Flagellated parasitic protozoa of the Leishmania genus are responsible for the neglected tropical disease known as leishmaniasis. This worldwide disease causes between 20,000 and 30,000 deaths per year in about 97 countries (Global Health Observatory data from the World Health Organization, September 22, 2017). Available treatments are limited owing to toxicity, mode of administration, cost, and drug resistance. In response to various stimuli, Leishmania cells present a phenotype similar to that of apoptotic mammalian cells. Cell rounding up can be observed, as well as chromatin condensation, oligonucleosomal DNA fragmentation, and mitochondrial depolarization1. Since the term “apoptosis” describes a type of cell death defined by its morphological aspects according to the Nomenclature Committee on Cell Death2 and these features are encountered in Leishmania, as cited above, we can talk about not only cell death but also apoptosis in this unicellular parasite. Physiologically, apoptosis has been described in the parasite as a selfish altruism, regulating parasite densities within the vector and the mammalian host, and avoiding hyperparasitism3. Apoptosis in Leishmania may also permit successful infection by modulating host immunity3.
Classically, two main apoptotic pathways are described in mammalian cells: (i) the extrinsic pathway that is activated by recognition between an extracellular ligand and a death receptor and (ii) the intrinsic pathway activated by intracellular signals, which involves the mitochondrion and pro-apoptotic and anti-apoptotic molecules. Both pathways lead to a cascade of activations of specific proteins known as caspases, which are cysteine proteases. In Leishmania, the apoptotic pathway remains largely unknown, due to the lack of knowledge concerning the proteins involved, since the classic mammalian key proteins of apoptosis are not found in this parasite. For example, no death receptor has been identified in Leishmania4 and the presence of pro-apoptotic and anti-apoptotic molecules is still a matter of debate5. Previously, we studied the metacaspase LmjMCA, a cysteine peptidase present in L. major that shares similarities with caspases but has different substrate specificity6. We showed that LmjMCA is involved in apoptosis induced by the anti-Leishmania drug miltefosine, having a role similar to that of caspases. The purpose of this study is to investigate whether apoptosis in Leishmania consistently involves LmjMCA, regardless of the stimulus. To do so, we tested five molecules: four anti-Leishmania drugs (amphotericin B, curcumin, miltefosine, and pentamidine) and another molecule (H2O2). We recently showed that all these five molecules induce L. major apoptosis7.
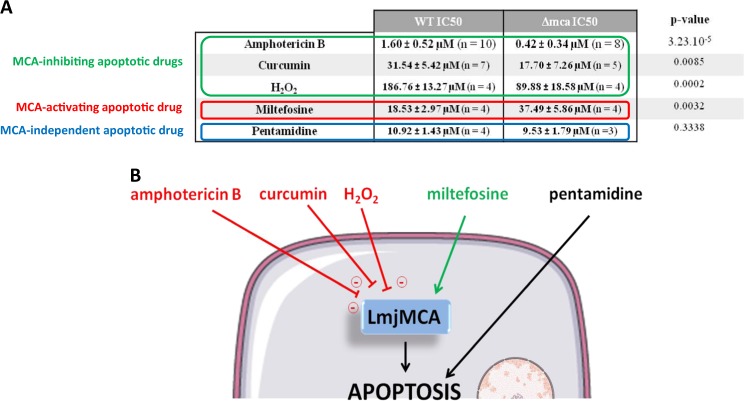
To assess whether the apoptotic pathway induced by the different molecules tested involved LmjMCA, we carried out a methyl thiazol tetrazolium (MTT) assay with the five molecules on the WT strain and on the LmjMCA-deficient L. major strain. We calculated the inhibitory concentration 50 (IC50), i.e., the molecule concentration inhibiting 50% of cell growth in comparison with a control without any molecule. As shown in Fig. 1a, we observed that the IC50 was significantly lower in the LmjMCA-deficient strain (Δmca) than in the wild-type (WT) strain for amphotericin B (0.42 and 1.60 µM, respectively), for curcumin (17.70 and 31.54 µM, respectively), and for H2O2 (89.88 and 186.76 µM, respectively). This suggests that amphotericin B, curcumin, and H2O2 induce LmjMCA inhibition. In contrast, the IC50 of miltefosine was significantly higher in the LmjMCA-deficient strain compared to the WT strain (37.49 and 18.53 µM, respectively), while no significant difference was observed between these two strains concerning pentamidine IC50 (about 10 µM). As a consequence, the miltefosine apoptotic pathway seems to involve LmjMCA activation, while the apoptotic pathway induced by pentamidine did not involve LmjMCA.
Fig. 1. Different apoptosis pathways in Leishmania cells.
(A) In vitro antileishmanicidal activity of different drugs/molecules on WT and LmjMCA-deficient (Δmca) L. major cells. The IC50 of WT cells and Δmca L. major cells are presented as mean of n experiments ± standard deviation. For statistical analysis, unpaired t-tests were done comparing the Δmca strain values with the WT strain values (BioStaTGV). The p-value of the different tests is written is the last column. (B) Summary of the different apoptosis pathways in Leishmania, regarding LmjMCA.
These results showed that the five molecules had different effects on LmjMCA. However, as recommended by Carmona-Gutierrez et al.8, “neologisms should be introduced with care and only when the characterization of a lethal process that bears new functional and biochemical aspects requires it. Otherwise, new expressions should be avoided to limit confusion”. Consequently, we prefer to talk of different apoptosis pathways induced by the different molecules rather than different types of cell deaths. More precisely, we identified three apoptosis pathways drawn in Fig. 1b, concerning LmjMCA: (i) an apoptosis pathway in which LmjMCA is activated (induced by miltefosine); (ii) a pathway in which LmjMCA is inhibited (amphotericin B, curcumin, and H2O2); and (iii) an LmjMCA-independent apoptosis pathway (pentamidine). In mammals, apoptosis is classically described as involving caspase activation. Hence, miltefosine would appear to induce the classic form of apoptosis. In 1996, Xiang et al.9 gave the first clear demonstration of caspase-independent regulated cell death. Since then, several articles have shown that regulated cell death can occur in the complete absence of caspases (reviewed in Bröker et al.10). In this case, several other proteases have been suggested to be involved, such as calpains, cathepsins, and serine proteases. In Leishmania, non-caspase proteases have been suggested to be involved in the cell death of the parasite, as the cysteine proteases calpains as reviewed in Branquinha et al.11. Moreover, El-Fadili et al.12 demonstrated that the cathepsin B-like enzyme LmjCPC is involved in L. major cell death. The third apoptosis pathway underlined in this article appears to be more original: the apoptosis pathway in which LmjMCA is inhibited. Two hypotheses can be formulated concerning this pathway: (i) either LmjMCA inhibition directly induces apoptosis, which could be explained by the role of LmjMCA in the cell survival process autophagy6 and by the fact that inhibiting autophagy induces cell apoptosis in stress conditions13, or (ii) apoptosis induced by amphotericin B, curcumin, and H2O2 involves an LmjMCA-independent pathway, and the inhibition of LmjMCA is not the cause of apoptosis.
These results support other articles which have already suggested different apoptosis pathways in Leishmania. For example, Vergnes et al.14 demonstrated that antimonial and miltefosine induced different cell death pathways in Leishmania. Foucher et al.15 have shown that amphotericin B and miltefosine induced different morphologic phenotypes in Leishmania, which is highlighted by the absence of cell shrinkage with amphotericin B while all other apoptotic markers were found with this drug7. In conclusion, this article highlights multiple apoptotic pathways in Leishmania in response to the addition of different molecules, while apoptosis is essential for successful survival of the population and for parasite infectivity3. However, other studies must be conducted to demonstrate whether these pathways are physiologically encountered in Leishmania cells and to better understand the entire pathway. To do this, genetic tools must be used, such as mutants deficient in a protein, rather than pharmacological inhibitors that might block the activity of several factors/pathways. In all cases, the study of an ancestral eukaryote such as Leishmania will contribute towards a better understanding of the evolution of regulated cell death in eukaryotes in general. These results will also possibly help the design of efficient new drugs based on their mode of killing of Leishmania parasites. Finally, identifying different cell death pathways will enable the combination of different drugs inducing different pathways in order to avoid or delay the appearance of drug resistance.
Acknowledgements
We thank the CNR Leishmania (Montpellier, France) for the L. major strain and Jeremy Mottram for providing us with the LmjMCA-deficient strain. We also thank Nicolas Fasel for helpful discussion. This work was supported by the French Government under the “Investissements d’avenir” program managed by the Agence Nationale de la Recherche (reference: Méditerranée Infection 10-IAHU-03). It was also supported by the PHC Germaine de Staël (project no. 35021RJ).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/10/2019
Due to a technical error, content intended for publication in Volume 4 (2018) published in Volume 5 (2019). The content has been moved into the correct volume, and the citation information was updated accordingly.
References
- 1.Jiménez-Ruiz A, et al. Apoptotic markers in protozoan parasites. Parasit. Vectors. 2010;3:104. doi: 10.1186/1756-3305-3-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kroemer G, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lüder CG, Campos-Salinas J, Gonzalez-Rey E, van Zandbergen G. Impact of protozoan cell death on parasite-host interactions and pathogenesis. Parasit. Vectors. 2010;3:116. doi: 10.1186/1756-3305-3-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Proto WR, Coombs GH, Mottram JC. Cell death in parasitic protozoa: regulated or incidental? Nat. Rev. Microbiol. 2013;11:58–66. doi: 10.1038/nrmicro2929. [DOI] [PubMed] [Google Scholar]
- 5.Genes CM, et al. A functional BH3 domain in an aquaporin from Leishmania infantum. Cell Death Discov. 2016;2:16043. doi: 10.1038/cddiscovery.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casanova M, et al. Implication of different domains of the Leishmania major metacaspase in cell death and autophagy. Cell Death Dis. 2015;6:e1933. doi: 10.1038/cddis.2015.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basmaciyan, L., Berry, L., Gros, J., Azas, N. & Casanova, M. Temporal analysis of the autophagic and apoptotic phenotypes in Leishmania parasites. Microb. Cell (2018). [DOI] [PMC free article] [PubMed]
- 8.Carmona-Gutierrez D, et al. Guidelines and recommendations on yeast cell death nomenclature. Microb. Cell. 2018;5:4–31. doi: 10.15698/mic2018.01.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiang J, Chao DT, Korsmeyer SJ. BAX-induced cell death may not require interleukin 1 beta-converting enzyme-like proteases. Proc. Natl Acad. Sci. USA. 1996;93:14559–14563. doi: 10.1073/pnas.93.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bröker LE, Kruyt FAE, Giaccone G. Cell death independent of caspases: a review. Clin. Cancer Res. 2005;11:3155–3162. doi: 10.1158/1078-0432.CCR-04-2223. [DOI] [PubMed] [Google Scholar]
- 11.Branquinha MH, et al. Calpains: potential targets for alternative chemotherapeutic intervention against human pathogenic trypanosomatids. Curr. Med. Chem. 2013;20:3174–3185. doi: 10.2174/0929867311320250010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Fadili AK, et al. Cathepsin B-like and cell death in the unicellular human pathogen Leishmania. Cell Death Dis. 2010;1:e71. doi: 10.1038/cddis.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mariño G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014;15:81–94. doi: 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vergnes B, et al. A proteomics screen implicates HSP83 and a small kinetoplastid calpain-related protein in drug resistance in Leishmania donovani clinical field isolates by modulating drug-induced programmed cell death. Mol. Cell. Proteom. 2007;6:88–101. doi: 10.1074/mcp.M600319-MCP200. [DOI] [PubMed] [Google Scholar]
- 15.Foucher AL, et al. Apoptotic marker expression in the absence of cell death in staurosporine-treated Leishmania donovani. Antimicrob. Agents Chemother. 2013;57:1252–1261. doi: 10.1128/AAC.01983-12. [DOI] [PMC free article] [PubMed] [Google Scholar]