Abstract
Rapid immune reconstitution (IR) following stem cell transplantation (SCT) is essential for a favorable outcome. The optimization of graft composition should not only enable a sufficient IR but also improve graft vs. leukemia/tumor effects, overcome infectious complications and, finally, improve patient survival. Especially in haploidentical SCT, the optimization of graft composition is controversial. Therefore, we analyzed the influence of graft manipulation on IR in 40 patients with acute leukemia in remission. We examined the cell recovery post haploidentical SCT in patients receiving a CD34+-selected or CD3/CD19-depleted graft, considering the applied conditioning regimen. We used joint model analysis for overall survival (OS) and analyzed the dynamics of age-adjusted leukocytes; lymphocytes; monocytes; CD3+, CD3+CD4+, and CD3+CD8+ T cells; natural killer (NK) cells; and B cells over the course of time after SCT. Lymphocytes, NK cells, and B cells expanded more rapidly after SCT with CD34+-selected grafts (P = 0.036, P = 0.002, and P < 0.001, respectively). Contrarily, CD3+CD4+ helper T cells recovered delayer in the CD34 selected group (P = 0.026). Furthermore, reduced intensity conditioning facilitated faster immune recovery of lymphocytes and T cells and their subsets (P < 0.001). However, the immune recovery for NK cells and B cells was comparable for patients who received reduced-intensity or full preparative regimens. Dynamics of all cell types had a significant influence on OS, which did not differ between patients receiving CD34+-selected and those receiving CD3/CD19-depleted grafts. In conclusion, cell reconstitution dynamics showed complex diversity with regard to the graft manufacturing procedure and conditioning regimen.
Keywords: immune reconstitution, allogeneic stem cell transplantation, CD34 selection, CD3/19 depletion, children
Introduction
The transplantation of allogeneic hematopoietic stem cells [stem cell transplantation (SCT)] provides a curative treatment option for patients suffering from high-risk acute leukemia and other hematological malignancies (1–4). The success of SCT is dependent on the rapid reconstitution of immune-competent cells, which is influenced by different factors (5–10). Adequate immune recovery may result in the eradication of residual malignant cells (11) and can protect the patient from infectious complications (12, 13). Allogeneic SCT is mostly performed from an HLA-matched related or unrelated donor. For patients who lack a suitable donor, a haploidentical SCT and umbilical cord blood SCT (14) serve as an alternative. Haploidentical SCT (haplo-SCT) carries an increased risk of both graft vs. host disease (GvHD) and graft rejection compared to HLA-matched SCT but shows a decreased relapse rate (2, 15–17). GvHD is mainly induced by donor T cells (18, 19). Therefore, T cells need to be depleted prior to the infusion of the haploidentical graft. Different strategies for the purification of haploidentical grafts are available, such as the positive selection of CD34+ stem cells leading to indirect T-cell depletion (2, 15, 17) and the direct depletion of CD3+ T cells or TCR alpha/beta T cells, both in combination with CD19+ B-cell removal (20–23). CD34+-selected grafts contain highly purified stem cells and can be grafted in the absence of GvHD prophylaxis (24). CD3/CD19 or TCR alpha/beta-depleted grafts contain hematopoietic progenitors, natural killer (NK) cells, and antigen-presenting cells, probably leading to an enhanced graft vs. leukemia effect (16, 20, 23).
To date, studies about cellular immune reconstitution (IR) in pediatric patients post haplo-SCT are mainly done as single center studies and have long-term monitoring intervals, leading to small and heterogeneous patient cohorts. Therefore, in a retrospective multicenter study (Frankfurt, Ulm, Wuerzburg), we examined the IR post haplo-SCT in a relatively homogeneous group of 40 pediatric patients suffering from acute leukemia. We analyzed and compared the regeneration of various leukocyte subpopulations from children receiving CD34+-selected and CD3/CD19-depleted grafts. Our study considered age-adjusted leukocytes subpopulations as well as the individual progress of IR of each patient. In this respect, we were able to set up a joint model for IR, which allowed modeling of the continuous recovery process over time post SCT.
Patients and Methods
Patients
In this retrospective study, we included 40 pediatric patients suffering from high-risk leukemia transplanted in complete remission (CR) with HLA-haploidentical peripheral blood (PB) stem cells between 1997 and 2012. Informed consent was obtained from all patients/parents, and the retrospective study was approved by the local ethic committee (Frankfurt, D Ethical No: 499/16). Inclusion criteria for patient recruitment in the three centers were as follows: (1) patients with acute lymphoblastic (ALL) or acute myeloblastic leukemia (AML), (2) a PBSC graft from a haploidentical donor processed with either positive CD34+ immunomagnetic selection (PBSCCD34sel) or CD3/CD19 depletion (PBSCCD3/CD19dep) and (3) in case of multiple transplantation, inclusion criteria requires a >6-month interval between the last and penultimate transplant. Only immune monitoring after the last transplantation was considered in these cases.
Depending on the underlying disease, either myeloablative conditioning regimens (MAC) based on total body irradiation (12 Gy TBI) or busulphan, or a reduced-intensity conditioning regimen (RIC) with fludarabine, thiotepa, and melphalan were used. The positive selection of CD34+ PB stem cells with immunomagnetic microbeads and the direct depletion of T and B cells with-coated microbeads under good manufacturing practice (GMP) were performed with-coated microbeads using a CliniMACS (Miltenyi Biotec, Bergisch-Gladbach, Germany) device under GMP as we described previously (23, 25). For prophylaxis of GvHD, OKT3 or anti-thymocyte globulin was given. Posttransplantation, the GvHD prophylaxis consisted of mycophenolate mofetil in cases of patients receiving PBSCCD3/CD19dep grafts, while for patients transplanted with PBSCCD34sel stem cells, no GvHD prophylaxis was given. The grade of acute GvHD was defined according to published consensus criteria (26). Engraftment of neutrophils was defined as the first of 3 days with a neutrophil count >500 cells/μl.
Flow Cytometric Analyses for Monitoring of IR
In all three centers, PB samples were collected regularly post haplo-SCT (>10 times/year) using EDTA tubes and analyzed the same day to monitor IR. The absolute numbers of leukocytes, CD14+ monocytes, lymphocytes, CD3+ T cells, CD3+CD4+ helper T cells, CD3+CD8+ cytotoxic T cells, CD3−CD56+ NK cells and CD19+ B cells were determined by flow cytometry in a lyse-no-wash procedure as we described previously (8, 9, 25, 27). Briefly, two tubes with 100 µl of PB were labeled with tetraCHROME (Frankfurt) or manually pipetted (Wuerzburg, Ulm) with combinational monoclonal antibody reagents for CD45/CD4/CD8/CD3 and CD45/CD56/CD19/CD3 conjugated with fluorescein isothiocyanate, phycoerythrin (PE), phycoerythrin Texas red (ECD), phycoerythrin-cyanine 5.1 (PC5), PerCp, and APC, respectively. The accredited tetraCHROME antibodies included anti-CD45 (clone: B3821F4A), anti-CD4 (clone: SFCI12T4D11), anti-CD8 (clone: SFCI21Thy2D3), anti-CD3 (clone: UCHT1), anti-CD56 (clone: N901) and anti-CD19 (clone: J3-119). Monocytes (clone: RMO52) were detected by adding phycoerythrin-cyanine 7 (PC7) labeled anti-CD14 monoclonal antibodies to the first tube or by a separate CD45/CD14 analysis. All reagents are purchased from Beckman Coulter® Immunotech (Marseilles, France) or BD/Pharmigen (Heidelberg). The samples were measured with an FC500™ (Coulter) or a FACSCalibur/Canto-II (BD) flow cytometer. Absolute cell counts were calculated from the percentage values using a dual-platform approach.
For assessment of quality control, flow-set™ fluorospheres or similar products were used to set up the photomultiplier tube values weekly. Fluorescence overlap was compensated for, and both the flow cytometer optical alignment and the fluidic stability were tested daily as we described previously. Control cells (i.e., Immuno-Trol™) were used for verification (27). During the study, the values of all controls remained within the designated limits.
Statistical Analysis
Sequential measurements of eight leukocyte subpopulations after haplo-SCT were used to compare the dynamics of IR between patient cohorts receiving PBSCCD34sel or PBSCCD3/CD19dep grafts in relation to outcome. We characterized the IR dynamics as a continuous process over time and took the strong age-dependency of IR into account (27–35) by normalizing each quantification value with the corresponding age-specific expected mean value (see Table S1 in Supplementary Material). Furthermore, the regression models used normalized levels of the leukocyte subpopulations after log-transformation to obtain an approximately Gaussian distribution for the residuals.
Then, each of the eight leukocyte subpopulations and patient survival were analyzed with a joint model for longitudinal and time-to-event data (36). Thereby, the whole recovery process over time post SCT was assessed. As immune recovery after SCT is not linear in time, the longitudinal submodel was a linear mixed-effect model using B-splines of the third order with two inner knots (37). The time-to-event outcome was overall survival (OS), which was assessed with a Cox proportional hazard submodel (38). Furthermore, the Kaplan-Meier method, standard Cox proportional hazard regression and the log-rank test were used for analyzing OS.
In addition, patient characteristics are described by median and range. Fisher’s exact test, the Wilcoxon-Mann-Whitney test and the Kruskal-Wallis test were used to compare characteristics between the two groups.
All tests were two sided, and P < 0.05 was considered significant. Statistics analysis used the R-3.2.5 (R Foundation for Statistical Computing, Vienna, Austria) with the JM package for model fitting (39) and ‘BIAS for Windows’ v11.02 (Epsilon-Verlag, Frankfurt, Germany).
Results
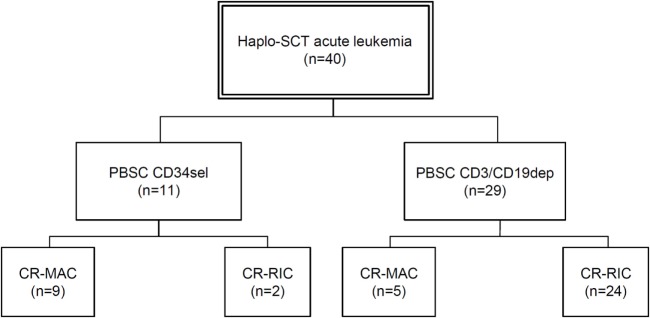
The study cohort included 40 patients, with 11 patients receiving PBSCCD34sel and 29 patients receiving PBSCCD3/CD19dep in CR at transplantation time. Table 1 and Figure 1 give detailed information about both the patients and the transplant characteristics.
Table 1.
Patient’s characteristics.
| CD34 sel | CD3/CD19 dep | P | |
|---|---|---|---|
| n = 11 | n = 29 | ||
| SCT time period | August 1997–January 2005 | April 2005–March 2012 | |
| IR follow-up, median (range) (months) | 5.7 (2.3–160.3) | 8 (1.4–138) | |
| Survival follow-up, median (range) (months) | 149 (72–160.3) | 101 (44–138) | |
| Patient-related factors | |||
| Age at SCT, median (range) years | 7.5 (3.5–23) | 10.6 (1.3–26) | 0.437 |
| Sex (male/female) | 8/3 | 20/9 | 1 |
| BMI at SCT, median (range) kg/m2 | 17.9 (14.3–20.2) | 17.3 (11.9–28.7) | 0.802 |
| Disease-related factors | |||
| Diagnosis | 0.158 | ||
| AML | 2 | 13 | |
| M0/M1/M2/M4/M5/M6/M7 | 0/0/1/1/0/0/0 | 2/2/3/1/2/1 | |
| No data | 2 | ||
| ALL | 9 | 16 | |
| T-ALL/BCP-ALL/Bipheno-ALL | 3/6/0 | 7/7/2 | |
| Status at SCT | |||
| CR1/CR2/ ≥ CR3 | 2/5/4 | 8/12/9 | 0.820 |
| Time from diagnosis to SCT, median (range) years | 2.4 (0.7–4.2) | 1.3 (0.3–5.7) | 0.417 |
| Donor-related factors | |||
| Age, median (range) years | 38 (28–42) | 37 (24–51) | 0.628 |
| Sex (male/female) | 9/2 | 16/13 | 0.158 |
| Patient–donor sex | 0.547 | ||
| Female–female | 1 | 5 | |
| Male–male | 7 | 12 | |
| Male–female | 1 | 8 | |
| Female–male | 2 | 4 | |
| ABO compatibility | |||
| Compatible | 1 | 20 | |
| Incompatible | 3 | 7 | |
| No data | 7 | 2 | |
| CMV status (recipient–donor) | |||
| Positive–positive | 2 | 11 | |
| Negative–positive | – | 9 | |
| Positive–negative | – | 1 | |
| Negative–Negative | 2 | 4 | |
| No data | 7 | 4 | |
| Transplanted-related factors | |||
| SCT-number | 0.732 | ||
| First/second/third | 2/5/4 | 8/12/9 | |
| Conditioning regimen | <0.001a | ||
| Standard myeloablative | 9 | 5 | |
| TBI-based | 6 | 4 | |
| Chemo-based | 3 | 1 | |
| Reduced myeloablative | 2 | 24 | |
| Flud/TT/Mel | 2 | 24 | |
| Serotherapy | 0.005 | ||
| Without | – | 2 | |
| ATG | 8 | 5 | |
| OKT3 | 3 | 21 | |
| Graft vs. host disease prophylaxis | <0.001 | ||
| Without | 11 | 3 | |
| MMF | – | 25 | |
| CSA, MTX | – | 1 | |
| Graft’s composition | |||
| CD34+, median (range) × 106/kg BW | 15.3 (4.9–36.9) | 10.1 (4.8–20.7) | 0.009 |
| CD3+, median (range) × 103/kg BW | 1.0 (0.4–3) | 8.9 (0–5,000) | <0.001 |
| Treatment post-SCT | |||
| DLI | 3 | 12 | 0.415 |
| Number of DLI infusions 1/2/3/4/5 | 1/0/0/0/2 | 5/1/3/2/1 | |
SCT, stem cell transplantation; IR, immune reconstitution; BMI, body mass index; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; M0, AML minimally differentiated; M1, AML without maturation; M2, AML with granulocytic maturation; M4, acute myelomonocytic leukemia; M5, acute monoblastic leukemia or acute monocytic leukemia; M6, acute erythroid leukemia; M7, acute megakaryoblastic leukemia; T-ALL, T cell ALL; BCP-ALL, B-cell precursor; B pheno ALL, biphenotypic/bilinear ALL; CR1, first complete remission; CR2, second complete remission; CR3, third complete remission; CMV, cytomegalovirus; TBI, total body irradiation; Flud, fludarabine; TT, thiotepa; Mel, melphalan; ATG, antithymocyte globuline; OKT3, anti-CD3 antibody; MMF, mycophenolate mofetil; MTX, methotrexate; CSA, cycloyporin A; BW, body weight.
aP-value for comparison of standard vs. reduced myeloablative conditioning regimen.
Figure 1.
Flow chart with patient classification. Abbreviations: Haplo-SCT, haploidentical stem cell transplantation; PBSC, peripheral blood stem cells; CD34sel, CD34+ selection; CD3/CD19dep, CD3/CD19+ depletion; CR, complete remission; MAC, myeloablative conditioning; RIC, reduced-intensity conditioning.
Dynamics of Immune Recovery
Our joint modeling approach primarily compared two graft manipulations (PBSCCD34sel vs. PBSCCD3/CD19dep) considering the conditioning regimen (MAC vs. RIC).
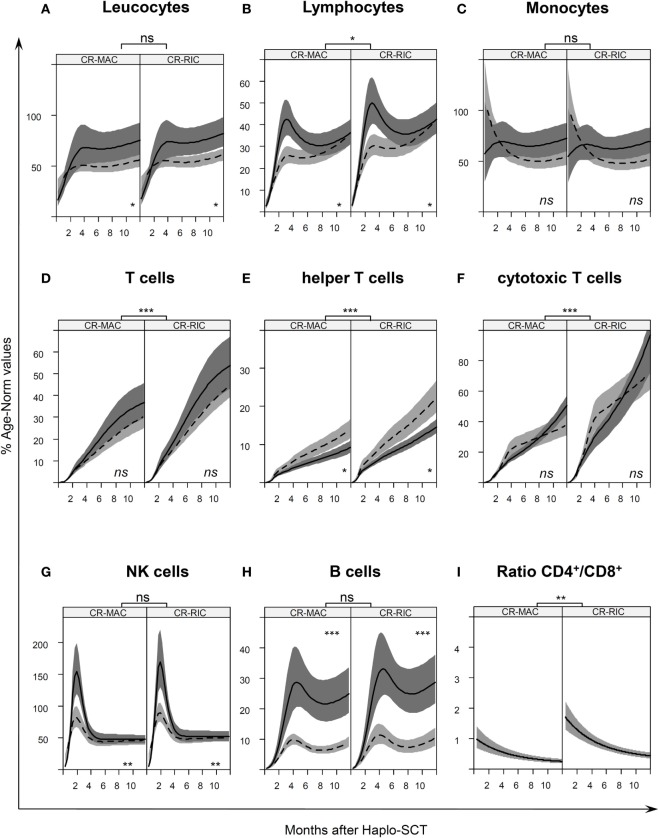
We fitted a joint model for OS and each of the following leukocyte subsets: overall leukocytes, lymphocytes, CD14+ monocytes, CD3+ T cells, CD3+CD4+ helper T cells, CD3+CD8+ cytotoxic T cells, CD3−CD56+ NK cells, and CD19+ B cells, as shown in Figure 2. Such modeling allowed a comparison of leukocyte subset recovery after PBSCCD34sel and PBSCCD3/CD19dep transplantations. Tables 2 and 3 present the respective immune recovery prognosis values of our models showing the predicted percent values (% of norm values) and their 95% confidence intervals on days +30, +60, +90, and +180 and +365 post haplo-SCT for children in CR after SCT. As an example, predicted values for the absolute numbers with 95% CI are shown for a 10-year-old child (Figure S1 and Tables S2A,B in Supplementary Material).
Figure 2.
Age-normalized immune reconstitution (IR) in the first year after haplo-stem cell transplantation (SCT) IR normalized by age-specific norm values (27) Table S1 in Supplementary Material, of (A) leukocytes, (B) lymphocytes, (C) monocytes, (D) T cells, (E) helper T cells, (F) cytotoxic T cells, (G) natural killer, and (H) B cells in the first year after transplantation. The curves illustrate the splines of the longitudinal trajectory of IR after SCT based on our joint models with point wise 95% confidence regions. The CR-MAC and CR-RIC panels (left and right panel, respectively) exhibit the predicted IR for patients in any remission prior to SCT according to the type of graft PBSCCD34sel (solid line with dark gray confidence regions) or PBSCCD3/CD19dep (dashed line with light gray confidence regions). (I) CD4/CD8 ratio of the absolute cell count of CD4+ helper T cells and CD8+ cytotoxic T cells at different time points after SCT. See also Figure S1 in Supplementary Material for a representation of absolute numbers for predicted values for a 10-year-old child. ns, not significant; *P < 0.05; **P < 0.01; ***P < 0.001.
Table 2.
Predicted cells immune reconstitution (% norm values) in patients who received myeloablative conditioning regimen.
| Cells | Day + 30 |
Day + 60 |
Day + 90 |
Day + 180 |
Day + 365 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD34sel | CD3/CD19dep | CD34sel | CD3/CD19dep | CD34sel | CD3/CD19dep | CD34sel | CD3/CD19dep | CD34sel | CD3/CD19dep | |
| Leukocytes | 34.22 | 39.98 | 52.49 | 47.05 | 64.26 | 50.23 | 66.80 | 49.21 | 75.69 | 56.14 |
| (25.94–45.15) | (34.91–45.79) | (38.82–70.98) | (41.29–53.60) | (47.11–87.64) | (44.15–57.14) | (53.09–84.04) | (43.78–55.31) | (61.47–93.19) | (49.47–63.72) | |
| Lymphocytes | 15.69 | 12.75 | 35.20 | 21.93 | 42.23 | 25.75 | 31.67 | 25.05 | 36.43 | 36.18 |
| (12.39–19.86) | (10.80–15.04) | (28.66–42.23) | (18.68–25.75) | (34.98–50.99) | (22.13–29.98) | (27.21–36.85) | (21.72–28.90) | (31.11–42.67) | (31.36–41.74) | |
| Monocytes | 64.81 | 86.29 | 68.52 | 71.44 | 68.99 | 62.25 | 64.91 | 51.04 | 72.76 | 54.14 |
| (48.21–87.14) | (72.61–102.56) | (53.69–87.44) | (60.32–84.61) | (53.85–88.39) | (52.62–73.64) | (54.22–77.71) | (44.01–59.19) | (60.36–87.71) | (46.34–63.26) | |
| CD3+ | 1.18 | 1.15 | 5.23 | 4.90 | 9.13 | 7.98 | 21.05 | 16.35 | 36.98 | 30.94 |
| (0.82–1.69) | (0.91–1.45) | (3.76–7.30) | (3.91–6.15) | (6.98–11.94) | (6.54–9.73) | (16.80–26.38) | (13.48–19.82) | (29.83–45.85) | (25.31–37.81) | |
| CD4+ | 0.91 | 1.32 | 2.32 | 3.22 | 3.10 | 4.44 | 5.15 | 7.68 | 9.27 | 14.08 |
| (0.72–1.16) | (0.94–1.86) | (1.90–2.85) | (2.46–4.21) | (2.59–3,72) | (3.63–5.44) | (4.29–6.18) | (6.18–9.54) | (7.79–11.04) | (11.81–16.77) | |
| CD8+ | 2.24 | 1.52 | 7.13 | 7.22 | 11.91 | 15.85 | 22.13 | 26.30 | 51.33 | 37.96 |
| (1.82–2.76) | (1.0–2.33) | (6.17–8.24) | (5.48–9.52) | (10.47–13.56) | (12.80–19.63) | (18.57–25.01) | (21.21–32.62) | (45.58–57.80) | (31.36–45.94) | |
| CD3−CD56+ | 88.93 | 65.10 | 145.31 | 78.72 | 85.19 | 58.80 | 47.80 | 44.25 | 47.66 | 46.51 |
| (68.93–115.23) | (53.59–79.07) | (114.59–184.27) | (65.0–94.34) | (72.35–100.31) | (49.56–69.77) | (40.37–56.60) | (37.23–52.59) | (41.79–54.35) | (39.65–54.55) | |
| CD19+ B | 2.82 | 1.71 | 10.57 | 5.09 | 21.46 | 8.59 | 24.52 | 7.30 | 25.49 | 9.35 |
| (1.86–4.26) | (1.40–2.09) | (7.12–15.70) | (4.23–6.13) | (14.45–31.89) | (7.10–10.40) | (18.55–32.40) | (6.16–8.65) | (18.98–34.24) | (7.77–11.26) | |
The table shows the predicted percent values (% of the norm values) arise from our joint model with 95% confidence interval at +30, +60, +90, +120, and +365 days after transplantation. The values were obtained for children in complete remission (CR) at time point of haplo-SCT. The percent values represent the fraction with respect to the predicted age-matched norm values by Huenecke et al. (27). See also Table S2A in Supplementary Material for a representation of absolute numbers for predicted values for a 10-year-old child.
Table 3.
Predicted cells immune reconstitution (% norm) in patients who received reduce-intensity conditioning regimen.
| Cells | Day + 30 |
Day + 60 |
Day + 90 |
Day + 180 |
Day + 365 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD34sel | CD3/CD19dep | CD34sel | CD3/CD19dep | CD34sel | CD3/CD19dep | CD34sel | CD3/CD19dep | CD34sel | CD3/CD19dep | |
| Leukocytes | 37.24 | 43.50 | 57.12 | 51.19 | 69.92 | 54.65 | 72.68 | 53.54 | 82.35 | 61.09 |
| (28.97–47.87) | (38.83–48.73) | (43.79–74.50) | (45.61–57.46) | (53.28–91.75) | (48.60–61.45) | (60.20–87.75) | (48.42–59.21) | (69.19–98.02) | (55.66–67.05) | |
| Lymphocytes | 18.39 | 14.94 | 41.27 | 25.71 | 49.52 | 30.20 | 37.13 | 29.38 | 42.72 | 42.42 |
| (14.35–23.57) | (12.68–17.62) | (32.9–51.77) | (21.8–30.34) | (40.17–61.03) | (25.72–35.45) | (31.71–43.48) | (25.17–34.28) | (36.15–50.48) | (36.78–48.94) | |
| Monocytes | 62.16 | 82.76 | 65.72 | 68.52 | 66.17 | 59.70 | 62.26 | 48.95 | 69.79 | 51.93 |
| (46.53–83.05) | (70.60–97.03) | (51.35–84.11) | (58.33–80.49) | (51.42–85.15) | (50.74–70.25) | (52.58–73.71) | (42.50–56.38) | (58.19–83.69) | (43.35–59.46) | |
| CD3+ | 1.72 | 1.68 | 7.65 | 7.17 | 13.35 | 11.66 | 30.77 | 23.90 | 54.07 | 45.23 |
| (1.2–2.45) | (1.4–2.01) | (5.49–10.66) | (5.97–8.61) | (10.24–17.40) | (10.04–13.55) | (24.79–38.19) | (20.74–27.54) | (43.42–67.32) | (39.53–51.75) | |
| CD4+ | 1.45 | 2.10 | 3.70 | 5.13 | 4.94 | 7.07 | 8.20 | 12.23 | 14.77 | 22.41 |
| (1.18–1.79) | (1.49–2.98) | (3.11–4.41) | (3.91–6.72) | (4.25–5.74) | (5.78–8.64) | (7.06–9.53) | (9.86–15.16) | (13.01–16.76) | (18.57–27.05) | |
| CD8+ | 4.28 | 2.91 | 13.62 | 13.80 | 22.76 | 30.28 | 42.26 | 50.25 | 98.05 | 72.51 |
| (3.29–5.56) | (1.91–4.43) | (11.07–16.77) | (10.51–18.12) | (18.76–27.62) | (24.53–37.37) | (34.92–51.16) | (40.89–61.75) | (80.66–119.18) | (61.50–85.49) | |
| CD3−CD56+ | 97.35 | 71.26 | 159.07 | 86.18 | 93.26 | 64.37 | 52.33 | 48.44 | 52.17 | 50.91 |
| (74.37–127.42) | (60.81–83.50) | (124.26–203.63) | (73.38–101.21) | (78.73–110.5) | (55.62–74.5) | (44.01–62.21) | (41.68–56.29) | (44.83–60.72) | (45.37–57.13) | |
| CD19+ B | 3.24 | 1.97 | 12.15 | 5.85 | 24.68 | 9.88 | 28.18 | 8.40 | 29.31 | 10.75 |
| (2.18–4.81) | (1.44–2.68) | (8.41–17.55) | (4.35–7.87) | (17.12–35.56) | (7.36–13.27) | (21.97–36.16) | (6.39–11.03) | (22.38–38.37) | (8.07–14.32) | |
The table shows the predicted percent values (% of the norm values) arise from our joint model with 95% confidence interval at +30, +60, +90, +120, and +365 days after transplantation. The values were obtained for children in complete remission (CR) at time point of haplo-SCT. The percent values represent the fraction with respect the predicted age-matched norm values by Huenecke et al. (27). See also Table S2B in Supplementary Material for a representation of absolute numbers for predicted values for a 10-year-old child.
Impact of Both Conditioning Regimen and Graft on IR of Patients in CR
The majority of the 29 patients transplanted in CR with a CD3/CD19-depleted graft received reduced intensity conditioning, while the majority of the 11 patients transplanted in CR with a CD34-selected graft underwent full myeloablative conditioning (see Figure 1 for full details). To separate the influence of conditioning regimen and graft manipulation, all four scenarios (CR-MAC PBSCCD34sel, CR-MAC PBSCCD3/CD19dep, CR-RIC PBSCCD34sel, and CR-RIC PBSCCD3/CD19dep) were compared.
The increase in overall leukocytes was similar in the RIC and MAC patients during the first year (Figure 2A). However, within the same conditioning regimen group, faster leukocyte proliferation was found in patients with PBSCCD34sel grafts than in patients with PBSCCD3/CD19dep grafts from the 6 months after SCT. Previously, the leukocyte populations were comparable between the two groups with graft manipulation.
The overall lymphocytes in patients receiving PBSCCD34sel grafts differed from those with PBSCCD3/CD19dep grafts, also showing a faster recovery in the early phase until the sixth month after haplo-SCT (Figure 2B, P = 0.036). This was mainly due to a faster reconstitution of NK cells and B cells in patients receiving PBSCCD34sel vs. PBSCCD3/CD19dep grafts. Afterward, the course of lymphocyte values was comparable in both groups. Concerning the conditioning regimen, we observed a faster expansion of the overall lymphocytes in the RIC group than in the MAC group. At the end of the first year, the RIC and MAC groups reached approximately 42 and 36% of the normal reference value, respectively.
Monocyte expansion was similar in RIC and MAC recipients, but the number of cells did not exhibit significant differences with respect to graft purification. For the overall monocytes, we observed a rapid IR, with approximately 80% of their age norm value already at 30 days after SCT (Figure 2C).
CD3+ T cells, CD3+CD4+ helper T cells, and the CD3+CD8+ cytotoxic T cells recovered significantly quicker in the patients receiving the RIC regimen than in those patients receiving MAC regimen (Figures 2D–F, P < 0.001). In the RIC group, the frequency values were nearly 1.5 times higher at each time point compared to the respective kind of manipulation receiving the MAC regimen during the first year post haplo-SCT. No significant differences were detected in the recovery of CD3+ T cells and the CD3+CD8+ cytotoxic T cells owing to the kind of graft purification, PBSCCD34sel, and PBSCCD3/CD19dep, among patients undergoing the same regimen of conditioning. The CD3+ T cells increased from 1% of the reference values at day 30 to 37% at the end of the first year and from 1 to 31% for PBSCCD34sel and PBSCCD3/CD19dep, respectively, with both groups receiving MAC (Table 2). In patients with the RIC regimen, the CD3+ T cells increased from 1.7% of the reference values at day 30 to 54% at the end of the first year and from 1 to 45% for PBSCCD34sel and PBSCCD19dep, respectively (Table 3).
In contrast, the CD3+CD4+ helper T cells recovered significantly delayed in patients receiving PBSCCD34sel grafts than in those transplanted with PBSCCD3/CD19dep grafts from approximately 6th to 24th months after transplantation for both the RIC and MAC regimens (P < 0.01).
In both the RIC and MAC groups, the ratio of CD4+/CD8+ trended downward because the cytotoxic T cells recovered faster than the helper T cells (Figure 2I).
As expected, in vivo NK expansion post haplo-SCT was very rapid (Figure 2G). No differences in the NK cell recovery were found between the RIC and MAC groups. Interestingly, in the first 4 months, NK cell recovery was faster in patients receiving PBSCCD34sel grafts than in those transplanted with a PBSCCD3/CD19dep graft (P = 0.002). Afterward, the in vivo NK cell proliferation was very similar between the patient groups.
Recovery of CD19+ B cells showed no differences in the IR regarding the conditioning regimen (Figure 2H). With regard to graft manipulation, B-cell recovery was faster in the PBSCCD34sel patient group than in the PBSCCD3/CD19dep group (P < 0.001). This difference was very pronounced in the first year after haplo-SCT. At the end of the first year, the B cells in the group PBSCCD34sel with RIC had already achieved 30% of the age-matched reference values. In contrast, in the PBSCCD3/CD19dep with MAC group, the B cells had only reached 9% of the age reference values.
Clinical Outcome and Survival
The medians (range) OS follow-up time were 149 (72–160.3) and 101 (44–138) months in patients receiving PBSCCD34se and PBSCCD3/CD19dep, respectively.
The incidence of aGvHD grades I and II was 45% (5/11) in patients with PBSCCD34sel grafts and 48% (14/29) in patients with PBSCCD3/CD19dep grafts. The incidence of aGvHD grades III and IV was 18% (2/11) and 14% (4/29) in the cohorts with PBSCCD34sel vs. PBSCCD3/CD19dep grafts, respectively. The incidence of aGvHD did not significantly differ between both groups. Table 4 presents details of the clinical outcome.
Table 4.
Clinical outcome.
| CD34 sel | CD3/CD19 dep | P | |
|---|---|---|---|
| n = 11 | n = 29 | ||
| Engraftment | |||
| Neutrophils engraftment | 0.054 | ||
| Median (range) days | 17 (11–22) | 12 (10–24) | |
| Graft vs. host disease | |||
| Acute GvHD | 1 | ||
| Without | 4 | 11 | |
| Grade I/II | 2/3 | 12/2 | |
| Grade III/IV | 1/1 | 3/1 | |
| Site of aGVHD | |||
| Skin | 4 | 13 | |
| Skin, GI | 1 | 2 | |
| Skin, GI, liver | – | 1 | |
| Skin, GI, lung | 1 | 2 | |
| No data | 1 | 0 | |
| Chronic GvHD | 3 | 2 | 0.117 |
| Cause of death | |||
| Relapse | 4 | 9 | |
| Treatment-related mortality | 3 | 5 | |
GvHD, graft vs. host disease; GI, gastrointestinal. P-value was calculated using Fisher-Exact test or Mann–Whitney–Wilcoxon test.
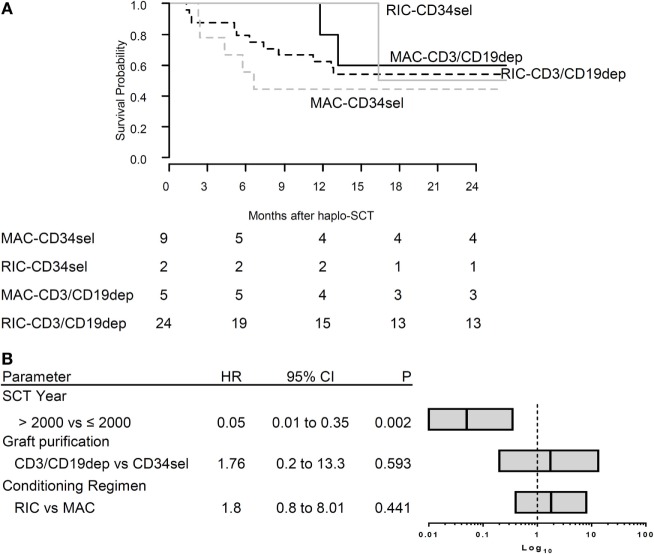
Overall, 21/40 patients died. The main cause of death was relapse (n = 13), followed by non-relapse mortality (NRM, n = 8). The Kaplan–Meier curves for OS were compared between the different groups (Figure 3A). The log rank test was not significant for differences between the PBSCCD34sel and the PBSCCD3/CD19dep groups (Table S3 in Supplementary Material). Likewise, there were no significant differences in the causes of death between the PBSCCD34sel and the PBSCCD3/CD19dep patient groups.
Figure 3.
(A) Survival overall survival (OS). The Kaplan–Meier curve of OS for CR patients according to type of graft (CD34sel, CD3/CD19dep) combined with conditioning regimen (MAC, RIC). (B) Multivariable analysis of clinical characteristics affecting OS. The plot shows the hazard ratio (HR) of multivariable Cox regression. The stem cell transplantation year was significant correlated with OS. The bar in each box represents the HR, the box the corresponding 95% confidence interval. The vertical line represents HR = 1 for reference.
Using standard Cox regression, we analyzed the association between transplant-associated factors and the probability of OS without considering the evolution of IR after haplo-SCT. In the univariable analysis, age, gender, diagnosis, TBI (yes/no), conditioning regimen, graft purification, graft composition, and engraftment day were not associated with OS. In the multivariable regression considering graft purification, conditioning regimen and year of transplantation (≤2001 vs. >2001), only the year of transplantation (P = 0.002) significantly influenced OS. The conditioning regimen had no significant impact on OS in our cohort (P = 0.441) (Figure 3B).
Discussion
To date, there are ongoing discussions about optimization of graft composition in haplo-SCT in order to reach a sufficient IR with an additional goal to improve the GVL/GVT effect and, finally, the survival of the patients. Various factors affecting IR after SCT, especially haplo-SCT (5–10), have been identified, such as age, diagnosis, stage of disease, remission status at transplantation, conditioning regimen, HLA-typing, and graft manipulation (6, 40–42).
Investigation of IR post SCT, especially haplo-SCT, has been done in single-center studies, mostly in heterogeneous patient groups using univariate analyses. Here, we compared the dynamics of IR in patients receiving PBSCCD34sel vs. PBSCCD3/CD19dep when used for haplo-SCT in a homogeneous pediatric patient group with acute leukemia from a retrospective multicenter study. To investigate the complexity of various factors on IR, we were able to develop joint models for longitudinal and time-to-event data. To our knowledge, this is the first time that the influence of multivariable clinical factors, including RIC, MAC, and age-related dependence of leukocytes and their subpopulations, has been evaluated in detail in haplo-SCT from pediatric patients suffering leukemia.
Surprisingly, IR for T cells did not differ between patients receiving PBSCCD34sel or PBSCCD3/CD19dep grafts with the exception of CD4+ helper T cells, which showed much slower recovery after transplantation using a PBSCCD34selp graft. NK and B cells had a faster reconstitution after transfusion of the PBSCCD34sel graft. In this respect, it has to be noted that direct comparisons of IR after transplantation with PBSCCD3/CD19dep compared with PBSCCD34sel grafts have to be handled with caution because patients receiving the latter normally do not need immunosuppressive therapy. However, the most important factor for T-cell and T subsets recovery was the conditioning regimen. For patients in CR receiving either PBSCCD34sel or PBSCCD3/CD19dep grafts, IR was much faster after the RIC regimen than after the MAC regimen. However, it has to be mentioned that the main limitations of our study are its retrospective nature and the small number of patients, although it is a multicenter study.
Comparison of our results with other haploidentical pediatric studies in the literature showed that most of the other studies about IR were using very heterogeneous patient groups (43–45) with only some of them treated with haplo-SCT (2, 46–52) or analyzed IR including patients with PR/NR (45, 53). Other investigations are not comparable with our study due to the administration of fixed addback (54, 55), or reported about other malignant (56) or nonmalignant diseases (57).
A high dose of CD34 cells is required in the haploidentical setting for a successful transplant and therefore rapid and sustained engraftment (58). Despite the differences in the CD34 doses between the CD34 selected group and the CD3/CD19-depleted group, no differences in the neutrophil engraftment or aGVHD was found.
In contrast to our findings that the IR of the CD3+ T cells and CD8+ cytotoxic T cells was comparable between recipients receiving haplo-PBSCCD3/CD19dep and haplo-PBSCCD34sel if both groups received the same conditioning regimen, other researchers reported slower expansion of T cells, CD4+, and CD8+ cells in recipients receiving mismatch/haplo-PBSCCD34sel than in recipients receiving haplo-PBSCCD3/CD19dep (41, 49). A possible reason for this discrepancy may be that the mentioned studies did not differentiate between the influences of the graft and the conditioning on IR. The majority of patients received PBSCCD34sel combined with the MAC regimen or PBSCCD3/CD19dep combined with the RIC regimen. When we compared the IR between the PBSCCD34sel vs. PBSCCD3/CD19dep without considering conditioning, our results were consistent with these previous findings (data not shown).
In our study, there was no difference in the CD3+CD4+ helper T-cell recovery between patients receiving depleted or selected grafts until the sixth month after haplo-SCT. Therefore, it seems unlikely that better expansion of helper T cells at late time points is explained by residual T cells in the CD3/CD19-depleted graft. However, after the sixth month, the CD3+CD4+ helper T cells recovered significantly faster in patients receiving CD3/CD19-depleted grafts than in those receiving CD34-selected grafts, regardless of the type of conditioning. This could also be the effect of other therapeutic agents applied combined with the CD3/CD19-depleted graft.
It is well known that RIC facilitates rapid reconstitution of T cells and its subsets in PBSC transplantation. However, the RIC method is often not strong enough to eradicate the malignant cells, and it is associated with a higher incidence of mixed chimerism (59), higher relapse, and mortality risk (60). After SCT, non-remission rates found in studies with patients who received haplo-PBSCCD3/CD19dep combined with an RIC regimen were 50.2% (61), 68% (45), and 57% (53), but note that 31 of 61 adult patients did not achieve CR in the first study, 22 of 26 analyzed childhood patients in the second study suffered from malignancies, and 43% of patients from the third study had active disease at the time of transplantation.
Surprisingly, we observed higher expansion of NK cells in the early phase of SCT in patients receiving a PBSCCD34sel graft than in those patients who received a PBSCCD3/CD19dep graft, despite the fact that PBSCCD3/CD19dep grafts contain considerably higher numbers of NK cells. Furthermore, we did not find any differences between the immune recoveries of NK cells when comparing positive selection or negative depletion with conditioning preparative treatments MAC vs. RIC. Our results about NK expansion in the PBSCCD34sel combined with MAC are in conformity with the study from Lang et al. (50). In that trial (n = 47), most of the pediatric patients suffered from acute leukemia (n = 37; 78%) were transplanted from a haploidentical donor (n = 29; 62%) and underwent haplo-PBSCCD34sel after MAC. The NK cells shown a pick with a median of 300 cells/μl reported by Lang et al, we found a peak with a median of 323 cells/μl as shown exemplarily for a 10-year-old recipient (Table S2A in Supplementary Material).
Pfeiffer et al. reported a comparable NK cell reconstitution between patients receiving a haplo-PBSCCD3/CD19dep graft with RIC and those patients receiving mismatch-PBSCCD34sel grafts with MAC in pediatric patients. They found that only at day +14 was there a significant difference advantageous for PBSCCD3/CD19dep recipients (45). However, the HLA-disparity and related donors were different between the PBSCCD34sel groups in our and the mentioned study, and the latter included heterogeneous diseases with 64% cases of acute leukemia in the mismatch-PBSCCD34sel group and only 42% cases of acute leukemia in the haplo-PBSCCD3/CD19dep group. Additionally, Pfeiffer reported a rate of 42% with active diseases (PR/NR) prior to SCT in the CD3/CD19-depleted group, while our study included patients in CR only.
Significantly higher NK cell proliferation in patients receiving PBSCCD3/CD19dep grafts than in patients undergoing PBSCCD34sel (both groups combined with RIC) was presented by Gonzalez-Vicent et al. (51). However, in this investigation, 74% of patients with PBSCCD34sel had a matched donor, while all patients with PBSCCD3/CD19dep received a transplant from mismatched donors.
Alternatively, unmanipulated blood and marrow transplantation is another feasible option in haploidentical transplantations. Marek et al. (62) examined lymphocyte recovery of haploidentical in vivo (n = 19) vs. in vitro T-cell depletion (n = 53, 89% haploidentical donors, 81% acute leukemia, 76% children). The in vitro T-cell depletion was mostly PBSCCD34sel (94%) with the MAC regimen (78%). They reported faster IR not only of the NK cells but also of all lymphocyte subpopulations in patients receiving grafts with in vitro T-cell depletion than observed in our results. Nevertheless, they used a MAC regimen based on cyclophosphamide + TBI instead of VP16 + TBI as in our study.
There are no studies comparing B-cell recovery in pediatric patients who received RIC vs. MAC regimens after haplo-SCT. However, in ALL childhood patients, the chemotherapy strongly affects B cells (63). In adult studies, there is no consensus about this point (7, 64–67).
Concerning the graft manipulation technique, similar to our results, Bethge et al. (20) reported faster B-cell expansion in adult patients receiving haplo-PBSCCD34sel than in those who received haplo-PBSCCD3/CD19dep grafts. The influence of serotherapy on the IR especially on the B cells is reported in several studies (68–70). In our study, the size of the cohort did not allow the inclusion of this factor in our model.
Booth et al. reported the unpublished observations from Lawson and Darbyshire (71). They found no significant difference between the CD34 selection compared to CD3/CD19 depletion in terms of engraftment, IR, viral infections, or non-relapse mortality.
Conclusion
We analyzed a homogeneous pediatric group suffering from high-risk acute leukemia in CR, who underwent in vitro haplo-PBSCT in a retrospective study. A limitation of our study is the small number of patients, although it is a multicenter study.
Comparing transplantation with CD34-positive selected and CD3/CD19-depleted grafts patients did not show marked differences in the IR, while differences were stronger between the conditioning regimens. For further optimization of the treatment of acute childhood leukemia, conditioning regimen, graft purification, and their interplay should always be considered, particularly for novel techniques of graft manipulation and engineering, such as TCR alpha/beta depletion and in vivo T-cell depletion, as well as genetically modified T-cell products.
Ethics Statement
Informed consent was obtained from all patients/parents, and the retrospective study was approved by the respective local ethic committee (Frankfurt EK 499/16, 50/07), Würzburg (EK 133/04) and has been included in part in the registered studies for both, transplantation with CD3/CD19 depleted grafts (EudraCT-No.: 2006-000393-76) as well as the ALL-SCT study (register No. 3352).
Author Contributions
Study design and wrote the manuscript: UK, EH, and ES-M. Provided clinical data: ME, AS, and PB. Analyzed the data: RE, MB, and SH. Coordinated the research: UK and EH. Organized and data collection: ES-M, MS, and MB. Performed statistical analyses: ES-M. Revised the manuscript: UK, EH, SH, MB, ME, and PB. Supervised the research: EH, UK, and TK. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Sibille Betz, Stephanie Erben, Olga Zimmermann, and Andrea Quaiser for the excellent technical support. We thank Gudrun Sachs for the expert data management.
Footnotes
Funding. This project was supported by “Frankfurter Stiftung für krebskranker Kinder”, “Hilfe für krebskranke Kinder e.V.” and BMF FKZ 01FP09120B. In addition, part of the work was funded by the Integrated Research and Treatment Center Transplantation (IFB-Tx, Ref. No. 01EO0802 and 01EO1302). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fimmu.2018.01841/full#supplementary-material.
References
- 1.Devine SM, Adkins DR, Khoury H, Brown RA, Vij R, Blum W, et al. Recent advances in allogeneic hematopoietic stem-cell transplantation. J Lab Clin Med (2003) 141:7–32. 10.1067/mlc.2003.5 [DOI] [PubMed] [Google Scholar]
- 2.Klingebiel T, Handgretinger R, Lang P, Bader P, Niethammer D. Haploidentical transplantation for acute lymphoblastic leukemia in childhood. Blood Rev (2004) 18:181–92. 10.1016/S0268-960X(03)00063-8 [DOI] [PubMed] [Google Scholar]
- 3.Barrett AJ, Horowitz MM, Pollock BH, Zhang MJ, Bortin MM, Buchanan GR, et al. Bone marrow transplants from HLA-identical siblings as compared with chemotherapy for children with acute lymphoblastic leukemia in a second remission. N Engl J Med (1994) 331:1253–8. 10.1056/NEJM199411103311902 [DOI] [PubMed] [Google Scholar]
- 4.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood (1990) 75:555–62. [PubMed] [Google Scholar]
- 5.Storek J, Dawson MA, Storer B, Stevens-Ayers T, Maloney DG, Marr KA, et al. Immune reconstitution after allogeneic marrow transplantation compared with blood stem cell transplantation. Blood (2001) 97:3380–9. 10.1182/blood.V97.11.3380 [DOI] [PubMed] [Google Scholar]
- 6.Kalwak K, Gorczyńska E, Toporski J, Turkiewicz D, Slociak M, Ussowicz M, et al. Immune reconstitution after haematopoietic cell transplantation in children: immunophenotype analysis with regard to factors affecting the speed of recovery. Br J Haematol (2002) 118:74–89. 10.1046/j.1365-2141.2002.03560.x [DOI] [PubMed] [Google Scholar]
- 7.Morecki S, Gelfand Y, Nagler A, Or R, Naparstek E, Varadi G, et al. Immune reconstitution following allogeneic stem cell transplantation in recipients conditioned by low intensity vs myeloablative regimen. Bone Marrow Transplant (2001) 28:243–9. 10.1038/sj.bmt.1703118 [DOI] [PubMed] [Google Scholar]
- 8.Koehl U, Bochennek K, Zimmermann SY, Lehrnbecher T, Sörensen J, Esser R, et al. Immune recovery in children undergoing allogeneic stem cell transplantation: absolute CD8+ CD3+ count reconstitution is associated with survival. Bone Marrow Transplant (2007) 39:269–78. 10.1038/sj.bmt.1705584 [DOI] [PubMed] [Google Scholar]
- 9.Koenig M, Huenecke S, Salzmann-Manrique E, Esser R, Quaritsch R, Steinhilber D, et al. Multivariate analyses of immune reconstitution in children after allo-SCT: risk-estimation based on age-matched leukocyte sub-populations. Bone Marrow Transplant (2010) 45:613–21. 10.1038/bmt.2009.204 [DOI] [PubMed] [Google Scholar]
- 10.Schwinger W, Weber-Mzell D, Zois B, Rojacher T, Benesch M, Lackner H, et al. Immune reconstitution after purified autologous and allogeneic blood stem cell transplantation compared with unmanipulated bone marrow transplantation in children. Br J Haematol (2006) 135:76–84. 10.1111/j.1365-2141.2006.06244.x [DOI] [PubMed] [Google Scholar]
- 11.Powles R, Singhal S, Treleaven J, Kulkarni S, Horton C, Mehta J. Identification of patients who may benefit from prophylactic immunotherapy after bone marrow transplantation for acute myeloid leukemia on the basis of lymphocyte recovery early after transplantation. Blood (1998) 91:3481–6. [PubMed] [Google Scholar]
- 12.Daley JP, Rozans MK, Smith BR, Burakoff SJ, Rappeport JM, Miller RA. Retarded recovery of functional T cell frequencies in T cell-depleted bone marrow transplant recipients. Blood (1987) 70:960–4. [PubMed] [Google Scholar]
- 13.Einsele H, Ehninger G, Steidle M, Fischer I, Bihler S, Gerneth F, et al. Lymphocytopenia as an unfavorable prognostic factor in patients with cytomegalovirus infection after bone marrow transplantation. Blood (1993) 82:1672–8. [PubMed] [Google Scholar]
- 14.Castillo N, García-Cadenas I, Barba P, Canals C, Díaz-Heredia C, Martino R, et al. Early and long-term impaired T lymphocyte immune reconstitution after cord blood transplantation with antithymocyte globulin. Biol Blood Marrow Transplant (2017) 23(3):491–7. 10.1016/j.bbmt.2016.11.014 [DOI] [PubMed] [Google Scholar]
- 15.Aversa F, Velardi A, Tabilio A, Reisner Y, Martelli MF. Haploidentical stem cell transplantation in leukemia. Blood Rev (2001) 15:111–9. 10.1054/blre.2001.0157 [DOI] [PubMed] [Google Scholar]
- 16.Bethge WA, Haegele M, Faul C, Lang P, Schumm M, Bornhauser M, et al. Haploidentical allogeneic hematopoietic cell transplantation in adults with reduced-intensity conditioning and CD3/CD19 depletion: fast engraftment and low toxicity. Exp Hematol (2006) 34:1746–52. 10.1016/j.exphem.2006.08.009 [DOI] [PubMed] [Google Scholar]
- 17.Lang P, Greil J, Bader P, Handgretinger R, Klingebiel T, Schumm M, et al. Long-term outcome after haploidentical stem cell transplantation in children. Blood Cells Mol Dis (2004) 33:281–7. 10.1016/j.bcmd.2004.08.017 [DOI] [PubMed] [Google Scholar]
- 18.Ball LM, Egeler RM. Acute GvHD: pathogenesis and classification. Bone Marrow Transplant (2008) 41(Suppl 2):64. 10.1038/bmt.2008.56 [DOI] [PubMed] [Google Scholar]
- 19.Ferrara JLM, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet (2009) 373:1550–61. 10.1016/S0140-6736(09)60237-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bethge WA, Faul C, Bornhäuser M, Stuhler G, Beelen DW, Lang P, et al. Haploidentical allogeneic hematopoietic cell transplantation in adults using CD3/CD19 depletion and reduced intensity conditioning: an update. Blood Cells Mol Dis (2008) 40:13–9. 10.1016/j.bcmd.2007.07.001 [DOI] [PubMed] [Google Scholar]
- 21.Federmann B, Hägele M, Pfeiffer M, Wirths S, Schumm M, Faul C, et al. Immune reconstitution after haploidentical hematopoietic cell transplantation: impact of reduced intensity conditioning and CD3/CD19 depleted grafts. Leukemia (2011) 25:121–9. 10.1038/leu.2010.235 [DOI] [PubMed] [Google Scholar]
- 22.Handgretinger R. Negative depletion of CD3(+) and TcRαβ(+) T cells. Curr Opin Hematol (2012) 19:434–9. 10.1097/MOH.0b013e3283582340 [DOI] [PubMed] [Google Scholar]
- 23.Huenecke S, Bremm M, Cappel C, Esser R, Quaiser A, Bonig H, et al. Optimization of individualized graft composition: CD3/CD19 depletion combined with CD34 selection for haploidentical transplantation. Transfusion (2016) 56:2336–45. 10.1111/trf.13694 [DOI] [PubMed] [Google Scholar]
- 24.Gordon PR, Leimig T, Mueller I, Babarin-Dorner A, Holladay MA, Houston J, et al. A large-scale method for T cell depletion: towards graft engineering of mobilized peripheral blood stem cells. Bone Marrow Transplant (2002) 30:69–74. 10.1038/sj.bmt.1703619 [DOI] [PubMed] [Google Scholar]
- 25.Koehl U, Bochennek K, Esser R, Brinkmann A, Quaritsch R, Becker M, et al. ISHAGE-based single-platform flowcytometric analysis for measurement of absolute viable T cells in fresh or cryopreserved products: CD34/CD133 selected or CD3/CD19 depleted stem cells, DLI and purified CD56+CD3- NK cells. Int J Hematol (2008) 87:98–105. 10.1007/s12185-007-0018-7 [DOI] [PubMed] [Google Scholar]
- 26.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant (1995) 15:825–8. [PubMed] [Google Scholar]
- 27.Huenecke S, Behl M, Fadler C, Zimmermann SY, Bochennek K, Tramsen L, et al. Age-matched lymphocyte subpopulation reference values in childhood and adolescence: application of exponential regression analysis. Eur J Haematol (2008) 80:532–9. 10.1111/j.1600-0609.2008.01052.x [DOI] [PubMed] [Google Scholar]
- 28.Comans-Bitter WM, de Groot R, van den Beemd R, Neijens HJ, Hop WC, Groeneveld K, et al. Immunophenotyping of blood lymphocytes in childhood. Reference values for lymphocyte subpopulations. J Pediatr (1997) 130:388–93. 10.1016/S0022-3476(97)70200-2 [DOI] [PubMed] [Google Scholar]
- 29.van Gent R, van Tilburg CM, Nibbelke EE, Otto SA, Gaiser JF, Janssens-Korpela PL, et al. Refined characterization and reference values of the pediatric T- and B-cell compartments. Clin Immunol (2009) 133:95–107. 10.1016/j.clim.2009.05.020 [DOI] [PubMed] [Google Scholar]
- 30.Shearer WT, Rosenblatt HM, Gelman RS, Oyomopito R, Plaeger S, Stiehm ER, et al. Lymphocyte subsets in healthy children from birth through 18 years of age: the pediatric AIDS clinical trials group P1009 study. J Allergy Clin Immunol (2003) 112:973–80. 10.1016/j.jaci.2003.07.003 [DOI] [PubMed] [Google Scholar]
- 31.Tosato F, Bucciol G, Pantano G, Putti MC, Sanzari MC, Basso G, et al. Lymphocytes subsets reference values in childhood. Cytometry A (2015) 87:81–5. 10.1002/cyto.a.22520 [DOI] [PubMed] [Google Scholar]
- 32.Schatorjé EJH, Gemen EFA, Driessen GJA, Leuvenink J, van Hout RWNM, van der Burg M, et al. Age-matched reference values for B-lymphocyte subpopulations and CVID classifications in children. Scand J Immunol (2011) 74:502–10. 10.1111/j.1365-3083.2011.02609.x [DOI] [PubMed] [Google Scholar]
- 33.Schatorjé EJH, Gemen EFA, Driessen GJA, Leuvenink J, van Hout RWNM, de Vries E. Paediatric reference values for the peripheral T cell compartment. Scand J Immunol (2012) 75:436–44. 10.1111/j.1365-3083.2012.02671.x [DOI] [PubMed] [Google Scholar]
- 34.Duchamp M, Sterlin D, Diabate A, Uring-Lambert B, Guérin-El Khourouj V, Le Mauff B, et al. B-cell subpopulations in children: National reference values. Immun Inflamm Dis (2014) 2:131–40. 10.1002/iid3.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiener D, Shah S, Malone J, Lowell N, Lowitt S, Rowlands DT. Multiparametric analysis of peripheral blood in the normal pediatric population by flow cytometry. J Clin Lab Anal (1990) 4:175–9. 10.1002/jcla.1860040305 [DOI] [PubMed] [Google Scholar]
- 36.Henderson R, Diggle P, Dobson A. Joint modelling of longitudinal measurements and event time data. Biostatistics (2000) 1:465–80. 10.1093/biostatistics/1.4.465 [DOI] [PubMed] [Google Scholar]
- 37.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York: Springer; (2009). [Google Scholar]
- 38.Therneau TM, Grambsch PM. Modeling survival Data. Extending the Cox Model. New York: Springer; (2000). [Google Scholar]
- 39.Rizopoulos D. Joint Models for Longitudinal and Time-to-Event Data. With Applications in R. Boca Raton: CRC Press; (2012). [Google Scholar]
- 40.Behringer D, Bertz H, Schmoor C, Berger C, Dwenger A, Finke J. Quantitative lymphocyte subset reconstitution after allogeneic hematopoietic transplantation from matched related donors with CD34+ selected PBPC grafts unselected PBPC grafts or BM grafts. Bone Marrow Transplant (1999) 24:295–302. 10.1038/sj.bmt.1701889 [DOI] [PubMed] [Google Scholar]
- 41.Lang P, Handgretinger R. Haploidentical SCT in children: an update and future perspectives. Bone Marrow Transplant (2008) 42(Suppl 2):9. 10.1038/bmt.2008.285 [DOI] [PubMed] [Google Scholar]
- 42.Marks DI, Khattry N, Cummins M, Goulden N, Green A, Harvey J, et al. Haploidentical stem cell transplantation for children with acute leukaemia. Br J Haematol (2006) 134:196–201. 10.1111/j.1365-2141.2006.06140.x [DOI] [PubMed] [Google Scholar]
- 43.Eyrich M, Lang P, Lal S, Bader P, Handgretinger R, Klingebiel T, et al. A prospective analysis of the pattern of immune reconstitution in a paediatric cohort following transplantation of positively selected human leucocyte antigen-disparate haematopoietic stem cells from parental donors. Br J Haematol (2001) 114:422–32. 10.1046/j.1365-2141.2001.02934.x [DOI] [PubMed] [Google Scholar]
- 44.Bader P, Soerensen J, Jarisch A, Ponstingl E, Krenn T, Faber J, et al. Rapid immune recovery and low TRM in haploidentical stem cell transplantation in children and adolescence using CD3/CD19-depleted stem cells. Best Pract Res Clin Haematol (2011) 24:331–7. 10.1016/j.beha.2011.04.005 [DOI] [PubMed] [Google Scholar]
- 45.Pfeiffer MM, Feuchtinger T, Teltschik H-M, Schumm M, Müller I, Handgretinger R, et al. Reconstitution of natural killer cell receptors influences natural killer activity and relapse rate after haploidentical transplantation of T- and B-cell depleted grafts in children. Haematologica (2010) 95:1381–8. 10.3324/haematol.2009.021121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Handgretinger R, Klingebiel T, Lang P, Schumm M, Neu S, Geiselhart A, et al. Megadose transplantation of purified peripheral blood CD34(+) progenitor cells from HLA-mismatched parental donors in children. Bone Marrow Transplant (2001) 27:777–83. 10.1038/sj.bmt.1702996 [DOI] [PubMed] [Google Scholar]
- 47.Ortín M, Raj R, Kinning E, Williams M, Darbyshire PJ. Partially matched related donor peripheral blood progenitor cell transplantation in paediatric patients adding fludarabine and anti-lymphocyte gamma-globulin. Bone Marrow Transplant (2002) 30:359–66. 10.1038/sj.bmt.1703667 [DOI] [PubMed] [Google Scholar]
- 48.Eyrich M, Leiler C, Croner T, Lang P, Schumm M, Mascher B, et al. Impaired T-cell activation and cytokine productivity after transplantation of positively selected CD34+ allogeneic hematopoietic stem cells. Hematol J (2004) 5:329–40. 10.1038/sj.thj.6200397 [DOI] [PubMed] [Google Scholar]
- 49.Lang P, Schumm M, Greil J, Bader P, Klingebiel T, Müller I, et al. A comparison between three graft manipulation methods for haploidentical stem cell transplantation in pediatric patients: preliminary results of a pilot study. Klin Padiatr (2005) 217:334–8. 10.1055/s-2005-872529 [DOI] [PubMed] [Google Scholar]
- 50.Lang P, Pfeiffer M, Teltschik HM, Schlegel P, Feuchtinger T, Ebinger M, et al. Natural killer cell activity influences outcome after T cell depleted stem cell transplantation from matched unrelated and haploidentical donors. Best Pract Res Clin Haematol (2011) 24:403–11. 10.1016/j.beha.2011.04.009 [DOI] [PubMed] [Google Scholar]
- 51.Gonzalez-Vicent M, Perez A, Abad L, Sevilla J, Ramirez M, Diaz MA. Graft manipulation and reduced-intensity conditioning for allogeneic hematopoietic stem cell transplantation from mismatched unrelated and mismatched/haploidentical related donors in pediatric leukemia patients. J Pediatr Hematol Oncol (2010) 32:90. 10.1097/MPH.0b013e3181cf813c [DOI] [PubMed] [Google Scholar]
- 52.Pérez-Martínez A, González-Vicent M, Valentín J, Aleo E, Lassaletta A, Sevilla J, et al. Early evaluation of immune reconstitution following allogeneic CD3/CD19-depleted grafts from alternative donors in childhood acute leukemia. Bone Marrow Transplant (2012) 47:1419–27. 10.1038/bmt.2012.43 [DOI] [PubMed] [Google Scholar]
- 53.Lang P, Teltschik HM, Feuchtinger T, Müller I, Pfeiffer M, Schumm M, et al. Transplantation of CD3/CD19 depleted allografts from haploidentical family donors in paediatric leukaemia. Br J Haematol (2014) 165:688–98. 10.1111/bjh.12810 [DOI] [PubMed] [Google Scholar]
- 54.Bunin N, Aplenc R, Grupp S, Pierson G, Monos D. Unrelated donor or partially matched related donor peripheral stem cell transplant with CD34+ selection and CD3+ addback for pediatric patients with leukemias. Bone Marrow Transplant (2006) 37:143–9. 10.1038/sj.bmt.1705211 [DOI] [PubMed] [Google Scholar]
- 55.Dvorak CC, Gilman AL, Horn B, Oon C-Y, Dunn EA, Baxter-Lowe LA, et al. Haploidentical related-donor hematopoietic cell transplantation in children using megadoses of CliniMACs-selected CD34(+) cells and a fixed CD3(+) dose. Bone Marrow Transplant (2013) 48:508–13. 10.1038/bmt.2012.186 [DOI] [PubMed] [Google Scholar]
- 56.Woodard P, Cunningham JM, Benaim E, Chen X, Hale G, Horwitz E, et al. Effective donor lymphohematopoietic reconstitution after haploidentical CD34+-selected hematopoietic stem cell transplantation in children with refractory severe aplastic anemia. Bone Marrow Transplant (2004) 33:411–8. 10.1038/sj.bmt.1704358 [DOI] [PubMed] [Google Scholar]
- 57.Lang P, Klingebiel T, Bader P, Greil J, Schumm M, Schlegel PG, et al. Transplantation of highly purified peripheral-blood CD34+ progenitor cells from related and unrelated donors in children with nonmalignant diseases. Bone Marrow Transplant (2004) 33:25–32. 10.1038/sj.bmt.1704303 [DOI] [PubMed] [Google Scholar]
- 58.Aversa F, Prezioso L, Manfra I, Galaverna F, Spolzino A, Monti A. Immunity to infections after haploidentical hematopoietic stem cell transplantation. Mediterr J Hematol Infect Dis (2016) 8(1):e2016057. 10.4084/mjhid.2016.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perez A, Gonzalez-Vicent M, Ramirez M, Sevilla J, Madero L, Diaz MA. Intentional induction of mixed haematopoietic chimerism as platform for cellular therapy after HLA-matched allogeneic stem cell transplantation in childhood leukaemia patients. Br J Haematol (2008) 140:340–3. 10.1111/j.1365-2141.2007.06911.x [DOI] [PubMed] [Google Scholar]
- 60.Peccatori J, Ciceri F. Allogeneic stem cell transplantation for acute myeloid leukemia. Haematologica (2010) 95:857–9. 10.3324/haematol.2010.023184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Federmann B, Bornhauser M, Meisner C, Kordelas L, Beelen DW, Stuhler G, et al. Haploidentical allogeneic hematopoietic cell transplantation in adults using CD3/CD19 depletion and reduced intensity conditioning: a phase II study. Haematologica (2012) 97:1523–31. 10.3324/haematol.2011.059378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marek A, Stern M, Chalandon Y, Ansari M, Ozsahin H, Güngör T, et al. The impact of T-cell depletion techniques on the outcome after haploidentical hematopoietic SCT. Bone Marrow Transplant (2014) 49:55–61. 10.1038/bmt.2013.132 [DOI] [PubMed] [Google Scholar]
- 63.Koskenvuo M, Ekman I, Saha E, Salokannel E, Matomäki J, Ilonen J, et al. Immunological reconstitution in children after completing conventional chemotherapy of acute lymphoblastic leukemia is marked by impaired B-cell compartment. Pediatr Blood Cancer (2016) 63:1653–6. 10.1002/pbc.26047 [DOI] [PubMed] [Google Scholar]
- 64.Maris M, Boeckh M, Storer B, Dawson M, White K, Keng M, et al. Immunologic recovery after hematopoietic cell transplantation with nonmyeloablative conditioning. Exp Hematol (2003) 31:941–52. 10.1016/S0301-472X(03)00201-7 [DOI] [PubMed] [Google Scholar]
- 65.Schulenburg A, Fischer M, Kalhs P, Mitterbauer M, Rabitsch W, Greinix HT, et al. Immune recovery after conventional and non-myeloablative allogeneic stem cell transplantation. Leuk Lymphoma (2005) 46:1755–60. 10.1080/10428190500264496 [DOI] [PubMed] [Google Scholar]
- 66.Jiménez M, Martínez C, Ercilla G, Carreras E, Urbano-Ispízua A, Aymerich M, et al. Reduced-intensity conditioning regimen preserves thymic function in the early period after hematopoietic stem cell transplantation. Exp Hematol (2005) 33:1240–8. 10.1016/j.exphem.2005.06.016 [DOI] [PubMed] [Google Scholar]
- 67.Busca A, Lovisone E, Aliberti S, Locatelli F, Serra A, Scaravaglio P, et al. Immune reconstitution and early infectious complications following nonmyeloablative hematopoietic stem cell transplantation. Hematology (2003) 8:303–11. 10.1080/10245330310001612125 [DOI] [PubMed] [Google Scholar]
- 68.Roll P, Muhammad K, Stuhler G, Grigoleit U, Einsele H, Tony HP. Effect of ATG-F on B-cell reconstitution after hematopoietic stem cell transplantation. Eur J Haematol (2015) 95(6):514–23. 10.1111/ejh.12524 [DOI] [PubMed] [Google Scholar]
- 69.Fuji S, Kim SW, Yano S, Hagiwara S, Nakamae H, Hidaka M, et al. A prospective multicenter study of unrelated bone marrow transplants using a reduced-intensity conditioning regimen with low-dose ATG-F. Bone Marrow Transplant (2015) 51(3):451–3. 10.1038/bmt.2015.268 [DOI] [PubMed] [Google Scholar]
- 70.Zand MS. B-cell activity of polyclonal antithymocyte globulins. Transplantation (2006) 82(11):1387–95. 10.1097/01.tp.0000244063.05338.27 [DOI] [PubMed] [Google Scholar]
- 71.Booth C, Lawson S, Veys P. The current role of T cell depletion in paediatric stem cell transplantation. Br J Haematol (2013) 162:177–90. 10.1111/bjh.12400 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.