Abstract
Cognitive dysfunction in fibromyalgia has been reported, especially memory. Anodal transcranial direct current stimulation (tDCS) over the dorsolateral prefrontal cortex (DLPFC) has been effective in enhancing this function. We tested the effects of eight sessions of tDCS and cognitive training on immediate and delayed memory, verbal fluency and working memory and its association with brain-derived neurotrophic factor (BDNF) levels. Forty females with fibromyalgia were randomized to receive eight sessions of active or sham tDCS. Anodal stimulation (2 mA) was applied over the DLPFC and online combined with a working memory training (WMT) for 20 minutes. Pre and post-treatment neurocognitive tests were administered. Data analysis on deltas considering years of education and BDNF as covariates, indicated active-tDCS + WMT significantly increased immediate memory indexed by Rey Auditory Verbal Learning Test score when compared to sham. This effect was dependent on basal BDNF levels. In addition, the model showed active stimulation increased orthographic and semantic verbal fluency scores (Controlled Oral Word Association Test) and short-term memory (Forward Digit Span). The combination of both techniques seemed to produce effects on specific cognitive functions related to short-term and long-term episodic memory and executive functions, which has clinical relevance for top-down treatment approaches in FM.
Introduction
Fibromyalgia (FM) is a chronic pain condition with 2 to 5% prevalence in general population, being more frequent in women1,2. It comprises widespread chronic pain, fatigue, depression, anxiety, disrupted sleep and other somatic complaints, as well as impaired cognition3. In general, the most frequent complains related to cognitive aspects include a poorer recall, difficulty with concentration and attention. Despite the pathophysiology of FM is not completely understood, an imbalance in the excitatory/inhibitory central nervous system (CNS) has being considered4. This imbalance comprises a phenomenon of central sensitization syndrome (CCS). The CSS is an amplification of neural signaling within the central nervous system associated with hypersensitivity to pain5. In fact, the CCS includes psychological distress, sleep disturbance, allodynia and hyperalgesia6. The impaired sustained attention was associated positively with deep-tissue hyperalgesia and deficient conditioned pain modulation7. Furthermore, a higher score in the Central Sensitization Inventory (CSI) in chronic pain was positively correlated with level of dysfunction in the descending pain modulatory system, as well as with higher levels of serum brain-derived neurotrophic factor (BDNF)8.
Although multiple mechanisms of synaptic plasticity are involved in the CCS, the BDNF has a central role in strengthening glutamatergic synapses, while it weakens GABAergic synapses. The increase of this neurotrophic factor inverts the polarity of GABA currents in dorsal horn neurons9. Also, convergent pieces of evidence suggest that BDNF is essential for maintaining the network activity in the prefrontal cortex (PFC)10. This region has neurons with intrinsic properties that allow them to initiate, maintain and terminate sustained non-adapting firing. The PFC is provided of extensive dopaminergic projections and other inputs for tuning the state of a network sensible to detect triggers and to initiate activity. Due to the fact that this region is responsible for many functions, it has been extensively used as a target for cognitive enhancement approaches via transcranial direct current stimulation (tDCS)11–13. Specifically, the dorsolateral prefrontal cortex (DLPFC) has been targeted for anodal stimulation due to its functional role in updating and maintaining goal-directed representations for context information (which is necessary for episodic memory) and task-related demands14.
Accordingly, the anodal tDCS applied to the DLPFC improved working memory (WM) learning curves in healthy adults, who trained on a visual/spatial and verbal adaptive n-Back Task15. Similarly, two studies with healthy subjects showed that the anodal stimulation on the left DLPFC improved the WM performance16. More robust effects have been reported with the combination of stimulation and a cognitive task11. One of the most used tests for working memory training is with the n-Back Task. Andrews et al.11 found that the tDCS associated with a WM task produced a better performance in another equivalent task applied at a later time. Additionally, recently our research group showed an additive effect of tDCS on DLPFC combined with a task that induces the activation of inhibitory control pathways in FM13. The study also shows that the combination of interventions improved performance of attention networks associated with an increase in pain threshold. We hypothesize tDCS may modulate prefrontal circuits, enhancing tolerance and minimizing the emotional component of pain experience. However, there is a gap in terms of exploring baseline neuroplasticity characteristics that could be related to tDCS’s effect on the DLPFC combined with a WM training. Moreover, multiples sessions of this combined treatment may have advantages over a singular session.
In this explanatory trial, we aimed to test if a treatment with active-tDCS combined with a working memory training (WMT) would increase immediate and delayed memory scores, as well as working memory, verbal fluency and divided attention capacity, when compared to sham-tDCS + WMT. We also aimed to test if the treatment effect is dependent on the serum BDNF levels. We hypothesize that neuroplasticity state measured by BDNF has a modulatory role for the effect of tDCS in cognitive performance. In other words, the higher the BDNF serum levels, the larger the anodic tDCS effects on memory and the other cognitive functions.
Results
Demographic and clinical characteristics
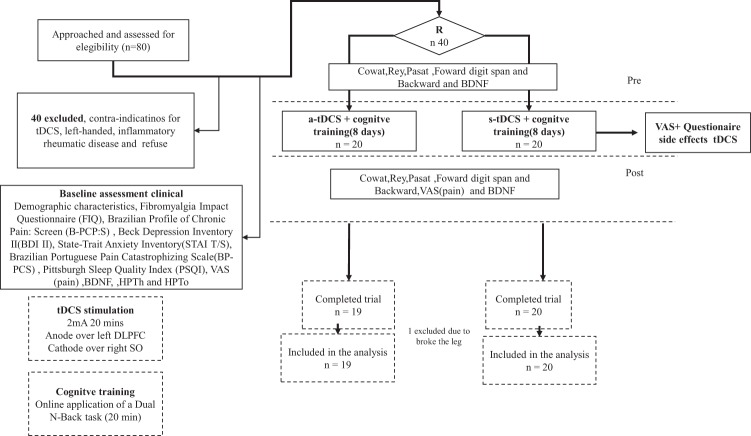
Thirty-nine patients completed the study (one patient had dropout from the a-tDCS group due to a leg injury). Clinical and demographic characteristics of the sample according to the intervention group are shown in Table 1.
Table 1.
Demographic and Clinical characteristics (n = 40).
| Active-tDCS (n = 20) | Sham-tDCS (n = 20) | p | |
|---|---|---|---|
| Demographic | |||
| Age (years) | 49.15 (8.43) | 50.05 (11.19) | 0.77 |
| Body weight (Kg) | 28.47 (4.18) | 27.73(5.20) | 0.62 |
| Years of education | 10.60(4.36) | 10.75(2.86) | 0.89 |
| Clinical | |||
| Clinical comorbidity (yes /%) | 11(55) | 15 (75) | 0.18 |
| Hypertension (n/%) | 6 (30) | 7(35) | |
| Hypothyroidism (n/%) | 1 (5) | 3(15) | |
| Asthma (n/%) | 1 (5) | 2(10) | |
| Gastritis (n/%) | 2 (10) | 0(0) | |
| Diabetes(n/%) | 1 (5) | 0(0) | |
| Other (n/%) | 0(0) | 3(15) | |
| Beck Depression Inventory – BDI – II | 24(9.84) | 28(12.96) | 0.22 |
| Brazilian Portuguese version of the Pain Catastrophizing Scale (BP-PCS) | 30.65(11.89) | 31.30(14.91) | 0.88 |
| Fibromyalgia Impact questionnaire(FIQ) | 63.43(18.29) | 66.16(15.31) | 0.61 |
| State-Trait Anxiety Inventory – STAI | |||
| STAI – State | 33.45(6.31) | 34.30(7.61) | 0.70 |
| STAI – Trait | 25.25(4.64) | 26.85(6.17) | 0.36 |
| Brazilian Profile of Chronic Pain: Screening (B-PCP:S) | 69.01(14.15) | 70.57(16.10) | 0.76 |
| Pittsburgh Sleep Quality Index – PSQI | 12.05(4.34) | 11.60(4.07) | 0.73 |
| Alcohol Consumption (yes/%) | 8(40) | 6(30) | 0.50 |
| Smoking (yes/%) | 6(30) | 6(30) | 1 |
| Biochemical | |||
| Serum BDNF | 28.70(12.43) | 30.41(12.48) | 0.67 |
| Measures of pain | |||
| Pain score on VAS (0 to100 cm) | 7.30(1.66) | 7.21(1.66) | 0.86 |
| QST: Heat Pain threshold | 33.13(1.13) | 33.19(1.05) | 0.85 |
| QST: Pain tolerance | 44.29(3.19) | 44(3.0) | 0.76 |
| Psychiatric disorder according to the MINI * | |||
| Major depressive episode | 11(55%) | 16(80%) | 0.91 |
| Major depressive episode with dysthymia | 7(35%) | 6(30%) | 0.73 |
| Maniac-depressive disorder | 3(15%) | 3(15%) | 1 |
| Post-traumatic stress disorder | 3(15%) | 3(15%) | 1 |
| Generalized anxiety disorder | 6(30%) | 9(45%) | 0.32 |
| Medication | |||
| Analgesic use (yes/%) | 12(60) | 10(50) | 0.52 |
| >4 times a week in the last 3 months t (n/%) | 5(25) | 7(35) | 0.24 |
| <4 times a week (n/%) | 7(35) | 3(15) | 0.36 |
| Aminophen/Dipirone(n/%) | 3 (15) | 5 (25) | |
| Non-steroidal anti-inflammatory drugs(n/%) | 9 (45) | 5 (25) | |
| Central nervous system active medication(yes/%) | 16(80) | 16(80) | |
| Antidepressant (n/%) | 9(45) | 10(50) | |
| Anticonvulsant (n/%) | 5 (25) | 4(20) | |
| Benzodiazepine (n/%) | 2 (10) | 2(10) | |
Notes. QST = Quantitative Sensory Testing; VAS: visual analog scale; BDNF = Brain-derived neurotrophic factors. Values are given as mean (standard deviation) or frequency (%). Independent samples t-Tests for mean values and Chi-Squared or Fisher’s tests for frequency values. *Most frequent Psychiatric disorder according to the Minnesota International Neuropsychiatric Invetory (MINI – DSM-IV).
Effect of treatment on immediate and delayed memory (primary outcome), verbal fluency and WM (secondary outcomes)
Immediate and delayed recall of episodic memory assessed with the Rey Auditory-Verbal Learning Test (RAVLT), orthographic and semantic category verbal fluency assessed with the Controlled Oral Word Association Test (COWAT), the Paced Auditory Serial Addiction Test (PASAT) and short-term and WM assessed with the Forward (FDS) and Backward Digit Span (BDS) were compared between treatment groups. Table 2 reports the results.
Table 2.
Independent t-Tests Between Active and Sham-tDCS + WMT Groups for the Differences (Deltas) of Cognitive Scores from Pre to Post-Treatment (n = 39).
| Cognitive Measures | Active-tDCS + WMT (n = 19) M(SD) | Sham-tDCS + WMT (n = 20) M(SD) | Between-groups P values for Δs | ||||
|---|---|---|---|---|---|---|---|
| Pre-treatment | Pos-treatment | Delta (Δ) | Pre-treatment | Pos-treatment | Delta (Δ) | ||
| Δ COWAT Orthographic | 28.80(11.24) | 34.05(9.65) | 23.46(27.94) | 31.30(10.88) | 34.31(11.88) | 10.74(19.70) | 0.11 |
| Δ COWAT Semantic | 16.80(6.07) | 18.90(5.85) | 14.08(23.78) | 17.55(5.66) | 17.26(5.15) | 0.95(16.32) | 0.52 |
| Δ RAVLT A1 | 6.50(1.67) | 9.20(2.56) | 45.55(40.60) | 6.80(2.09) | 8.26(1.96) | 25.52(35.96) | 0.11 |
| Δ RAVLT A1_A5 | 50.10(10.29) | 58.40(8.74) | 17.30(15.01) | 45.70(10.15) | 53.26(9.21) | 18.03(15.33) | 0.88 |
| Δ RAVLT A7 | 9.65(2.88) | 12.20(2.30) | 28.37(23.67) | 8.70(2.57) | 10.94(2.73) | 25.23(24.13) | 0.68 |
| Δ RAVLT Recognition | 13.30(1.78) | 14.10(1.20) | 6.17(13.21) | 13.50(1.27) | 13.89(1.41) | 2.72(10.75) | 0.37 |
| Δ PASAT | 28.73(13.04) | 33.50(12.61) | 18.42(26.93) | 28.15(11.30) | 32.72(12.10) | 18.21 | 0.98 |
| Δ FDS | 6.90(1.74) | 6.45(1.50) | −4.39(18.29) | 6.90(2.29) | 6.00(1.69) | −9.62 | 0.45 |
| Δ BDS | 4.60(1.35) | 5.05(1.82) | 15.66(49.17) | 4.30(1.62) | 4.73(2.02) | 12.36(39.61) | 0.81 |
Data presented as mean (M) and standard deviation (SD).
Notes. Δ = deltas; COWAT = Controlled Word Association Test; PASAT = Paced Auditory Serial Addiction Test; RAVLT = Rey Auditory Verbal Learning Test; FDS = Forward Digit Span; BDS = Backward Digit Span. P-value is the comparison of the deltas. Significance level was P < 0.05.
Effect of treatment on primary and secondary outcomes considering BDNF levels and years of education as covariates
The regression analysis exploring variables that influence BDNF levels showed presence of psychiatric diagnosis (B = −3.64; P < 0.007) and number of medications (B = 9.30; P < 0.019) significantly explained BDNF levels (R² = 0.22). Thus, the adjusted values of BDNF were used for the following analyses. The MANCOVA revealed that treatment had a significant influence in the model [Wilk’s Lambda = 0.488; F(9,24) = 2.80; P = 0.021; η² = 0.512], as well as the interaction term treatment*BDNF adjusted index [Wilk’s Lambda = 0.264; F(9, 24) = 2.52; P = 0.005; η² = 0.486]. Years of education was non-significant (P = 0.069). The influence of the factor treatment and covariates (years of education and BDNF adjusted index) together for each cognitive score is presented in Table 3. Accordingly, the model was significant for verbal fluency measures (COWAT orthographic and semantic), immediate recall (RAVLT A1_A5) and short-term memory (with the FDS score), considering Δs.
Table 3.
Analysis of Covariance (ANCOVA) Models for the Association of Treatment, years of education and BDNF adjusted index on Deltas of Cognitive Scores (n = 39).
| Cognitive Measures | Type III Sum of Squares | df | Mean Square | F | p | η²partial |
|---|---|---|---|---|---|---|
| Δ COWAT Orthographic | 6257.07 | 4 | 1564.27 | 3.26 | 0.024 | 0.29 |
| Δ COWAT Semantic | 4241.91 | 4 | 1060.48 | 3.05 | 0.031 | 0.28 |
| Δ RAVLT A1 | 8996.26 | 4 | 2249.07 | 1.47 | 0.233 | 0.16 |
| Δ RAVLT A1_A5 | 2534.90 | 4 | 633.72 | 3.42 | 0.020 | 0.30 |
| Δ RAVLT A7 | 6695.88 | 4 | 1673.97 | 1.80 | 0.152 | 0.18 |
| Δ RAVLT Recognition | 151.84 | 4 | 37.96 | 0.23 | 0.921 | 0.03 |
| Δ PASAT | 3699.63 | 4 | 924.91 | 1.50 | 0.225 | 0.16 |
| Δ FDS | 5716.00 | 4 | 1429.00 | 4.01 | 0.010 | 0.33 |
| Δ BDS | 12014.31 | 4 | 3003.58 | 1.54 | 0.213 | 0.16 |
Notes: df = degrees of freedom; Δ = deltas; COWAT = Controlled Word Association Test; PASAT = Paced Auditory Serial Addiction Test; RAVLT = Rey Auditory Verbal Learning Test; FDS = Forward Digit Span; BDS = Backward Digit Span. Statistics refer to the Corrected Model, with Treatment (active and sham-TDCS + WMT) and Treatment*BDNF adjusted index as factors and years of education as covariate. Significance level was P < 0.05.
In Table 4, we investigated in depth how group factor and covariates are associated with the cognitive scores, using univariate linear regression analyses as parameters estimates. As can be seen, belonging to the a-tDCS + WM training group was associated with an increased change in orthographic verbal fluency and immediate recall, independently of the educational and the BDNF level. Year of study was negatively associated with changes in orthographic verbal fluency score. Moreover, BDNF adjusted index correlated negatively with changes in immediate memory recall for the active tDCS and positively with the sham-tDCS group, while it correlated negatively with changes in FDS for the sham tDCS group only.
Table 4.
Univariate Linear Regression Models for the Effects of Treatment Groups (Active and Sham-tDCS + WMT), Years of education (as a Covariate) and the Interaction Treatment*BDNF on Deltas of Cognitive Measures (n = 39).
| Dependent Variable | B | SEM | F | P |
|---|---|---|---|---|
| Δ COWAT Orthographic | ||||
| Intercept | 1.12 | 28.32 | 0.04 | 0.969 |
| Active tDCS | 75.36 | 35.77 | 2.10 | 0.043 |
| Sham tDCSa | . | . | . | . |
| Education (years) | −2.43 | 1.01 | −2.39 | 0.022 |
| Active tDCS*index BDNF | −0.91 | 0.77 | −1.18 | 0.244 |
| Sham tDCS*index BDNF | 1.54 | 0.90 | 1.27 | 0.211 |
| Δ COWAT Semantic | ||||
| Intercept | −17.56 | 24.14 | −0.72 | 0.472 |
| Active tDCS | 45.25 | 30.49 | 1.49 | 0.145 |
| Sham tDCSa | . | . | . | . |
| Education (years) | 1.45 | 0.86 | 1.68 | 0.103 |
| Active tDCS*index BDNF | −0.93 | 0.65 | −1.42 | 0.164 |
| Sham tDCS*index BDNF | −0.01 | 0.77 | −0.01 | 0.999 |
| Δ RAVLT A1_A5 | ||||
| Intercept | −6.37 | 17.62 | −0.31 | 0.720 |
| Active tDCS | 74.29 | 22.26 | 3.33 | 0.002 |
| Sham tDCSa | . | . | . | . |
| Education (years) | −0.80 | 0.63 | −1.28 | 0.210 |
| Active tDCS*index BDNF | −1.39 | 0.47 | −2.90 | 0.007 |
| Sham tDCS*index BDNF | 1.15 | 0.53 | 2.04 | 0.049 |
| Δ FDS | ||||
| Intercept | 78.12 | 24,430 | 3,198 | 0.003 |
| Active tDCS | −46.00 | 30.85 | −1.49 | 0.146 |
| Sham tDCSa | . | . | . | . |
| Education (years) | −1.49 | 0.87 | −1.31 | 0.199 |
| Active tDCS*index BDNF | −0.77 | 0.66 | −1.16 | 0.251 |
| Sham tDCS*index BDNF | −2.61 | 0.78 | −3.35 | 0.002 |
Notes: df = degrees of freedom; SEM = standard error of the mean; COWAT = Controlled Word Association Test; RAVLT = Rey Auditory Verbal Learning Test; FDS = Forward Digit Span.
aComparative group, to which values are referenced to. Significance level was P < 0.05.
Associations between BDNF adjusted index and episodic memory scores
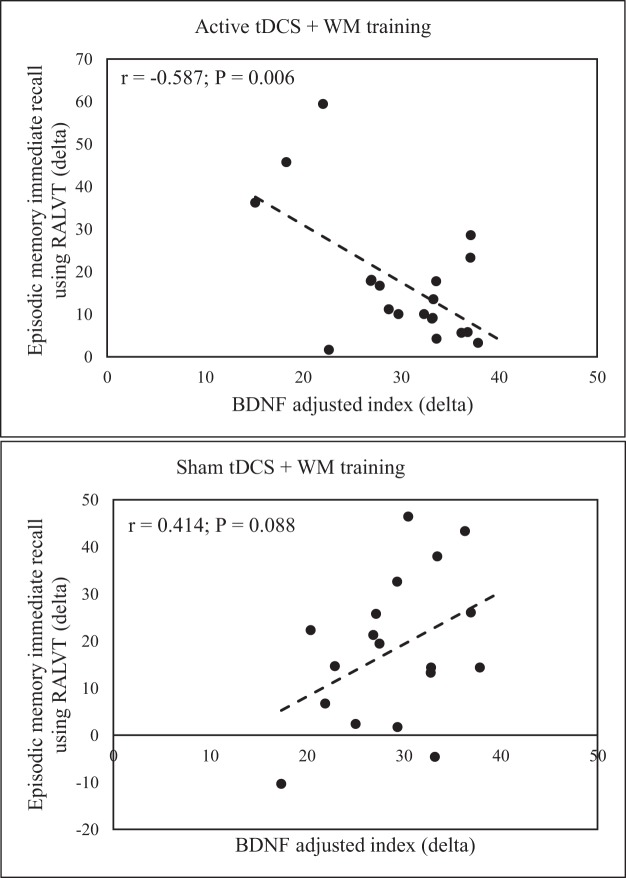
Figure 1 depicts this interaction, where the correlation was only significant for the active tDCS group.
Figure 1.
Scatter plots indicating the Pearson (r) correlations between changes in episodic memory immediate recall assess with the Rey Auditory Verbal Learning Test (RALVT) and changes in BDNF adjusted index.
Discussion
The present study aimed to test if a treatment tDCS coupled with a WM cognitive task would have additive effects that benefit memory, attention and executive functions for patients with FM when compared to cognitive training alone. In fact, data suggests this was the case regarding the higher increase in immediate memory capacity and verbal fluency after active treatment compared to sham. Interestingly, the effect of active tDCS on the short-term memory was partially dependent on baseline levels of serum BDNF. This neurotrophin was associated with changes in RAVLT A1_A5 score only for the active tDCS. Nevertheless, BNDF mediated changes in short-term digit span memory only for the placebo group. Also, years of education did not influence significantly the effect of interventions.
Our findings are congruent with other studies that found a better effect of tDCS combined with a cognitive training on WM and other cognitive performances17–19. Recent studies have showed that tDCS combined with a cognitive training task is more efficient to improve the pain threshold in FM compared to sham stimulation20. Particularly, in Silva et al.’s (2017)13 study, anodal tDCS applied on the DLPFC coupled with a training task for inhibitory control (a Go No-Go task) improved the executive and orienting attentional networks performance after a single session. In addition, previous researchers have found that for healthy volunteers the DLPFC anodal stimulation combined with an adapted verbal n-Back Task for training improved recall performance of word pairs21–23. It is possible that the impact of treatment observed in our study may also be associated with the lateralization of verbal material processing. This hypothesis is plausible since our sample comprises right-hand subjects only, which have mostly formal aspects of language being processed by the left-hemisphere23. Considering this rationale, the stimulus modality (verbal and visual) of the WM training task may interacts in a particular way with the site of the stimulation (left or right DLPFC). For example, the tDCS task-congruent intervention had a stronger and long-lasting enhancement of cognitive outcomes23. However, the effect reported by some authors23,24 was observed in healthy subjects. Our study comprised only verbal tasks for cognitive assessment. So, further studies would be necessary to test the hemisphere lateralization hypothesis. Using a similar methodology of the present study, Elmasry, Loo, and Martin (2015)25 concluded that ten sessions of online tDCS combined with a cognitive training (Dual n-Back Task) were not able to change neither WM nor executive function measures significantly. However, active tDCS improved the Dual n-Back discrimination ability25. This finding is quite similar to our univariate data, where no significant difference between groups was found. So, it is possible to argue that baseline factors associated with the central neurophysiological state are likely to influence this sort of treatment. This argument is supported by a growing body of evidence suggesting that tDCS produces a state-dependent impact when considering cognitive outcomes26. In this line, we found that a factor closely related to neuroplasticity state (BDNF) have influenced the effectiveness of treatment.
Baseline BDNF had a relevant effect on short-term memory indices. The RAVLT A1_A5, which assess the cumulative short storage capacity after a word list is presented five times to the patient, is a renowned instrument for episodic memory assessment27. Our data suggests tDCS induced a higher increase in this function from pre to post-intervention compared to sham. However, this effect was only significant when the interaction term, considering BDNF levels, was included. According to Table 2 t-tests, changes due to active or sham treatment have roughly the same magnitude. But when the interaction between Treatment and BDNF is considered (Table 4), it becomes clearer this neurotrophine had an opposite effect for the groups. Figure 1 illustrates that higher levels of BDNF at baseline assessment were associated with smaller changes from pre to post-intervention.
We expected that higher BDNF serum level would be related with better performance on memory tests. There is some literature indicating a positive relation between BDNF level with verbal memory and learning capacity in healthy subjects28,29. Moreover, there is evidence that the volume of the left hippocampus mediates the association between BDNF and spatial memory30. It should be considered FM is known as a syndrome that comprises a central sensitization process associated with higher levels of BDNF compared to controls8. Simultaneously, higher levels of BDNF have been related to both higher pain scores and disability in FM31. Therefore, especially for this population, and perhaps other similar pain syndromes, higher levels of this neurotrophin may impact negatively on the tDCS effect, leading to smaller changes in cognitive outcomes. This effect occurs in the hippocampus region, which is central to memory processes, such as consolidation32. BDNF is also widespread in central and peripheral nervous system, and present in many neural systems33 and its concentration may affect each neural network differently. Therefore, it seems plausible that in patients with FM, high levels of BDNF are not only associated with pain increase and maintenance but may also may be associated with an adverse neurophysiological environment for therapeutic approaches. Another measure of immediate recall evaluated here is the FDS. The BDNF was inversely correlated with the change in this test only for the sham group. Considering that no simple effect for treatment was found, we deductively concluded that a higher BDNF level at baseline could reduce the changes from pre to post-intervention. However, it remains unclear yet why this relationship was observed only for the sham group. First point to be raised concerns the differences between tasks. Despite evaluating similar recall abilities in both cases, RAVLT involves meaningful stimuli (words), learning curves (by repeating the stimuli), a free-order recall method and complex associative strategies, whereas FDS is an auditory test that classically measures phonological components of short-term memory34. This idea may help to understand the different role of BDNF for the RAVLT A1_A5 score for the sham group, which had a positive relation. When we consider the associations between BDNF and cognitive measures for the sham group, it should be highlighted these patients did received an intervention, a cognitive training. Besides, it should be considered that serum BDNF accounts only partially for the central nervous system concentration of the neurotrophin35,36.
We have also found effects of our treatment in other cognitive systems, and that was independent of BDNF levels. The COWAT is a measure of verbal fluency and covers a wide range of cognitive functions, including verbal ability and executive control37. Some authors reviewed cognitive processes evaluated with verbal fluency tasks. They suggest category fluency tasks, such as the semantic COWAT, reflect better the verbal ability, while letter fluency, on which our orthographic test is based, reveals more executive aspects38. Semantic verbal fluency is associated with more anterior-ventrally localized networks of the frontal cortex, while letter fluency is located more posterior-dorsally. Thus, it is plausible to consider that a DLPC anodal stimulation combined with a WM task that equally recruits the DLPFC area (apart from other regions, see Constantinidis and Klingberg 2016)39 would have a more salient impact over executive functions, than language-related functions. Patients with FM are known to have executive attention and WM difficulties40 what indicates a more clinically relevant effect of our treatment. On the other hand, the diffuse effect of tDCS should not be neglected, which means DLPFC stimulations may increase excitability in various regions of the frontal lobe. Because fluency tasks have time restrictions, higher general processing speed associated with increased excitability would benefit the active group.
In overall, the effect of tDCS observed in the current study may suggest that the active-tDCS combined with a WMT induced an increase functioning of the inhibitory system. This hypothesis might be plausibility if we considered that the FM is the prototypical syndrome of CSS41, which encompasses an impaired function of neurons and circuits in nociceptive pathways, with an increase in either membrane excitability and synaptic efficacy, and reduced inhibition42. In fact, the neurobiological mechanism of FM28 involves an imbalance between excitatory and inhibitory systems, by a dysfunction in the GABAergic and glutamatergic pathways43. In this sense, therapeutic use of active-tDCS may induce long-lasting after-effects. It was found long-term potentiation and depression and involvement of NMDA-receptor channels related to the tDCS effects, as well as domaminergic and cholinergic systems44. The stimulation is able to change the neuronal calcium influx, protein synthesis, blood flow, the level of brain oxygenation. The results can, however, differ between healthy and individuals with some central nervous system dysfunction, such as FM patients45.
Also, the present study represents progress to the question of non-invasive treatment in FM patients about transfer effects. As we found performance enhancements in functions other than WM tasks, transfer effects to other cognitive processes are plausible to be considered. RAVLT, COWAT, and FDS measure functions other than WM. However, this idea should be regarded cautiously, because we have not found effects for PASAT or backward digit span scores, which measure different aspects of WM40,46. Another limitation of our study was that the Dual n-Back Task used for training purposes is a highly demanding task, especially for older patients not familiarized with the computer. Even considering the adaptive version, starting at 1-back and increasing according to accuracy performance, none of the patients was able to achieve more than 2-back WM load. This task may have exhausted the limits of WM and cognitive processing, not allowing performance gains. These inferences are also limited due to the lack of a healthy control group and a sham cognitive training. Also, we had a sample of women, which limits our conclusions to this gender, although it should be highlighted that FM has a higher prevalence in females1.
Overall, our results highlight two important conclusions. First, eight session of anodal tDCS over the left DLPFC combined with WM training has a modulatory effect on short-term memory capacity and verbal fluency after active treatment compared to sham stimulation. The secondary effect BDNF had a relevant effect in our model when we consider short-term memory indices. Also, these findings suggest that the effects of tDCS combined with a WM training relation to transfer effects to other cognitive processes are plausible to be considered.
Methods
Design, setting and participants
The methods and results sections are reported according to the CONSORT guidelines. All subjects provided written informed consent before participating in this randomized, double blind, sham-controlled, two arm parallel design with allocation ratio of 1:1. The study was approved by the Research Ethics Committee at Hospital de Clínicas de Porto Alegre (HCPA) (Institutional Review Board IRB 140369). The current controlled trial is registered at Clinical Trial (No. NCT02880917; End date: August 26, 2016).
We recruited 40 outpatients of the HCPA, all women aged between 18 and 65 years-old. They were invited via advertisement to participate from November 2015 to July 2017. Sample size was calculated based on previous with 0.25 effect size compare the effect of active tDCS and sham with alpha level of 0.05 and 80% power. FM was diagnosed according to American College of Rheumatology criteria47. Subjects were required to have a score at least 50 mm on the 0–100 mm visual analogue scale for pain during most of the days over the last three months48. Subjects were allowed to remain on analgesic medications, including drugs for which they were refractory, and these medications could not be adjusted during the study. Major depressive disorders were accepted as secondary to FM. Subjects with history of substance abuse or evidence of other pain-related disorder were excluded. Females pregnant, in breast-feeding or with a history of neurologic or oncologic disease and ischemic heart disease, kidney or hepatic insufficient were also excluded.
Intervention
The intervention of tDCS (TCT, Hong Kong, China) combined with a cognitive training task was applied for eight consecutive days (working days). tDCS was delivered using the anode electrode positioned over the left DLPFC (F3 according to the 10–20 system for EEG) and the cathode electrode at right supraorbital region (Fp2). The electrodes were placed into–35 cm2 sponges soaked in saline solution for better current conductivity. Rubber bandages were used to hold the electrodes in place for the duration of stimulation. The active-tDCS condition, a constant current of 2 mA was applied for 20 min. For sham stimulation the electrodes were placed in the same position, but the stimulator was turned off after a ramp-up of 30 s of stimulation49. To evaluate the safety of tDCS, we used questionnaire based on previously reported adverse events.
The cognitive training consisted of an online application of a Dual N-Back task50. A laptop (15 inches screen, distance of ~60 cm ahead) with software E-Prime 2.0 Standard presented two types of stimuli, simultaneously. Visual stimuli were green squares presented in eight different positions, and auditory stimuli presented binaurally via headphones. Patients had to decide for each trial if the stimuli were the same as n-trials before (memory workload), by pressing the keyboard “A” button for visual and “L” for auditory (and do not press any button when none of the alternatives apply). The task had 20 blocks with 20 trials each, of which 10 were “no target”, 4 were “visual target only”, 4 “auditory target only” and 2 “dual target”, and had duration of about 25 minutes. The memory workload information was presented in the beginning of each block, and a feedback (percentage of correct responses) was presented in the end of each block. Because of the adaptive nature of the cognitive training, workload level increased when the previous block had 90% correct responses or higher and decreased when less than 70% was achieved. (Fig. 2).
Figure 2.

The Dual n-Back Task scheme.
Randomization
Before the recruitment phase, the randomization was generated using a computer system by researchers who did not administer the intervention. They put the sequence in separately opaque sealed envelopes. The simple randomization method was applied, with patients assigned to the one of the two groups with a rate of 1:1.
Blinding
Envelopes containing the patients’ protocol numbers were opened by an auxiliary researcher, who also programmed the tDCS device for active or sham stimulation.Allocation concealment was assured by intervention being assigned only after enrollment.Furthermore, to assess whether blinding was effective, at the end of the experiment subjects were asked to guess whether they had received a-tDCS or sham and to rate their confidence level using a 5-point Likert scale.
Baseline instruments and assessments
All tests used have been validated for the Brazilian population. At the baseline, the instruments used were: Pittsburgh Sleep Quality Index51 to assess the sleep quality; Beck Depression Inventory-II (BDI-II)52, for the assessment of depressive symptoms; The Brazilian validated version of the Fibromyalgia Impact Questionnaire (FIQ)53, to assess quality of life of FM patients; the Brazilian Portuguese version of the Pain Catastrophizing Scale (BP-PCS)54, for the catastrophic thinking, State-Trait Anxiety Inventory (STAI) for the assessment of Anxiety55; Brazilian Profile of Chronic Pain: Screen (B-PCP:S)56 to characterize functional limitations related to severity of pain, emotional stress and pain interference in life; Pain level was assessed with a visual-analogue scale (VAS); Mini-International Neuropsychiatric Interview (M.I.N.I.)57 to detect psychiatric disorder; Medical comorbidities and demographic data were assessed using a standardized questionnaire. Heat pain threshold; Heat pain tolerance; BDNF marker of plasticity.
Outcomes and instruments of assessment
The primary outcome was the performance of the Rey Auditory-Verbal Learning Test (RAVLT). The second outcomes were performance of the Paced Auditory Serial Addiction Test (PASAT)58, Controlled Oral Word Association Test (COWAT), Forward Digit Span (FDS), Backward Digit Span(BDS) and serum level of BDNF.
The Rey Auditory-Verbal Learning Test
RAVLT is a test for the evaluation of episodic memory, with components related to short- and long-term memory and recognition. The 15 words of the test were read slowly, and patients were asked to recall them regardless of the order (A1). The same procedure was repeated in the following steps A2, A3, A4 and A5. A second list of words (B1) was then applied and patients were asked to evoke them immediately. Afterwards, patients were asked to recall the first list (A6). About 20 to 30 minutes later, patients had to recall the words from the first list (A7) once more. Finally, a list of 50 words was presented and patients should judge whether the word belonged or not to the first list27.
Controlled Oral Word Association Test (COWAT)
Involves word fluency for two categories: orthographic and semantic. In the orthographic category, patients were instructed to say aloud as many words having F, A or S as the first letter as possible in 1 minute. In the semantic category, subjects were instructed to say aloud as many animal names as possible in 1 minute59.
Forward and backward digit span
The test consists of arrays of algorisms, presented each a time, with a gradual increase in the array (starting with two digits) for direct order (eight arrays; FDS) and for reverse order (seven arrays; BDS). Patients were instructed to recall the numbers immediately and in a serial order (FDS) or in inverse order (BDS). FDS was applied first, followed by BDS46. The maximum score is 16 points for the FDS and 14 points for the BDS.
Paced Auditory Serial Addiction Test (PASAT)
It evaluates sustained and divided attention and working memory. In this test, the stimuli are numbers from one to nine, presented in random and predetermined sequence. The task was to perform the sum of the numbers presented, two by two, disregarding the result of the calculation. The test started by displaying the numerical sequence every 3 seconds. It comprises equivalent versions A and B. In this project we use version A in the first assessment phase and B in the second one to prevent habituation. Maximum number of correct answers is 60 in each version.
Serum levels of brain-derived neurotrophic factor (BDNF)
Serum BDNF was determined by the Enzyme-Linked Immunoabsorbent Assay (ELISA) using a ChemiKine BDNF Sandwich ELISA Kit, CYT306 (Chemicon/Millipore, Billerica, MA, USA). The lower detection limit of the kit is 7.8 pg/mL for BDNF.
General Procedure
Participants initially volunteered by signing the consent form. Following this, they responded to the baseline assessments and were checked for necessary exclusion criteria. Then, they were randomly allocated to one of the experimental groups, either receiving sham or active stimulation.Measures of working memory through the Dual N-back were obtained during the tDCS. Figure 3 presents the flowchart of the study.
Figure 3.
Flowchart showing recruitment and progress through the study. Controlled Word Association Test (Cowat); Rey Auditory Verbal Learning Test (RAVLT); Paced Auditory Serial Addiction Test (PASAT); Brain-derived neurotrophic factor(BDNF); Heat pain threshold (HPTh) and tolerance (HPTo).
Statistical plan of analysis
Descriptive analysis were performed using mean, standard deviation and frequency. Inferential tests for demographic and clinical measures, as well as for the cognitive outcomes, were based on independent samples t-Tests for continuous variables and Chi-Squared or Fisher’s tests for categorical variables. To avoid baseline differences, we used deltas (Δ) based on the mean differences calculation [(post-test – pre-test)/pre-test] for cognitive outcomes. In order to test the influence of BDNF levels as a modulator for the treatment’s effect, we used a multivariate analysis of covariance (MANCOVA) for the cognitive scores as dependent variables. Due to many factors may influence serum BDNF level, we have adjusted its value in a linear regression model with stepwise method, which tested the influence of age, number of medications and frequency of medication use, presence of psychiatric diagnosis and baseline depressive symptoms (using the BDI-II) and functional incapacity (using the B-PCP:S). We also included years of education as a covariate because raw scores were used (instead of standardized scores), which may be affected by educational level. We tested single effects for treatment (active-tDCS + WM training and sham-tDCS + WM training) and years of education, and interaction between treatment and BDNF adjusted index, followed by Bonferroni correction and univariate linear regression analyses when significant main effects were found. Finally, for exploratory purposes, Pearson correlations between BDNF adjusted index and immediate memory recall scores were applied for each treatment group.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgements
This research was supported by grants and material support from the following Brazilian agencies: Committee for the Development of Higher Education Personnel – CAPES - PNPD/CAPES and material support. National Council for Scientific and Technological Development - CNPq (grants to Dr. I.L.S. Torres, Dr. W. Caumo). Postgraduate Program in Medical Sciences at the School of Medicine of the Federal University of Rio Grande do Sul (material support). Postgraduate Research Group at the Hospital de Clínicas de Porto Alegre – FIPE HCPA (material support). Foundations for Support of Research at Rio Grande do Sul (FAPERGS) (material support).
Author Contributions
V.S.S., M.Z. and W.C. conceived and designed the study, participated in the sequence alignment, performed the statistical analysis, and coordinated and drafted the manuscript; R.L. and C.C.S.N. collected and registered the data; J.S. helped in the data analysis; S.C. and J.L. helped conceive and design the study and drafted the manuscript; I.L.S.T., P.U.C., F.F. conceived the study, participated in its design and coordinated and reviewed the manuscript; A.S. helped with the blood laboratorial analyses and reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wolfe F, Brähler E, Hinz A, Häuser W. Fibromyalgia prevalence, somatic symptom reporting, and the dimensionality of polysymptomatic distress: Results from a survey of the general population. Arthritis Care Res. 2013;65:777–785. doi: 10.1002/acr.21931. [DOI] [PubMed] [Google Scholar]
- 2.Jones GT, et al. The prevalence of fibromyalgia in the general population: A comparison of the American College of Rheumatology 1990, 2010, and modified 2010 classification criteria. Arthritis Rheumatol. 2015;67:568–575. doi: 10.1002/art.38905. [DOI] [PubMed] [Google Scholar]
- 3.Volz MS, Suarez-Contreras V, Portilla ALS, Fregni F. Mental imagery-induced attention modulates pain perception andcortical excitability. BMC Neurosci. 2015;16:15. doi: 10.1186/s12868-015-0146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meeus, M. & Nijs, J. Central sensitization: A biopsychosocial explanation for chronic widespread pain in patients with fibromyalgia and chronic fatigue syndrome. 26, 465–473(2007). [DOI] [PMC free article] [PubMed]
- 5.Cuesta-Vargas, et al. Dimensionality and Reliability of the Central Sensitization Inventory (CSI) in a Pooled MultiCountry Sample. J Pain. 2018;19:317–329. doi: 10.1016/j.jpain.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Yunus MB. Central Sensitivity Syndromes: A New Paradigm and Group Nosology for Fibromyalgia and Overlapping Conditions, and the Related Issue of Disease versus Illness. Semin Arthritis Rheum. 2008;37:339–352. doi: 10.1016/j.semarthrit.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Coppieters I, et al. Cognitive Performance Is Related to Central Sensitization and Health-related Quality of Life in Patients with Chronic Whiplash-Associated Disorders and Fibromyalgia. Pain Physician. 2015;18:389–401. [PubMed] [Google Scholar]
- 8.Caumo W, et al. The Central Sensitization Inventory validated and adapted for a Brazilian population: psychometric properties and its relationship with brain-derived neurotrophic factor. J Pain Res. 2017;10:2109–2122. doi: 10.2147/JPR.S131479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coull JAM, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 10.Galloway EM, Woo NH, Lu B. Persistent neural activity in the prefrontal cortex: A mechanism by which BDNF regulates working memory? Prog Brain Res. 2008;169:251–66. doi: 10.1016/S0079-6123(07)00015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrews SC, Hoy KE, Enticott PG, Daskalakis ZJ, Fitzgerald PB. Improving working memory: The effect of combining cognitive activity and anodal transcranial direct current stimulation to the left dorsolateral prefrontal cortex. Brain Stimul. 2011;4:84–89. doi: 10.1016/j.brs.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Fregni F, et al. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp Brain Res. 2005;16:23–30. doi: 10.1007/s00221-005-2334-6. [DOI] [PubMed] [Google Scholar]
- 13.Silva AF, et al. Anodal transcranial direct current stimulation over the left dorsolateral prefrontal cortex modulates attention and pain in fibromyalgia: randomized clinical trial. Sci Rep. 2017;7:135. doi: 10.1038/s41598-017-00185-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunoni AR, Vanderhasselt MA. Working memory improvement with non-invasive brain stimulation of the dorsolateral prefrontal cortex: a systematic review and meta-analysis. Brain Cogn. 2014;86:1–9. doi: 10.1016/j.bandc.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Ruf SP, Fallgatter AJ, Plewnia C. Augmentation of working memory training by transcranial direct current stimulation (tDCS) Sci Rep. 2017;7:876. doi: 10.1038/s41598-017-01055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaehle T, Sandmann P, Thorne JD, Jäncke L, Herrmann CS. Transcranial direct current stimulation of the prefrontal cortex modulates working memory performance: combined behavioural and electrophysiological evidence. BMC Neurosci. 2011;12:1–11. doi: 10.1186/1471-2202-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oldrati V, Colombo B, Antonietti A. Combination of a short cognitive training and tDCS to enhance visuospatial skills: A comparison between online and offline neuromodulation. Brain Res. 2018;1678:32–39. doi: 10.1016/j.brainres.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Fazeli PL, Woods AJ, Pope CN, Vance DE, Ball KK. Effect of transcranial direct current stimulation combined with cognitive training on cognitive functioning in older adults with HIV: A pilot study. Appl Neuropsychol Adult. 2017;16:1–12. doi: 10.1080/23279095.2017.1357037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawrence BJ, Gasson N, Bucks RS, Troeung L, Loftus AM. Cognitive Training and Noninvasive Brain Stimulation for Cognition in Parkinson’s Disease: A Meta-analysis. Neurorehabil Neural Repair. 2017;31:597–608. doi: 10.1177/1545968317712468. [DOI] [PubMed] [Google Scholar]
- 20.Powers A. et al. Effects of Combining a Brief Cognitive Intervention with Transcranial Direct Current Stimulation on Pain Tolerance: A Randomized Controlled Pilot Study. Pain Med. 10.1093/pm/pnx098 (2017). [DOI] [PubMed]
- 21.Hoy KE, et al. Testing the limits: Investigating the effect of tDCS dose on working memory enhancement in healthy controls. Neuropsychologia. 2013;51:1777–1784. doi: 10.1016/j.neuropsychologia.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Horvath JC, Forte JD, Carter O. Quantitative review finds no evidence of cognitive effects in healthy populations from single-session transcranial direct current stimulation (tDCS) Brain Stimul. 2015;8:535–550. doi: 10.1016/j.brs.2015.01.400. [DOI] [PubMed] [Google Scholar]
- 23.Meiron O, Lavidor M. Unilateral Prefrontal Direct Current Stimulation Effects are Modulated by Working Memory Load and Gender. Brain Stimul. 2013;6:440–447. doi: 10.1016/j.brs.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Cattaneo Z, Pisoni A, Papagno C. Transcranial direct current stimulation over Broca’s region improves phonemic and semantic fluency in healthy individuals. Neuroscience. 2011;183:64–70. doi: 10.1016/j.neuroscience.2011.03.058. [DOI] [PubMed] [Google Scholar]
- 25.Elmasry J, Loo C, Martin D. A systematic review of transcranial electrical stimulation combined with cognitive training. Restor Neurol Neurosci. 2015;33:263–278. doi: 10.3233/RNN-140473. [DOI] [PubMed] [Google Scholar]
- 26.Hsu WY, Ku Y, Zanto TP, Gazzaley A. Effects of noninvasive brain stimulation on cognitive function in healthy aging and Alzheimer’s disease: a systematic review and meta-analysis. Neurobiol Aging. 2015;36:2348–2359. doi: 10.1016/j.neurobiolaging.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magalhães SS, Hamdan AC. The Rey Auditory Verbal Learning Test: normative data for the Brazilian population and analysis of the in Àuence of demographic variables. Psychol Neurosci. 2010;3:85–91. doi: 10.3922/j.psns.2010.1.011. [DOI] [Google Scholar]
- 28.Antal A., Keeser, D., Priori, A., Padberg F. & Nitsche, M. A. Conceptual and Procedural Shortcomings of the Systematic Review “Evidence That Transcranial Direct Current Stimulation (tDCS) Generates Little-to-no Reliable Neurophysiologic Effect Beyond MEP Amplitude Modulation in Healthy Human Subjects: A Systematic Review” by Horvath and Co-workers. 8, 27–31(2015). [DOI] [PubMed]
- 29.Komulainen P, et al. BDNF is a novel marker of cognitive function in ageing women: The DR’s EXTRA Study. Neurobiol Learn Mem. 2008;90:596–603. doi: 10.1016/j.nlm.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Erickson KI, et al. Brain-Derived Neurotrophic Factor Is Associated with Age-Related Decline in Hippocampal Volume. J Neurosci. 2010;30:5368–75. doi: 10.1523/JNEUROSCI.6251-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zanette SA, et al. Higher serum S100B and BDNF levels are correlated with a lower pressure-pain threshold in fibromyalgia. Mol Pain. 2014;10:46. doi: 10.1186/1744-8069-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray, P. S. & Holmes, P. V. An Overview of Brain-Derived Neurotrophic Factor and Implications for Excitotoxic Vulnerability in the Hippocampus. Int J Pept. 10.1155/2011/654085 (2011). [DOI] [PMC free article] [PubMed]
- 33.Wu YJ, et al. Nerve growth factor, brain-derived neurotrophic factor, and neurotrophin-3 are sorted to dense-core vesicles and released via the regulated pathway in primary rat cortical neurons. J Neurosci Res. 2004;75:825–834. doi: 10.1002/jnr.20048. [DOI] [PubMed] [Google Scholar]
- 34.Baddeley A. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- 35.Savli H, Gulkac MD, Esen N. The effect of stimulated microglia conditioned media on bdnf gene expression of striatal astrocytes: quantification by real-time pcr. Int J Neurosci. 2004;114:1601–1612. doi: 10.1080/00207450490476138. [DOI] [PubMed] [Google Scholar]
- 36.Schinder A. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000;23:639–645. doi: 10.1016/S0166-2236(00)01672-6. [DOI] [PubMed] [Google Scholar]
- 37.Becker N, Salles JFde. Methodological Criteria for Scoring Clustering and Switching in Verbal Fluency Tasks. Psico-USF. 2016;21:445–457. doi: 10.1590/1413-82712016210301. [DOI] [Google Scholar]
- 38.Shao Z, Janse E, Visser K, Meyer AS. What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Front Psychol. 2014;5:772. doi: 10.3389/fpsyg.2014.00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Constantinidis C, Klingberg T. The neuroscience of working memory capacity and training. Nat Rev Neurosci. 2016;26:438–449. doi: 10.1038/nrn.2016.43. [DOI] [PubMed] [Google Scholar]
- 40.Glass JM. Review of Cognitive Dysfunction in Fibromyalgia: A Convergence on Working Memory and Attentional Control Impairments. Rheum Dis Clin North Am. 2009;35:299–311. doi: 10.1016/j.rdc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Clauw DJ. Fibromyalgia: a clinical review. Jama. 2014;311:1547–55. doi: 10.1001/jama.2014.3266. [DOI] [PubMed] [Google Scholar]
- 42.Latremoliere A, Woolf CJ. Central Sensitization: A Generator of Pain Hypersensitivity by Central Neural Plasticity. J pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mhalla A, de Andrade DC, Baudic S, Perrot S, Bouhassira D. Alteration of cortical excitability in patients with fibromyalgia. Pain. 2010;149:495–500. doi: 10.1016/j.pain.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 44.Stagg CJ, Nitsche MA. Physiological Basis of Transcranial Direct Current Stimulation. Neurosci. 2011;17:37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- 45.Burgmer M, et al. Altered brain activity during pain processing in fibromyalgia. Neuroimage. 2009;44:502–508. doi: 10.1016/j.neuroimage.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 46.De FVLM, Nascimento Do E. Desempenhos nas duas tarefas do subteste dígitos do WISC-III e do WAIS-III. Psicol Teor e Pesqui. 2007;23:313–318. doi: 10.1590/S0102-37722007000300010. [DOI] [Google Scholar]
- 47.Wolfe F, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62:600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 48.Bennett R. Fibromyalgia: present to future. Curr Rheumatol Rep. 2004;7:371–376. doi: 10.1007/s11926-005-0022-y. [DOI] [PubMed] [Google Scholar]
- 49.Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117:845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 50.Jaeggi SM, et al. On how high performers keep cool brains. Cogn Affect Behav Neurosci. 2007;7:75–89. doi: 10.3758/CABN.7.2.75. [DOI] [PubMed] [Google Scholar]
- 51.Bertolazi AN, et al. Validation of the Brazilian Portuguese version of the Pittsburgh Sleep Quality Index. Sleep Med. 2011;12:70–75. doi: 10.1016/j.sleep.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 52.Gorenstein, C., Pang, W., Argimon, I. & Werlang, B. Manual do Inventário de depressão de Beck - BDI-II. (Casa do Psicólogo, 2011).
- 53.Marques AP, et al. Validação da versão Brasileira do Fibromyalgia Impact Questionnaire (FIQ) Rev Bras Reumatol. 2006;46:24–31. [Google Scholar]
- 54.Sehn F, et al. Cross-Cultural Adaptation and Validation of the Brazilian Portuguese Version of the Pain Catastrophizing Scale. Pain Med. 2012;13:1425–1435. doi: 10.1111/j.1526-4637.2012.01492.x. [DOI] [PubMed] [Google Scholar]
- 55.Kaipper MB, Chachamovich E, Hidalgo MPL, da Silva Torres IL, Caumo W. Evaluation of the structure of Brazilian State-Trait Anxiety Inventory using a Rasch psychometric approach. J Psychosom Res. 2010;68:223–233. doi: 10.1016/j.jpsychores.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 56.Caumo W, et al. Cross-Cultural Adaptation and Validation of the Profile of Chronic Pain: Screen for a Brazilian Population. Pain Med. 2013;14:52–61. doi: 10.1111/j.1526-4637.2012.01528.x. [DOI] [PubMed] [Google Scholar]
- 57.Amorim P. Mini International Neuropsychiatric Interview (MINI): validação de entrevista breve para diagnóstico de transtornos mentais. Rev Bras Psiquiatr. 2000;22:106–115. doi: 10.1590/S1516-44462000000300003. [DOI] [Google Scholar]
- 58.Haase VG, Lima E, de P, Lacerda SS, Lana-Peixoto MA. Development of the Brazilian version of the Multiple Sclerosis Functional Composite Measure (MSFC-BCTRIMS): pilot study. Arq Neuropsiquiatr. 2004;62:363–369. doi: 10.1590/S0004-282X2004000200033. [DOI] [PubMed] [Google Scholar]
- 59.Strauss, E., Sherman, E. M. S. & Spreen. A compendium of neuropsychological tests: Administration, norms, and commentary. (eds Strauss, E. & Spreen) 500–510 (Oxford, 2006).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.