Abstract
A transgenic maize event ZD12-6 expressing a Bacillus thuringiensis (Bt) fusion protein Cry1Ab/Cry2Aj and a modified 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) protein G10 was characterized and evaluated. Southern blot analysis indicated that ZD12-6 is a single copy integration event. The insert site was determined to be at chromosome 1 by border sequence analysis. Expression analyses of Bt fusion protein Cry1Ab/Cry2Aj and the EPSPS protein G10 suggested that they are both expressed stably in different generations. Insect bioassays demonstrated that the transgenic plants are highly resistant to Asian corn borer (Ostrinia furnacalis), cotton boll worm (Helicoverpa armigera), and armyworm (Mythimna separata). This study suggested that ZD12-6 has the potential to be developed into a commercial transgenic line.
Keywords: Transgenic maize, Bacillus thuringiensis (Bt), Insect resistance, Glyphosate tolerance
1. Introduction
Maize (Zea mays L.) is one of the most important food, feed, and energy crops worldwide. With the growth of global population and the steady decrease in the amount of arable land, the demand for corn is increasing sharply. The major lepidopteran pests on maize, such as Ostrinia furnacalis, Helicoverpa armigera, and Mythimna separata, cause significant yield loss constantly (Du et al., 2014; Shen et al., 2016). Transgenic corn has been rapidly adopted worldwide for insect resistance and herbicide tolerance (James, 2015). Benefits of transgenic Bacillus thuringiensis (Bt) crops include effective management of target pests, decreased use of conventional insecticides, and reduced harm to non-target creatures (Huang et al., 2005; Cattaneo et al., 2006; Hunt et al., 2007; Hutchison et al., 2010).
However, chemical insecticides are the main method for controlling corn insect pests in China as it has not yet implemented transgenic insect-resistant corn commercially. We believe that the transgenic insect control method will be adopted in China in the near future and that this will greatly benefit China’s agricultural practices. We previously engineered transgenic corn expressing a fusion Bt protein Cry1Ab/Cry2Aj and a modified 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) protein G10 (Chang et al., 2013). Here, we report the molecular characterization and efficacy evaluation of a transgenic event ZD12-6, a candidate for deregulation study for future commercial planting.
2. Materials and methods
2.1. Integration of transgenic traits into an elite corn line
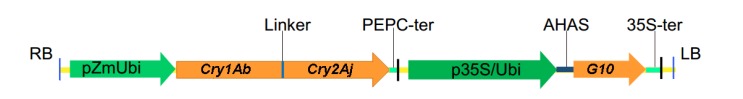
“Hi-II” corn line was used as the recipient for Agrobacterium-mediated transformation to integrate the transferred DNA (T-DNA) encoding a fusion Bt insecticidal protein Cry1Ab/Cry2Aj and a glyphosate-tolerant EPSPS protein G10 (Fig. 1). The truncated Cry1Ab encoding the N-terminal 648 amino acids of active Cry1Ab endotoxin was fused with the full-length Cry2Aj encoding a 65-kDa protein by a linker encoding peptide PGKGGG. Thus, the fusion gene encodes a fusion protein about 130 kDa with two functional Bt toxin cores. The fusion gene Cry1Ab/Cry2Aj was under the control of maize ubiquitin promoter (pZmUbi), and the G10 gene was under the control of a chimeric promoter consisting of cauliflower mosaic virus 35S promoter and the maize ubiquitin promoter. Elite maize inbred line Zheng58 was used as the recurrent parent. By successively backcrossing 6 times, we obtained a transgenic maize line ZD12-6.
Fig. 1.
Schematic diagram of the T-DNA
pZmUbi, maize constitutive promoter based on the maize ubiquitin gene; Cry1Ab and Cry2Aj, synthetic Bt insecticidal genes; p35S/Ubi, the fusion promoter of 35S promoter from cauliflower mosaic virus and maize ubiquitin gene promoter; G10, EPSPS gene; Linker, DNA fragment encoding peptide PGKGGG; PEPC-ter, maize phosphoenolpyruvate carboxylase gene terminator; AHAS, signal peptide of maize acetohydroxyacid synthase; 35S-ter, Cauliflower mosaic virus 35S terminator; LB, left border of T-DNA; RB, right border of T-DNA
2.2. Detection of target genes
Genomic DNA was isolated from leaf tissue of ZD12-6 and the non-transgenic inbred line Zheng58 using the cetyltrimethylammonium bromide (CTAB) method. Total genomic DNA was used as the template for polymerase chain reaction (PCR). Specific primers were designed as in Table 1. NJB-F and NJB-R were used to detect the insect-resistant gene Cry1Ab/Cry2Aj, and G10-F and G10-R were used to detect the glyphosate-tolerant gene G10. PCR was conducted in 25 µl reactions using the following parameters: 94 °C for 2 min; 30 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 2 min; 72 °C for 5 min.
Table 1.
Primers used in PCR
| Primer | Sequence (5'→3') | |
| NJB-F | GGAGTTCACCTGGAAGAGGGAGTAGAGG | |
| NJB-R | CAGTGCCGAGTTCAACAACATCATCCC | |
| G10-F | CAGCGAGGTGAGCAGAGCCAGTCACG | |
| G10-R | ACTTTCGTGACCGACTACCCGGACTC | |
| P1Ab-F | GACAACAACCCCAACATCAACGAGTG | |
| P1Ab-R | ATGTGGTAGTCGGTCACGTCGGTCTT | |
| P10-F | CACCTTCGACGTGATCGTGCATCCA | |
| P10-R | CGAGGTGAGCGAAGAACTGAGGGTAGGA | |
| RB-SPI | CGTGACTGGGAAAACCCTGGCGTT | |
| RB-SPII | ACGATGGACTCCAGTCCGGCCCAACTTAATCGCCTTGCAGCACATC | |
| RB-SPIII | GAAGAGGCCCGCACCGATCGCCCTT | |
| AD4L | AGGTTATGCTANTCAGSTWTSGWGWT | |
| LB-1K | GCCGTACGTTTCCCAGCC | |
| LB-SP4 | CTAAAACCAAAATCCAGTACTAAAATCC | |
| RB-600 | CGTACAGGGAGCTTAGGGGG | |
F, forward; R, reverse
2.3. Determination of the T-DNA insertion site by hiTAIL-PCR
High-efficiency thermal asymmetric interlaced-PCR (hiTAIL-PCR) was carried out as specified by Liu and Chen (2007). Primers used are listed in Table 1. Three runs of PCR reaction were carried out: the primary PCR with RB-SPI and AD4L primers, the secondary PCR with RB-SPII and AD4L primers, and the tertiary PCR with RB-SPIII and AD4L primers. The product of the tertiary PCR was recovered and sequenced. Primer sets that span the joint sequence of the maize genomic and T-DNA border were designed to validate the insertion site. LB-SP4 and LB-1K (Table 1) were used to amplify the left border to validate the insertion site. RB-600 and LB-SP4 were used to validate the right border.
2.4. Southern blot analysis
To demonstrate the stability of the DNA insert in ZD12-6, Southern blot analysis was performed using genomic DNA isolated from three generations of ZD12-6. Southern blot was carried out as described in Molecular Cloning (Sambrook and Russell, 2001). The genomic DNA (100 g) was digested overnight with restriction enzyme. The digested genomic DNA was separated in 7 g/L agarose gel at 30 V over 8 h and transferred onto a Hybond N+membrane (Amersham, UK). The hybridization probes, which were specific to the G10 gene and the Cry1Ab gene, were prepared as described in the DIG-High Prime DNA Labeling and Detection Starter Kit II (Roche, Basel, Switzerland). The templates for producing Cry1Ab probes and G10 probes were generated by PCR using primers P1Ab-F/P1Ab-R and P10-F/P10-R, respectively. The blots were visualized in a Gel Logic 2200 imaging system (Kodak, USA).
2.5. Western blot analysis
The Western blot analysis method was used to detect the expression of G10 and Cry1Ab/Cry2Aj in transgenic plants. Western blot analysis was conducted as previously described (Zhao et al., 2014). Leaves of three generations of ZD12-6 as well as non-transgenic control maize leaves were ground to powder and then suspended in 500 µl phosphate-buffered saline (PBS) buffer. Supernatants were collected after centrifugation at 12 000g for 15 min. The supernatants were mixed with 5×protein loading buffer and boiled for 10 min. After centrifugation at 12 000g for 5 min, the soluble fractions of these samples were separated by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto a nitrocellulose membrane. After blocking with 50 mg/ml bovine serum albumin (BSA) at ambient temperature, the membranes were then incubated with primary antibody against either Cry2Ab or G10. The polyclonal antisera against Cry2Ab and G10 EPSPS were prepared from New Zealand white rabbits immunized with purified recombinant Cry2Ab and G10 from Escherichia coli, respectively. Three washes of TBST (a mixture of Tris-buffered saline (TBS) and Tween 20) were applied after primary antibody incubation. The membranes were then incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (Promega, Wisconsin, USA). The blots were visualized with a diaminobenzidine (DAB) substrate.
2.6. Protein quantification of Cry1Ab and G10
The expression levels of Cry1Ab and G10 in the transgenic maize were determined by enzyme-linked immunosorbent assay (ELISA) as previously described (Zhao et al., 2014). Whorl leaf, pollen, silk, husk, and root of three different generations of ZD12-6 were collected. The Cry1Ab protein in the collected transgenic maize plant tissues was quantified by ELISA using the Cry1Ab/Cry1Ac QuantiPlate Kit (EnviroLogix, Portland, OR, USA). The expression of G10 in the collected tissues was quantified by ELISA using the QuantiPlate Kit for G10-EPSPS (Youlong Biotech, Shanghai, China). Samples prepared from non-transgenic maize were used to eliminate basal absorption at 450 nm.
2.7. Laboratory bioassays
Insect bioassays were conducted with Asian corn borer (O. furnacalis), cotton boll worm (H. armigera), and oriental armyworm (M. separata). Detached leaf bioassay was carried out as in the previous study (Chen et al., 2010). Leaf blades from three generations of ZD12-6 and non-transgenic isogenic line Zheng58 were collected at 2‒3 cm length at whorl stage and placed in a 70-mm diameter petri dish lined with a pre-moistened filter paper. The leaf samples in the petri dishes were infested with 10 newly hatched neonates per plate. The petri dishes were then sealed with parafilm and placed in the dark at 28 °C. The number of surviving larvae was recorded at 48, 72, and 96 h post infestation. Eggs of O. furnacalis, H. armigera, and M. separata were obtained from Genralpest Biotech (Beijing, China). Each assay was repeated 10 times.
2.8. Field insect resistance evaluation
The efficacy study of ZD12-6 against the Asian corn borer using non-transgenic isogenic line Zheng58 as control was conducted at the Agricultural Experimental Station of Zhejiang University in Hangzhou, China, during the spring and summer crop season of 2015. Seeds were planted in two-row plots with rows spaced 40 cm apart and seed placed at 5 seeds per meter in the row. Rows were 5 m long with an 80-cm alley between plots. Therefore, each plot included 80 individual plants. Plots were arranged in a completely randomized block design with three replicates. When plants developed to the V6 whorl stage, each plant of ZD12-6 and Zheng58 was artificially infested with 60 newly hatched Asian corn borer neonates. Infestation was repeated once 5 d post the initial infestation. Leaf damage was checked and recorded 2 weeks after the second infestation. Leaf damage was rated on a scale of 1 to 9, with 1 representing the most resistant and 9 representing the most susceptible (He et al., 2000). When plants developed to silking stage, young silks were infested with Asian corn borer twice in the same way as at the V6 whorl stage. Damage ratings were collected at physiological maturity (R6). Maize plants were dissected to record surviving larvae or pupae, and the number and the length of tunnels in the stalks and cobs. Ear-feeding was rated on a scale of 0 to 9, with 0 representing no ear damage (He et al., 2000).
2.9. Glyphosate tolerance trial
Greenhouse glyphosate spraying was carried out as described by Zhang et al. (2013). Propyl amine salt of glyphosate (410 g/L; Roundup, Monsanto, La Conner, USA) diluted at 1:100 (10 ml/L) was sprayed onto three generations of ZD12-6 maize and non-transgenic isogenic line Zheng58 at the rate of 45 ml/m². The results were recorded 14 d post spray.
The field trial was conducted for glyphosate tolerance of ZD12-6 using isogenic line Zheng58 as control. The field trial followed a split block design (plots of 5-m twin rows spaced 0.76 m apart) with three replications. ZD12-6 and non-transgenic maize were sprayed with Roundup diluted at 1:100 (10 ml/L) and at the rate of 45 ml/m2 at the V4 leaf stage. Plots were visually inspected at 14 d post application.
3. Results
3.1. PCR and Southern blot analysis of transgenic maize
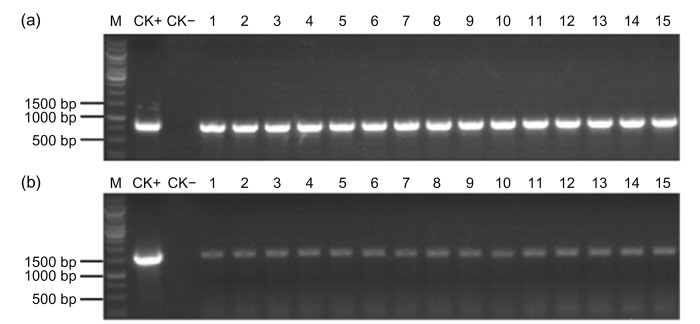
To determine if the T-DNA is stably inherited, PCR analysis was performed to detect the Cry1Ab/Cry2Aj gene and the G10 gene among the ZD12-6 maize plants of different generations. A single band with the expected size of 800 bp of the G10 gene was obtained using primers G10-F and G10-R (Fig. 2a), and a single band with the expected size of 1500 bp of the Cry1Ab/Cry2Aj gene was obtained by PCR using primers NJB-F and NJB-R (Fig. 2b). No amplicon was observed in non-transgenic plants under identical PCR conditions. This result suggested that the T-DNA with both genes was stably integrated into the maize genome.
Fig. 2.
PCR analysis of the DNA insert in three generations of ZD12-6 maize
(a) PCR products for a G10 fragment of about 800 bp in the transgenic maize plants; (b) PCR products for a fragment of the Cry1Ab/Cry2Aj gene in the transgenic maize plants. M, DNA marker; CK+, plasmid DNA as positive control; CK−, non-transgenic corn control; Lanes 1–5, 6–10, and 11–15 represent T4, T5, and T6 generations of ZD12-6 maize, respectively
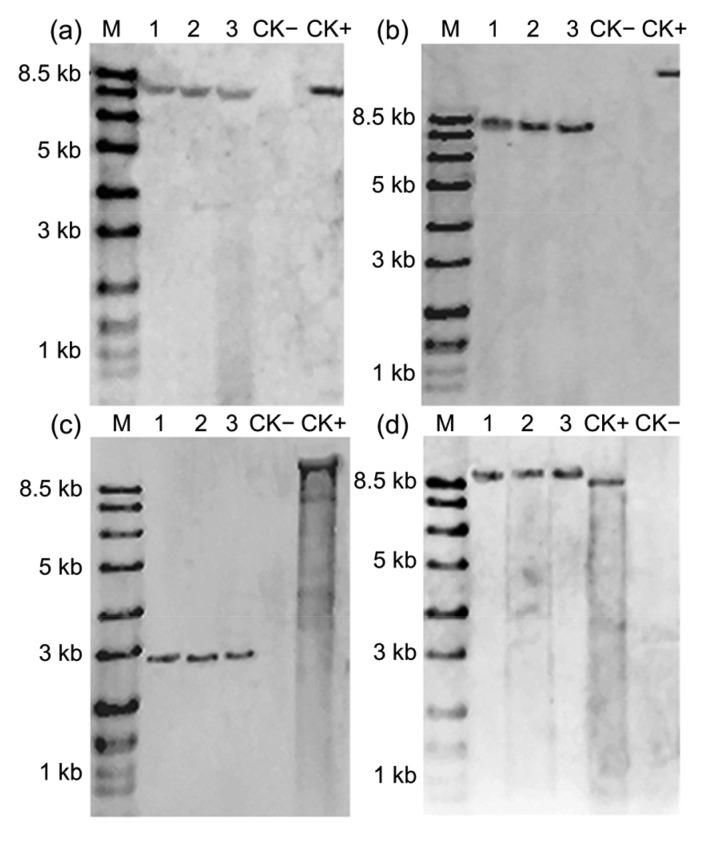
To determine the copy number and the stability of the T-DNA insert in ZD12-6, Southern blot analysis was performed using genomic DNA isolated from three generations of ZD12-6. The genomic DNA digested with SmaI and SacI hybridized with a Cry1Ab probe produced a single band of about 7.5 kb (Fig. 3a) and 8.0 kb (Fig. 3b), respectively. The genomic DNA digested with BamHI and XbaI hybridized with G10 probe produced a single band of about 2.7 kb (Fig. 3c) and 9.0 kb (Fig. 3d), respectively. The Southern blot analysis suggested that ZD12-6 is a single copy T-DNA insert event and its T-DNA is stably inherited over the generations.
Fig. 3.
Southern blot analyses of ZD12-6 maize
The event ZD12-6 was analyzed using probe against Cry1Ab (a, b) and G10 (c, d). (a) The restriction enzyme used for genomic DNA digested was SmaI; (b) The restriction enzyme used for genomic DNA digested was SacI; (c) The restriction enzyme used for genomic DNA digested was BamHI; (d) The restriction enzyme used for genomic DNA digested was XbaI. M, DNA ladder; CK+, plasmids as positive control; CK−, non-transgenic maize as negative control; Lanes 1, 2, and 3 represent T4, T5, and T6 generations of ZD12-6 maize, respectively
3.2. Determination of the T-DNA insertion site
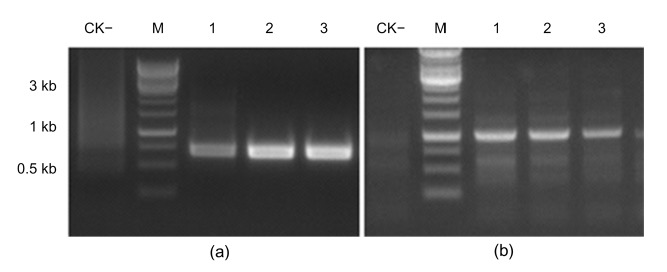
To determine the insertion site of the T-DNA at the maize genome, hiTAIL-PCR was performed to obtain the genomic sequence that borders the T-DNA of ZD12-6 maize. By searching the maize genome database, the insertion site of the right border was located at position 268 930 576 on chromosome 1. Specific PCR primers were designed on the maize chromosome at the position of about 600 bp outside the right border of the T-DNA and about 1000 bp outside the left border of the T-DNA to verify the insertion site. PCR products of expected size were obtained from both the left and right border sequences (Fig. 4). Sequencing of the PCR products of the junction sequences suggested that there was no deletion of the maize genomic sequence due to T-DNA insertion. Moreover, bioinformatic analysis indicated that no annotated or putative genes were located at or close to the insertion site, suggesting that the impact of the T-DNA insertion on maize gene expression would likely be minimal.
Fig. 4.
PCR validation of the right and left border sequences
The PCR was performed with RB600 and LB-SP4 to validate the right border (a), and with LB-1K and LB-SP4 to validate the left border (b). M, DNA marker; CK−, non-transgenic maize as negative control; Lanes 1, 2, and 3 represent T4, T5, and T6 generations of ZD12-6 maize, respectively
3.3. Expression analysis of transgenes
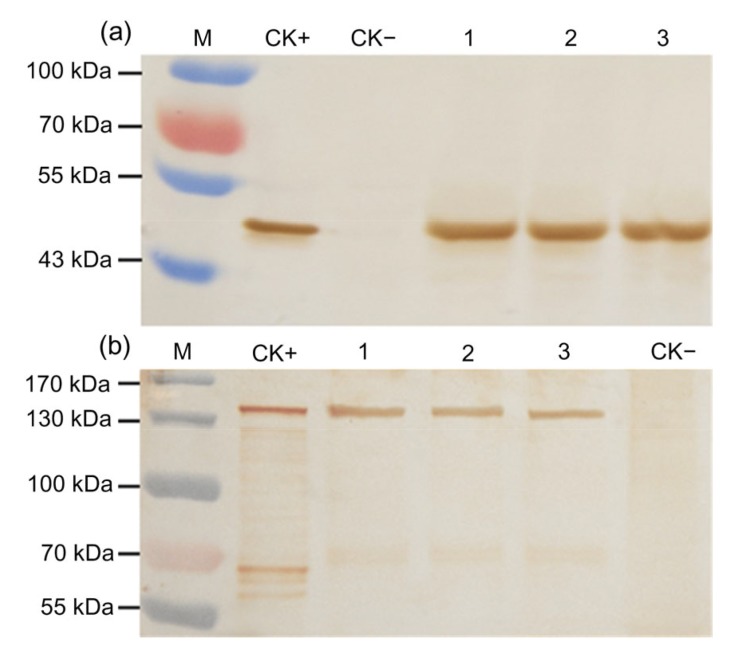
Western blot analyses showed that both G10 and Cry1Ab/Cry2Aj proteins were expressed in the plants from three generations of ZD12-6. When G10 antiserum was used to conduct Western blot analysis, an estimated size of about 48 kDa was detected. This size is very close to its calculated size (Fig. 5a). When detected by antiserum against Cry2Ab, a band of about 130 kDa was detected in different generations of transgenic maize (Fig. 5b). The same size of band was detected when Cry1Ab antiserum was used against the fusion protein (data not shown). Western blot analysis showed that the Cry1Ab/Cry2Aj protein is not self-cleaving in transgenic maize.
Fig. 5.
Western blot analyses of transgenic maize in three generations
Each sample was detected with antiserum against G10 (a) and Cry2Ab (b). M, pre-stained protein ladder; CK+, G10 protein expressed by E. coli (a) and Cry1Ab/Cry2Aj expressed by E. coli (b) were used as positive control; CK−, non-transgenic maize as negative control; Lanes 1, 2, and 3 represent T4, T5, and T6 generations of ZD12-6 maize, respectively
The ELISA method was used to measure the expression levels of G10 and Cry1Ab/Cry2Aj. The average concentrations of Cry1Ab/Cry2Aj were 17.56 µg/g (protein per fresh tissue weight, fwt) in ZD12-6 leaves, 11.12 µg/g in pollen, 2.54 µg/g in silk, 2.62 µg/g in husk, and 3.42 µg/g in root (Fig. 6a). The average concentrations of G10 were 13.83 µg/g (fwt) in ZD12-6 leaves, 7.30 µg/g in pollen, 1.61 µg/g in silk, 1.14 µg/g in husk, and 1.71 µg/g in root (Fig. 6b). The expression levels of insect-resistant protein were similar in T4, T5, and T6 generations of ZD12-6 plants, indicating that the expression of fusion gene Cry1Ab/Cry2Aj was stable (Fig. 6).
Fig. 6.
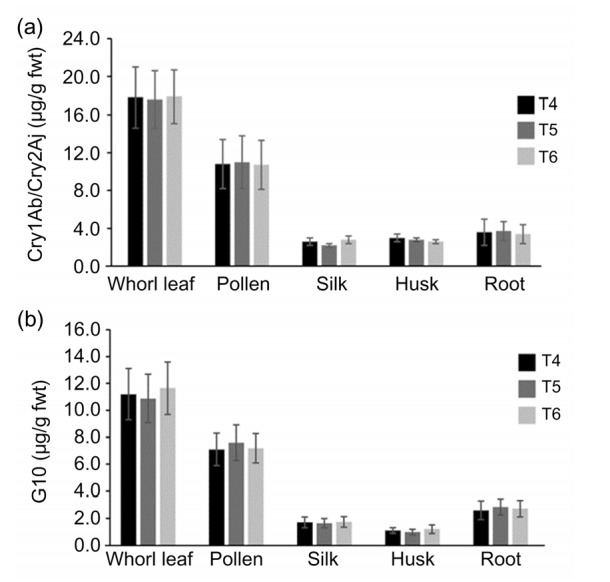
Concentrations of Cry1Ab (a) and G10 (b) in different tissues of three generations of transgenic ZD12-6 maize
The data in all samples are expressed as mean±standard deviation (n=5). fwt, fresh weight
3.4. Insect resistance of ZD12-6
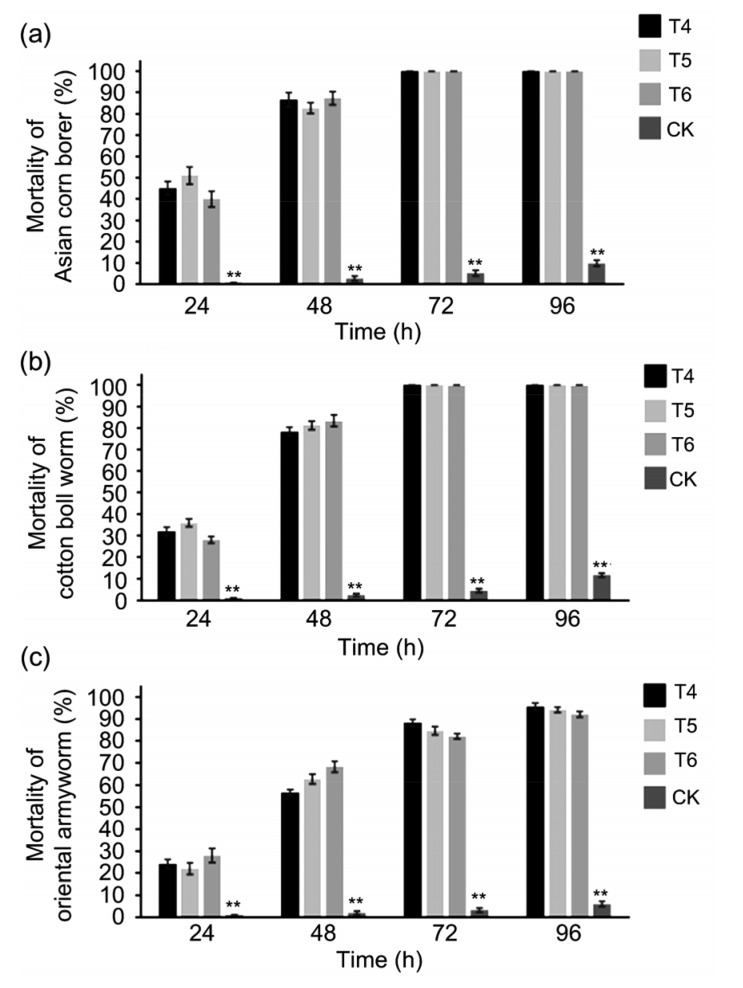
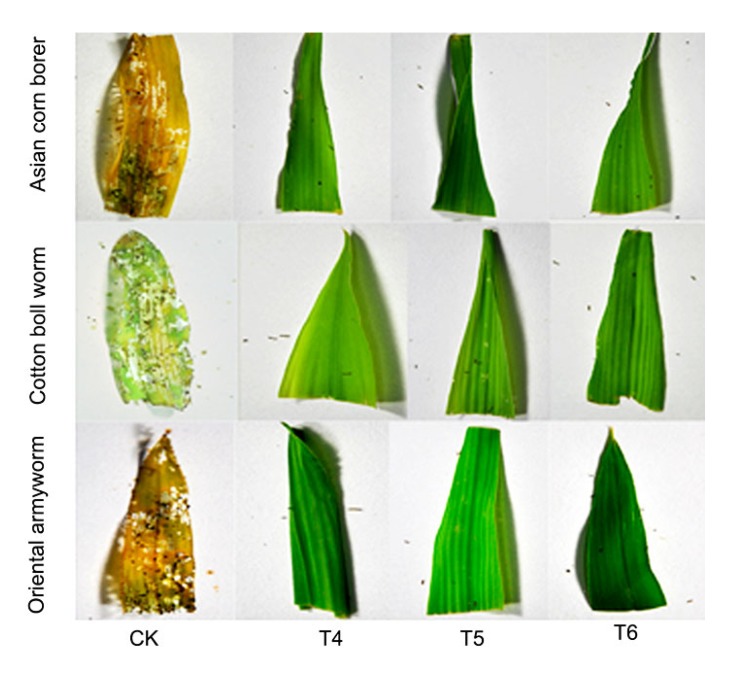
To determine the insecticidal activity of the ZD12-6 maize, neonates of O. furnacalis, H. armigera, and M. separata were used for the bioassay. Mortalities of O. furnacalis, H. armigera, and M. separata on ZD12-6 leaves were 100%, 100%, and 95% at 96 h post infestation. The M. separata was severely inhibited in growth, and eventually died. The average mortality rates on the non-transgenic maize were 9.1%, 11.6%, and 6.0% for the three insects (Fig. 7). The leaves from ZD12-6 maize were only slightly bitten by O. furnacalis, H. armigera, and M. separata, while non-transgenic maize had suffered significant damage (Fig. 8). These results demonstrated that ZD12-6 was highly insect-resistant and the insecticidal activity was stable over different generations.
Fig. 7.
Mortalities of O. furnacalis (a), H. armigera (b), and M. separata (c) feeding on three generations of ZD12-6
Non-transgenic maize at the same growing stage was used as the control (CK). Data are expressed as mean±standard deviation (n=10) and analyzed with nonparametric test in SPSS. ** indicates extremely significant difference between CK and ZD12-6 (P<0.01)
Fig. 8.
Insect bioassays for three generations of ZD12-6 with O. furnacalis, H. armigera, and M. separata
Non-transgenic maize at the same growing stage was used as the control (CK)
The field tests of the artificial infestation of O. furnacalis at V6 stage showed minimal leaf feeding damage to ZD12-6. The leaf feeding ratings were drastically lower than those of the non-transgenic variety Zheng58 (Table 2). The field tests at silking stage showed that the stalks and ears on non-transgenic Zheng58 were severely damaged by Asian corn borer, while no significant damage was found on ears or in stalks of ZD12-6 in both spring and summer cropping seasons. The number of tunnels per plant was significantly higher in the non-Bt control than in the Bt maize: over three larvae or pupae were found on each plant in control plots (Table 2). These results indicated that the ZD12-6 provided a higher protection against Asian corn borer under field conditions.
Table 2.
Damage rating of maize ZD12-6 and non-transgenic isogenic line Zheng58 under artificial infestations of O. furnacalis under field conditions
| Cropping season | Variety | Mid-whorl leaf stage leaf feeding rating | Silking stage |
|||
| Ear damage rating | No. of survived larvae or pupae per plant | No. of holes found in tunnels or stalks | Tunnel length (cm) | |||
| Spring maize | ZD12-6 | 1.00±0.00** | 0.00±0.00** | 0.00±0.00** | 0.00±0.00** | 0.00±0.00** |
| Zheng58 | 4.30±0.31 | 4.30±0.31 | 4.10±0.39 | 3.95±0.46 | 8.58±1.61 | |
| Summer maize | ZD12-6 | 1.00±0.00** | 0.00±0.00** | 0.00±0.00** | 0.00±0.00** | 0.00±0.00** |
| Zheng58 | 5.50±0.51 | 6.50±0.57 | 3.60±0.34 | 4.20±0.41 | 13.94±1.69 | |
The data are expressed as mean±standard error (SE) (n=30) and analyzed with nonparametric test in SPSS.
indicates extremely significant difference between CK (Zheng58) and ZD12-6 (P<0.01)
3.5. Glyphosate tolerance evaluation
To determine the efficacy of glyphosate tolerance of ZD12-6, maize transgenic plants in greenhouse at V4 stage were sprayed with 410 g/L propyl amine salt of glyphosate diluted 100 times at the rate of 45 ml/m2, which is twice the recommended dose of corn field application. After 14 d, we found that all the non-transgenic maize plants were dead, while no obvious damage was observed on all plants of three generations of ZD12-6 (Fig. 9a). The results demonstrated that glyphosate tolerance of ZD12-6 was high and stable over generations.
Fig. 9.
Glyphosate-resistant assay of ZD12-6
(a) The plants were sprayed with 410 g/L propyl amine salt of glyphosate diluted at 1:100. (b) Glyphosate spray tolerance of field trial plants. Non-transgenic maize at the same growing stage was used as a control (CK). Non-transgenic maize and ZD12-6 were sprayed with glyphosate diluted at 1:100 or were sprayed with water. The pictures were taken two weeks after spraying
A field trial was conducted for glyphosate tolerance of ZD12-6. The plants were sprayed at the V4 stage with twice the recommended dose for corn. All the non-transgenic maize plants died, while the transgenic plants showed no visual damage. The plant height did not differ significantly from water-sprayed transgenic control (Fig. 9b). The results suggested that maize ZD12-6 showed excellent tolerance to glyphosate and spraying with twice the recommended dose of glyphosate has no negative impact on ZD12-6.
4. Discussion
Genetic engineering (GE) technology provides an effective way for corn insect pest control. Since the first commercialization in the United States in 1996, GE corn has been widely and rapidly adopted worldwide. However, intensive planting of Bt crops inevitably creates strong selection pressure on the target insect pests. Development of the resistance of insects to these Bt crops is a major threat to the durability of Bt crops (Tabashnik, 1994; Gould, 1998; Tabashnik et al., 2008; Huang et al., 2011). So far, a large number of studies have been reported regarding the development of insect resistance to transgenic maize (Coll et al., 2009; Carrillo et al., 2011). Multiple strategies have been developed to control Bt resistance pests. One such strategy is “pyramid”, which simultaneously expresses two or more different types of insect-resistant genes. The use of two or more insect control genes may slow down the development of pest resistance in addition to expanding the insecticide spectrum and enhancing efficacy (Zhao et al., 2003; Xu et al., 2010; Yang et al., 2011). The Cry2A protein is highly effective against many lepidopteron pests and has a different receptor binding site from Cry1A proteins (Hernández-Rodríguez et al., 2008, 2013). Therefore, Cry2A has been used extensively in pyramided transgenic crops (Kota et al., 1999; Chen et al., 2005; Gouffon et al., 2011; Yang et al., 2011; Sohail et al., 2012; Hernández-Rodríguez et al., 2013; Zhao et al., 2014). As ZD12-6 contains both Cry1A and Cry2A, this transgenic maize event could provide a better resistance management strategy than plants with only a single Bt protein.
Stable expression of Bt proteins in plants is the basis for constant high efficacy in controlling target pests, and is also important for delaying the development of Bt resistance of target pests. Our study indicated that the fusion gene Cry1Ab/Cry2Aj and G10 were expressed stably at high levels in maize ZD12-6 over different generations. The average expression levels of Cry1Ab/Cry2Aj in ZD12-6 were 17.56 µg/g (fwt) in leaves, and 2.54 µg/g in silk. These levels are comparable to the leading commercial Bt corn event MON810, which expresses a single Cry1Ab protein at 9.56 μg/g (fwt) in leaves (He et al., 2004). Our study in the greenhouse proved that ZD12-6 was highly effective to the O. furnacalis, H. armigera, and M. separata. The field trial results clearly demonstrated that ZD12-6 was effective in resistance to the O. furnacalis. We anticipate that ZD12-6 will be a highly effective lepidopteran insect-resistant event.
Transgenic maize with more than one trait will better meet the demand under complex farming conditions. ZD12-6 is not only resistant to insects but also highly tolerant to glyphosate, which will help farmers develop an efficient insect control and weed management system. The molecular stacking of the Bt gene and the glyphosate-tolerant gene by constructing them in a single T-DNA can greatly simplify the breeding process by eliminating the need for stacking by hybridization. We are currently doing a deregulation study on the event to push for eventual commercial planting.
Footnotes
Project supported by the Fundamental Research Funds for the Central Universities (No. 2017FZA6011) and the National Key Transgenic Research Projects (No. 2016ZX08010003) of China
Compliance with ethics guidelines: Miao-miao LIU, Xiao-jing ZHANG, Yan GAO, Zhi-cheng SHEN, and Chao-yang LIN declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of authors.
References
- 1.Carrillo L, Martinez M, Ramessar K, et al. Expression of a barley cystatin gene in maize enhances resistance against phytophagous mites by altering their cysteine-proteases. Plant Cell Rep. 2011;30(1):101–112. doi: 10.1007/s00299-010-0948-z. [DOI] [PubMed] [Google Scholar]
- 2.Cattaneo MG, Yafuso C, Schmidt C, et al. Farm-scale evaluation of the impacts of transgenic cotton on biodiversity, pesticide use, and yield. Proc Natl Acad Sci USA. 2006;103(20):7571–7576. doi: 10.1073/pnas.0508312103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang X, Liu GG, He KL, et al. Efficacy evaluation of two transgenic maize events expressing fused proteins to Cry1Ab-susceptible and -resistant Ostrinia furnacalis (Lepidoptera: Crambidae) J Econ Entomol. 2013;106(6):2548–2556. doi: 10.1603/EC13100. [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Tang W, Xu CG, et al. Transgenic indica rice plants harboring a synthetic cry2A * gene of Bacillus thuringiensis exhibit enhanced resistance against lepidopteran rice pests. Theor Appl Genet. 2005;111(7):1330–1337. doi: 10.1007/s00122-005-0062-8. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Tian JC, Shen ZC, et al. Transgenic rice plants expressing a fused protein of Cry1Ab/Vip3H has resistance to rice stem borers under laboratory and field conditions. J Econ Entomol. 2010;103(4):1444–1453. doi: 10.1603/EC10014. [DOI] [PubMed] [Google Scholar]
- 6.Coll A, Nadal A, Collado R, et al. Gene expression profiles of MON810 and comparable non-GM maize varieties cultured in the field are more similar than are those of conventional lines. Transgenic Res. 2009;18(5):801–808. doi: 10.1007/s11248-009-9266-z. [DOI] [PubMed] [Google Scholar]
- 7.Du DX, Geng CJ, Zhang XB, et al. Transgenic maize lines expressing a cry1C * gene are resistant to insect pests. Plant Mol Biol Rep. 2014;32(2):549–557. doi: 10.1007/s11105-013-0663-3. [DOI] [Google Scholar]
- 8.Gouffon C, van Vliet A, van Rie J, et al. Binding sites for Bacillus thuringiensis Cry2Ae toxin on heliothine brush border membrane vesicles are not shared with Cry1A, Cry1F, or Vip3A toxin. Appl Environ Microbiol. 2011;77(10):3182–3188. doi: 10.1128/AEM.02791-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gould F. Sustainability of transgenic insecticidal cultivars: integrating pest genetics and ecology. Annu Rev Entomol. 1998;43:701–726. doi: 10.1146/annurev.ento.43.1.701. [DOI] [PubMed] [Google Scholar]
- 10.He KL, Wang ZY, Zhou DR, et al. Methodologies and criterions for evaluating maize resistance to Asian maize borer. J Shenyang Agric Univ. 2000;31(5):439–443. doi: 10.3969/j.issn.1000-1700.2000.05.009. (in Chinese) [DOI] [Google Scholar]
- 11.He KL, Wang ZY, Wen LP, et al. Transgenic maize evaluated for resistance to the Asian corn borer (Lepidoptera: Pyralidae) Chin Agric Sci Bull. 2004;20(6):240–242. 240. doi: 10.3969/j.issn.1000-6850.2004.06.077. (in Chinese) [DOI] [Google Scholar]
- 12.Hernández-Rodríguez CS, van Vliet A, Bautsoens N, et al. Specific binding of Bacillus thuringiensis Cry2A insecticidal proteins to a common site in the midgut of Helicoverpa species. Appl Environ Microbiol. 2008;74(24):7654–7659. doi: 10.1128/AEM.01373-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernández-Rodríguez CS, Hernández-Martínez P, van Rie J, et al. Shared midgut binding sites for Cry1A.105, Cry1Aa, Cry1Ab, Cry1Ac and Cry1Fa proteins from Bacillus thuringiensis in two important corn pests, Ostrinia nubilalis and Spodoptera frugiperda . PLoS ONE. 2013;8(7):e68164. doi: 10.1371/journal.pone.0068164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang FN, Andow DA, Buschman LL. Success of the high-dose/refuge resistance management strategy after 15 years of Bt crop use in North America. Entomol Exp Appl. 2011;140(1):1–16. doi: 10.1111/j.1570-7458.2011.01138.x. [DOI] [Google Scholar]
- 15.Huang JK, Hu RF, Rozelle S, et al. Insect-resistant GM rice in farmers’ fields: assessing productivity and health effects in China. Science. 2005;308(5722):688–690. doi: 10.1126/science.1108972. [DOI] [PubMed] [Google Scholar]
- 16.Hunt TE, Buschman LL, Sloderbeck PE. Insecticide use in Bt and non-Bt field corn in the western corn belt: as reported by crop consultants in a mail survey. Am Entomol. 2007;53(2):86–93. doi: 10.1093/ae/53.2.86. [DOI] [Google Scholar]
- 17.Hutchison WD, Burkness EC, Mitchell PD, et al. Areawide suppression of European corn borer with Bt maize reaps savings to non-Bt maize growers. Science. 2010;330(6001):222–225. doi: 10.1126/science.1190242. [DOI] [PubMed] [Google Scholar]
- 18.James C. Global Status of Commercialized Biotech/GM Crops: 2015. ISAAA Brief No. 51, International Service for the Acquisition of Agri-biotech Applications (ISAAA), Ithaca, NY, USA; 2015. [Google Scholar]
- 19.Kota M, Daniell H, Varma S, et al. Overexpression of the Bacillus thuringiensis (Bt) Cry2Aa2 protein in chloroplasts confers resistance to plants against susceptible and Bt-resistant insects. Proc Natl Acad Sci USA. 1999;96(5):1840–1845. doi: 10.1073/pnas.96.5.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu YG, Chen YL. High-efficiency thermal asymmetric interlaced PCR for amplification of unknown flanking sequences. BioTechniques. 2007;43(5):649–656. doi: 10.2144/000112601. [DOI] [PubMed] [Google Scholar]
- 21.Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual, 3rd Ed. Cold Spring Harbor Laboratory Press, New York; 2001. [Google Scholar]
- 22.Shen P, Zhang QY, Lin YH, et al. Thinking to promote the industrialization of genetically modified corn of our country. China Biotechnol. 2016;36(4):24–29. (in Chinese) [Google Scholar]
- 23.Sohail MN, Karimi SM, Asad S, et al. Development of broad-spectrum insect-resistant tobacco by expression of synthetic cry1Ac and cry2Ab genes. Biotechnol Lett. 2012;34(8):1553–1560. doi: 10.1007/s10529-012-0923-6. [DOI] [PubMed] [Google Scholar]
- 24.Tabashnik BE. Evolution of resistance to Bacillus thuringiensis . Annu Rev Entomol. 1994;39:47–79. doi: 10.1146/annurev.en.39.010194.000403. [DOI] [Google Scholar]
- 25.Tabashnik BE, Gassmann AJ, Crowder DW, et al. Insect resistance to Bt crops: evidence versus theory. Nat Biotechnol. 2008;26(2):199–202. doi: 10.1038/nbt1382. [DOI] [PubMed] [Google Scholar]
- 26.Xu L, Wang Z, Zhang J, et al. Cross-resistance of Cry1Ab-selected Asian corn borer to other Cry toxins. J Appl Entomol. 2010;134(5):429–438. doi: 10.1111/j.1439-0418.2010.01517.x. [DOI] [Google Scholar]
- 27.Yang Z, Chen H, Tang W, et al. Development and characterisation of transgenic rice expressing two Bacillus thuringiensis genes. Pest Manag Sci. 2011;67(4):414–422. doi: 10.1002/ps.2079. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Q, Yu H, Zhang FZ, et al. Expression and purification of recombinant human serum albumin from selectively terminable transgenic rice. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2013;14(10):867–874. doi: 10.1631/jzus.B1300090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao JZ, Cao J, Li YX, et al. Transgenic plants expressing two Bacillus thuringiensis toxins delay insect resistance evolution. Nat Biotechnol. 2003;21(12):1493–1497. doi: 10.1038/nbt907. [DOI] [PubMed] [Google Scholar]
- 30.Zhao QC, Liu MH, Tan MM, et al. Expression of Cry1Ab and Cry2Ab by a polycistronic transgene with a self-cleavage peptide in rice. PLoS ONE. 2014;9(10):e110006. doi: 10.1371/journal.pone.0110006. [DOI] [PMC free article] [PubMed] [Google Scholar]