Abstract
The storage and transportation of raw milk at low temperatures promote the growth of psychrotrophic bacteria and the production of thermo-stable enzymes, which pose great threats to the quality and shelf-life of dairy products. Though many studies have been carried out on the spoilage potential of psychrotrophic bacteria and the thermo-stabilities of the enzymes they produce, further detailed studies are needed to devise an effective strategy to avoid dairy spoilage. The purpose of this study was to explore the spoilage potential of psychrotrophic bacteria from Chinese raw milk samples at both room temperature (28 °C) and refrigerated temperature (7 °C). Species of Yersinia, Pseudomonas, Serratia, and Chryseobacterium showed high proteolytic activity. The highest proteolytic activity was shown by Yersinia intermedia followed by Pseudomonas fluorescens (d). Lipolytic activity was high in isolates of Acinetobacter, and the highest in Acinetobacter guillouiae. Certain isolates showed positive β-galactosidase and phospholipase activity. Strains belonging to the same species sometimes showed markedly different phenotypic characteristics. Proteases and lipases produced by psychrotrophic bacteria retained activity after heat treatment at 70, 80, or 90 °C, and proteases appeared to be more heat-stable than lipases. For these reasons, thermo-stable spoilage enzymes produced by a high number of psychrotrophic bacterial isolates from raw milk are of major concern to the dairy industry. The results of this study provide valuable data about the spoilage potential of bacterial strains in raw milk and the thermal resistance of the enzymes they produce.
Keywords: Spoilage enzyme, Psychrotrophic bacteria, Raw milk, Thermo-stability
1. Introduction
Raw milk serves as an ideal medium for the growth of bacteria due to its high nutritional value (Champagne et al., 1994). A cold chain system is commonly used for controlling the growth of bacteria in raw milk during storage and transportation. Psychrotrophic bacteria are ubiquitous organisms that have the ability to grow at 7 °C or below, regardless of their higher optimal growth temperature (Sørhaug and Stepaniak, 1997). These bacteria usually account for less than 10% of the total microflora of raw milk, but invariably become predominant during the transportation and prolonged storage of raw milk at low temperatures (Sørhaug and Stepaniak, 1997).
Raw milk can be contaminated with psychrotrophic bacteria from a variety of sources including air, water, soil, and milking equipment (Vacheyrou et al., 2011). Among psychrotrophic bacteria, genera of Pseudomonas, Acinetobacter, Flavobacterium, Chryseobacterium, and Serratia are the most frequently isolated from raw milk (Vithanage et al., 2016; Yuan et al., 2017).
Increasing global demand for dairy products requires dairy manufacturers to produce products with high quality and prolonged shelf-life. The dairy industry is facing constraints related to maintaining high quality and avoiding losses as a result of microbial spoilage. Mesophilic and thermophilic spore forming bacteria have the potential to contaminate processed dairy products, which may then fail to comply with specifications for spore content (Sadiq et al., 2016). However, psychrotrophic bacteria present more serious challenges to the dairy industry. Raw milk is contaminated with heat-stable enzymes produced by a broad spectrum of psychrotrophic bacteria at low temperatures, which can survive all successive processing conditions and remain active in processed dairy products (Vithanage et al., 2016). For example, the decimal reduction time (D-value) of proteases produced by Pseudomonas spp. was 416.67 min at 75 °C and 250 min at 85 °C, indicating that these enzymes are difficult to inactivate by normal thermal processing techniques (Machado et al., 2016). Consequently, these heat-stable enzymes may lead to unacceptable biochemical changes, a decrease in nutritional value, and reduced shelf-life of dairy products (Stoeckel et al., 2016). Lipases catalyze the hydrolysis of triglycerides which cause rancid, butyric, or soapy flavors and also may lead to a reduction in milk foaming properties (Chen et al., 2003; Bekker et al., 2016). Proteases hydrolyze casein fractions and produce defects described as bitter off-flavors and result in age gelation (Stoeckel et al., 2016). Phospholipases degrade the integrity of the milk fat globule membrane, facilitating more lipolysis by milk’s natural lipases (Lilbaek et al., 2007). β-Galactosidases catalyze the hydrolysis of β-1,4-galactosidic bonds in lactose of milk (Deeth et al., 2002; Chen et al., 2009). Therefore, spoilage caused by psychrotrophic bacteria and their enzymes is a major concern in the dairy industry.
In our previous study (Yuan et al., 2017), a diverse range of psychrotrophic bacteria were found in Chinese raw milk, but their ability to produce spoilage enzymes or the thermo-stability patterns of these enzymes were not studied. Thus, the objective of this study was to explore the enzymatic characteristics of all bacterial isolates in Chinese raw milk at both room temperature (28 °C) and refrigerated temperature (7 °C), and to evaluate the thermo-resistance of spoilage enzymes, thereby enhancing knowledge of the spoilage potential of psychrotrophic bacteria.
2. Materials and methods
2.1. Bacterial strains
Four-hundred and eighty strains previously isolated from Chinese raw milk samples were studied (Yuan et al., 2017). These strains, representing 24 genera, 74 species, and 85 different random amplified polymorphic DNA (RAPD)-based genetic groups, were maintained in nutrient broth containing 20% glycerol (Sinopharm Chemical Reagent Co., Ltd., China) at −80 °C.
2.2. Screening for enzyme production
All strains were checked for their abilities to produce protease, lipase, β-galactosidase, and phospholipase on the following screening media: plate count agar supplemented with 6% (0.06 g/ml) skimmed milk powder (Difco, USA) for protease; tributyrin agar (Sigma-Aldrich, USA) for lipase; nutrient agar plate spread with 35 μl 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal; Sigma-Aldrich, USA) and 20 μl isopropyl-β-D-thiogalactopyranoside (IPTG; Sigma-Aldrich, USA) for β-galactosidase; and nutrient agar plate supplemented with 8% (v/v) egg yolk emulsion (Oxoid, UK) for phospholipase. Each test was performed at 7 °C for 7 d and 28 °C for 2 d.
2.3. Quantitative assessment of total proteolytic activity
Strains that showed positive protease production on screening medium were selected to quantify their total proteolytic activity. Strains were first grown in nutrient broth for 36 h on a shaking incubator at 150 r/min. They were then diluted in 50 ml commercial ultra-heat-treated (UHT) milk to a final concentration of 1×104 colony-forming unit (CFU)/ml, corresponding to the psychrotrophic bacterial cell density found in our previous study (Yuan et al., 2017), and incubated on a shaking incubator at 120 r/min under two different conditions: 7 °C for 7 d and 28 °C for 2 d.
Samples were centrifuged at 12 000g for 10 min at 4 °C after the incubation. Proteolytic activity was measured using azocasein (Sigma, USA) as a substrate following the protocol described by Santos et al. (1996) with some modifications. To initialize the reaction, 100 μl cell-free supernatant was mixed with 500 μl phosphate buffer saline (PBS; 50 mmol/L, pH 7.2, Sangon Biotech Co., Ltd., China). Then, 100 μl azocasein (1.5% (15 g/L), dissolved in 50 mmol/L PBS) was added. The mixture was incubated at 37 °C for 60 min and the reaction was stopped by adding 500 μl of 20% (0.2 g/ml) trichloroacetic acid (Aladdin, China). After centrifugation at 12 000g for 5 min at 4 °C, absorbance at 366 nm was measured using a spectrometer-based absorbance microplate reader (Thermo Fisher Multiskan FC, USA). A control was included, in which 100 μl of sterile PBS was used in place of supernatant. The proteolytic activity is expressed as the increase of absorption at 366 nm per hour and milliliter (∆A h−1·ml−1). Each assay was performed in triplicate and with three replicates.
2.4. Quantitative assessment of total lipolytic activity
Strains that showed positive lipase production on screening medium were selected to quantify the total lipolytic activity. Strains were grown in nutrient broth for 36 h on a shaking incubator at 150 r/min, and then diluted in 50 ml UHT milk to a concentration of 1×104 CFU/ml. The milk samples were then incubated on a shaking incubator at 120 r/min under two different conditions: 7 °C for 7 d and 28 °C for 2 d.
After incubation, samples were centrifuged at 15 000g for 20 min at 4 °C, and the supernatants were filtered using 0.45-μm cellulose acetate filter units to avoid the interference of milk proteins (Abdou, 2003; Chen et al., 2003). Lipolytic activity was measured using p-nitrophenol palmitate (p-NPP; Sigma, USA) as a substrate following the method described by Teh et al. (2013) with slight modifications. To initialize the reaction, 100 μl of cell-free supernatant was added to 900 μl substrate solution containing 3 mg of p-NPP dissolved in 1 ml isopropanol (Aladdin, China), 9 ml of 50 mmol/L Tris-HCl (pH 8) solution containing 40 mg of Triton X-100 (Aladdin, China), and 1 mg of arabic gum (Aladdin, China). The mixture was incubated at 37 °C for 20 min and the reaction was stopped by adding 500 μl 95% ethanol (Sinopharm Chemical Reagent Co., Ltd., China). After centrifugation at 15 000g for 5 min at 4 °C, absorbance at 410 nm was measured using a spectrometer-based absorbance microplate reader. A control was included, in which 100 μl sterile PBS was used in place of the supernatant. The lipolytic activity was expressed as the amount of p-nitrophenol (μmol) released per minute per milliliter (μmol/(min·ml)) of milk sample under the assay conditions described above. Each assay was performed in triplicate and with three replicates.
2.5. Heat resistance of proteases and lipases
Strains that proved to be positive for protease or lipase production were selected to determine the thermo-resistance of their enzymes. Strains were grown in nutrient broth for 36 h on a shaking incubator at 150 r/min and then diluted in 50 ml UHT milk to a concentration of 1×104 CFU/ml, followed by incubation on a shaking incubator at 120 r/min and 7 °C for 7 d or 28 °C for 2 d. Samples were heat-treated in a water bath at 70 °C for 15 min. After heat treatment, samples were cooled immediately in an ice bath followed by the addition of 0.01% (0.1 g/L) sodium azide (Sinopharm Chemical Reagent Co., Ltd., China) and 0.025% (0.25 g/L) bronopol (Aladdin, China) to inhibit bacterial growth (Machado et al., 2016). Subsequently, milk samples were incubated at 37 °C for 5 d. Proteolytic and lipolytic activity was measured as described above.
Strains shown to produce heat-stable enzymes in the tests described above were selected for further thermal inactivation trials. Milk samples divided into aliquots of 5 ml in sterile tubes were heat-treated under the following time-temperature conditions. For protease, three different temperature-time combinations were used: 70 °C for 180 min, 80 °C for 150 min, and 90 °C for 100 min. For lipases, different temperature-time combinations were used: 70 °C for 80 min, 80 °C for 70 min, and 90 °C for 60 min. After each heat treatment, samples were cooled immediately in an ice bath followed by the addition of 0.01% (0.1 g/L) sodium azide and 0.025% (0.25 g/L) bronopol. Subsequently, milk samples were incubated at 37 °C for 5 d. Log of percentage residual activity (R) was plotted against heating time and expressed as the thermo-stability. The rate constant k for first-order inactivation, activation energy (E a), half-life (t 1/2), D-value, change in enthalpy (∆H #), change in entropy (∆S #), and change in free energy (∆G #) were calculated according to Olusesan et al. (2011).
3. Results
3.1. Screening for enzyme production
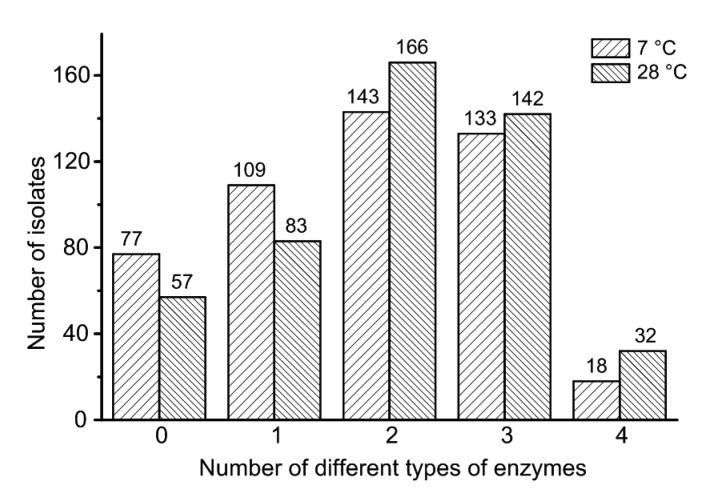
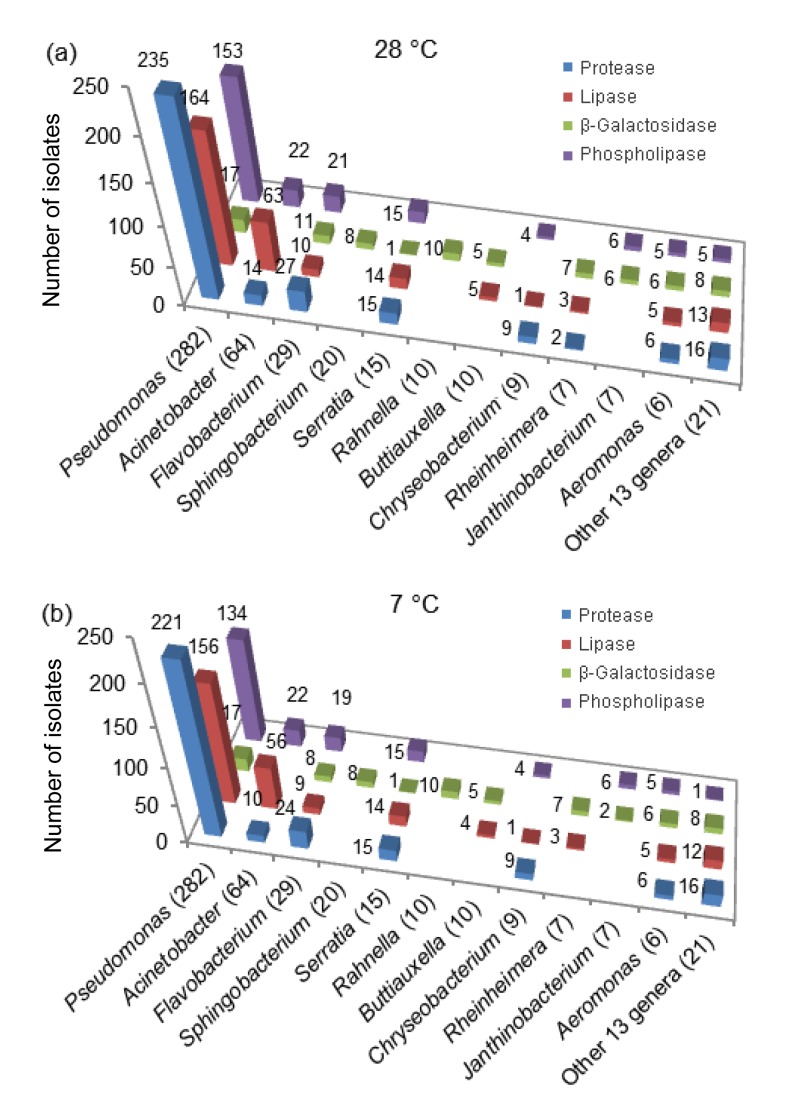
A total of 423 isolates incubated at 28 °C showed the ability to produce at least one type of enzyme, while 166, 142, and 32 isolates produced two, three, and four different enzymes, respectively (Fig. 1). Isolates of Pseudomonas, Acinetobacter, Flavobacterium, Chryseobacterium, Serratia, and Aeromonas were not only the most predominant in raw milk samples, but also proved to be high enzyme producers. Among the Pseudomonas isolates, 235 showed proteolytic activity and 164 showed lipolytic activity (Fig. 2a). Also, 153 isolates showed phospholipase activity and 17 showed β-galactosidase activity. Species of Acinetobacter exhibited high lipolytic (63 out of 64 strains) but weak proteolytic (14 out of 64) and phospholipase (22 out of 64) activity, and none of them produced β-galactosidase. Of the 29 Flavobacterium isolates, 27, 10, 11, and 21 isolates showed proteolytic, lipolytic, β-galactosidase, and phospholipase activity, respectively. Isolates belonging to Serratia displayed a strong tendency to produce proteases (15 out of 15 strains), lipases (14 out of 15), and phospholipases (15 out of 15), but a weak ability to produce β-galactosidases (1 out of 15). Species belonging to Aeromonas produced all four types of enzymes, indicating their high spoilage potential. Sphingobacterium and Rahnella produced only β-galactosidases, and Janthinobacterium showed negative protease and lipase production
Fig. 1.
Number of isolates that produce different types of enzymes at 7 and 28 °C
Fig. 2.
Abilities of isolates to produce protease, lipase, β-galactosidase, and phospholipase at 28 °C (a) and 7 °C (b)
The number in the bracket implies the total number of strains for each genus
Similar results were obtained at 7 °C, but slightly fewer isolates belonging to each genus showed spoilage potential. A total of 403 isolates showed the ability to produce at least one type of enzyme, while 143, 133, and 18 isolates produced two, three, and four different enzymes, respectively (Fig. 1). Among the Pseudomonas isolates, 221, 156, 17, and 134 isolates showed proteolytic, lipolytic, β-galactosidase, and phospholipase activity (Fig. 2b), respectively. Species of Acinetobacter exhibited high lipolytic (56 out of 64 strains), but weak proteolytic (10 out of 64) and phospholipase (22 out of 64) activity. Among Flavobacterium isolates, 24, 9, 8, and 19 isolates showed proteolytic, lipolytic, β-galactosidase, and phospholipase activity, respectively. The abilities of isolates belonging to Serratia, Aeromonas, Sphingobacterium, and Rahnella to produce each enzyme were the same as those observed at 28 °C.
3.2. Quantitative assessment of total proteolytic activity
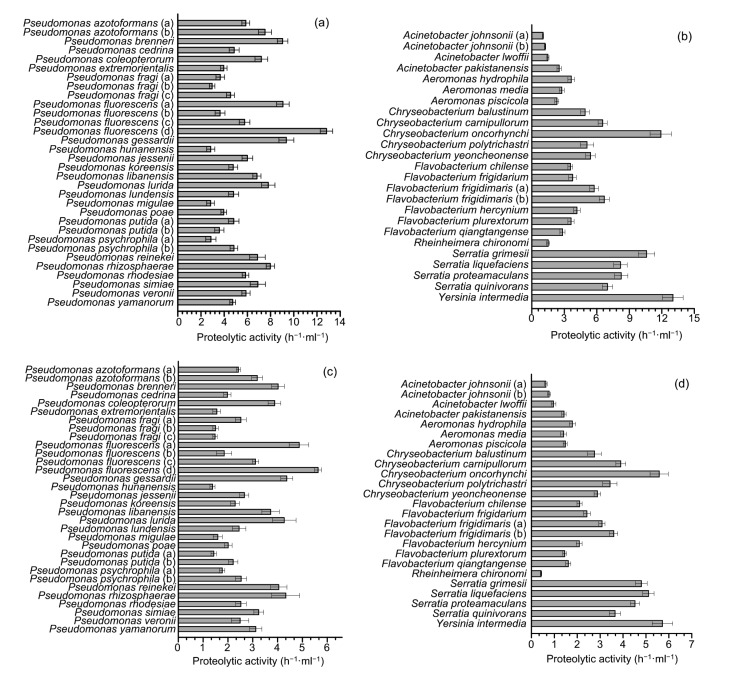
The proteolytic activity of the predominant psychrotrophic bacteria is shown in Fig. 3. The range of proteolysis was 1.23–13.02 h−1·ml−1 at 28 °C and 0.42–5.72 h−1·ml−1 at 7 °C. At 28 °C, Yersinia intermedia showed the highest proteolytic activity followed by Pseudomonas fluorescens (d), Chryseobacterium oncorhynchi, Serratia grimesii, and Pseudomonas gessardii. Species of Acinetobacter showed low proteolytic activity, and the lowest proteolytic activity was shown by Acinetobacter johnsonii (a). Isolates belonging to Serratia showed high proteolytic activity with a mean value of 8.51 h−1·ml−1.
Fig. 3.
Proteolytic activity of psychrotrophic bacteria isolated from raw milk
(a) Isolates belonging to Pseudomonas incubated at 28 °C; (b) Isolates belonging to other predominated species incubated at 28 °C; (c) Isolates belonging to Pseudomonas incubated at 7 °C; (d) Isolates belonging to other predominated species incubated at 7 °C. Data are expressed as mean±standard deviation (n=3)
The proteolytic activity of isolates incubated at 7 °C was lower than that at 28 °C. The highest protease activity was shown by Y. intermedia, followed by P. fluorescens (d), C. oncorhynchi, and Serratia liquefaciens. Rheinheimera chironomi showed the lowest protease activity followed by A. johnsonii.
3.3. Quantitative assessment of total lipolytic activity
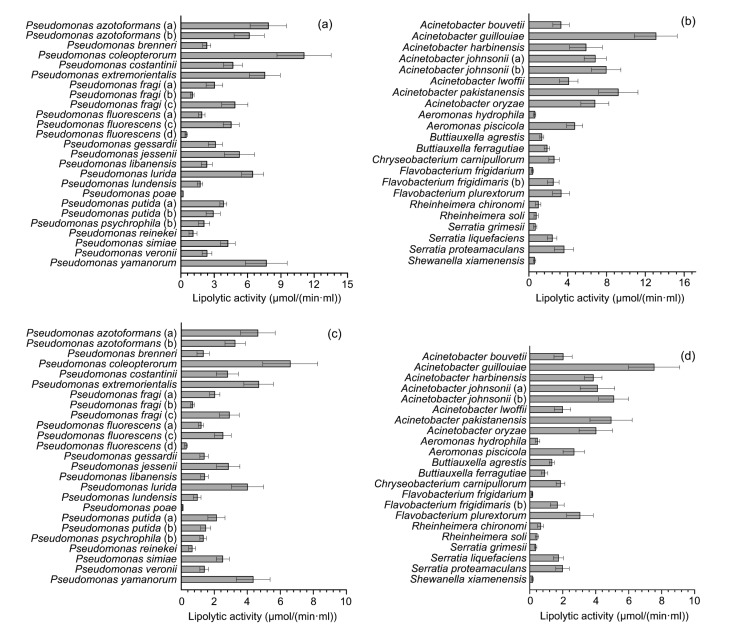
The lipolytic activity of the predominant psychrotrophic bacteria is shown in Fig. 4. The range of lipolytic activity was 0.20–13.09 μmol/(min·ml) at 28 °C and 0.11–7.54 μmol/(min·ml) at 7 °C. At 28 °C, Acinetobacter guillouiae showed the highest lipolytic activity followed by Pseudomonas coleopterorum, Acinetobacter pakistanensis, and A. johnsonii (b). The lowest lipase activity was shown by Pseudomonas poae. Isolates belonging to Acinetobacter showed relatively high lipase activity with a mean value of 7.15 μmol/(min·ml).
Fig. 4.
Lipolytic activity of psychrotrophic bacteria isolated from raw milk
(a) Isolates belonging to Pseudomonas incubated at 28 °C; (b) Isolates belonging to other predominated species incubated at 28 °C; (c) Isolates belonging to Pseudomonas incubated at 7 °C; (d) Isolates belonging to other predominated species incubated at 7 °C. Data are expressed as mean±standard deviation (n=3)
The lipolytic activity of each isolate incubated at 7 °C was also lower than that of corresponding bacteria incubated at 28 °C. The highest lipolytic activity was shown by A. guillouiae followed by P. coleopterorum, A. johnsonii (b), and Pseudomonas extremorientalis. P. poae showed the lowest lipolytic activity followed by Flavobacterium frigidarium.
3.4. Heat resistance of proteases and lipases
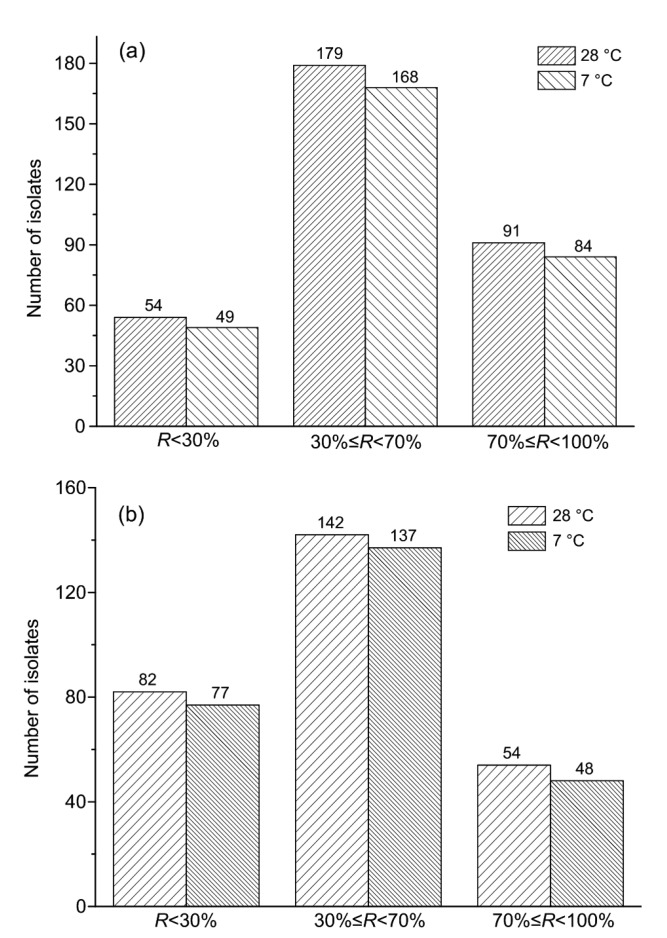
Milk samples incubated at 7 °C were heat-treated at 70 °C for 15 min. As shown in Fig. 5, 49 isolates showed a residual proteolytic activity of <30%. The highest proportion of isolates exhibited a residual activity from 30% to 70%. Eighty-four isolates had a residual activity of ≥70%. For lipases, residual activity was <30% for 77 isolates, ≥70% for 48 isolates, and from 30% to 70% for 137 isolates.
Fig. 5.
Distribution of isolates based on their residual activity (R) of proteases (a) and lipases (b) after the heat treatment at 70 °C for 15 min
Residual activity was compared after the incubation of samples at 28 °C. Fifty-four isolates showed <30%, 91 showed ≥70%, and 179 showed from 30% to 70% residual activity. For lipases, 82 isolates showed <30%, 54 showed ≥70%, and 142 showed from 30% to 70% residual activity.
Strains that showed high (≥70%) residual proteolytic activity (P. fluorescens (d), Pseudomonas azotoformans (a), S. grimesii, Chryseobacterium polytrichastri, and Flavobacterium frigidimaris (b)) or lipolytic activity (A. johnsonii (b), A. pakistanensis, P. fluorescens (c), and P. azotoformans (a)) were selected for evaluating kinetic parameters of thermal inactivation. An increase in enzyme inactivation correlated well with temperature and heating time. D-values and t 1/2 decreased with increasing temperature (Table 1). At 7 °C, the D-values of proteases produced by these five isolates ranged from 116.34 to 220.51 min at 70 °C, 87.40 to 133.59 min at 80 °C, and 59.83 to 77.13 min at 90 °C. E a ranged from 31.33 to 53.72 kJ/mol, and ∆S # from −171.23 to −111.14 J/(mol·K) at 70 °C, −164.48 to −104.29 J/(mol·K) at 80 °C, and −157.55 to −97.65 J/(mol·K) at 90 °C. No significant differences in these kinetic parameters were observed when samples were incubated at 28 °C. Proteases produced by S. grimesii proved to be the most resistant, both at 28 and 7 °C.
Table 1.
Thermodynamic parameters of proteases produced by representative isolates cultured at 28 and 7 °C
| Cultured temp (°C) | Isolate | Temp |
1/temp (×10−3 K−1) | k (min−1) | D (min) | t 1/2 (min) | E a (kJ/mol) | ∆H # (kJ/mol) | ∆S # (J/(mol·K)) | ∆G # (kJ/mol) | |
| (°C) | (K) | ||||||||||
| 28 | Pseudomonas fluorescens (d) | 70 | 343 | 2.92 | 0.013 | 171.17 | 50.48 | 48.33 | 45.48 | −124.86 | 88.24 |
| 80 | 353 | 2.83 | 0.021 | 107.82 | 30.91 | 45.40 | −117.95 | 87.08 | |||
| 90 | 363 | 2.75 | 0.035 | 65.79 | 18.81 | 45.32 | −111.38 | 85.82 | |||
| Pseudomonas azotoformans (a) | 70 | 343 | 2.92 | 0.012 | 177.80 | 52.78 | 49.44 | 46.59 | −122.23 | 88.45 | |
| 80 | 353 | 2.83 | 0.021 | 111.17 | 30.71 | 46.51 | −114.81 | 87.08 | |||
| 90 | 363 | 2.75 | 0.033 | 69.40 | 18.67 | 46.43 | −108.74 | 85.97 | |||
| Serratia grimesii | 70 | 343 | 2.92 | 0.010 | 225.75 | 66.66 | 53.72 | 50.87 | −111.14 | 88.93 | |
| 80 | 353 | 2.83 | 0.017 | 135.19 | 39.30 | 50.79 | −104.29 | 87.64 | |||
| 90 | 363 | 2.75 | 0.030 | 77.59 | 22.03 | 50.71 | −97.65 | 86.22 | |||
| Chryseobacterium polytrichastri | 70 | 343 | 2.92 | 0.019 | 122.27 | 33.66 | 33.64 | 30.79 | −164.89 | 87.26 | |
| 80 | 353 | 2.83 | 0.026 | 88.22 | 22.18 | 30.71 | −157.94 | 86.52 | |||
| 90 | 363 | 2.75 | 0.038 | 59.87 | 15.56 | 30.63 | −151.20 | 85.61 | |||
| Flavobacterium frigidimaris (b) | 70 | 343 | 2.92 | 0.017 | 133.05 | 37.16 | 36.72 | 33.35 | −158.26 | 87.55 | |
| 80 | 353 | 2.83 | 0.026 | 90.28 | 23.61 | 33.27 | −149.79 | 86.52 | |||
| 90 | 363 | 2.75 | 0.036 | 63.42 | 16.44 | 33.19 | −144.54 | 85.75 | |||
|
| |||||||||||
| 7 | Pseudomonas fluorescens (d) | 70 | 343 | 2.92 | 0.014 | 166.37 | 47.73 | 44.92 | 42.07 | −134.26 | 88.05 |
| 80 | 353 | 2.83 | 0.022 | 106.67 | 30.59 | 41.99 | −127.24 | 86.95 | |||
| 90 | 363 | 2.75 | 0.035 | 65.21 | 18.23 | 41.91 | −120.75 | 85.82 | |||
| Pseudomonas azotoformans (a) | 70 | 343 | 2.92 | 0.013 | 176.21 | 51.21 | 46.90 | 44.05 | −129.03 | 88.24 | |
| 80 | 353 | 2.83 | 0.021 | 110.53 | 30.07 | 43.97 | −122.00 | 87.08 | |||
| 90 | 363 | 2.75 | 0.034 | 68.41 | 18.05 | 43.89 | −115.53 | 85.90 | |||
| Serratia grimesii | 70 | 343 | 2.92 | 0.010 | 220.51 | 58.46 | 53.72 | 50.87 | −111.14 | 88.93 | |
| 80 | 353 | 2.83 | 0.017 | 133.59 | 37.70 | 50.79 | −104.29 | 87.64 | |||
| 90 | 363 | 2.75 | 0.030 | 77.13 | 21.58 | 50.71 | −97.65 | 86.22 | |||
| Chryseobacterium polytrichastri | 70 | 343 | 2.92 | 0.020 | 116.34 | 32.00 | 31.33 | 28.48 | −171.23 | 87.12 | |
| 80 | 353 | 2.83 | 0.026 | 87.40 | 21.36 | 28.40 | −164.48 | 86.52 | |||
| 90 | 363 | 2.75 | 0.038 | 59.83 | 15.24 | 28.32 | −157.55 | 85.61 | |||
| Flavobacterium frigidimaris (b) | 70 | 343 | 2.92 | 0.018 | 130.05 | 35.46 | 34.23 | 31.38 | −163.58 | 87.40 | |
| 80 | 353 | 2.83 | 0.026 | 90.02 | 22.71 | 31.30 | −156.27 | 86.52 | |||
| 90 | 363 | 2.75 | 0.036 | 63.53 | 16.24 | 31.22 | −149.96 | 85.75 | |||
Lipases showed less heat resistance than proteases (Table 2). At 7 °C, D-values of lipases produced by the four isolates ranged from 44.31 to 72.74 min at 70 °C, 35.41 to 57.44 min at 80 °C, and 28.98 to 40.69 min at 90 °C. E a ranged from 21.03 to 28.20 kJ/mol, and ∆S # from −194.04 to −176.81 J/(mol·K) at 70 °C, −186.89 to −170.17 J/(mol·K) at 80 °C, and −180.62 to −163.27 J/(mol·K) at 90 °C. Similarly, no significant differences in these kinetic parameters were observed when samples were incubated at 28 °C. Lipase produced by A. pakistanensis showed the highest heat resistance, both at 28 and 7 °C.
Table 2.
Thermodynamic parameters of lipases produced by representative isolates cultured at 28 and 7 °C
| Cultured temp (°C) | Isolate | Temp |
1/temp (×10−3 K−1) | k (min−1) | D (min) | t 1/2 (min) | E a (kJ/mol) | ∆H # (kJ/mol) | ∆S # (J/(mol·K)) | ∆G # (kJ/mol) | |
| (°C) | (K) | ||||||||||
| 28 | Acinetobacter johnsonii (b) | 70 | 343 | 2.92 | 0.033 | 70.17 | 21.56 | 27.24 | 24.39 | −179.38 | 85.82 |
| 80 | 353 | 2.83 | 0.041 | 56.64 | 15.46 | 24.31 | −172.69 | 85.33 | |||
| 90 | 363 | 2.75 | 0.058 | 39.41 | 10.60 | 24.23 | −165.77 | 84.51 | |||
| Acinetobacter pakistanensis | 70 | 343 | 2.92 | 0.031 | 75.43 | 23.96 | 28.73 | 25.45 | −176.75 | 85.98 | |
| 80 | 353 | 2.83 | 0.040 | 58.23 | 16.32 | 25.37 | −169.88 | 85.40 | |||
| 90 | 363 | 2.75 | 0.056 | 41.06 | 11.40 | 25.29 | −163.10 | 84.60 | |||
| Pseudomonas fluorescens (c) | 70 | 343 | 2.92 | 0.051 | 45.55 | 13.44 | 21.51 | 18.66 | −192.81 | 84.69 | |
| 80 | 353 | 2.83 | 0.064 | 36.13 | 10.10 | 18.58 | −185.65 | 84.18 | |||
| 90 | 363 | 2.75 | 0.079 | 29.07 | 8.11 | 18.50 | −169.62 | 83.74 | |||
| Pseudomonas azotoformans (a) | 70 | 343 | 2.92 | 0.041 | 56.42 | 16.87 | 25.94 | 23.09 | −181.51 | 85.25 | |
| 80 | 353 | 2.83 | 0.055 | 41.59 | 11.67 | 23.01 | −174.21 | 84.57 | |||
| 90 | 363 | 2.75 | 0.070 | 32.74 | 8.60 | 22.94 | −167.97 | 84.02 | |||
|
| |||||||||||
| 7 | Acinetobacter johnsonii (b) | 70 | 343 | 2.92 | 0.033 | 69.92 | 21.31 | 27.24 | 24.39 | −179.38 | 85.82 |
| 80 | 353 | 2.83 | 0.041 | 56.13 | 15.20 | 24.31 | −172.69 | 85.33 | |||
| 90 | 363 | 2.75 | 0.058 | 39.55 | 10.62 | 24.23 | −165.77 | 84.51 | |||
| Acinetobacter pakistanensis | 70 | 343 | 2.92 | 0.032 | 72.74 | 21.66 | 28.20 | 25.35 | −176.81 | 85.90 | |
| 80 | 353 | 2.83 | 0.040 | 57.44 | 15.77 | 25.27 | −170.17 | 85.40 | |||
| 90 | 363 | 2.75 | 0.057 | 40.69 | 11.28 | 25.19 | −163.27 | 84.56 | |||
| Pseudomonas fluorescens (c) | 70 | 343 | 2.92 | 0.052 | 44.31 | 12.78 | 21.03 | 18.18 | −194.06 | 84.64 | |
| 80 | 353 | 2.83 | 0.065 | 35.41 | 9.67 | 18.10 | −186.89 | 84.14 | |||
| 90 | 363 | 2.75 | 0.079 | 28.98 | 7.77 | 18.02 | −180.62 | 83.70 | |||
| Pseudomonas azotoformans (a) | 70 | 343 | 2.92 | 0.042 | 55.34 | 16.23 | 25.46 | 22.61 | −182.73 | 85.19 | |
| 80 | 353 | 2.83 | 0.056 | 41.19 | 11.53 | 22.53 | −175.43 | 84.52 | |||
| 90 | 363 | 2.75 | 0.071 | 32.60 | 8.47 | 22.45 | −169.21 | 83.98 | |||
4. Discussion
Heat-stable enzymes produced by psychrotrophic bacteria cannot be completely inactivated by thermal processing techniques and pose threats to the quality and shelf-life of dairy products. Some studies have reported results of screening for enzymes produced by psychrotrophic bacteria isolated from raw milk (von Neubeck et al., 2015; Vithanage et al., 2016). However, quantitative assessments and the thermo-stabilities of proteases and lipases have rarely been reported. The objective of this work was to explore the spoilage potential of psychrotrophic bacteria and the thermo-stabilities of proteases and lipases they produced.
A wide variety of psychrotrophic bacteria can produce spoilage enzymes during late log or stationary growth phases (Rajmohan et al., 2002). Most isolates in this study had enzymatic activity at low temperatures. Pseudomonas is reported to be the most frequently isolated genus in raw milk, probably because of its short generation time (von Neubeck et al., 2015; Yuan et al., 2017). The spoilage potential of Pseudomonas has been extensively studied and those bacteria tend to produce proteases combined with lipases (Capodifoglio et al., 2016; Vithanage et al., 2016).
In this study, most Pseudomonas species, such as P. fluorescens (strains b and d), P. gessardii, P. lurida, and P. brenneri, showed high protease activity, while P. azotoformans (a), P. coleopterorum, P. extremorientalis, P. fragi (c), P. fluorescens (c), and P. lurida showed high lipase activity. P. fluorescens, P. lurida, and P. simiae can produce all four of the different enzymes screened in this work, indicating their great spoilage potential. The aprX gene coding for the protease is located at the beginning of a polycistronic operon with the lipA gene that encodes the lipase (Woods et al., 2001). Thus, most isolates belonging to to Pseudomonas have the ability to produce proteases and lipases due to the simultaneous expression of both genes. However, expression of enzymes can be tightly regulated, which may explain the absence of one or both enzymes in some isolates. In this work, some isolates belonging to P. fluorescens and P. fragi
did not show proteolytic or lipolytic activity. This phenotypic variation within the same species is consistent with previous findings (Wiedmann et al., 2000; Caldera et al., 2016). For example, only 36% of isolates belonging to P. fragi from different foods showed proteolytic activity (Caldera et al., 2016). For this reason, in the future, whole genome sequencing of these isolates will be carried out to differentiate their spoilage potential.
Acinetobacter is considered to be the predominant bacterial genus in raw milk (von Neubeck et al., 2015; Yuan et al., 2017). This genus is ubiquitous in nature and characterized by the tendency to tolerate dry conditions and multidrugs (Gurung et al., 2013). In the current work, all isolates belonging to Acinetoba showed high lipolytic activity and low proteolytic activity, which is in agreement with previous studies (von Neubeck et al., 2015; Vithanage et al., 2016). However, a high number of Acinetobacter isolates with proteolytic activity were found in Mozzarella cheese. Such isolates can carry out the hydrolysis of α-casein and spoil the cheese (Baruzzi et al., 2012). This might be explained by horizontal gene transfer from proteolytic species or phenotypic variation among isolates. Chryseobacterium also appears as a dominant member of raw milk microbiota (Yuan et al., 2017) and some species show a greater spoilage ability than P. fluorescens based on their proteolytic and lipolytic activity (Bekker et al., 2015, 2016). In this study, species of Chryseobacterium showed more proteolytic than lipolytic activity, and C. oncorhynchi showed higher proteolytic activity than many isolates of Pseudomonas. To our knowledge, few studies have reported the spoilage potential of Flavobacterium. The results of our study suggest that it has high proteolytic but weak lipolytic activity. Species of Serrati are also characterized as predominant milk spoilers due to their ability to produce heat-stable proteases and lipases (Machado et al., 2016). In this study, Serratia was shown to have high spoilage potential based on its ability to produce protease, lipase, β-galactosidase, and phospholipase. However, isolates belonging to other predominant genera (Sphingobacterium, Rahnella, Buttiauxella, and Janthinobacterium) lacked the ability to produce both proteases and lipases. Thus, the population of psychrotrophic bacteria plays a key role in the determination of the quality of dairy products.
The productions of phospholipases and β-galactosidases have been less frequently reported than those of proteases and lipases, since proteases and lipases pose more serious threats to the quality and shelf-life of dairy products. Phospholipase activity was found among the isolates belonging to the genera Pseudomonas, Acinetobacter, Flavobacterium, Serratia, Chryseobacterium, Janthinobacterium, and Aeromonas, consistent with their ability to cause sweet curdling defects or bitter cream in milk as a result of fat globule aggregation (Titball, 1993; Vithanage et al., 2016). β-Galactosidase is an important indicator of lactose fermentation in dairy products, and may catalyze the hydrolysis of β-1,4-galactosidic bonds in lactose (Chen et al., 2009). The production of β-galactosidase by psychrotrophic bacteria has seldom been reported. In this study, β-galactosidases were produced by isolates of the genera Pseudomonas, Flavobacterium, Sphingobacterium, Rahnella, Buttiauxella, Rheinheimera, Sphingobacterium, and Aeromonas.
The production of enzymes is a temperature-dependent process (Buchon et al., 2000). As reported by Decimo et al. (2014), enzymatic activity is highly influenced by the incubation temperature as results obtained at 30 °C differ from those obtained at 7 °C. In our work, more isolates showed positive results for each enzymatic activity, and activity of proteases and lipases was higher at 28 °C than at 7 °C, indicating that the storage and transportation of raw milk at low temperatures could be effective to some extent in controlling the spoilage of raw milk.
Considering the potential effects of enzymes on dairy products, appropriate practices should be adopted to minimize the quality losses, by either limiting the growth of psychrotrophic bacteria or inactivation of their enzymes by thermal processing. Adequate addition of CO2 to raw milk has been shown to reduce proteolysis and lipolysis by limiting microbial growth and the production of microbial proteases (Ma et al., 2003). Sophisticated dairy farm management systems, such as Good Manufacturing Practice (GMP) and Hazard Analysis and Critical Control Point (HACCP), are also effective ways to limit the initial number of psychrotrophic bacteria in raw milk (Cusato et al., 2014). However, the effects of these practices on the heat stability of enzymes are unknown. The heat stability of enzymes originating from psychrotrophs is a limiting factor in maintaining the quality and shelf-life of dairy products. Thus, better knowledge of their heat stability is needed.
Heat treatments adopted by the dairy industry are insufficient to completely inhibit the activity of enzymes produced by psychrotrophic bacteria (Glück et al., 2016; Vithanage et al., 2016). Reducing the activity and limiting the secretion of heat-stable enzymes are scientific challenges. In this work, proteases produced by a large number of predominant isolates still remained active after heat treatment at 70 °C for 15 min, and some even showed residual activity of ≥70%. Kinetic parameters for the thermal inactivation of P. fluorescens (d), P. azotoformans (a), and Serratia quinivorans highlight the high potential of these enzymes to cause dairy spoilage. In previous studies, the thermo-stability of proteases produced by Pseudomonas or Serratia has been extensively reported. The D-values of proteases produced by S. liquefaciens and Pseudomonas sp. were 96.15 and 80.00 min, respectively, at 90 °C (Machado et al., 2016). The D-values of proteases produced by Pseudomonas proteolytica were 6.3, 4.0, and 1.3 min at 120, 130, and 140 °C, respectively (Stoeckel et al., 2016). The residual activity of protease produced by P. fluorescens was 97.2% after heat treatment at 80 °C for 30 min (Zhang and Lv, 2014). The purified protease Ser2 secreted by S. liquefaciens was highly heat-stable in skimmed, semi-skimmed, and whole milk at 140 °C, with D-values of 2.4, 3.9, and 4.5 min, respectively, and the presence of milk fat increased the heat-stability of the protease (Baglinière et al., 2017). The heat inactivation of the purified protease from Chryseobacterium indologenes did not follow first-order kinetics, but showed biphasic inactivation curves, and this protease is much more heat-labile than other psychrotrophic proteases (Venter et al., 1999). In our study, the kinetic parameters for inactivation of the protease secreted by C. polytrichastri suggested that this protease is also heat-resistant. This may be because the crude enzyme is more heat-stable than the purified enzyme, and the different methods for estimating heat resistance should also be taken into account. Kinetic thermal inactivation of proteases produced by species belonging to Flavobacterium has not been reported previously, and our results suggest that the protease produced by F. frigidimaris (b) is thermo-stable.
Many studies have determined the heat stability of proteases, but the heat stability of lipases is less well known. It has been reported that lipases produced by various Pseudomonas isolates showed residual activity ranging from 55% to 100% after heat treatment at 63 °C for 30 min (Law et al., 1976). The D-value of lipase produced by P. fluorescens was 23.5 min after heat treatment at 100 °C (Andersson et al., 1979). Vithanage et al. (2016) reported that more than 30% of strains belonging to Pseudomonas isolated from raw milk showed 50% to 75% residual lipase activity after heat treatment at 142 °C for 4 s. In this study, many isolates showed high residual lipase activity after heat treatment at 70 °C for 15 min. Kinetic parameters showed that lipases produced by A. johnsonii (b), A. pakistanensis, P. fluorescens (c), and P. azotoformans (a) are heat-resistant and can survive after pasteurization. Surprisingly, lipases were less heat-resistant than proteases. For example, the D-value of the most heat-stable lipase produced by A. pakistanensis was 41.06 min at 90 °C, which is much smaller than that of the most heat-stable protease produced by S. grimesii. Moreover, enzymes are more heat-stable in synthetic milk salt solutions than in phosphate buffer due to the protective effect of milk components in the milk system (Baur et al., 2015).
The inactivation of enzymes fits first-order kinetics, and kinetic parameters indicate that while a higher temperature and longer heat treatment period may result in a higher reduction of enzymatic activity, the destruction and inactivation of milk constituents will be increased. Results for the Gibbs free energy (G #), enthalpy (H #), and entropy (S #) of thermal inactivation of enzymes have rarely been reported. Hydrophobic interactions, hydrogen bonds, disulfide bridges, and electrostatic interactions are the forces that stabilize a protein and are determined by ∆G #, while ∆H # and ∆S # provide a measure of structural disorder upon protein folding associated with the stability of an enzyme. High levels of ∆G # and ∆H #, and low values of ∆S # indicate the structural stability of enzymes in this study. It is difficult to compare the results of heat stability of enzymes provided by different laboratories due to different time-temperature combinations of heat treatments and because heat resistance varies at the species or strain level. The thermo-stability patterns exhibited by proteases and lipases demonstrate the importance of exploring the combination of optimal temperature and time for heat treatments in the dairy industry.
The cold-chain of storage and transportation reduces the production and activity of spoilage enzymes. However, no significant difference in thermo-stability was observed between low and high incubation temperatures in this study. This can be explained by the fact that the thermo-stability of enzymes depends on the genetics of the specific isolates tested.
It is important to stress that this work focused only on enzymes produced by planktonic cells. The amount of proteolysis and lipolysis produced is higher in biofilms than in planktonic cultures, and the thermo-stability of enzymes can be enhanced by the protective matrix of EPS (Teh et al., 2012, 2013). This suggests that biofilms formed by spoilage psychrotrophs may pose more serious threats to the dairy industry. To our knowledge, few studies have reported any possible link between spoilage enzymes and biofilm formation by psychrotrophic bacteria. This will be taken into consideration in the future research.
5. Conclusions
The production of heat-resistant spoilage enzymes presents a formidable challenge to today’s dairy industry. A high number of predominant bacteria isolated from Chinese dairy milk can produce spoilage enzymes. Species of Pseudomonas, Serratia, and Chryseobacterium are highly proteolytic, while isolates of Acinetobacter and Pseudomonas can produce highly active lipases. Isolates belonging to certain genera show positive β-galactosidase and phospholipase activity. Most enzymes are heat-resistant as they can survive after different heat treatments applied in the manufacture of dairy products. There is a vast amount of scientific knowledge about the ability of psychrotrophic bacteria to produce spoilage enzymes, but no effective strategy has yet been presented to control this problem. Thus, the heat stability and molecular characteristics of enzymes and the development of novel screening methods should be addressed in the future.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 31772080) and the Major Science and Technology Projects of Zhejiang Province (No. 2015C02039), China
Compliance with ethics guidelines: Lei YUAN, Faizan A. SADIQ, Tong-jie LIU, Yang LI, Jing-si GU, Huan-yi YANG, and Guo-qing HE declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Abdou AM. Purification and partial characterization of psychrotrophic Serratia marcescens lipase. J Dairy Sci. 2003;86(1):127–132. doi: 10.3168/jds.S0022-0302(03)73591-7. [DOI] [PubMed] [Google Scholar]
- 2.Andersson RE, Hedlund CB, Jonsson U. Thermal inactivation of a heat-resistant lipase produced by the psychrotrophic bacterium Pseudomonas fluorescens . J Dairy Sci. 1979;62(3):361–367. doi: 10.3168/jds.S0022-0302(79)83252-X. [DOI] [PubMed] [Google Scholar]
- 3.Baglinière F, Salgado RL, Salgado CA, et al. Biochemical characterization of an extracellular heat-stable protease from Serratia liquefaciens isolated from raw milk. J Food Sci. 2017;82(4):952–959. doi: 10.1111/1750-3841.13660. [DOI] [PubMed] [Google Scholar]
- 4.Baruzzi F, Lagonigro R, Quintieri L, et al. Occurrence of non-lactic acid bacteria populations involved in protein hydrolysis of cold-stored high moisture Mozzarella cheese. Food Microbiol. 2012;30(1):37–44. doi: 10.1016/j.fm.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Baur C, Krewinkel M, Kutzli I, et al. Isolation and characterisation of a heat-resistant peptidase from Pseudomonas panacis withstanding general UHT processes. Int Dairy J. 2015;49:46–55. doi: 10.1016/j.idairyj.2015.04.009. [DOI] [Google Scholar]
- 6.Bekker A, Steyn L, Charimba G, et al. Comparison of the growth kinetics and proteolytic activities of Chryseobacterium species and Pseudomonas fluorescens . Can J Microbiol. 2015;61(12):977–982. doi: 10.1139/cjm-2015-0236. [DOI] [PubMed] [Google Scholar]
- 7.Bekker A, Jooste P, Steyn L, et al. Lipid breakdown and sensory analysis of milk inoculated with Chryseobacterium joostei or Pseudomonas fluorescens . Int Dairy J. 2016;52:101–106. doi: 10.1016/j.idairyj.2015.09.003. [DOI] [Google Scholar]
- 8.Buchon L, Laurent P, Gounot AM, et al. Temperature dependence of extracellular enzymes production by psychrotrophic and psychrophilic bacteria. Biotechnol Lett. 2000;22(19):1577–1581. doi: 10.1023/A:1005641119076. [DOI] [Google Scholar]
- 9.Caldera L, Franzetti L, van Coillie E, et al. Identification, enzymatic spoilage characterization and proteolytic activity quantification of Pseudomonas spp. isolated from different foods. Food Microbiol. 2016;54:142–153. doi: 10.1016/j.fm.2015.10.004. [DOI] [Google Scholar]
- 10.Capodifoglio E, Vidal AMC, Lima JAS, et al. Lipolytic and proteolytic activity of Pseudomonas spp. isolated during milking and storage of refrigerated raw milk. J Dairy Sci. 2016;99(7):5214–5223. doi: 10.3168/jds.2015-10453. [DOI] [PubMed] [Google Scholar]
- 11.Champagne CP, Laing RR, Roy D, et al. Psychrotrophs in dairy products: their effects and their control. Crit Rev Food Sci Nutr. 1994;34(1):1–30. doi: 10.1080/10408399409527648. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Daniel RM, Coolbear T. Detection and impact of protease and lipase activities in milk and milk powders. Int Dairy J. 2003;13(4):255–275. doi: 10.1016/S0958-6946(02)00171-1. [DOI] [Google Scholar]
- 13.Chen W, Chen H, Xia Y, et al. Immobilization of recombinant thermostable β-galactosidase from Bacillus stearothermophilus for lactose hydrolysis in milk. J Dairy Sci. 2009;92(2):491–498. doi: 10.3168/jds.2008-1618. [DOI] [PubMed] [Google Scholar]
- 14.Cusato S, Gameiro AH, Sant'Ana AS, et al. Assessing the costs involved in the implementation of GMP and HACCP in a small dairy factory. Qual Assur Saf Crops Foods. 2014;6(2):135–139. doi: 10.3920/QAS2012.0195. [DOI] [Google Scholar]
- 15.Decimo M, Morandi S, Silvetti T, et al. Characterization of Gram-negative psychrotrophic bacteria isolated from Italian bulk tank milk. J Food Sci. 2014;79(10):M2081–M2090. doi: 10.1111/1750-3841.12645. [DOI] [PubMed] [Google Scholar]
- 16.Deeth HC, Khusniati T, Datta N, et al. Spoilage patterns of skim and whole milks. J Dairy Res. 2002;69(2):227–241. doi: 10.1017/S0022029901005301. [DOI] [PubMed] [Google Scholar]
- 17.Glück C, Rentschler E, Krewinkel M, et al. Thermostability of peptidases secreted by microorganisms associated with raw milk. Int Dairy J. 2016;56:186–197. doi: 10.1016/j.idairyj.2016.01.025. [DOI] [Google Scholar]
- 18.Gurung M, Nam HM, Tamang MD, et al. Prevalence and antimicrobial susceptibility of Acinetobacter from raw bulk tank milk in Korea. J Dairy Sci. 2013;96(4):1997–2002. doi: 10.3168/jds.2012-5965. [DOI] [PubMed] [Google Scholar]
- 19.Law BA, Sharpe ME, Chapman HR. The effect of lipolytic Gram-negative psychrotrophs in stored milk on the development of rancidity in Cheddar cheese. J Dairy Res. 1976;43(3):459–468. doi: 10.1017/S0022029900016046. [DOI] [Google Scholar]
- 20.Lilbaek HM, Fatum TM, Ipsen R, et al. Modification of milk and whey surface properties by enzymatic hydrolysis of milk phospholipids. J Agric Food Chem. 2007;55(8):2970–2978. doi: 10.1021/jf062705b. [DOI] [PubMed] [Google Scholar]
- 21.Ma Y, Barbano DM, Santos M. Effect of CO2 addition to raw milk on proteolysis and lipolysis at 4°C. J Dairy Sci. 2003;86(5):1616–1631. doi: 10.3168/jds.S0022-0302(03)73747-3. [DOI] [PubMed] [Google Scholar]
- 22.Machado SG, Heyndrickx M, de Block J, et al. Identification and characterization of a heat-resistant protease from Serratia liquefaciens isolated from Brazilian cold raw milk. Int J Food Microbiol. 2016;222:65–71. doi: 10.1016/j.ijfoodmicro.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Olusesan AT, Azura LK, Forghani B, et al. Purification, characterization and thermal inactivation kinetics of a non-regioselective thermostable lipase from a genotypically identified extremophilic Bacillus subtilis NS 8. New Biotechnol. 2011;28(6):738–745. doi: 10.1016/j.nbt.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Rajmohan S, Dodd CER, Waites WM. Enzymes from isolates of Pseudomonas fluorescens involved in food spoilage. J Appl Microbiol. 2002;93(2):205–213. doi: 10.1046/j.1365-2672.2002.01674.x. [DOI] [PubMed] [Google Scholar]
- 25.Sadiq FA, Li Y, Liu TJ, et al. The heat resistance and spoilage potential of aerobic mesophilic and thermophilic spore forming bacteria isolated from Chinese milk powders. Int J Food Microbiol. 2016;238:193–201. doi: 10.1016/j.ijfoodmicro.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Santos JA, González CJ, López-Díaz TM, et al. Extracellular protease production by dairy strains of Aeromonas hydrophila as affected by growth media and incubation temperature. Food Microbiol. 1996;13(1):47–51. doi: 10.1006/fmic.1996.0006. [DOI] [Google Scholar]
- 27.Sørhaug T, Stepaniak L. Psychrotrophs and their enzymes in milk and dairy products: quality aspects. Trends Food Sci Technol. 1997;8(2):35–41. doi: 10.1016/S0924-2244(97)01006-6. [DOI] [Google Scholar]
- 28.Stoeckel M, Lidolt M, Achberger V, et al. Growth of Pseudomonas weihenstephanensis, Pseudomonas proteolytica and Pseudomonas sp. in raw milk: impact of residual heat-stable enzyme activity on stability of UHT milk during shelf-life. Int Dairy J. 2016;59:20–28. doi: 10.1016/j.idairyj.2016.02.045. [DOI] [Google Scholar]
- 29.Teh KH, Flint S, Palmer J, et al. Proteolysis produced within biofilms of bacterial isolates from raw milk tankers. Int J Food Microbiol. 2012;157(1):28–34. doi: 10.1016/j.ijfoodmicro.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Teh KH, Lindsay D, Palmer J, et al. Lipolysis within single culture and co-culture biofilms of dairy origin. Int J Food Microbiol. 2013;163(2-3):129–135. doi: 10.1016/j.ijfoodmicro.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Titball RW. Bacterial phospholipases C. Microbiol Rev. 1993;57(2):347–366. doi: 10.1128/mr.57.2.347-366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vacheyrou M, Normand AC, Guyot P, et al. Cultivable microbial communities in raw cow milk and potential transfers from stables of sixteen French farms. Int J Food Microbiol. 2011;146(3):253–262. doi: 10.1016/j.ijfoodmicro.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 33.Venter H, Osthoff G, Litthauer D. Purification and characterization of a metalloprotease from Chryseobacterium indologenes Ix9a and determination of the amino acid specificity with electrospray mass spectrometry. Protein Expr Purif. 1999;15(3):282–295. doi: 10.1006/prep.1998.1020. [DOI] [PubMed] [Google Scholar]
- 34.Vithanage NR, Dissanayake M, Bolge G, et al. Biodiversity of culturable psychrotrophic microbiota in raw milk attributable to refrigeration conditions, seasonality and their spoilage potential. Int Dairy J. 2016;57:80–90. doi: 10.1016/j.idairyj.2016.02.042. [DOI] [Google Scholar]
- 35.von Neubeck M, Baur C, Krewinkel M, et al. Biodiversity of refrigerated raw milk microbiota and their enzymatic spoilage potential. Int J Food Microbiol. 2015;211:57–65. doi: 10.1016/j.ijfoodmicro.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Wiedmann M, Weilmeier D, Dineen SS, et al. Molecular and phenotypic characterization of Pseudomonas spp. isolated from milk. Appl Environ Microbol. 2000;66(5):2085–2095. doi: 10.1128/AEM.66.5.2085-2095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woods RG, Burger M, Beven CA, et al. The aprX-lipA operon of Pseudomonas fluorescens B52: a molecular analysis of metalloprotease and lipase production. Microbiology. 2001;147(2):345–354. doi: 10.1099/00221287-147-2-345. [DOI] [PubMed] [Google Scholar]
- 38.Yuan L, Sadiq FA, Liu TJ, et al. Psychrotrophic bacterial populations in Chinese raw dairy milk. LWT. 2017;84:409–418. doi: 10.1016/j.lwt.2017.05.023. [DOI] [Google Scholar]
- 39.Zhang SW, Lv JP. Purification and properties of heat-stable extracellular protease from Pseudomonads fluorescens BJ-10. J Food Sci Technol. 2014;51(6):1185–1190. doi: 10.1007/s13197-012-0620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]