Abstract
Diacylglyceryltrimethylhomo-Ser (DGTS) is an abundant lipid in the membranes of many algae, lower plants, and fungi. It commonly has an inverse concentration relationship with phosphatidylcholine, thus seemingly capable of replacing this phospholipid in these organisms. In some places this replacement is complete; Chlamydomonas reinhardtii is such an organism, and was used for these investigations. We have assayed headgroup incorporation to form DGTS in vitro. The precursor for both the homo-Ser moiety and the methyl groups was found to be S-adenosyl-L-Met. DGTS formation was associated with microsomal fractions and is not in plastids. By analogy with phosphatidylcholine and phosphatidylethanolamine biosynthesis in higher plants, the microsomal activity probably is associated with the endoplasmic reticulum. The pH optimum for the total reaction was between 7.5 and 8.0, and the best temperature was 30°C. The apparent Km and Vmax for S-adenosyl-L-Met in the overall reaction were 74 and 250 μm, respectively.
Diacylglyceryltrimethylhomo-Ser [DGTS; diacylglyceryl-O-4′-(N,N,N-trimethyl) homo-Ser] is a primary membrane lipid of Chlamydomonas reinhardtii (Giroud et al., 1988). Although it is not a phospholipid, DGTS appears to replace phosphatidylcholine (PtdCho) in C. reinhardtii; PtdCho has been reported not to occur in this organism (Giroud et al., 1988). This situation is not unusual, however, because DGTS is widely distributed, occurring in pteridophytes, bryophytes, and many algae (Sato, 1992; Eichenberger, 1993; Dembitsky, 1996; Kato et al., 1996), where its concentrations relative to PtdCho generally are inversely proportional. DGTS substitutes for PtdCho more completely than simply as a membrane component of these organisms because it has been demonstrated that oleic acid esterified to DGTS might be desaturated to linoleic acid and an isomer of linolenic acid in C. reinhardtii (Schlapfer and Eichenberger, 1983; Giroud and Eichenberger, 1989). It has been proposed, based on the fatty acid composition of DGTS, that this lipid occurs and is synthesized in membranes outside the plastids (Eichenberger, 1993). This combination of abundance, broad distribution, and apparent substitution for PtdCho in lower plants and algae indicates the importance of this lipid with respect to understanding membrane lipid function and evolution.
Biosynthesis of the DGTS headgroup has been investigated by in vivo feeding of radiolabeled methionine to C. reinhardtii (Sato, 1988), Marchantia polymorpha (Sato and Kato, 1988), Ochromonas danica (Vogel and Eichenberger, 1992), Cryptomonas sp. CR-1 (Sato and Murata, 1991), and Rhodobacter sphaeroides (Hofmann and Eichenberger, 1997). In all cases, Met served as an effective precursor for both the homo-Ser moiety of the DGTS headgroup and the methyl units bonded to the amine group of the homo-Ser. Although the methyl units are likely to be contributed by S-adenosyl-L-Met (SAM) by analogy with other lipid syntheses (Moore, 1976), neither these contributions nor a possible role as the precursor to the homo-Ser moiety have been directly demonstrated. We describe here the first in vitro assays for DGTS biosynthesis and provide evidence that the form of Met utilized for the synthesis of both the homo-Ser and methylation contributions is SAM.
RESULTS
Compartmentalization
Biosynthesis of DGTS was measured predominantly in two fractions of C. reinhardtii homogenate following differential centrifugation (Table I). The primary activity was found in the lowest speed fraction where the plastids and cell fragments are found. The second highest DGTS synthetic activity was in the same high-speed microsomal fraction that contained the majority of PtdCho synthesis, an activity demonstrated to be associated with the endoplasmic reticulum in plant systems (Moore, 1976). The microsomal fraction had the highest specific activity, and therefore the greatest purity, and so was chosen as the enzyme source for most of the remaining experiments.
Table I.
Sub-fractionation of organelles from Chlamydomonas reinhardtii
| Fraction | Chlorophyll | Protein | Rubisco | Cyt c Oxidase | Choline P Transferase | PEP Carboxylase | DGTS Synthesis, Total Activity | DGTS Synthesis, Specific Activity |
|---|---|---|---|---|---|---|---|---|
| % of total | ||||||||
| 760g ppt | 88.27 | 50.50 | 76.10 | 64.15 | 0.95 | 5.38 | 66.46 | 19.64 |
| 3,000g ppt | 6.21 | 5.26 | 2.54 | 11.96 | 9.80 | 0.95 | 5.42 | 12.30 |
| 10,000g ppt | 3.14 | 2.87 | 1.03 | 17.48 | 6.83 | 0.85 | 4.90 | 19.05 |
| 100,000g ppt | 2.38 | 4.72 | 2.43 | 4.16 | 82.25 | 3.13 | 23.22 | 49.00 |
| 100,000g aup | 0.00 | 36.35 | 17.89 | 2.25 | 0.16 | 89.70 | 0.00 | 0.00 |
Homogenization, organelle fractionation, and enzyme assays were as described in “Material and Methods.” Activities are presented as percentage of total activity in all fractions. Cyt c, Cytochrome c; PEP, phosphoenolpyruvate; ppt, precipitate; sup, supernatant.
In an effort to further define the location of the activity in the low-speed fraction, plastids were purified (Mason et al., 1991) and fractionated (Douce and Joyard, 1980). No activity was found in either the envelope or inner membrane fractions (data not included). Therefore, it seems unlikely that the activity is associated with the chloroplasts. Recentrifugation at 10,000g of resuspended low-speed fraction resulted in virtually all the activity appearing in the supernatant, and this activity in turn could be precipitated by centrifugation at 100,000g. This result indicates that the activity in the low-speed precipitate is an artifact resulting from nonspecific trapping or binding of microsomal membranes.
Source of Homo-Ser
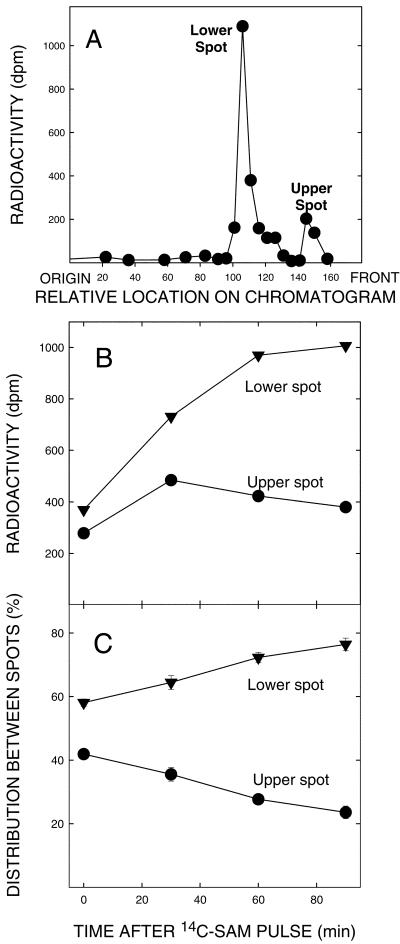
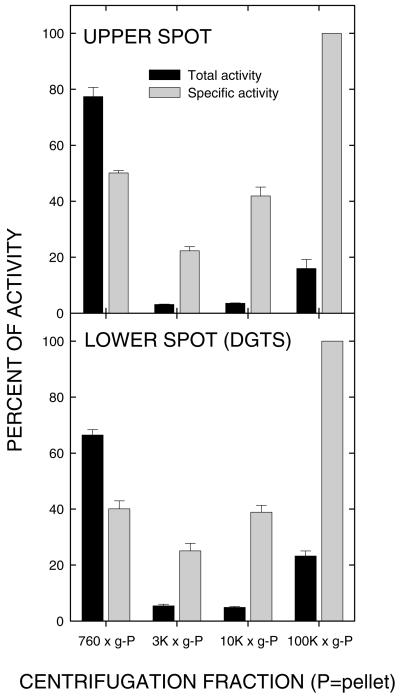
The data presented in Table I provide support for the homo-Ser moiety of DGTS being derived from SAM. The precursor used was SAM radiolabeled in the carboxyl group, so the incorporated radioactivity was not due to methylation. A more detailed examination of this possibility is presented in Figure 1. In Figure 1A, the distribution of products of [14C-carboxyl]-SAM incorporation into the microsomal fraction is presented. Two obvious peaks are present; the lower peak cochromatographs with DGTS. The upper spot remains unidentified because the concentration is low; however, the pulse-chase data presented in Figure 1, B and C, allow the hypothesis that it is an intermediate in DGTS synthesis, the radiolabel increasing in the lower spot while decreasing in the upper during the chase period. The occurrence of a shoulder above the lower spot following continuous feeding suggests a second intermediate, but its appearance was variable under the conditions used and so it probably turns over quickly. Continuous feeding of SAM to the low-speed and microsomal fractions resulted in relative proportions of radioactivity in the upper and lower spots, expressed as both total and specific activities, which were quite similar in both (Fig. 2), further supporting them having the same intracellular origin.
Figure 1.
Precursor product relationship of two radiolabeled spots from microsomes separated on thin-layer chromatography (TLC). A, The radioactivity in products separated by one-dimensional TLC (see below for details) after a continuous 1-h assay for DGTS synthesis. B, The changes in radioactivity in the upper and lower products after the onset of a chase period. C, The relative activity expressed in percent remaining in each spot. The pulse-chase procedures involved presenting microsomal fractions with 130 μm [14C-carboxyl]- SAM (specific activity = 11 mCi/mmol) for 30 min, followed by the addition of 1.3 mm nonradioactive SAM. Aliquots were taken at indicated intervals after the latter addition, the lipids extracted as described in “Materials and Methods,” and the chloroform-soluble fraction was chromatographed on silica gel G TLC plates using chloroform:methanol:7 n NH4OH (65:35:4, v/v). Spots were visualized with I2 vapors and those of interest were scraped from the plates into scintillation vials for the determination of radioactivity.
Figure 2.
Relative total and specific activities for synthesis of each of the products described in Figure 1 by the active fractions described in Table I. Note the parallel in relative rates of synthesis in each of the fractions, thus further supporting a precursor-product relationship between the upper and lower spots.
Because the addition of radiolabeled L-Met (Met) to intact cells leads to incorporation of radioactivity into DGTS in C. reinhardtii (Sato, 1988), and Met might be a degradation product of SAM, we tested for competition by Met with radiolabel incorporation from SAM, and also for direct incorporation of Met into the lipids under the conditions of our standard assay. Met was found to inhibit the incorporation of radiolabeled SAM; however, the greatest inhibition in our experiments was with 0.5 mm Met in the presence of 0.13 mm SAM. The inhibition under these conditions was about 38%. When radiolabeled Met was used in place of SAM, no radioactivity was detected in the chloroform-soluble fraction following the assay.
Substrates and General Assay Requirements
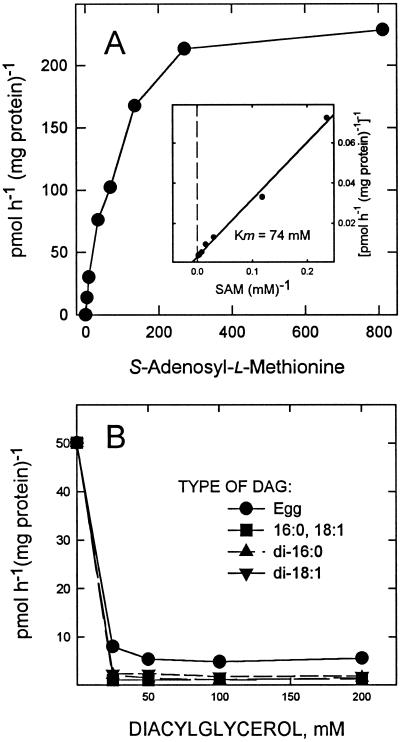
Synthesis of DGTS by the microsomal fraction demonstrates saturation kinetics with SAM, with an apparent Km of 74 μm and Vmax of about 250 μm; SAM was absolutely required for the synthesis of DGTS (Fig. 3A and Inset).
Figure 3.
Effects of possible substrates on DGTS synthesis. A, The effects of increasing SAM concentrations on DGTS synthesis. Carboxyl-radiolabeled SAM was used and the radioactivity in the DGTS spot determined after chromatography. The apparent Km was estimated to be 74 μm and the Vmax to be about 250 μm. B, Results on DGTS synthesis by the microsomal fraction resulting from increasing concentrations of a possible lipid precursor, diacylglycerol (DAG). Egg DAG, derived from egg PtdCho, was added as a naturally occurring mixture of DAG species. DAG-16:0, 18:1 is a possible natural precursor of DGTS synthesis.
The lipid substrate for this reaction remains elusive. Addition of several molecular species of DAG, the lipid precursor for PtdCho synthesis (Moore, 1976) and therefore a candidate for DGTS synthesis, at all concentrations tested resulted in strong inhibition of the reaction (Fig. 3B). Additions of CDP-DAG or phosphatidate did not inhibit the reaction at concentrations tested, but also did not appear to be incorporated into DGTS (data not shown). Further experiments with possible lipid precursors, perhaps requiring at least partial solubilization of the enzyme, will be necessary to elucidate the lipid substrate.
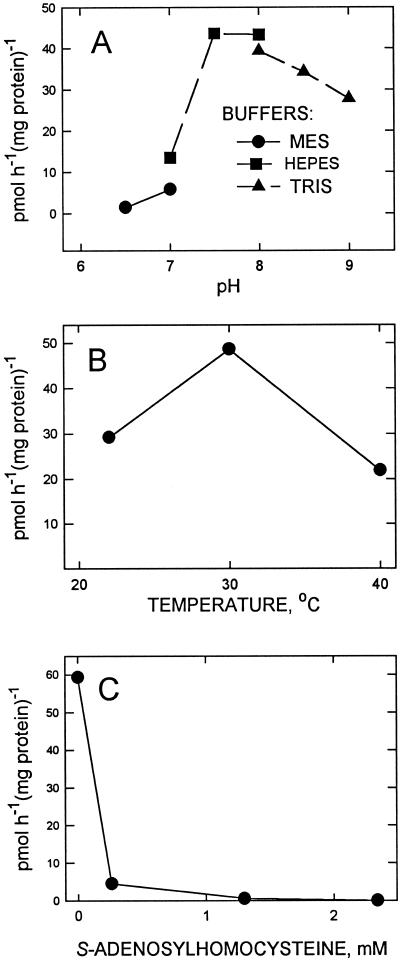
The pH optimum was found to be between 7.5 and 8.0 (Fig. 4A), and for temperature the maximum activity was obtained at about 30°C (Fig. 4B). The addition of S-adenosylhomo-Cys, an inhibitor of reactions utilizing SAM, strongly inhibited the activity (Fig. 4C). This inhibition included not only methylation, but also introduction of the homo-Ser headgroup. This inhibition was evidenced by the absence of any radioactive peaks when chloroform-soluble fractions of assays including 0.5 mm of S-adenosylhomo-Cys were chromatographed.
Figure 4.
Effects of pH (A), temperature (B), and S-adenosylhomo-Cys (C) on biosynthesis of DGTS by the microsomal fraction. The inhibitor was added prior to the addition of radiolabeled SAM. Other conditions were as described in “Materials and Methods” except as indicated in the figure.
Methylation
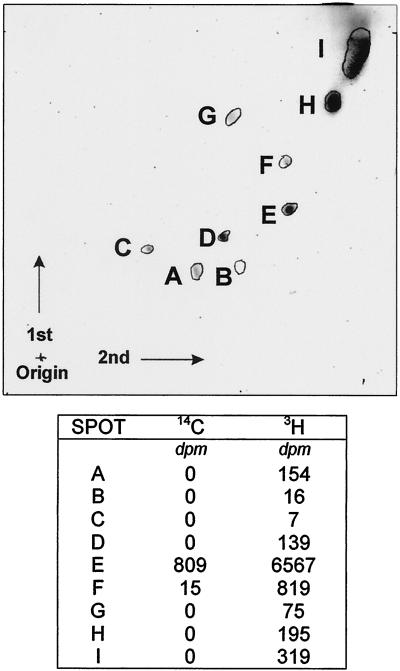
Radiolabeled methyl units of SAM were incorporated into several chloroform-soluble fractions obtained by two-dimensional chromatography (Fig. 5). One of the more strongly labeled fractions was DGTS (I2-visualized spot E in Fig. 5). The carboxyl-labeled precursor was more specific, the 14C occurring only in DGTS and not the other visualized spots. It is probable that the precursor seen in Figure 1 did not occur at a sufficiently strong concentration to be detected by the iodine vapors.
Figure 5.
Distributions of radiolabel in chloroform-soluble products following introduction of either [14C]carboxyl- or [3H]methyl-labeled SAM. The assays were performed as described in the materials and methods and the chloroform-soluble material was chromatographed with the second two-dimensional system described in “Materials and Methods.” The chloroform-soluble compounds were visualized with iodine vapors after chromatography (top) and visible spots were scraped into scintillation vials for determination of radioactivity (bottom).
CONCLUSIONS AND DISCUSSION
The data presented herein define the in vitro assay of DGTS synthesis and indicate that SAM is the form that donates both the homo-Ser and the methyl groups. Thus the role of Met in formation of the entire trimethylhomo-Ser moiety, as indicated by the results from the laboratories of Sato (Sato, 1988; Sato and Kato, 1988; Sato, 1992) and Eichenberger (Vogel and Eichenberger, 1992; Hofmann and Eichenberger, 1997) is confirmed and expanded to indicate the form of Met utilized, which is SAM. It is not surprising that multiple products in addition to DGTS were obtained with both the carboxyl- and methyl-labeled precursors, and some of them appear to be short-lived intermediates of DGTS synthesis. In particular, one specific compound separated from DGTS by one-dimensional TLC (Fig. 1) appears to be a precursor of DGTS synthesis based on pulse-chase kinetics. The occurrence of tritium in this compound from methyl-labeled SAM (data not shown) indicates that at least one methyl group already has been incorporated, suggesting that the diacylglycerylhomo-Ser intermediate is short lived. Although other candidates for roles of short-lived intermediates of DGTS headgroup synthesis occasionally were obtained upon two-dimensional TLC, no intermediate was found that would qualify as being diacyglycerylhomo-Ser. After 1 h, most of the radioactivity from [14C-carboxyl]-SAM occurred in a spot on the chromatograms that corresponded to DGTS. Thus methylation occurs rapidly after the homo-Ser addition. We expect that at least two enzymes are involved in formation of the total headgroup, one for homo-Ser addition and at least one for methylation, but we are unable to estimate the total number of enzymes involved in the overall pathway at this time.
The lipid substrate was anticipated to be DAG, based on its role in PtdCho synthesis and the fact that DGTS appears to replace multiple roles of PtdCho in C. reinhardtii (Vogel and Eichenberger, 1992). However, our repeated efforts to measure stimulation by various forms of DAG resulted only in inhibition of the reaction. Such responses to putative lipid precursors in membrane systems is not unusual and might arise from disruption of the membrane or other perturbations resulting in detrimental environments for the enzymes (Moore, 1976). Neither phosphatidic acid nor CDP-DAG had any consistent effect on the synthesis at the concentrations tested, although they did not inhibit activity. Therefore, partial enzyme purification might be necessary to elucidate the proper lipid substrate.
The reactions for complete synthesis of DGTS from SAM and a lipid precursor occur in a microsomal fraction, and by analogy with biosynthesis of membrane lipids in other systems (Kinney, 1993) probably are associated with the endoplasmic reticulum. This compartmentalization would be in agreement with the eukaryotic nature of DGTS described by Giroud et al. (1988). The release of activity from the low-speed centrifugation fraction by resuspension followed by higher speed centrifugation, which packs the larger membranes more tightly and apparently forces the smaller particles out of an association with this fraction, results in the activity becoming microsomal.
In summary, the DGTS headgroup is synthesized utilizing SAM for both the homo-Ser and methyl moieties. The activity is associated with the microsomal fraction and does not occur in the plastids.
MATERIALS AND METHODS
Organism and Culture
Chlamydomonas reinhardtii, CC400 cw-15 mt+, a wall-deficient mutant, was originally from the Duke University (Durham, NC) culture collection and obtained by us from J. Moroney (Department of Biological Sciences, Louisiana State University, Baton Rouge). Cells normally were grown in liquid cultures in a phosphate-buffered medium containing CO2 as the carbon source (minimal medium; Sueoka, 1960). The cultures were maintained by aeration with 5% (v/v) CO2 in air with continuous reciprocal shaking, and illumination was with 300 μE m−2 s−1 of white fluorescent light.
Methods
Homogenization and Fractionation
The cells were collected by centrifugation at 2,000 to 3,000 rpm in a Beckman (Fullerton, CA) J2–21 centrifuge using a JA-10 rotor, then washed with 20 mm of ice-cold HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid] buffer (pH 7.5) and concentrated with a final centrifugation. The concentrated cells were rapidly diluted to a chlorophyll content of 0.3 mg/mL with a solution comprised of 300 mm sorbitol, 50 mm HEPES-KOH (pH 7.5), 2 mm EDTA (pH 7.5), 1 mm MgCl2, and 1 mm dithiothreitol. The resulting suspension was placed in a Parr pressure bomb and held at 1500 lbs/in2 pressure for 4 min, after which the contents were released slowly into a container on ice (Mason et al., 1991).
The resultant homogenate was subjected to sequential centrifugation at 2,500 rpm (760g) for 10 min, 5,000 rpm (3,000g) for 20 min, 9,500 rpm (10,000g) for 30 min in a Beckman J2–21 centrifuge with a JA-20 rotor, and finally 37K rpm (100,000g) for 1 h in a Sorvall (Newtown, CT) OTD-65B ultracentrifuge with a T-875 rotor. The locations of cellular components in the precipitates and final supernatant were identified through the use of marker enzymes for the cytosol (PEP carboxylase; Belknap and Togasaki, 1981), mitochondria (cytochrome oxidase; Wigge and Gardestrom, 1987; Eriksson et al., 1995), endoplasmic reticulum (DAG:CDPcholine cholinephosphotransferase; Moore, 1976), intact chloroplasts (Rubisco; Spreitzer et al., 1988), the chloroplast envelope (monogalactosyldiacylglycerol synthase; Douce and Joyard, 1980), and chloroplast thylakoids (chlorophyll; Mason et al., 1991).
DGTS Assay
DGTS synthesis was routinely assayed with a mixture of 50 mm HEPES (pH 7.5), 5 mm EGTA (pH 7.5), and 130 μm [14C-carboxyl]-SAM in a final volume of 0.5 mL. The assay was conducted for 60 min at 30°C. The reaction was terminated by the addition of 3.3 mL of chloroform:methanol:water (10:20:3, v/v), incubated at room temperature for 30 min, and the lipids partitioned into chloroform following the addition of 1.0 mL of chloroform, three washes with 1 m KCl and a final wash with water (based on Bligh and Dyer, 1959, as modified by Bjerve et al., 1974). The chloroform fraction was either placed into scintillation vials for direct measurement of radioactivity after drying, or dried and chromatographed.
Following most assays, DGTS was separated from other radiolabeled compounds in one dimension on silica gel 60 TLC plates (EM Separations Technology, Darnstadt, Germany) using chloroform:methanol:7 n NH4OH (65:35:4, v/v). This provided a relatively rapid and efficient separation of DGTS for measuring radioactivity (see below).
Chromatography
Two two-dimensional TLC systems were used, both using silica gel 60 TLC plates. The first solvent system (slightly modified from Giroud et al., 1988) was: first dimension, chloroform:methanol:7 n NH4OH (65:35:4, v/v) and second dimension, chloroform:methanol:acetic acid:water (170:25:25:2, v/v). The second solvent system (Vogel and Eichenberger, 1992) was: first dimension, chloroform:methanol:water (65:25:4, v/v) and second dimension, chloroform:methanol:isopropylamine:concentrated NH4OH (650:350:5:50, v/v).
Measurement of Radioactivity
Radioactivity was measured either after drying the total chloroform extract in 3.0-mL scintillation vials or after scraping spots visualized with I2 vapors from TLC plates into the vials. The scintillation cocktail was Cytoscint (ICN, Costa Mesa, CA) and the scintillation counter was a Beckman LS-8000.
ACKNOWLEDGMENTS
The authors would like to thank Dr. James Moroney and Cathy Mason for valuable assistance during this work. They also would like to thank Ashley Blouin and Candice Chenier for technical help and discussions during the progress of the research.
Footnotes
This work was supported by the National Science Foundation (grant no. MCB–9603626 to T.S.M.).
LITERATURE CITED
- Belknap W, Togasaki R. Chlamydomonas reinhardtii cell preparation with altered permeability toward substrates of organellar reactions. Proc Natl Acad Sci USA. 1981;778:2310–2314. doi: 10.1073/pnas.78.4.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerve K, Daae L, Bremer J. The selective loss of lysophospholipids in some commonly used lipid-extraction procedures. Anal Biochem. 1974;58:238–245. doi: 10.1016/0003-2697(74)90463-1. [DOI] [PubMed] [Google Scholar]
- Bligh E, Dyer W. A rapid method of total lipid extractions and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Dembitsky V. Betaine ether-linked glycerolipids: chemistry and biology. Prog Lipid Res. 1996;35:1–51. doi: 10.1016/0163-7827(95)00009-7. [DOI] [PubMed] [Google Scholar]
- Douce R, Joyard J. Chloroplast envelope lipids: detection and biosynthesis. Methods Enzymol. 1980;69:290–301. [Google Scholar]
- Eichenberger W. Betaine lipids in lower plants: distribution of DGTS, DGTA and phospholipids, and the intracellular localization and site of biosynthesis of DGTS. Plant Physiol Biochem. 1993;31:213–221. [Google Scholar]
- Eriksson M, Gardestrom P, Samuelsson G. Isolation, purification, and characterization of mitochondria from Chlamydomonas reinhardtii. Plant Physiol. 1995;107:479–483. doi: 10.1104/pp.107.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroud C, Eichenberger W. Lipids of Chlamydomonas reinhardtii: incorporation of [14C]acetate, [14C]palmitate and [14C]oleate into different lipids and evidence for lipid-linked desaturation of fatty acids. Plant Cell Physiol. 1989;30:121–128. [Google Scholar]
- Giroud C, Gerber A, Eichenberger W. Lipids of Chlamydomonas reinhardtii: analysis of molecular species and intracellular site(s) of biosynthesis. Plant Cell Physiol. 1988;29:587–595. [Google Scholar]
- Hofmann M, Eichenberger W. Biosynthesis of diacylglyceryl-N,N,N-trimethylhomoserine (DGTS) in Rhodobacter sphaeroides and evidence for lipid-linked N-methylation. In: Williams J, Khan M, Lem N, editors. Physiology, Biochemistry, and Molecular Biology of Plant Lipids. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1997. pp. 116–118. [Google Scholar]
- Kato M, Sakai S, Adachi K, Ikemoto H, Sano H. Distribution of betaine lipids in marine algae. Phytochemistry. 1996;42:1341–1345. [Google Scholar]
- Kinney A. Phospholipid head groups. In: Moore T, editor. Lipid Metabolism in Plants. Boca Raton, FL: CRC Press; 1993. pp. 259–284. [Google Scholar]
- Mason C, Matthews S, Bricker T, Moroney J. Simplified procedure for the isolation of intact chloroplasts from Chlamydomonas reinhardtii. Plant Physiol. 1991;97:1576–1580. doi: 10.1104/pp.97.4.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T. Phosphatidylcholine synthesis in castor bean endosperm. Plant Physiol. 1976;57:383–386. doi: 10.1104/pp.57.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N. Dual role of methionine in the biosynthesis of diacylglyceryltrimethylhomoserine in Chlamydomonas reinhardtii. Plant Physiol. 1988;86:931–934. doi: 10.1104/pp.86.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N. Betaine lipids. Bot Mag Tokyo. 1992;105:185–197. [Google Scholar]
- Sato N, Kato K. Analysis and biosynthesis of diacylglyceryl-N,N,N-trimethylhomoserine in the cells of Marchantia in suspension culture. Plant Sci. 1988;55:21–25. [Google Scholar]
- Sato N, Murata N. Transition of lipid phase in aqueous dispersions of diacylglyceryltrimethylhomoserine. Biochim Biophys Acta. 1991;1082:108–111. doi: 10.1016/0005-2760(91)90306-3. [DOI] [PubMed] [Google Scholar]
- Schlapfer P, Eichenberger W. Evidence for the involvement of diacylglyceryl(N,N,N-trimethyl)-homoserine in the desaturation of oleic and linoleic acids in Chlamydomonas reinhardtii (Chlorophyceae) Plant Sci. 1983;32:243–252. [Google Scholar]
- Spreitzer R, Al-Abed S, Huether M. Temperature sensitive photosynthesis-deficient mutants of Chlamydomonas reinhardtii. Plant Physiol. 1988;86:773–777. doi: 10.1104/pp.86.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N. Mitotic replication of deoxyribonucleic acids in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 1960;46:83–91. doi: 10.1073/pnas.46.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel G, Eichenberger W. Betaine lipids in lower plants. Biosynthesis of DGTS and DGTA in Ochromonas danica (Chrysophyceae) and the possible role of DGTS in lipid metabolism. Plant Cell Physiol. 1992;33:427–436. [Google Scholar]
- Wigge B, Gardestrom P. The effects of different ionic conditioning on the activity of cytochrome c oxidase in purified plant mitochondria. In: Moore A, Beachy R, editors. Plant Mitochondria. New York: Plenum Press; 1987. pp. 127–130. [Google Scholar]