Abstract
Targeting Induced Local Lesions IN Genomes (TILLING) is a reverse genetics strategy for the high-throughput screening of induced mutations. γ radiation, which often induces both insertion/deletion (Indel) and point mutations, has been widely used in mutation induction and crop breeding. The present study aimed to develop a simple, high-throughput TILLING system for screening γ ray-induced mutations using high-resolution melting (HRM) analysis. Pooled rice (Oryza sativa) samples mixed at a 1:7 ratio of Indel mutant to wild-type DNA could be distinguished from the wild-type controls by HRM analysis. Thus, an HRM-TILLING system that analyzes pooled samples of four M2 plants is recommended for screening γ ray-induced mutants in rice. For demonstration, a γ ray-mutagenized M2 rice population (n=4560) was screened for mutations in two genes, OsLCT1 and SPDT, using this HRM-TILLING system. Mutations including one single nucleotide substitution (G→A) and one single nucleotide insertion (A) were identified in OsLCT1, and one trinucleotide (TTC) deletion was identified in SPDT. These mutants can be used in rice breeding and genetic studies, and the findings are of importance for the application of γ ray mutagenesis to the breeding of rice and other seed crops.
Keywords: Mutation screening, High-resolution melting (HRM) analysis, Targeting Induced Local Lesions IN Genomes (TILLING), Mutant, γ ray, Rice
1. Introduction
Mutants are an important genetic resource for both functional genomics and crop breeding. For the fast and efficient screening of induced mutations, McCallum et al. (2000) developed a novel system, now known as TILLING (Targeting Induced Local Lesions IN Genomes), which can be used to screen for mutations in a target region in pooled samples instead of in individual plants. TILLING has since been applied in investigations of a great number of animal and plant species (Taheri et al., 2017). TILLING was initially developed for screening point mutations induced by chemical mutagenesis, mostly by ethyl methanesulfonate (EMS) (McCallum et al., 2000; Till et al., 2003). A TILLING system for the screening of large deletions induced by fast neutrons was developed and referred to as deletion TILLING (De-TILLING) (Rogers et al., 2009). In addition, new TILLING variants, such as individualized TILLING (iTILLING) (Bush and Krysan, 2010), TILLING by sequence (Seq-TILLING) (Tsai et al., 2011; Kumar et al., 2017), high-resolution melting (HRM)-TILLING (Dong et al., 2009; Gady et al., 2009), compressed sequencing (ComSeq)-TILLING (Nida et al., 2016), and denaturing high-performance liquid chromatography (DHPLC)-based TILLING (Colasuonno et al., 2016), were developed for simplicity or higher throughput levels. However, these systems have been applied only to EMS or fast neutron mutagenesis.
HRM curve analysis, which is based on fluorescence changes during the melting of the DNA duplex, is a simple, cost-effective, and high-throughput method for mutation screening and genotyping (Ririe et al., 1997). HRM has already been widely used in plant research for high-throughput genotyping, mapping genes, and testing food products and seeds (Simko, 2016). It has also been used in combination with TILLING (HRM-TILLING) for screening EMS-mutagenized populations of tomato (Gady et al., 2009), wheat (Dong et al., 2009; Lochlainn et al., 2011), and grapevine (Acanda et al., 2014). As with most other TILLING systems, HRM-TILLING has been used only for the screening of EMS-induced mutations.
γ irradiation has been used widely as an efficient tool for mutation induction in about 200 economically important plant species (http://mvd.iaea.org). A great number of mutants have been generated for genetic studies and for the breeding of new cultivars that have been grown on billions of hectares (Ahloowalia et al., 2004; Shu et al., 2012). Both small insertion/deletions (Indels) and point mutations can be induced by γ ray (Nawaz and Shu, 2014; Li et al., 2016), which makes it a unique mutagen. As with other mutagens, γ radiation induces mutations only at low frequencies, such as 7.5×10−6 in rice (Li et al., 2016). Therefore, a TILLING system suitable for the high-throughput screening of both Indels and point mutations is needed to increase the selection efficiency of γ ray-induced mutations. Sato et al. (2006) reported a simplified version of TILLING for the identification of γ ray-induced mutants in rice, in which crude extracts of Brassica rapa petioles were used as mismatch-specific endonucleases instead of celery endonuclease I (CELI), and DNA fragments were separated on agarose gels instead of using the expensive LI-COR system. However, its throughput capacity was still very low. Recently, Hwang et al. (2017) identified a number of mutations in membrane transport genes in a γ ray-mutagenized rice population using TILLING. Thus, there is a need to establish new TILLING platforms suitable for more efficient and simpler screening of γ ray-induced mutations.
In this study, we first investigated the power of HRM analysis for the detection of various rice (Oryza sativa) deletion mutations in pooled samples. Then, we applied the optimized system to screening for mutations in two genes in an M2 rice population exposed to γ radiation.
2. Materials and methods
2.1. Plant materials
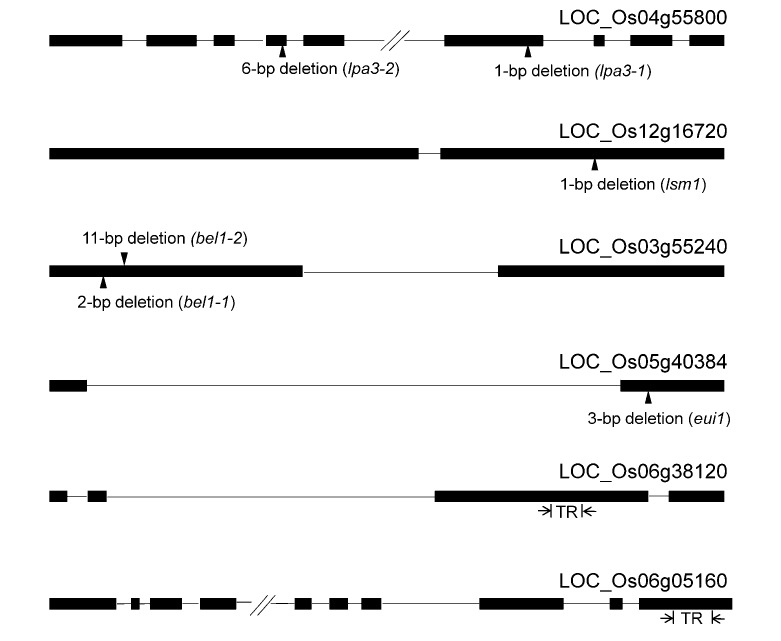
Because γ rays can induce deletion mutations of different sizes, mutants with 1–11-bp deletions together with their wild-type (WT) parents were used for the HRM analysis of pooled samples (Table 1, Fig. 1). The mutant lines included two low phytic acid lines (MH86-lpa and Z9B-lpa), one lesion mimic mutant (JZBm), two bentazon susceptible mutants (GZ63m1 and GZ63m2), and one elongated uppermost internode mutant (Elong B).
Table 1.
Rice materials used for establishing an HRM-TILLING system
| Mutant | WT parent | Gene (LOC number) | Deletion | Primer (5'→3') | T m (°C) | Amplicon (WT) (bp) | Reference |
| Mutants and WT parents | |||||||
| MH86-lpa | Minghui86 (MH86) | OsSultr3;3 (Os04g55800) | G | F:CGACTTGAAGAAATCAACAGAGAAGCCTG | 56.3 | 179 | Zhao et al., 2008 |
| R:GGGGTTTACTATGCCACATAACT | |||||||
| JZBm | Jiazhe B (JZB) | CYP71A1 (Os12g16720) | G | F:CTGGGACAACCCGCTGGAGT | 62.0 | 288 | Lu et al., 2016 |
| R:TTCCTGACGGCGACGAAGA | |||||||
| GZ63m1 | Guangzhan 63 (GZ63) | CYP81A6 (Os03g55240) | CG | F:GAAGCCGATGCACGCCACC | 60.0 | 192 | Wang et al., 2012 |
| R:GCGCCGTTGAACGAGACCAG | |||||||
| Elong B (ELB) | Kelong B (KLB) | CYP714D1 (Os05g40384) | TCT | F:AAGCCCAAGTACCTCCAGAA | 54.8 | 294 | Fu et al., 2008 |
| R:CGAGTAATCACTCCCAAAGC | |||||||
| Z9B-lpa | Zhong 9B (Z9B) | OsSultr3;3 (Os04g55800) | AAATCC | F:GGTGCCCAGCTACTCCTTCTC | 59.0 | 98 | Zhao et al., 2008 |
| R:GCGAAGATTATATCATTGCCTG | |||||||
| GZ63m2 | Guangzhan 63 (GZ63) | CYP81A6 (Os03g55240) | ACCTTCGCGAA | F:GAAGCCGATGCACGCCACC | 60.0 | 192 | Wang et al., 2012 |
| R:GCGCCGTTGAACGAGACCAG | |||||||
|
| |||||||
| M2 population | |||||||
| DS552 M2 | DS552 | OsLCT1 (Os06g38120) | F:CTCGATGTTAAGCATGCTCC | 61.0 | 195 | Uraguchi et al., 2011 | |
| R:AGAGTCAGGAACGCGGCTAC | |||||||
| SPDT (Os06g05160) | F:TTCTCGGAGGAGGCTAAT | 52.0 | 259 | Yamaji et al., 2017 | |||
| R:CCACGCATTCTGGTTACAT | |||||||
WT: wild-type; F: forward; R: reverse; T m: melting temperature
Fig. 1.
Rice genes with the wild-type and known mutation position for establishing HRM-TILLING, and with the target region (TR) for mutation screening in rice
Exons and introns are to scale and are indicated by filled boxes and solid lines, respectively, for each gene, with deletion mutations represented by black triangles
The M2 population of the japonica rice line DS552 was developed through γ ray (137Cs) irradiation at the Irradiation Centre of the Zhejiang Academy of Agricultural Sciences (Hangzhou, China). A total dose of 100 Gy was applied for the treatment of dried seeds at a dose rate of about 1 Gy/min. Irradiated seeds were germinated and grown as an M1 population at the experimental farm of Jiaxing Academy of Agricultural Sciences (Jiaxing, Zhejiang, China). M2 seeds were bulk-harvested from the M1 plants. For mutation screening, about 5000 M2 seeds were grown into M2 seedlings under hydroponic culture conditions with a culture solution modified from Yoshida et al. (1976) in a glasshouse with a 12-h photoperiod (day (30±2) °C and night (24±2) °C). Seeds harvested from mutated M2 plants were used to produce M3 populations.
For mutation screening, we selected two rice genes: rice low-affinity cation transporter 1 (OsLCT1, LOC_Os06g38120), which encodes a cadmium (Cd) transporter (Uraguchi et al., 2011), and SULTR-like phosphorus (P) distribution transporter gene (SPDT, LOC_Os06g05160), which controls the allocation of P to the rice grain (Yamaji et al., 2017).
2.2. DNA extraction
To establish the HRM-TILLING and genotyping of M3 plants, genomic DNA was extracted from leaf tissues using a modified cetyltrimethylammonium bromide (CTAB) method according to Li et al. (2016) and adjusted to a final concentration of about 50 ng/µl after quantification using a Nanodrop 2000 (Thermo Scientific, USA). For mutation screening, DNA of M2 seedlings was extracted using a simple, safe, and fast DNA extraction protocol adopted from Tan et al. (2016). Briefly, leaf disks (diameter about 2 mm) were collected from samples of four M2 seedlings using a hole puncher, and then mixed and extracted into 96-well polymerase chain reaction (PCR) plates.
2.3. HRM analysis
PCR primers for the amplification of fragments encompassing the mutations of six deletion materials (Table 1) were designed based on the genome sequence of the rice cultivar Nipponbare (http://www.gramene.org). Based on the gene information on OsLCT1 and SPDT from the Gramene database (Fig. 1), primer pairs were designed in their respective exonic regions (Table 1). All of the primers were designed using Primer Premier 5 software and synthesized by Shanghai Sangong Biological Engineering Technology & Services Co., Ltd., China.
To establish the HRM-TILLING, pooled DNA templates were produced by mixing WT and mutants at 1:1, 3:1, 7:1, and 15:1 ratios. For the mutation screening, pooled DNA extracted directly from each batch of four M2 plants was used. PCRs were performed in a 10-µl volume with 25 ng of mixed DNA, 5 µl of 2× Master Mix (containing 2× PCR buffer, 4 mmol/L MgCl2, 0.4 mmol/L 2'-deoxyribonucleoside triphosphates (dNTPs), and 50 U/ml Taq DNA polymerase; Toyobo Co., Ltd., Japan), 0.2 µl each of 10 μ mol/L primers, and 1 µl of 10× EvaGreen (Biotium, USA), covered with a drop of mineral oil to prevent solution evaporation. The WT and mutants were used in each run as controls. The following PCR conditions were used: 5 min at 94 °C, followed by 40 cycles of 30 s at 94 °C, 30 s at 52–62 °C, and 30 s at 72 °C, with a final extension at 72 °C for 8 min and a hold at 16 °C. The annealing temperature was optimized for each particular gene and fragment (Table 1).
Following PCR, plates were transferred to a LightScanner (Idaho Technology Inc., USA) and subjected to HRM analysis according to Tan et al. (2016). In brief, the temperature was ramped up from 55 to 95 °C at 0.1 °C/s, and data were analyzed using the proprietary software, Call ITTM 2.0 (Idaho Technology Inc., USA) after normalization and temperature shifting of the melting curves according to the LightScanner Operator’s Manual (Idaho Technology Inc.). The WTs were used as references, and samples with relative fluorescence differences (∆F) of >0.05 were considered to be significantly different from the reference (Hofinger et al., 2009).
2.4. Validation by Sanger sequencing
For mixed samples that had ∆F values >0.05 when compared with the WT parent “DS552”, the genomic DNA of each of the four plants was individually extracted using the CTAB method. The target fragments were amplified by PCR in a 50-µl volume, separated on 1.0% (0.01 g/ml) agarose gels, recovered using a DNA Gel Extraction Kit (Axygen Biotechnology Co., Ltd., Hangzhou, China), and subsequently sequenced by Sanger sequencing by Tsingke Biological Technology Co., Ltd. (Hangzhou, China). Because the sequencing results showed mixed peaks, clone sequencing was performed.
3. Results
3.1. Establishment of an HRM-TILLING system for screening deletion mutations
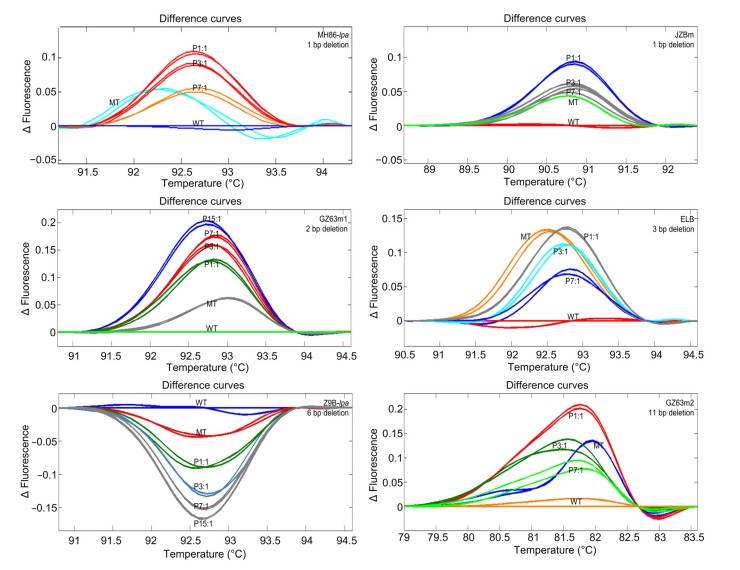
To establish an HRM-TILLING system suitable for screening Indel mutants in pooled DNA samples, the identification of a proper pooling ratio of Indel mutants to WT samples was key. Thus, the DNA of each of six Indel mutants was mixed with WT DNA in different ratios and subjected to HRM analyses. All six mutants, except the mutant of Zhong 9B (Z9B-lpa), had HRM curves significantly different from that of the WT, with peak fluorescence differences (∆F values) equal to or greater than 0.05. Z9B-lpa had an HRM curve most similar to its WT, with the ∆F being only about 0.03 (Fig. 2).
Fig. 2.
HRM analysis of pooled rice samples at different wild-type (WT):mutant (MT) DNA ratios
WT samples were used as references for each analysis and are shown as horizontal lines. Fluorescence difference curves are automatically grouped by the HRM system and are indicated by different colors. P1:1, 3:1, 7:1, and 15:1 stand for the mixture ratios of WT:MT
HRM curves of the pooled samples were all significantly different from those of the WTs, with ∆F values equal to or greater than 0.05 when the ratio was less than 7:1. In most cases, the differences between the pooled and the WT samples were greater than those between the mutants and the WT lines (Fig. 2). There were two types of differences between pooled and WT samples. The first type consisted of mutant line samples that showed decreasing ∆F values from WT as their proportion in the pool decreased, including MH86-lpa, JZBm, ELB, and GZ63m2. These samples had ∆F values close to 0.05 at the 7:1 (WT: mutant) ratio. The second type included mutant lines GZ63m1 and Z9B-lpa. Their pooled samples showed increasing ∆F values when compared with the WT, as their proportion in the pool decreased (Fig. 2). The sharp difference between the two types of samples appeared to be mutation/amplicon specific, because samples within each type had different deletion sizes and amplicon lengths (Table 1). Furthermore, except for GZ63m2, the ∆F values resulting from the deletions were mostly revealed at high temperatures of 90.0–94.0 °C. In addition, all but one mutant, Z9B-lpa, had positive ∆F values, suggesting that these deletion mutations resulted in more fluorescence dye integrating into the amplicons.
These results demonstrated that pooled samples with 1/8 mutant DNA could be differentiated from WT DNA by HRM analysis. Because M2 plants could be heterozygous for induced mutations, pooling of four M2 plants was suitable for HRM-based mutation screening.
3.2. HRM-TILLING of OsLCT1 and SPDT
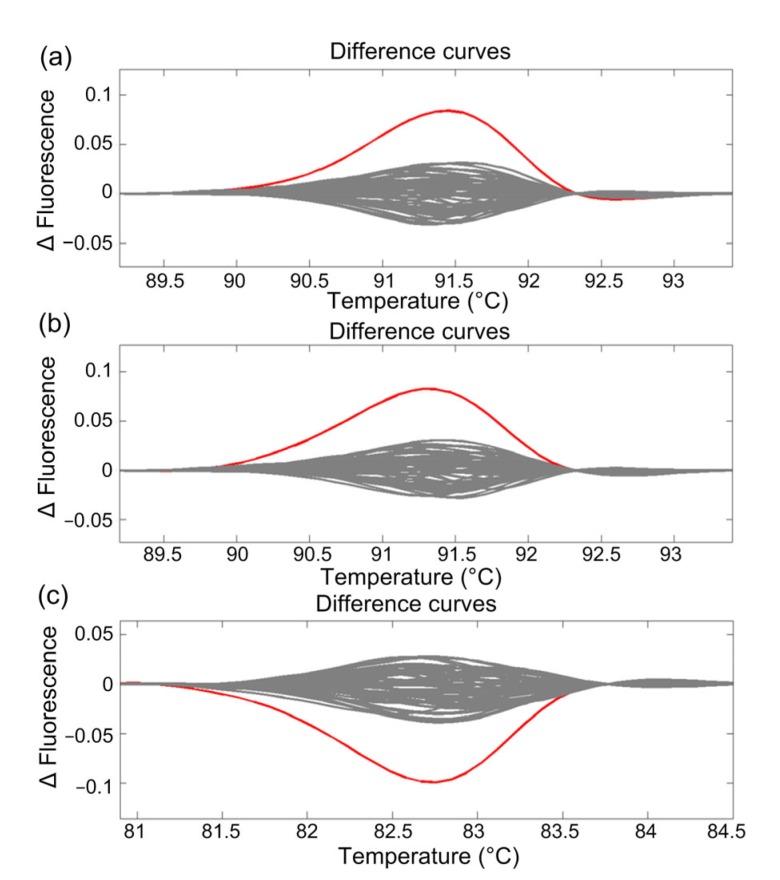
From about 5000 M2 seeds, 4560 seedlings were established and grown hydroponically. A total of 1140 pooled DNA samples were produced and subjected to HRM analysis. Most samples had melting curves not significantly different from “DS552” (∆F<0.05); however, three samples, two for OsLCT1 and one for SPDT, showed significantly different HRM curves with ∆F values of >0.05 at temperatures of 90.0–92.0 °C and 81.5–83.5 °C, respectively (Fig. 3).
Fig. 3.
HRM analysis of pooled M2 rice seedlings for mutations in OsLCT1 (a, b) and SPDT (c) genes
The wild-type (WT, horizontal line) was chosen as the reference for the development of fluorescence difference curves. The mutants (red lines) were automatically sorted by the system because their HRM curves had ∆F values of >0.05 from the WT curves
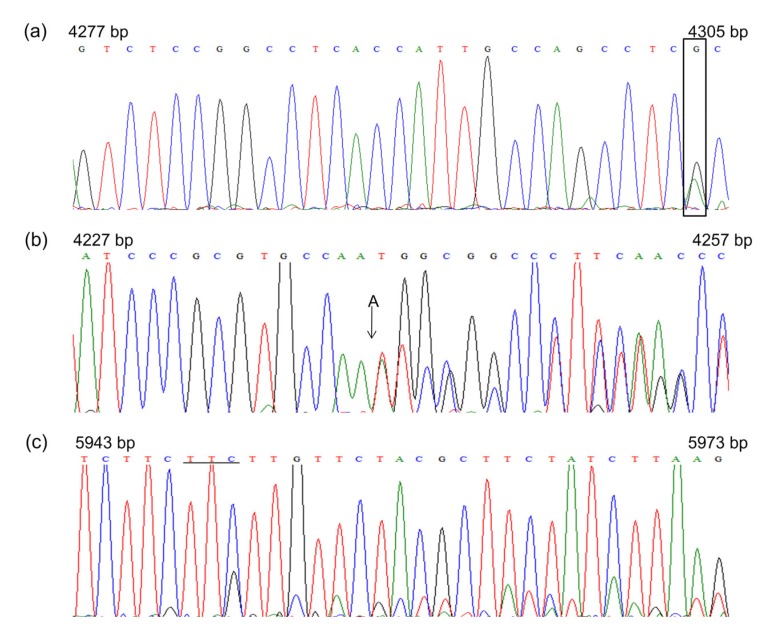
The 12 seedlings represented in these three pooled samples were sequenced for the respective fragments. Three seedlings had sequencing chromatograms that contained either a single double peak (Fig. 4a) or double peaks after a particular nucleotide (Figs. 4b and 4c), suggesting that they carried mutations in the heterozygous state. Thus, the amplicons of these seedlings were subjected to clone sequencing. Finally, one G→A single nucleotide substitution at the 4304 bp and one single nucleotide A insertion at the 4240 bp of OsLCT1 were identified. Also, one TTC trinucleotide deletion was identified at the position of 5948–5950 bp of SPDT. Based on their positions in their respective genes, the G→A mutation is a synonymous mutation that does not change the amino acid; the single A insertion causes a frame shift, resulting in a truncated protein due to an early stop codon at 4399 bp; and the TTC deletion of SPDT occurs in the 3' untranslated region (3' UTR).
Fig. 4.
Sequencing chromatograms of three pooled M2 rice samples
(a, b) OsLCT1; (c) SPDT. The rectangular box in (a) indicates a heterozygous site with a mutation of G→A; a single A insertion in (b) is indicated by an arrow, and a TTC deletion in (c) is indicated by a black line
3.3. Isolation of homozygous M3 mutant plants
An M3 population was developed from seeds harvested from an M2 plant carrying a heterozygous A insertion mutation and was subjected to HRM analysis, together with the WT parent “DS552”. Plants were divided into three groups based on their |ΔF| values compared with the WT. Group I plants were indistinguishable from “DS552”, suggesting that they were homozygous WT lines; Group II plants were similar to the M2 plant, suggesting that they were heterozygous mutant lines; and Group III plants differed from Groups I and II, suggesting that they were homozygous mutant lines (Fig. 5).
Fig. 5.
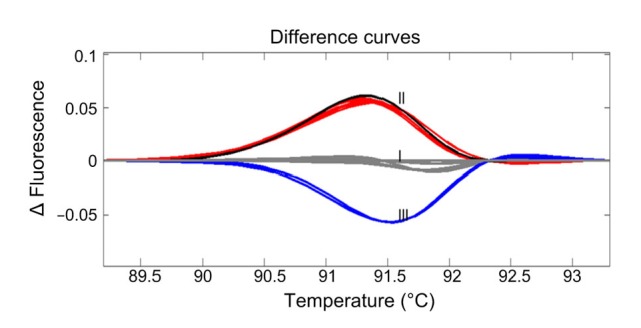
HRM analysis of M3 plants derived from an M2 plant with a heterozygous A insertion in OsLCT1
The wild-type parent “DS552” was used as the reference for fluorescence curve development and is shown as the horizontal line (Group I). The curve of the M2 plant is indicated in black (Group II), curves of heterozygous M3 plants in red, and curves of homozygous M3 plants in blue (Group III)
4. Discussion
The invention of TILLING has significantly advanced functional genomics by efficiently incorporating classical mutagenesis into a modern gene function analysis. However, its application in γ ray mutagenesis has, to date, been limited. In this study we have established an HRM-based TILLING system and demonstrated its suitability for screening mutations in γ ray-mutagenized M2 plants.
4.1. Detection of Indel mutations in pooled samples
The detection of mutations in pooled samples is at the core of TILLING systems. In mismatch cleavage-based systems (using CELI or other endonucleases), the mutation detection limit is about 1 in 16. Thus, pooling eight M2 plants is recommended because mutations often exist in the heterozygous state (Colbert et al., 2001). In De-TILLING (Rogers et al., 2009) and Seq-TILLING (Tsai et al., 2011), more M2 plants can be pooled because these techniques have a greater capability for identifying mutations in pooled samples. Although HRM analysis has the advantage of being high throughput, its capability for identifying single nucleotide polymorphisms (SNPs) in pooled samples is not as good as that of CELI-based mismatch detection. Thus, the pooling of samples of four M2 plants was recommended for detecting mutations in EMS-mutagenized populations (Lochlainn et al., 2011).
In the present study, we first demonstrated that Indel mutations could be efficiently identified in pooled samples when the WT:mutant ratio was higher than 7:1. Unexpectedly, the detection power was much higher for certain mutations. For example, GZ63m1 and Z9B-lpa had HRM curves that were even more different from WT when the WT:mutant ratio was lower (Fig. 2). This appears to be unique to Indel mutations because this phenomenon was never reported in HRM-TILLING studies characterizing single base-pair substitutions (Gady et al., 2009; Botticella et al., 2011; Lochlainn et al., 2011; Acanda et al., 2014; Bovina et al., 2014). However, in HRM analysis of nine Indel mutations using mixed samples, Cousins et al. (2013) also observed one mutation (1-bp deletion) that performed similarly to GZ63m1 and Z9B-lpa, i.e. the HRM difference between the mixture samples and the WT increased as the WT:mutant ratio decreased from 1:1 to 1:9. While its underlying mechanism is still unknown, this characteristic makes HRM-TILLING more powerful for detecting Indel mutations than for detecting point mutations in pooled samples.
A number of factors can affect the HRM curves, including DNA amplicon size and sequence context, and GC content and distribution (Mader et al., 2008). In the present study, the amplicons had lengths from 98 bp (Z9B-lpa) to 294 bp (ELB) and GC content from 39.66% (MH86-lpa) to 70.14% (JZBm), representing the most targets that may be searched using an HRM-TILLING analysis (Reed et al., 2007). This was also reflected by the different shapes and differentiating temperature regimes of fluorescence in the present study (Fig. 2). Because HRM analysis is not suitable for long amplicons, the target fragment size of HRM-TILLING is often less than 400 bp (Wittwer et al., 2003), which is only about 1/4 to 1/3 the length of target fragments analyzed by classical CELI-based TILLING (Till et al., 2003). However, DNA extraction, PCR, and melting curve analyses can all be performed in 96-well plates in HRM-TILLING, which enables very high-throughput mutation screening. For example, it took only 18 d to complete the analysis of the two genes in the 4560 M2 seedlings in the present study.
4.2. Type, frequency, and potential uses of identified mutations
From the 4560 M2 seedlings, two Indel mutations, which were both heterozygous, were identified by HRM-TILLING. Based on the length of the amplicons, 195 bp for OsLCT1 and 259 bp for SPDT, and the size of rice genome, about 373 Mbp, this amounts to a mutation rate of 0.97×10–6 per nucleotide. This mutation rate is similar to those reported for Indels in γ ray-induced M2 plants (1.1×10−6–1.3×10−6) (Li et al., 2016). Only one nucleotide substitution was identified in the present study; therefore, there was a substitution mutation rate of 0.33×10−6 per nucleotide. The substitution mutation rate was much lower than those estimated by whole genome sequencing (4.7×10−6–7.9×10−6) (Li et al., 2016). Also, it is possible that a small proportion of substitution mutations were missed because certain mutations are difficult to identify using a routine HRM analysis (Zhang et al., 2014). However, this does not suggest that our HRM-TILLING method missed a large proportion of base substitution mutations. Because mutations do not occur evenly in all genomic regions, mutation frequencies, when estimated on the basis of individual genes or genomic fragments, could vary greatly for different genes in the same mutated population. For example, Hwang et al. (2017) observed mutation frequencies from 0 to 3.38×10−6 for different genes (1.52×10−6 on average) in a γ ray-irradiated rice mutant population. Variation in mutation frequency among different genes was also reported in EMS-mutagenized rice populations, such as frequencies of 0.87×10−6–6.08×10−6 per nucleotide for the eight genes screened by Till et al. (2007).
Among the 41 mutations identified by Hwang et al. (2017), only 1 was a deletion mutation (1 bp). The low proportion of this type of mutation and its single base-pair deletion suggest that CELI-based TILLING might be more suited to identifying base substitution mutations. However, more studies are needed to ascertain whether CELI-based TILLING could miss deletion mutations.
In the present study, OsLCT1 and SPDT were chosen to demonstrate the effectiveness of our technique because mutations of both genes are expected to generate “promising alleles” for breeding healthy rice grains. OsLCT1 is crucial for Cd transport into rice grains and, thus, a mutation of OsLCT1 could block transport and generate “low-Cd rice” (Uraguchi et al., 2011). SPDT is responsible for channeling P from nodes to rice grains, and knockout or knockdown mutations of SPDT reduces the total P and phytic acid content of rice grains (Yamaji et al., 2017). The 1-bp insertion mutation truncates OsLCT1 and thus would be valuable for breeding low-Cd rice. While the 3' UTR plays an important role in messenger RNA (mRNA) stability and translation efficiency in mammals (Matoulkova et al., 2012), further studies are needed to assess the actual effect of the 3' UTR deletion mutation of SPDT and its value in the development of low total P and phytic acid rice.
In conclusion, our present study not only demonstrated that HRM analysis is particularly useful as a fast and high-throughput screen for Indel mutations induced by γ rays, but also generated valuable mutants with the potential to be used directly as new cultivars or in future breeding programs to breed healthier, more nutritional rice cultivars.
Footnotes
Project supported by the National Key Research and Development Program of China (No. 2016YFD0102103)
Compliance with ethics guidelines: Shan LI, Song-mei LIU, Hao-wei FU, Jian-zhong HUANG, and Qing-yao SHU declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Acanda Y, Martínez Ó, Prado MJ, et al. EMS mutagenesis and qPCR-HRM prescreening for point mutations in an embryogenic cell suspension of grapevine. Plant Cell Rep. 2014;33(3):471–481. doi: 10.1007/s00299-013-1547-6. [DOI] [PubMed] [Google Scholar]
- 2.Ahloowalia BS, Maluszynski M, Nichterlein K. Global impact of mutation-derived varieties. Euphytica. 2004;135(2):187–204. doi: 10.1023/B:EUPH.0000014914.85465.4f. [DOI] [Google Scholar]
- 3.Botticella E, Sestili F, Hernandez-Lopez A, et al. High resolution melting analysis for the detection of EMS induced mutations in wheat Sbella genes. BMC Plant Biol, 11:156. 2011 doi: 10.1186/1471-2229-11-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bovina R, Brunazzi A, Gasparini G, et al. Development of a TILLING resource in durum wheat for reverse-and forward-genetic analyses. Crop Pasture Sci. 2014;65(1):112–124. doi: 10.1071/cp13226. [DOI] [Google Scholar]
- 5.Bush SM, Krysan PJ. ITILLING: a personalized approach to the identification of induced mutations in Arabidopsis. Plant Physiol. 2010;154(1):25–35. doi: 10.1104/pp.110.159897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colasuonno P, Incerti O, Lozito ML, et al. DHPLC technology for high-throughput detection of mutations in a durum wheat TILLING population. BMC Genet, 17:43. 2016 doi: 10.1186/s12863-016-0350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colbert T, Till BJ, Tompa R, et al. High-throughput screening for induced point mutations. Plant Physiol. 2001;126(2):480–484. doi: 10.1104/pp.126.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cousins MM, Donnell D, Eshleman SH. Impact of mutation type and amplicon characteristics on genetic diversity measures generated using a high-resolution melting diversity assay. J Mol Diagn. 2013;15(1):130–137. doi: 10.1016/j.jmoldx.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong CM, Vincent K, Sharp P. Simultaneous mutation detection of three homoeologous genes in wheat by high resolution melting analysis and mutation surveyor®. BMC Plant Biol, 9:143. 2009 doi: 10.1186/1471-2229-9-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu HW, Li YF, Shu QY. A revisit of mutation induction by gamma rays in rice (Oryza sativa L.): implications of microsatellite markers for quality control. Mol Breed. 2008;22(2):281–288. doi: 10.1007/s11032-008-9173-7. [DOI] [Google Scholar]
- 11.Gady ALF, Herman FWK, van de Wal MHBJ, et al. Implementation of two high through-put techniques in a novel application: detecting point mutations in large EMS mutated plant populations. Plant Methods, 5:13. 2009 doi: 10.1186/1746-4811-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofinger BJ, Jing HC, Hammond-Kosack KE, et al. High-resolution melting analysis of cDNA-derived PCR amplicons for rapid and cost-effective identification of novel alleles in barley. Theor Appl Genet. 2009;119(5):851–865. doi: 10.1007/s00122-009-1094-2. [DOI] [PubMed] [Google Scholar]
- 13.Hwang JE, Jang DS, Lee KJ, et al. Identification of gamma ray irradiation-induced mutations in membrane transport genes in a rice population by TILLING. Genes Genet Syst. 2017;91(5):245–256. doi: 10.1266/ggs.15-00052. [DOI] [PubMed] [Google Scholar]
- 14.Kumar APK, McKeown PC, Boualem A, et al. TILLING by sequencing (TbyS) for targeted genome mutagenesis in crops. Mol Breed. 2017;37(2):14. doi: 10.1007/s11032-017-0620-1. [DOI] [Google Scholar]
- 15.Li S, Zheng YC, Cui HR, et al. Frequency and type of inheritable mutations induced by γ rays in rice as revealed by whole genome sequencing. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2016;17(12):905–915. doi: 10.1631/jzus.B1600125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lochlainn SÓ, Amoah S, Graham NS, et al. High resolution melt (HRM) analysis is an efficient tool to genotype EMS mutants in complex crop genomes. Plant Methods, 7:43. 2011 doi: 10.1186/1746-4811-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu HP, Zhang HL, Fu HW, et al. Identification and characterization of a novel lesion mimic mutant in rice. J Nucl Agric Sci. 2016;30(6):1037–1044. (in Chinese) [Google Scholar]
- 18.Mader E, Lukas B, Novak J. A strategy to setup codominant microsatellite analysis for high-resolution-melting-curve-analysis (HRM) BMC Genet, 9:69. 2008 doi: 10.1186/1471-2156-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matoulkova E, Michalova E, Vojtesek B, et al. The role of the 3' untranslated region in post-transcriptional regulation of protein expression in mammalian cells. RNA Biol. 2012;9(5):563–576. doi: 10.4161/rna.20231. [DOI] [PubMed] [Google Scholar]
- 20.McCallum CM, Comai L, Greene EA, et al. Targeting induced local lesions in genomes (TILLING) for plant functional genomics. Plant Physiol. 2000;123(2):439–442. doi: 10.1104/pp.123.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nawaz Z, Shu QY. Molecular nature of chemically and physically induced mutants in plants: a review. Plant Genet Res. 2014;12(S1):S74–S78. doi: 10.1017/S1479262114000318. [DOI] [Google Scholar]
- 22.Nida H, Blum S, Zielinski D, et al. Highly efficient de novo mutant identification in a Sorghum bicolor TILLING population using the ComSeq approach. Plant J. 2016;86(4):349–359. doi: 10.1111/tpj.13161. [DOI] [PubMed] [Google Scholar]
- 23.Reed GH, Kent JO, Wittwer CT. High-resolution DNA melting analysis for simple and efficient molecular diagnostics. Pharmacogenomics. 2007;8(6):597–608. doi: 10.2217/14622416.8.6.597. [DOI] [PubMed] [Google Scholar]
- 24.Ririe KM, Rasmussen RP, Wittwer CT. Product differentiation by analysis of DNA melting curves during the polymerase chain reaction. Anal Biochem. 1997;245(2):154–160. doi: 10.1006/abio.1996.9916. [DOI] [PubMed] [Google Scholar]
- 25.Rogers C, Wen JQ, Chen RJ, et al. Deletion-based reverse genetics in Medicago truncatula . Plant Physiol. 2009;151(3):1077–1086. doi: 10.1104/pp.109.142919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato Y, Shirasawa K, Takahashi Y, et al. Mutant selection from progeny of gamma-ray-irradiated rice by DNA heteroduplex cleavage using brassica petiole extract. Breed Sci. 2006;56(2):179–183. doi: 10.1270/jsbbs.56.179. [DOI] [Google Scholar]
- 27.Shu QY, Forster BP, Nakagawa H. Plant Mutation Breeding and Biotechnology. CABI Publishing, Wallingford, UK; 2012. pp. 123–134. [DOI] [Google Scholar]
- 28.Simko I. High-resolution DNA melting analysis in plant research. Trends Plant Sci. 2016;21(6):528–537. doi: 10.1016/j.tplants.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Taheri S, Lee Abdullah T, Jain SM, et al. TILLING, high-resolution melting (HRM), and next-generation sequencing (NGS) techniques in plant mutation breeding. Mol Breed, 37:40. 2017 doi: 10.1007/s11032-017-0643-7. [DOI] [Google Scholar]
- 30.Tan YY, Yu XM, Shu QY, et al. Development of an HRM-based, safe and high-throughput genotyping system for two low phytic acid mutations in soybean. Mol Breed, 36:101. 2016 doi: 10.1007/s11032-016-0529-0. [DOI] [Google Scholar]
- 31.Till BJ, Reynolds SH, Greene EA, et al. Large-scale discovery of induced point mutations with high-throughput TILLING. Genome Res. 2003;13(3):524–530. doi: 10.1101/gr.977903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Till BJ, Cooper J, Tai TH, et al. Discovery of chemically induced mutations in rice by TILLING. BMC Plant Biol, 7:19. 2007 doi: 10.1186/1471-2229-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai H, Howell T, Nitcher R, et al. Discovery of rare mutations in populations: TILLING by sequencing. Plant Physiol. 2011;156(3):1257–1268. doi: 10.1104/pp.110.169748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uraguchi S, Kamiya T, Sakamoto T, et al. Low-affinity cation transporter (OsLCT1) regulates cadmium transport into rice grains. Proc Natl Acad Sci USA. 2011;108(52):20959–20964. doi: 10.1073/pnas.1116531109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang QZ, Fu HW, Huang JZ, et al. Generation and characterization of bentazon susceptible mutants of commercial male sterile lines and evaluation of their utility in hybrid rice production. Field Crop Res. 2012;137:12–18. doi: 10.1016/j.fcr.2012.09.001. [DOI] [Google Scholar]
- 36.Wittwer CT, Reed GH, Gundry CN, et al. High-resolution genotyping by amplicon melting analysis using LCGreen. Clin Chem. 2003;49(6):853–860. doi: 10.1373/49.6.853. [DOI] [PubMed] [Google Scholar]
- 37.Yamaji N, Takemoto Y, Miyaji T, et al. Reducing phosphorus accumulation in rice grains with an impaired transporter in the node. Nature. 2017;541(7635):92–95. doi: 10.1038/nature20610. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida S, Forno DA, Cock J, et al. Laboratory Manual for Physiological Studies of Rice. The International Rice Research Institute, Los Banos, Manila, Philippines; 1976. [Google Scholar]
- 39.Zhang HL, Huang JZ, Chen XY, et al. Competitive amplification of differentially melting amplicons facilitates efficient genotyping of photoperiod-and temperature-sensitive genic male sterility in rice. Mol Breed. 2014;34(4):1765–1776. doi: 10.1007/s11032-014-0136-x. [DOI] [Google Scholar]
- 40.Zhao HJ, Liu QL, Ren XL, et al. Gene identification and allele-specific marker development for two allelic low phytic acid mutations in rice (Oryza sativa L.) Mol Breed. 2008;22(4):603–612. doi: 10.1007/s11032-008-9202-6. [DOI] [Google Scholar]