Abstract
Unaffected relatives of individuals with Lynch syndrome can be offered predictive genetic testing to guide surveillance recommendations. The decision-making process of those who decline testing, particularly those who do not attend a clinical genetics service, is poorly understood. We have addressed this gap by interviewing 33 individuals from Lynch syndrome mutation-carrying families, unaffected by cancer, who declined predictive genetic testing. Here, we analyse the data provided by 20 participants who unequivocally declined testing. Those who indicated they did not have enough information to make a decision or intended to undergo testing in the future were excluded. Analysis revealed that few decliners discussed their decision with general practitioners or genetic counsellors. Family members were commonly involved to varying degrees, with participants either (1) making group decisions with family members, (2) feeling persuaded by family members to either accept or decline testing, (3) discussing the test but making their own decision. A minority did not discuss testing with family members while making their decision. This research reveals the health communication activities of an understudied group, those declining predictive testing, and indicates that for many, health professionals play a minor role in the decision compared to family.
Keywords: Predictive genetic testing, Decision making, Communication, Decliners, Qualitative methods, Lynch syndrome
Introduction
Lynch syndrome is one of the most common inherited cancer syndromes accounting for 1–3% of all cases of colorectal cancer (CRC) [1]. Those who carry a mutation in a mismatch repair gene associated with Lynch syndrome (MLH1, MSH2, MSH6, PMS2) have a substantially increased lifetime risk of CRC estimated between 50 and 80% [2–4]. Relatives of those with Lynch syndrome can undergo predictive genetic testing to determine whether they are carrying a mutation. In Australia, an at-risk relative can be referred to a Family Cancer Clinic for genetic counselling about predictive genetic testing when a family member is diagnosed with Lynch syndrome, or if they have an unusually high family history of cancer. We focus on those with a family member carrying a Lynch syndrome mutation.
At-risk individuals are offered predictive testing to determine whether they carry a Lynch syndrome mutation. This allows for more accurate risk categorisation, enabling more appropriate surveillance and prevention, such as colonoscopy, estimated to reduce CRC risk by 56% and death by 65% [4]. If followed by appropriate screening, predictive genetic testing for Lynch syndrome has potential to reduce CRC morbidity and mortality. Therefore, it is important to understand uptake of predictive genetic testing.
Uptake of predictive genetic testing ranges from 13 to 64% [5]. Similarly, in our study of over 800 research participants in Australia who were offered predictive genetic testing, only half pursued it [6]. Those who declined testing perceived their risk of CRC to be substantially higher than their actual risk, however a higher perceived risk did not lead to more colonoscopy [7]. Reasons for declining testing included: no clear benefit, low priority, inconvenient, anxiety causing, negative life insurance implications, or potential decreased motivation for screening [8]. Other studies have shown similar reasons for declining [9]. However, little is known about how these reasons are formulated and from where information is gathered. In this study, we explore the role of genetic counsellors, general practitioners (GPs) and family members as sources of information and support.
In Australia, the majority of eligible, high risk individuals choosing to have a predictive test do so through publicly funded Familial Cancer Centres and receive pre- and post-test genetic counselling [10]. Genetic counsellors are trained to provide information about genetic testing and support the individual before and after genetic testing [11], with a strong emphasis on facilitating informed, autonomous decision-making [12, 13]. However, those who are offered testing often make their decision before seeing a genetic counsellor [14]. In fact, those who choose to decline may not pursue genetic counselling at all [8]. Participants who decline counselling immediately after being offered the test are often older [15, 16], have a lower perceived risk of mutation [15, 17], and perceive fewer benefits from genetic testing [15, 16, 18, 19]. Therefore, it is important to understand the information and support accessed by those who decline genetic testing outside of a clinical genetics service. This may be through discussions with general practitioners and family members who are already accessing genetic services, or by accessing information through the internet. Yet there is little research conducted on this question.
In this study, we use thematic analysis of semi-structured interviews to explore interactions with family and clinicians from the perspective of individuals who declined predictive genetic testing for Lynch syndrome. We consider whether individuals involve family, GPs and/or genetic counsellors in their decision-making, what the discussions entail, and what factors influence whether these discussions occur. Exploring the role of others in the decision to decline will provide a fuller understanding of the context in which predictive genetic testing is considered, particularly for those not accessing genetic services. Ultimately this research can contribute to the goal of improving and increasing informed decision-making.
Methods
This study was approved by the University of Melbourne Human Research Ethics Committee.
Sampling and recruitment
Participants for this study were recruited from the participants of the Australian Colorectal Cancer Family Registry (ACCFR), part of an international consortium, the Colon Cancer Family Registry. This large cohort contributes to research relating to the genetic and environmental causes of CRC. The ACCFR recruited recently diagnosed colorectal cancer cases via the Victorian Cancer Registry, patients from family cancer clinics (probands), and relatives of these probands. Participants were asked to donate a blood sample for DNA research. In the research setting of the ACCFR, probands were tested for germline mutations in MLH1, MSH2, MSH6 and PMS2 [20]. If a mutation was found in a proband, the available DNA of all participating relatives was tested for the proband’s mutation.
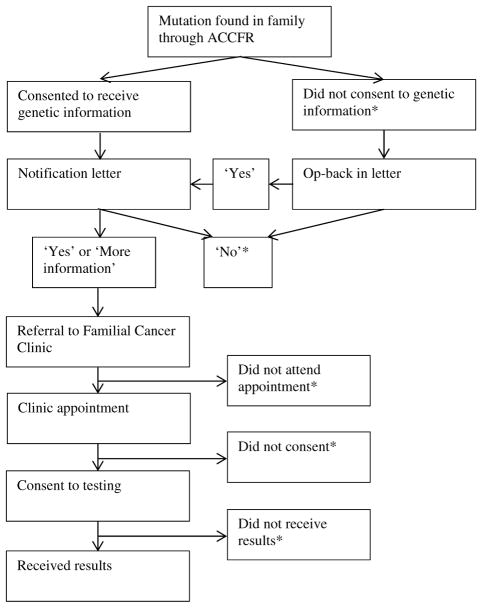
Independently of this testing, ACCFR participants were given the opportunity to learn of any clinically relevant genetic mutations, via a referral to a Family Cancer Clinic from their doctor. Participants in families that the ACCFR identified as carrying a mutation in a mismatch repair gene, and who consented to be informed of a clinically relevant result, were sent a ‘notification letter’ by the ACCFR investigators offering referral to a Family Cancer Clinic for a clinically accredited test, and to receive results. This ‘notification’ letter did not provide any results of testing. Relatives who did not consent to receive clinically relevant results were given a second letter that gave them a second opportunity to ‘opt back in’. Attendance at the clinic and whether individuals chose to receive their results was recorded. This process is summarised in Fig. 1 and described in detail elsewhere [21].
Fig. 1.
Process of testing relatives in mutation carrying families in the ACCFR (*Participants who declined at any point in this process were invited to participate in an interview.) [8]
Individuals who declined predictive genetic testing through ACCFR were invited to participate in our study. The inclusion criteria for this study were:
first or second-degree relative of an ACCFR participant found to carry a mutation associated with Lynch syndrome
no previous diagnosis of cancer under 70 years of age at the time of recruitment
declined the offer of genetic test results (actively or passively)
offered genetic information more than 12 months ago, but less than 10 years ago, to allow time for them to make a decision and to allow sufficient recall.
We purposively sampled to ensure those who declined at different stages of the process were represented. We also aimed to purposively sample for demographics (age, gender, rural vs. urban) to represent the decliners in the ACCFR.
Data collection
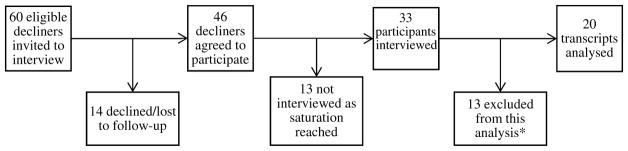
For this study, 60 suitable participants of the ACCFR were contacted by an ACCFR team member and invited to participate in a qualitative study in 2011 and 2012. Forty-six ACCFR participants agreed to be contacted, and their details were provided to the qualitative study team. Participants were posted a participant information sheet and a consent form. This was followed up by phone to determine whether they wished to participate, and to organise an interview.
Semi-structured interviews were conducted in person, or by telephone if necessary. Each interview lasted 60–90 min. The interview guide was developed after a review of the relevant literature on motivations and deterrents to predictive genetic testing, which identified the following domains of interest; cancer experience, genetic testing understanding and decision-making, health provider communication, risk perception, prevention and future intentions. Using these domains, a range of questions were developed and revised in consultation with a steering group including a consumer, genetic counsellors and researchers. Preliminary analysis of data, which has been presented elsewhere [8], was used to assess the saturation of themes. Recruitment ceased at 33 interviews. Interviews were digitally recorded and transcribed verbatim. The interviewer checked audio files against the transcripts to ensure accuracy. Identifying details were removed from the transcripts and participants were assigned pseudonyms.
Of the 33 interviews, 20 transcripts were deemed appropriate for this analysis. Using a typology described elsewhere [8], 13 participants were excluded from analysis. We excluded four ‘uninformed’ participants from this analysis because they were not aware that it was possible to have genetic testing for colorectal cancer at the time of the interview and were therefore unable to comment on the decision-making process. We also excluded those who intended to undergo testing but had not yet done so as they were not considered true decliners. Construction of the final sample of 20 transcripts is summarised in Fig. 2.
Fig. 2.
Construction of the sample of interview transcripts analysed. (*13 participants were excluded from this analysis because they did not have enough information to make a decision, or they intended to undergo testing, but had not yet done so)
Data analysis
The 20 relevant transcripts were imported into NVivo software [22]. Thematic analysis as described by Braun and Clarke [23] was used to determine and explain patterns in the data. The data was read multiple times to identify key words and concepts. This was used to create and develop a coding framework. The transcripts were coded using the framework, and codes and sub-themes were iteratively developed to ensure they reflected the entire dataset. This analysis aimed to explore how genetic counsellors, GPs and family members were involved in decision-making. An inductive approach was then used to explore what influenced discussion patterns.
Results
Participant characteristics
Demographics are displayed in Table 1. Mean age of participants was 47, of which 60% were female. There were more urban participants (80%) than rural; however rural participants were over-represented compared to the general population [24]. Family history of participants varied from no first-degree relatives (parents, children, siblings) to five.
Table 1.
Demographics
| Characteristics | Number (%) n = 20 |
|---|---|
| Mean age (range) | 46.8 (28–68) |
| Gender (%) | |
| Male | 8 (40) |
| Female | 12 (60) |
| Location (%) | |
| Urban | 16 (80) |
| Rural | 4 (20) |
| First degree relatives diagnosed with CRC (%) | |
| 0 | 6 (30) |
| 1 | 10 (50) |
| 2 | 2 (10) |
| ≥ 3 | 2 (10) |
| Reasons for declining testing (participants may have more than one reason) (%) | |
| Won’t alter anything | 8 (40) |
| Anxiety causing | 7 (35) |
| Insurance concerns | 6 (30) |
| Fear of decreased screening | 1 (5) |
The role of others in decision-making varied, with heavy to no involvement. Most participants involved family members to some degree (n = 14), however health professionals were underutilised as a source of information and support (n = 6). Five participants did not involve a health professional, or family member in their decision (Table 2).
Table 2.
Number (%) of participants whom involved a genetic counsellor, general practitioner or family member in their decision about predictive genetic testing
| Familya
|
|||
|---|---|---|---|
| Yes | No | Total | |
| Health professional | |||
| Yes (GCb) | 2 | 1 | 3 (15%) |
| Yes (GPc) | 3 | 0 | 3 (15%) |
| No | 9 | 5 | 14 (70%) |
| Total | 14 (70%) | 6 (30%) | 20 |
At least one first or second-degree relative
Genetic counsellor
General practitioner
Health professional involvement
Participants were offered referral to a genetic counsellor for more information about the test. Only three participants spoke with a genetic counsellor prior to their decision to decline testing. These participants all intended to undergo genetic testing, until learning about potential risks, in particular insurance.
I wanted to know because then I’d know myself and then I could be proactive about it… [genetic counsellor] explained to me about…life insurance… And I thought this is just not right…that’s sort of in a real quick snapshot how it got from yes to no to forget about it. (male, 48, declined due to insurance concerns)
In addition, only three participants discussed predictive genetic testing with a GP. Two participants felt that their GPs expressed opinions rather than providing information.
[GP] didn’t sort of add or suggest anything…he steered me away from it a bit…he sort of just sat on the fence either one way or the other. He didn’t seem to be that positive about it. No, I didn’t sort of get the message that he highly recommended it. (male, 44, declined because test won’t alter anything)
One participant brought up family history and the need for colonoscopy with her GP, who informed her that genetic counselling was also an option.
I think [GP] might have asked if I have had genetic counselling and I said I haven’t at this stage, I decided not to and he thinks it’s an area of you know, you know just—I think he was just making sure that I knew that I could if I wanted, you know that it was an option. (female, 29, declined to avoid anxiety)
In all three cases, participants had a GP that they saw regularly and the patient initiated discussion about cancer risk or genetic testing.
I went to a GP and asked for a referral to see someone for a colonoscopy…I chatted to him about it but I was a little bit clued up into that I needed to see someone. (female, 29, declined to avoid anxiety)
Although only three participants discussed the test with a GP, 15 participants discussed their general cancer risk with a GP.
I’ve certainly tried to give [GP] an overview of what some of the family history is…no [discussion about screening] other than when you reach a certain age it would be a good idea to have it. (female, 30, declined to avoid anxiety)
Five participants did not speak to a GP about any aspect of cancer risk. These participants did not have a GP that they saw on a regular basis.
I haven’t been going regularly to a GP that I could just have that conversation with, or any conversation for that matter, but I keep myself in reasonable health. (male, 44, declined because test results may lead to decreased screening)
One participant who did not bring up cancer risk or genetic testing with a doctor, was sceptical of doctors’ intentions.
[Doctor] would say get genetically tested and know for sure. It’s not their problem is it? (male, 44, declined because test results may lead to decreased screening)
Family involvement
Family members were involved in the decision to varying degrees. Two participants made group decisions to decline testing as a family. Both participants were influenced by their parents’ decision.
I think we must have made a list of pros and cons, and came to the decision as a family [participant, father, mother, brother] that it was best that we don’t get tested. But having said that I’m sure if I wanted to my whole family would have supported me in that decision. (male, 28, declined because test won’t change anything and insurance concerns)
Five participants felt pressured by family members to either accept or decline testing, despite also mentioning that family members would ultimately support the final decision.
My youngest [son] was very much like no mum…so he was very much not to go ahead with it. He said you’d be worried sick all the time…[but] they were sort of just supportive of whatever I did anyway. (female, 65, declined to avoid anxiety)
Seven participants discussed testing with family members before making their own decision.
[Husband is] quite pragmatic too about anything that you have tested. If you’re gonna have a test you have to work your way through the potential scenarios that the results of that will bring…so yeah I talked to him about it. (female, 54, declined because test won’t change anything)
Six participants did not involve family members in their decision. Two of the six participants did not discuss any aspect of the test with family. The remaining four participants discussed aspects of genetic testing, such as who in the family had undergone testing, or discussion after making the decision.
[Discussion] would have been post me making the decision, so I was talking to my cousin for example and we were talking about it and I, at that point, said I’m not going to do that, this is my reasoning, that was about it. (female, 30, declined to avoid anxiety)
Transcripts revealed various factors that may influence the degree to which family members are involved in genetic testing decision-making. Discussion was limited when participants were not close with certain family members.
The remainder of the family has become quite fragmented so I know when another one passes on, but I don’t know whether they took part in the study. (female, 54, declined to avoid anxiety)
There was also a desire to avoid negative topics and persuasion.
Why would I talk about it…we don’t have conversations about that. We have conversations about family. We don’t talk about what we’re dying of, we talk about what’s keeping us alive. (female, 55, declined because test won’t change anything)
Some participants prioritised other diseases or family members over cancer prevention.
We talk more specifically about dad’s health than about cancer in general. (female, 40, declined because test won’t change anything)
Lastly, family explanations of cancer may have influenced discussion of genetic testing. Those who did not believe genetics increased the risk of cancer, may not consider testing as an option.
I think we’re just an unlucky family. Like obviously there’s a lot of families around and it’s just there. So, and you can think oh God, you know, there’s that old, go and get some horrible person, you know, go and pick on some horrible family, just leave me alone. (female, 38, declined because test won’t change anything)
As well as these discussions, participants mentioned other sources of information about CRC and genetic testing: other medical specialists, study/employment, colleagues, friends, television, radio, books and information leaflets at medical centres.
Discussion
The decision to undergo predictive genetic testing is complex and influenced by several factors. As well as considering individual reasons for declining, it is important to understand the setting in which decision-making occurs. This will inform service providers and clinicians facilitating decision-making, particularly for those who do not access genetic services.
In Australia, a referral from a health care professional is required to access most genetic services. Participants in this study were offered referral to a genetic counsellor and were prompted to obtain referral from their GP. Interview transcripts revealed the way in which GPs were involved in decision making. Fifteen participants recalled discussing cancer risk with a GP, however only three participants discussed their decision about predictive genetic testing with a GP. Of the three participants who did involve a GP in their genetic testing decision, only one GP suggested the option of genetic counselling, whereas two GPs offered more directive opinions as to whether or not the participant should undergo testing. These participants did not recall attending a genetic counselling appointment. In this study, it is unclear as to whether discussions with GPs occurred before or after receiving the notification letter from the study, and whether this letter was taken to the appointment. Even so, these interactions may be reflective of the barriers to referral and risk assessment that GPs face.
GPs have identified challenges that they face in risk assessment and referral, such as the patient being unaware of family history, limited time to collect family history, lack of Lynch syndrome knowledge to assess risk and make recommendations and uncertainty regarding referral [25–28]. For example, most clinicians in an Australian study reported that if presented with an asymptomatic individual at risk of Lynch syndrome, they would assess family history and refer them to genetic services. However, when asked to consider a hypothetical at-risk patient, one-third of clinicians, particularly GPs, did not consider this patient to be at risk of CRC or endometrial cancer [26]. Surveys with Lynch syndrome-affected families demonstrate the impact of these barriers, with 41% of reporting that speaking to their GP was only somewhat or not useful, and qualitative responses illustrating the challenges of obtaining referral through a GP [29].
Despite the barriers, GPs are an important source of health information and can play a significant role in influencing health decisions [29–31]. Clinicians view recognising and referring at-risk individuals to genetic services as part of the GP role [26]. For example, Australian clinicians reported a slight preference toward oncologists and GPs compared to other clinicians when surveyed about who is responsible for the initial referral to genetic services for Lynch syndrome [26]. Research in this area suggests resources and GP education [25–28], however, it is also important to acknowledge that GPs may be reluctant to take on enhanced roles in genetics, with clinical genetics being viewed by GPs as ‘rare, complex, esoteric, and specialist’ [32]. It is important to consider perceived identities and roles of health professionals when addressing barriers to risk assessment, referral, and communication about genetics.
Our analysis highlights that decliners may not involve GPs in their genetic testing decisions. Research into GP-patient communication suggests that differing experiences of doctor–patient communication across these characteristics such as age, gender, ethnicity and ability to speak English may influence an individual’s trust and trust and confidence in health professionals [33, 34], as well as varying experiences of GP-patient communication [35].
Through this study, we suggest factors that influence GP communication specifically in the context of genetic testing decision making. The three participants who discussed their decision with a GP all initiated this discussion. It is unsurprising that patient enquiry is a strong predictor of recommendation to genetic services given the research regarding challenges GPs face when determining which patients should be referred to genetic services [36]. Although many participants may not have wished to involve a health professional, some may have felt unable to initiate a desired discussion with a GP. The three participants who involved a GP, had a GP that they saw regularly. Studies have suggested that having a regular doctor increases the use of GP services [37]. Those with a regular GP may feel more able to initiate discussions about genetic testing with a GP. One participant displayed scepticism about doctors’ intentions. Individuals who mistrust health professionals may feel unable to discuss their healthcare decisions with GPs, which may impact on uptake of health behaviours such as genetic testing. In fact, higher levels of trust in the information sources from which participants heard about genetic testing have been associated with increased uptake of the test [38]. This is particularly concerning because publicly funded genetic testing in Australia often requires a referral from a health professional.
Despite limited discussion with health professionals, most participants in this study discussed their decision with at least one family member, involving them to varying degrees. Transcripts revealed the ways in which individuals may involve family members in their decisions. Participants either made a joint decision with their family, felt persuaded by another family member to make a particular decision, involved family but made their own decision, or did not involve any family members in their decision. This typology can be viewed in the context of existing family patterns and rules about what is acceptable to talk about, when, and with whom. Family communication patterns theory [39–42] suggests that families create shared social realities which influence the way in which family members communicate and how they are involved in decision-making. This theory suggests that in some families, communication is open and warm, whereas in others it is restricted. Decision making may involve collaboration to make consensual decisions. Other families may communicate openly, but ultimately make individual decisions. In some families, authority figures make decisions, while in others, individuals make decisions in isolation from other family members. Decisions can be based on interests of the individual, or of the family. This spectrum was evident across our participants. The extent to which these natural communication patterns can be influenced is unknown. However, it is important to consider these patterns when facilitating family communication and decision-making.
Facilitating family communication is important to inform at-risk relatives about the possibility of testing. It has also been found that family communication may encourage at-risk individuals to attend familial cancer clinics, with family members who were persuaded to have counselling and testing being more likely to seek these services, and more likely to pursue them sooner [43]. On the other hand, barriers to family communication may impact the uptake of genetic testing in at-risk relatives of mutation carriers [44, 45]. Research in this area aims to improve the ability of health professionals to facilitate communication between family members, focusing on the diagnosed individual informing relatives of their risk and the option of predictive genetic testing [5, 46].
Our data highlights that family communication does not only occur at the point of disclosure. These situations are dynamic, and family conversations occur over extended periods of time, with further opportunities for family to be involved in decision-making. The degree to which an individual discusses testing with their family may be influenced by their engagement with their cancer risk at that given time. A study conducted in 2002, suggested that the degree of an individual’s engagement with cancer risk varies from partial to intense. Engagement can change over time, as they become more cognitively and emotionally involved with their cancer risk [47]. Further understanding of how families are involved in decision making over time will reveal more of the factors that may influence decision making.
Limitations
Participants in this study were interviewed 12 months to 10 years after their offer to allow enough time for a decision and to allow sufficient recall of the decision. We considered this variable when describing decliners and did not find there to be any patterns or impact on decision making.
Our study highlights the role of others in decision-making about predictive genetic testing, only from the perspective of an at-risk individual offered testing. However, our work complements previous research on communication about genetic testing from other perspectives, i.e. affected individuals and health professionals, leaving a clear gap in the research which we aimed to address. Furthermore, participants were offered predictive genetic testing through participation in a research study. Thus, experiences of these participants may not be reflective of decision-making and discussion about the test when first alerted by a family member. However, their experiences provide insight into the ongoing decision-making process of those who have declined genetic testing, after becoming aware of the option.
Conclusion
Our study highlights that decisions about predictive genetic testing are made in a social context, and provides insight into the ways in which health professionals and family members are involved in the decision to decline testing. Involvement of others in decision making is an ongoing process rather than a single instance, and further research to quantify how others influence decision making in the absence of genetic services is an important next step. Furthermore, some individuals may not involve anyone in their decision. Further research into the decision-making process of this group will allow a better understanding of the factors that may influence decision-making in the absence of information and support from families and health professionals.
Acknowledgments
This work was supported by the Victorian Cancer Agency (#EO109-33), National Cancer Institute, National Institutes of Health (RFA #CA-95-011), and through cooperative agreements with the Australasian Colorectal Cancer Family Registry (U01 CA097735). Findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Cancer Institute. We acknowledge the data collection conducted by Alison Rutstein and Heather Niven, and thank the participants for sharing their experiences.
References
- 1.Lynch HT, Smyrk T. Hereditary nonpolyposis colorectal cancer (Lynch syndrome): an updated review. Cancer. 1996;78(6):1149–1167. doi: 10.1002/(SICI)1097-0142(19960915)78:6<1149::AID-CNCR1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Dowty JG, Win AK, Buchanan DD, Lindor NM, Macrae FA, Clendenning M, Antill YC, Thibodeau SN, Casey G, Gallinger S. Cancer risks for MLH1 and MSH2 mutation carriers. Hum Mutat. 2013;34(3):490–497. doi: 10.1002/humu.22262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Senter L, Clendenning M, Sotamaa K, Hampel H, Green J, Potter JD, Lindblom A, Lagerstedt K, Thibodeau SN, Lindor NM. The clinical phenotype of Lynch syndrome due to germline PMS2 mutations. Gastroenterology. 2008;135(2):419. e411–428. e411. doi: 10.1053/j.gastro.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umar A, Boland CR, Terdiman JP, Syngal S, Chapelle AD, Rüschoff J, Fishel R, Lindor NM, Burgart LJ, Hamelin R. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96(4):261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaff CL, Clarke AJ, Atkinson P, Sivell S, Elwyn G, Iredale R, Thornton H, Dundon J, Shaw C, Edwards A. Process and outcome in communication of genetic information within families: a systematic review. Eur J Hum Genet. 2007;15(10):999–1011. doi: 10.1038/sj.ejhg.5201883. [DOI] [PubMed] [Google Scholar]
- 6.Keogh LA, Fisher D, Gorin SS, Schully SD, Lowery JT, Ahnen DJ, Maskiell JA, Lindor NM, Hopper JL, Burnett T. How do researchers manage genetic results in practice? The experience of the multinational Colon Cancer Family Registry. J Community Genet. 2014;5(2):99–108. doi: 10.1007/s12687-013-0148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flander L, Speirs-Bridge A, Rutstein A, Niven H, Win AK, Ouakrim DA, Hopper JL, Macrae F, Keogh L, Gaff C. Perceived versus predicted risks of colorectal cancer and self-reported colonoscopies by members of mismatch repair gene mutation-carrying families who have declined genetic testing. J Genet Couns. 2014;23(1):79–88. doi: 10.1007/s10897-013-9614-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keogh LA, Niven H, Rutstein A, Flander L, Gaff C, Jenkins M. Choosing not to undergo predictive genetic testing for hereditary colorectal cancer syndromes: expanding our understanding of decliners and declining. J Behav Med. 2017;40:583–594. doi: 10.1007/s10865-016-9820-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadley DW, Jenkins J, Dimond E, Nakahara K, Grogan L, Liewehr DJ, Steinberg SM, Kirsch I. Genetic counseling and testing in families with hereditary nonpolyposis colorectal cancer. Arch Intern Med. 2003;163(5):573–582. doi: 10.1001/archinte.163.5.573. [DOI] [PubMed] [Google Scholar]
- 10.Henderson BJ, Maguire BT, Gray J, Morrison V. How people make decisions about predictive genetic testing: an analogue study. Psychology Health. 2006;21(4):513–539. [Google Scholar]
- 11.Human Genetics Society of Australia. Process of genetic counseling. 2012. [Google Scholar]
- 12.Chadwick RF. What counts as success in genetic counselling? J Med Ethics. 1993;19(1):43–49. doi: 10.1136/jme.19.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skirton H, Goldsmith L, Jackson L, Tibben A. Quality in genetic counselling for presymptomatic testing—clinical guidelines for practice across the range of genetic conditions. Eur J Hum Genet. 2013;21(3):256. doi: 10.1038/ejhg.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brain K, Sivell S, Bennert K, Howell L, France L, Jordan S, Rogers M, Gray J, Sampson J. An exploratory comparison of genetic counselling protocols for HNPCC predictive testing. Clin Genet. 2005;68(3):255–261. doi: 10.1111/j.1399-0004.2005.00491.x. [DOI] [PubMed] [Google Scholar]
- 15.Schlich-Bakker KJ, ten Kroode HF, Wárlám-Rodenhuis CC, van den Bout J, Ausems MG. Barriers to participating in genetic counseling and BRCA testing during primary treatment for breast cancer. Genet Med. 2007;9(11):766–777. doi: 10.1097/gim.0b013e318159a318. [DOI] [PubMed] [Google Scholar]
- 16.Peters N, Domchek SM, Rose A, Polis R, Stopfer J, Armstrong K. Knowledge, attitudes, and utilization of BRCA1/2 testing among women with early-onset breast cancer. Genet Test. 2005;9(1):48–53. doi: 10.1089/gte.2005.9.48. [DOI] [PubMed] [Google Scholar]
- 17.Lerman C, Daly M, Masny A, Balshem A. Attitudes about genetic testing for breast-ovarian cancer susceptibility. J Clin Oncol. 1994;12(4):843–850. doi: 10.1200/JCO.1994.12.4.843. [DOI] [PubMed] [Google Scholar]
- 18.Geer KP, Ropka ME, Cohn WF, Jones SM, Miesfeldt S. Factors influencing patients’ decisions to decline cancer genetic counseling services. J Genet Couns. 2001;10(1):25–40. doi: 10.1023/a:1009451213035. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong K, Calzone K, Stopfer J, Fitzgerald G, Coyne J, Weber B. Factors associated with decisions about clinical BRCA1/2 testing. Cancer Epidemiol Prev Biomark. 2000;9(11):1251–1254. [PubMed] [Google Scholar]
- 20.Win AK, Young JP, Lindor NM, Tucker KM, Ahnen DJ, Young GP, Buchanan DD, Clendenning M, Giles GG, Winship I. Colorectal and other cancer risks for carriers and noncarriers from families with a DNA mismatch repair gene mutation: a prospective cohort study. J Clin Oncol. 2012;30(9):958–964. doi: 10.1200/JCO.2011.39.5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keogh LA, van Vliet CM, Studdert DM, Maskiell JA, Macrae FA, St John DJ, Gaff CL, Young MA, Southey MC, Giles GG. Is uptake of genetic testing for colorectal cancer influenced by knowledge of insurance implications? Med J Aust. 2009;191(5):255. doi: 10.5694/j.1326-5377.2009.tb02778.x. [DOI] [PubMed] [Google Scholar]
- 22.QSR International Pty Ltd. Nvivo qualitative data analysis software. 2010. [Google Scholar]
- 23.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101. [Google Scholar]
- 24.Baxter J, Hayes A, Gray M. Families in regional, rural and remote Australia (Fact sheet) 2011. [Google Scholar]
- 25.Tan YY, Fitzgerald LJ. Barriers and motivators for referral of patients with suspected Lynch syndrome to cancer genetic services: a qualitative study. J Pers Med. 2014;4(1):20–34. doi: 10.3390/jpm4010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan YY, Spurdle AB, Obermair A. Knowledge, attitudes and referral patterns of Lynch syndrome: a survey of clinicians in Australia. J Pers Med. 2014;4(2):218–244. doi: 10.3390/jpm4020218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vig HS, Armstrong J, Egleston BL, Mazar C, Toscano M, Bradbury AR, Daly MB, Meropol NJ. Cancer genetic risk assessment and referral patterns in primary care. Genet Test Mol Biomark. 2009;13(6):735–741. doi: 10.1089/gtmb.2009.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wideroff L, Freedman AN, Olson L, Klabunde CN, Davis W, Srinath KP, Croyle RT, Ballard-Barbash R. Physician use of genetic testing for cancer susceptibility. Cancer Epidemiol Prev Biomark. 2003;12(4):295–303. [PubMed] [Google Scholar]
- 29.Morris S, Rice T, O’Neill S, Raets E, Fairbank B. Misdiagnosed, misunderstood and missing out: Lynch syndrome Australia’s untold health story. 2017. [Google Scholar]
- 30.Ouakrim DA, Lockett T, Boussioutas A, Keogh L, Flander LB, Hopper JL, Jenkins MA. Screening participation predictors for people at familial risk of colorectal cancer: a systematic review. Am J Prev Med. 2013;44(5):496–506. doi: 10.1016/j.amepre.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 31.Singer E, Antonucci T, Van Hoewyk J. Racial and ethnic variations in knowledge and attitudes about genetic testing. Genet Test. 2004;8(1):31–43. doi: 10.1089/109065704323016012. [DOI] [PubMed] [Google Scholar]
- 32.Mathers J, Greenfield S, Metcalfe A, Cole T, Flanagan S, Wilson S. Family history in primary care: understanding GPs’ resistance to clinical genetics—qualitative study. Br J Gen Pract. 2010;60(574):e221–e230. doi: 10.3399/bjgp10X501868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Croker JE, Swancutt DR, Roberts MJ, Abel GA, Roland M, Campbell JL. Factors affecting patients’ trust and confidence in GPs: evidence from the English national GP patient survey. BMJ Open. 2013;3(5):e002762. doi: 10.1136/bmjopen-2013-002762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong ST, Black C, Cutler F, Brooke R, Haggerty JL, Levesque J-F. Patient-reported confidence in primary healthcare: are there disparities by ethnicity or language? BMJ Open. 2014;4(2):e003884. doi: 10.1136/bmjopen-2013-003884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burt J, Lloyd C, Campbell J, Roland M, Abel G. Variations in GP–patient communication by ethnicity, age, and gender: evidence from a national primary care patient survey. Br J Gen Pract. 2016;66(642):e47–e52. doi: 10.3399/bjgp15X687637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klitzman R, Thorne D, Williamson J, Marder K. The roles of family members, health care workers, and others in decision-making processes about genetic testing among individuals at risk for Huntington disease. Genet Med. 2007;9(6):358–371. doi: 10.1097/GIM.0b013e3180653c5a. [DOI] [PubMed] [Google Scholar]
- 37.Thanh NX, Rapoport J. Health services utilization of people having and not having a regular doctor in Canada. Int J Health Plan Manag. 2017;32(2):180–188. doi: 10.1002/hpm.2338. [DOI] [PubMed] [Google Scholar]
- 38.Roberts MC, Taber JM, Klein WM. Engagement with genetic information and uptake of genetic testing: the role of trust and personal cancer history. J Cancer Educ. 2017 doi: 10.1007/s13187-016-1160-9. [DOI] [PubMed]
- 39.Koerner AF, Fitzpatrick MA. Toward a theory of family communication. Commun Theory. 2002;12(1):70–91. [Google Scholar]
- 40.Koerner FA. Understanding family communication patterns and family functioning: the roles of conversation orientation and conformity orientation. Ann Int Commun Assoc. 2002;26(1):36–65. [Google Scholar]
- 41.Koerner AF, Fitzpatrick MA. Family communication patterns theory: a social cognitive approach. In: Braithwaite DO, Baxter LA, editors. Engaging theories in family communication: multiple perspectives. Sage; Thousand Oaks: 2006. pp. 50–65. [Google Scholar]
- 42.Koerner AF, Schrodt P. An introduction to the special issue on family communication patterns theory. J Fam Commun. 2014;14(1):1–15. [Google Scholar]
- 43.Peterson SK, Watts BG, Koehly LM, Vernon SW, Baile WF, Kohlmann WK, Gritz ER. How families communicate about HNPCC genetic testing: findings from a qualitative study. Am J Med Genet C. 2003;119:78–86. doi: 10.1002/ajmg.c.10010. [DOI] [PubMed] [Google Scholar]
- 44.McCann S, MacAuley D, Barnett Y, Bunting B, Bradley A, Jeffers L, Morrison PJ. Family communication, genetic testing and colonoscopy screening in hereditary non-polyposis colon cancer: a qualitative study. Psycho-Oncology. 2009;18(11):1208–1215. doi: 10.1002/pon.1487. [DOI] [PubMed] [Google Scholar]
- 45.Mesters I, Ausems M, Eichhorn S, Vasen H. Informing one’s family about genetic testing for hereditary non-polyposis colorectal cancer (HNPCC): a retrospective exploratory study. Fam Cancer. 2005;4(2):163–167. doi: 10.1007/s10689-004-7992-1. [DOI] [PubMed] [Google Scholar]
- 46.Hodgson J, Gaff C. Enhancing family communication about genetics: ethical and professional dilemmas. J Genet Couns. 2013;22(1):16–21. doi: 10.1007/s10897-012-9514-x. [DOI] [PubMed] [Google Scholar]
- 47.Mcallister M. Predictive genetic testing and beyond: a theory of engagement. J Health Psychol. 2002;7(5):491–508. doi: 10.1177/1359105302007005628. [DOI] [PubMed] [Google Scholar]