurrent cervical cancer, the GOG has also evaluated non-cytotoxic therapy in a series of single arm trials in order to determine potential activity of these novel therapies. Bevacizumab, a monoclonal antibody that targets vascular endothelial growth facto, was evaluated in patients with recurrent cervical cancer that had been treated with one or two prior lines of chemotherapy. GOG 227C was a trial of 46 patients, the majority of which (74%) had received only one line of chemotherapy and radiation (83%), of which 11 patients (23.9%) survived progression free for at least six months (37). Last year, results from GOG 265, a single arm phase 2 trial, which evaluated the use of a Listeria monocytogeneses immunotherapy agent targeted in patients with 1–3 prior lines of therapy was presented at the Society of Gynecologic Oncology Annual meeting (38). Of 50 evaluable patients, of which 52% had received 2 or 3 prior lines of therapy, 56% had received prior bevacizumab and 86% had undergone prior pelvic radiation, the 12-month OS rate was 38% with a median OS of 6.2 months (95% CI 4.4 – 12.3 months), including a patient with a confirmed complete response.
The encouraging single agent response to bevacizumab in GOG 227C, led to the development of the GOG 240 protocol, incorporating both bevacizumab and cytotoxic chemotherapy (39). In addition, secondary to the decreased response to platinum seen in GOG 169 and 179, a decision was made to evaluate non-platinum-based therapy in addition to the standard chemotherapy control arm of paclitaxel and cisplatin. In GOG 240, 452 patients were randomly assigned in a 2 × 2 factorial designed phase 3 clinical trial to receive one of two chemotherapeutic backbones: paclitaxel 135 or 175 mg/m2 with cisplatin 50mg/m2 or the non-platinum doublet of paclitaxel 175 mg/m2 on day 1 with topotecan 0.75mg/m2 days 1–3. In addition, a second randomization then assigned patients to either add or withhold bevacizumab 15mg/kg every 3 weeks. In addition to a greater response rate, 48% vs. 36% (p=0.008), OS was improved with the addition of bevacizumab to chemotherapy, 17.0 months vs. 13.3 months with a HR for death of 0.71 (98% CI 0.54–0.95, p= 0.004). This improvement in OS was confirmed in the final analysis, 16.8 vs. 13.3 months (HR 0.77, 95% CI 0.62–0.95, p=0.007) which was recently published (40). Comparisons of the key phase 3 trials from the GOG are presented in Table 3. Moreover, the Moore Criteria were evaluated in a prospective fashion by collection at patient enrollment, which confirmed the earlier discriminatory ability of the criteria to predict outcome (36, 41). In GOG 240, patients with High Risk factor Moore Criteria had an observed RR of 18.5% (p < 0.0001) with a median PFS of 4.7 months (p=0.005) and OS of 8.2 months (p < 0.001) with all of these outcomes inferior when compared to patients with either Mid Risk or Low Risk factors (41). Specifically, RR in Low and Mid Risk patients were 57.1% and 43.2% respectively, while PFS 9.2 vs. 6.9 months and OS 21.8 vs. 14.7 months, both respectively, were much improved when compared to High Risk factor patients. Nonetheless, patients with Low Risk factors did not appear to benefit from the addition of bevacizumab to chemotherapy in survival outcomes, although patients with Mid or High Risk disease had statistically superior PFS and OS when stratified for the use or lack of use of bevacizumab, with a 5.8 month improvement in OS seen in both these groups.
Table 3.
| Protocol |
Chemotherapy | N | RR | CR | PR | PFS (mos) |
OS (mos) |
|---|---|---|---|---|---|---|---|
| GOG 43 |
CP 50mg/m2 D1 CP 100mg/m2 D1 CP 20mg/m2 D1–5 |
150 166 128 |
20.7% 31.4%* 25% |
10% 12.7% 8.6% |
10.7% 18.7% 16.4% |
3.7 4.6 3.9 |
7.1 7.0 6.1 |
| GOG 110 |
CP CP + I CP + M |
140 151 147 |
17.8% 31.1%* 21.1% |
6.4% 12.6% 9.5% |
11.4% 18.5% 11.6% |
3.2 4.6* 3.3 |
8.0 8.3 7.3 |
| GOG 149 |
CP+I CP+I+B |
146 141 |
32.0% 31.2% |
NR NR |
NR NR |
4.6 5.1 |
8.5 8.4 |
| GOG 169 |
CP CP+P |
134 130 |
19% 35%* |
6% 15% |
13% 21% |
2.8 4.8* |
8.8 9.7 |
| GOG 179 |
CP CP+T |
146 147 |
13% 26.7%* |
2.9% 10.4% |
10.1% 16.3% |
2.9 4.6* |
6.5 9.4* |
| GOG 204 |
CP+P CP+T CP+G CP+V |
103 111 112 108 |
29.1% 23.4% 22.3% 25.9% |
2.9% 1.8% 0.9% 7.4% |
26.2% 21.6% 21.4% 18.5% |
5.8 4.6 4.7 4.0 |
12.9 10.3 10.3 10.0 |
| GOG 240 |
CP+P+Bev CP+P T+P+Bev T+P |
115 114 112 111 |
50% 46% 48% 25% |
16% 10% 12% 5% |
35% 36% 37% 20% |
8.2* 6.0° |
17.5* 15.0 16.2 12.0 |
N - number of evaluable patients per study arm; OS - overall survival; PFS - progression-free survival; RR - response rate; CR - complete response; PR - partial response; GOG - Gynecologic Oncology Group; D - day of the cycle; NR - not reported; CP - cisplatin; I - ifosfamide; M - mitolactol; B - bleomycin; P - paclitaxel; T - topotecan; G - gemcitabine; V - vinorelbine; BEV - bevacizumab; C - carboplatin
Conclusion
The GOG/NRG Oncology has had a very successful history in not only evaluating management strategies in women with cervical cancer, but in advancing the science for the care of women with this disease. While results from chemoradiation therapy trials are considered seminal, current investigations will continue to define and alter the treatment paradigms for these patients. Forthcoming studies will further guide fertility preserving surgery and likely limit patient morbidity, refine and further optimize chemoradiation for locally advanced disease and build upon the chemotherapy and bevacizumab backbone from GOG 240, which although the most active regimen in metastatic disease, is still not curative.
Cervical Cancer Review Highlights.
– The Gynecologic Oncology Group (GOG) has been fundamental in advancing the science of cervical cancer management
– Ongoing clinical trials will further define therapy in intermediate and high-risk cervical cancer after radical hysterectomy
– Chemoradiation is the cornerstone to improved outcomes in locally advanced cervical cancer
Acknowledgments
Grant Support: CAL was supported in part by the 3P30CA013148–43S3, U10CA18055 and P50 CA098252.
Footnotes
Conflict of Interests The authors affirm they have no conflict of interests for the current manuscript.
Author Contribution Section Conception and Design – Monk, Collection / Assembly of Data –Leath, Analysis / Interpretation - Leath, Monk, Manuscript Writing - Leath, Monk, Final Approval of Manuscript - Leath, Monk, Accountable for All Aspects - Leath, Monk
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7– 30. [DOI] [PubMed] [Google Scholar]
- 2.Huh WK, Joura EA, Giuliano AR, Iversen OE, de Andrade RP, Ault KA, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: a randomised, double-blind trial. Lancet. 2017;390(10108):2143–59. [DOI] [PubMed] [Google Scholar]
- 3.Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL 3rd, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340(15):1154–61. [DOI] [PubMed] [Google Scholar]
- 4.Peters WA 3rd, Liu PY, Barrett RJ 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18(8):1606–13. [DOI] [PubMed] [Google Scholar]
- 5.Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340(15):1144–53. [DOI] [PubMed] [Google Scholar]
- 6.Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, Fowler WC Jr., et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999;17(5):1339–48. [DOI] [PubMed] [Google Scholar]
- 7.Green J, Kirwan J, Tierney J, Symonds P, Fresco L, Williams C, et al. Concomitant chemotherapy and radiation therapy for cancer of the uterine cervix. Cochrane Database Syst Rev. 2001(4):CD002225. [DOI] [PubMed] [Google Scholar]
- 8.Green J, Kirwan J, Tierney J, Vale C, Symonds P, Fresco L, et al. Concomitant chemotherapy and radiation therapy for cancer of the uterine cervix. Cochrane Database Syst Rev. 2005(3):CD002225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green JA, Kirwan JM, Tierney JF, Symonds P, Fresco L, Collingwood M, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet. 2001;358(9284):781–6. [DOI] [PubMed] [Google Scholar]
- 10.Leath CA 3rd, Straughn JM Jr. Chemotherapy for advanced and recurrent cervical carcinoma: results from cooperative group trials. Gynecol Oncol. 2013; 129(1):251–7. [DOI] [PubMed] [Google Scholar]
- 11.Tewari KS, Monk BJ. Recent achievements and future developments in advanced and recurrent cervical cancer: trials of the Gynecologic Oncology Group. Semin Oncol. 2009;36(2):170–80. [DOI] [PubMed] [Google Scholar]
- 12.Rader JS, Sill MW, Beumer JH, Lankes HA, Benbrook DM, Garcia F, et al. A stratified randomized double-blind phase II trial of celecoxib for treating patients with cervical intraepithelial neoplasia: The potential predictive value of VEGF serum levels: An NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol. 2017;145(2):291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koh WJ, Greer BE, Abu-Rustum NR, Apte SM, Campos SM, Cho KR, et al. Cervical Cancer, Version 2.2015. J Natl Compr Canc Netw. 2015;13(4):395–404; quiz [DOI] [PubMed] [Google Scholar]
- 14.Delgado G, Bundy BN, Fowler WC Jr., Stehman FB, Sevin B, Creasman WT, et al. A prospective surgical pathological study of stage I squamous carcinoma of the cervix: a Gynecologic Oncology Group Study. Gynecol Oncol. 1989;35(3):314–20. [DOI] [PubMed] [Google Scholar]
- 15.Delgado G, Bundy B, Zaino R, Sevin BU, Creasman WT, Major F. Prospective surgical- pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 1990;38(3):352–7. [DOI] [PubMed] [Google Scholar]
- 16.Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A Gynecologic Oncology Group Study. Gynecol Oncol. 1999;73(2):177–83. [DOI] [PubMed] [Google Scholar]
- 17.Rotman M, Sedlis A, Piedmonte MR, Bundy B, Lentz SS, Muderspach LI, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiat Oncol Biol Phys. 2006;65(1):169–76. [DOI] [PubMed] [Google Scholar]
- 18.Bleker OP, Ketting BW, van Wayjen-Eecen B, Kloosterman GJ. The significance of microscopic involvement of the parametrium and/or pelvic lymph nodes in cervical cancer stages IB and IIA. Gynecol Oncol. 1983;16(1):56–62. [DOI] [PubMed] [Google Scholar]
- 19.Creasman WT, Soper JT, Clarke-Pearson D. Radical hysterectomy as therapy for early carcinoma of the cervix. Am J Obstet Gynecol. 1986;155(5):964–9. [DOI] [PubMed] [Google Scholar]
- 20.Carlson JW, Kauderer J, Hutson A, Carter J, Armer JA, Lockwood S, et al. GOG 244, the lymphedema and gynecologic cancer (LEG) study: Incidence and risk factors in newly diagnosed patients. Society of Gynecologic Oncology 49th Annual Meeting. 2018:Abstract 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanciano R, Calkins A, Bundy BN, Parham G, Lucci JA 3rd, Moore DH, et al. Randomized comparison of weekly cisplatin or protracted venous infusion of fluorouracil in combination with pelvic radiation in advanced cervix cancer: a gynecologic oncology group study. J Clin Oncol. 2005;23(33):8289–95. [DOI] [PubMed] [Google Scholar]
- 22.Monk BJ, Tian C, Rose PG, Lanciano R. Which clinical/pathologic factors matter in the era of chemoradiation as treatment for locally advanced cervical carcinoma? Analysis of two Gynecologic Oncology Group (GOG) trials. Gynecol Oncol. 2007;105(2):427–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atri M, Zhang Z, Dehdashti F, Lee SI, Ali S, Marques H, et al. Utility of PET-CT to evaluate retroperitoneal lymph node metastasis in advanced cervical cancer: Results of ACRIN6671/GOG0233 trial. Gynecol Oncol. 2016;142(3):413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rose PG, Ali S, Watkins E, Thigpen JT, Deppe G, Clarke-Pearson DL, et al. Long-term follow-up of a randomized trial comparing concurrent single agent cisplatin, cisplatin-based combination chemotherapy, or hydroxyurea during pelvic irradiation for locally advanced cervical cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25(19):2804–10. [DOI] [PubMed] [Google Scholar]
- 25.Stehman FB, Ali S, Keys HM, Muderspach LI, Chafe WE, Gallup DG, et al. Radiation therapy with or without weekly cisplatin for bulky stage 1B cervical carcinoma: follow-up of a Gynecologic Oncology Group trial. Am J Obstet Gynecol. 2007;197(5):503e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas G, Ali S, Hoebers FJ, Darcy KM, Rodgers WH, Patel M, et al. Phase III trial to evaluate the efficacy of maintaining hemoglobin levels above 12.0 g/dL with erythropoietin vs above 10.0 g/dL without erythropoietin in anemic patients receiving concurrent radiation and cisplatin for cervical cancer. Gynecol Oncol. 2008;108(2):317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DiSilvestro PA, Ali S, Craighead PS, Lucci JA, Lee YC, Cohn DE, et al. Phase III randomized trial of weekly cisplatin and irradiation versus cisplatin and tirapazamine and irradiation in stages IB2, IIA, IIB, IIIB, and IVA cervical carcinoma limited to the pelvis: a Gynecologic Oncology Group study. J Clin Oncol. 2014;32(5):458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunos CA, Radivoyevitch T, Waggoner S, Debernardo R, Zanotti K, Resnick K, et al. Radiochemotherapy plus 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, NSC #663249) in advanced-stage cervical and vaginal cancers. Gynecol Oncol. 2013;130(1):75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunos CA, Waggoner S, von Gruenigen V, Eldermire E, Pink J, Dowlati A, et al. Phase I trial of pelvic radiation, weekly cisplatin, and 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, NSC #663249) for locally advanced cervical cancer. Clin Cancer Res. 2010;16(4):1298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonomi P, Blessing JA, Stehman FB, DiSaia PJ, Walton L, Major FJ. Randomized trial of three cisplatin dose schedules in squamous-cell carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 1985;3(8):1079–85. [DOI] [PubMed] [Google Scholar]
- 31.Bloss JD, Blessing JA, Behrens BC, Mannel RS, Rader JS, Sood AK, et al. Randomized trial of cisplatin and ifosfamide with or without bleomycin in squamous carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol. 2002;20(7):1832–7. [DOI] [PubMed] [Google Scholar]
- 32.Omura GA, Blessing JA, Vaccarello L, Berman ML, Clarke-Pearson DL, Mutch DG, et al. Randomized trial of cisplatin versus cisplatin plus mitolactol versus cisplatin plus ifosfamide in advanced squamous carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 1997;15(1):165–71. [DOI] [PubMed] [Google Scholar]
- 33.Moore DH, Blessing JA, McQuellon RP, Thaler HT, Cella D, Benda J, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol. 2004;22(15):3113–9. [DOI] [PubMed] [Google Scholar]
- 34.Long HJ 3rd, Bundy BN, Grendys EC Jr., Benda JA, McMeekin DS, Sorosky J, et al. Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: a Gynecologic Oncology Group Study. J Clin Oncol. 2005;23(21):4626–33. [DOI] [PubMed] [Google Scholar]
- 35.Monk BJ, Sill MW, McMeekin DS, Cohn DE, Ramondetta LM, Boardman CH, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2009;27(28):4649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore DH, Tian C, Monk BJ, Long HJ, Omura GA, Bloss JD. Prognostic factors for response to cisplatin-based chemotherapy in advanced cervical carcinoma: a Gynecologic Oncology Group Study. Gynecol Oncol. 2010;116(1):44–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monk BJ, Sill MW, Burger RA, Gray HJ, Buekers TE, Roman LD. Phase II trial of bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol. 2009;27(7):1069–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huh WK, Brady WE, Dizon DS, Powell MA, Landrum LM, Leath CA 3rd, et al. A prospective phase II trial of the listeria-based human papillomavirus immunotherpay axalimogene filolisbac in second- and third-line metastatic cervical cancer: A NRG oncology group trial. Gynecol Oncol. 2017;145(Supplement 1):page 220, Late Breaking Abstract 2. [Google Scholar]
- 39.Tewari KS, Sill MW, Long HJ 3rd, Penson RT, Huang H, Ramondetta LM, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370(8):734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tewari KS, Sill MW, Penson RT, Huang H, Ramondetta LM, Landrum LM, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. 2017;390(10103):1654–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tewari KS, Sill MW, Monk BJ, Penson RT, Long HJ 3rd, Poveda A, et al. Prospective Validation of Pooled Prognostic Factors in Women with Advanced Cervical Cancer Treated with Chemotherapy with/without Bevacizumab: NRG Oncology/GOG Study. Clin Cancer Res. 2015;21(24):5480–7. [DOI] [PMC free article] [PubMed] [Google Scholar]