Abstract
Tomato genotypes selected for their high foliar zingiberene (ZGB) contents in a segregating F2 population were assessed to determine their effect on behavior and biology of Tetranychus urticae Koch, the putative resistance mechanisms involved and the role of trichomes on that resistance. Genotypes with contrasting ZGB content (RVTZ-09 = low ZGB, RVTZ-79 = high ZGB, RVTZ-142 = high ZGB, and RVTZ-331 = high ZGB) were selected from an interspecific cross between wild S. habrochaites var. hirsutum accession PI-127826 (high ZGB content and resistant to mites) and S. lycopersicum cv. Redenção (low ZGB content and susceptible to mites). To determine the effect of these genotypes on mite behavior and biology, free- and no-choice tests, as well as biological studies were performed. Types and densities of trichomes on the foliar surface and their correlation with ZGB contents was determined. Genotypes rich in ZGB (RVTZ-79, RVTZ-142, and RVTZ-331) presented a high number of types IV and VI glandular trichomes, and both type IV and VI densities were positively correlated with ZGB content. In the free-choice test, T. urticae showed a high preference toward S. lycopersicum cv. Redenção and the genotype RVTZ-09 (low ZGB content), whereas, genotypes with high ZBG content were less preferred. Moreover, on high ZGB genotypes, increase in the egg incubation period and in total mortality of nymphs, and decrease of fecundity rate were observed, indicating deleterious effects in mite biology. Results indicated that high ZGB/high glandular trichome densities genotypes present both non-preference and antibiosis mechanisms of resistance to the mite.
Keywords: Solanum lycopersicum, Solanum habrochaites var. hirsutum, zingiberene, glandular trichomes, biology, plant-resistance
Introduction
The two-spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae), is a phytophagous and polyphagous mite species (Clotuche et al., 2011). In tomato, Solanum lycopersicum L. (formerly Lycopersicon esculentum Mill.), this arthropod causes severe damage on leaves and on fruits, especially when they are grown in greenhouses, where the mites find favorable environmental conditions (Meck et al., 2013). Considering the need for reduction of chemical applications (i.e., synthetic acaricides) for mite control, tomato breeding programs aimed to develop resistant cultivars should be considered an important contribution for the integrated management of this pest.
Studies have shown the potential use of some accessions of S. lycopersicum as resistance sources against arthropod pests. For instance, S. lycopersicum var. cerasiforme (Dunal), known as cherry tomato, has been studied for this purpose (Sánchez-Peña et al., 2006; Lucini et al., 2016). However, wild Solanum (section Lycopersicon) species are the most exploited as sources of resistance genes that may be deployed in tomato cultivars (Resende et al., 2009; Silva et al., 2009; Maluf et al., 2010; Oliveira et al., 2012; Dias et al., 2013; Lima et al., 2015).
Wild tomato accessions have been shown to affect behavior and biology of lepidopterans (Gurr and McGrath, 2001; Dias et al., 2013; Lima et al., 2015), coleopterans (Carter et al., 1989), hemipterans (Simmons et al., 2003; Resende et al., 2009) and mites Tetranychus spp. (Carter and Snyder, 1985; Gonçalves et al., 2006; Resende et al., 2008; Lucini et al., 2015). The main factor associated with resistance to pests in wild tomatoes, is reportedly the presence of glandular trichomes (especially, types IV and VI), which are responsible by storing and releasing allelochemical compounds (Maluf et al., 2001; Freitas et al., 2002; Simmons and Gurr, 2005). The zingiberene (ZGB) (a sesquiterpene) is an allelochemical stored and exuded by type IV and VI glandular trichomes present on plant surface of Solanum habrochaites Knapp and Spooner var. hirsutum Dunal (Maluf et al., 2001; Freitas et al., 2002; Gonçalves et al., 2006). The repellent effect of this wild tomato species against mites, imparted by presence of glandular trichomes containing ZGB, has been reported (Weston et al., 1989; Gonçalves et al., 2006).
Behavioral and biological bioassays are required to determine the mechanism(s) involved in the resistance against pests, in order to provide information on the extent of genotype resistance to herbivory. In this study, we selected tomato genotypes which contrasting ZGB contents in the F2 generation of the interspecific cross S. lycopersicum cv. Redenção ×S. habrochaites var. hirsutum accession PI-127826, and evaluated their effects on behavior and biology of the spider mite T. urticae, the putative resistance mechanisms involved (i.e., antixenosis and/or antibiosis), and the role of trichomes in the resistance.
Materials and Methods
Spider Mite Population Maintenance
Adults of T. urticae were field-collected at the experimental area of the Universidade Estadual do Centro Oeste do Paraná, Guarapuava, Brazil (25°23′S; 51°29W). Mites were taken to the Laboratory of Entomology where a colony was established under controlled conditions (25 ± 2°C, photoperiod of 12L:12D hours). Plants of Canavalia ensiformis (L.) (Fabaceae), grown in plastic pots (5L), were used as mite food source, and replaced when necessary. For use in the laboratory bioassays, a mite colony was established in a BOD incubator chamber at 25 ± 2°C; 70 ± 10% relative humidity, and photoperiod of 12L:12D hours.
The tomato genotypes used in bioassays were the acessions S. lycopersicum cv. Redenção (low ZGB content and susceptible to mites), S. habrochaites var. hirsutum accession PI-127826 (wild accession rich in ZGB and resistant to mites), and additional genotypes selected from the interspecific cross between PI-127826 × cv. Redenção.
These additional genotypes comprised one F1 plant (Redenção × PI 127826), and four plants selected from the F2 (Redenção × PI 127826) population, three of which (RVTZ-79, RVTZ-142, RVTZ-331) selected for high ZGB contents and one (RVTZ-09) selected for low ZGB.
The selected genotypes were cloned through rooting of axillary shoots in a tray filled with commercial substrate that was kept moist. Seedlings were transplanted into 10L plastic pots containing a mixture of commercial substrate: soil (1:1) and fertilized with NPK (04:14:08). The plants were kept in a greenhouse with daily irrigation. Expanded leaflets of 40/50-day-old plants were sampled and used in the laboratory bioassays.
Zingiberene Contents and Trichomes Densities
In order to select for ZGB contents, 553 plants were analyzed [433 plants of the F2 generation (PI-127826 × cv. Redenção), 40 plants of the F1 generation, 40 plants of cv. Redenção and 40 plants of PI-127826]. The methodology proposed by Freitas et al. (2000) was used to quantify ZGB: six leaf disks (diameter 1 cm) were sampled from each plant, placed in tubes containing 2 ml of hexane, and then vortexed for 30 s. After that, the leaf disks were removed and the absorbance of the solution was measured using a spectrophotometer (Cary series – UV-Vis Spectrophotometer) at 270 nm of wavelength. The selection of genotypes was carried based on the absorbance values, which were above 0.700 for the three high ZGB genotypes, and below 0.250 for the low ZGB genotype.
Identification and quantification of trichomes on each genotype was based on images obtained using a scanning electron microscope (SEM) (Hitachi High-Tech TM3000 with tungsten filament, low vacuum and 15 kV). For this purpose, leaf disks (diameter 1 cm) taken from leaflets were inserted into the microscope. Luckwill’s (1943) and Toscano et al.’s (2001) classifications were followed to identify the trichome types.
Types IV and VI glandular trichomes, and type VIII non-glandular trichomes were counted separately. Counting was performed on both foliar surfaces (abaxial and adaxial) of four equal quadrants of each leaf disk, and numbers of trichomes per mm2 were recorded.
Free- and No-Choice Tests
For free- and no-choice test, arenas (petri dishes with 6 cm diameter) were coated inside with a layer of household sponge topped with a layer of cotton-wool, both moistened in distilled water. In the free-choice test, leaf disks (diameter 3 cm) of the different genotypes evaluated were placed in pairs (one pair per arena) onto the cotton wool layer with abaxial side up. Leaf disks in each pair were connected each other using a transparent plastic coverslip (18 × 18 mm). After that, six 10-day-old adult T. urticae females collected from the colony were transferred into the center of the coverslip under a stereomicroscope (Nikon SMZ745T), allowing for free choice and mite access to both leaf disks. Because there were seven treatments, a full replication comprised 21 pairwise combination of genotypes (i.e., 21 arenas). Five full replications, totaling 105 petri dishes, were used, in a completely randomized design. After 24 h, the number of mites on each leaf disk were counted, and used to calculate the mite preference (%) for the respective genotype.
In the no-choice test, leaf disks (diameter 3 cm) of each genotype were placed separately onto the cotton wool layer, with abaxial side up, into the arenas. Six 10-day-old adult T. urticae female mites collected from the colony were transferred to each leaf disk under a stereomicroscope, and kept for 24 h. After that period, the number of eggs laid on each leaf disk was recorded. This test was conducted in a completely randomized design with 10 replications per genotype, comprising a total of 210 arenas. For both tests, the arenas were kept in a walk-in chamber at 25 ± 2°C; 70 ± 10% relative humidity, and photoperiod of 12L:12D hours.
In addition, another no-choice experiment was performed in order to evaluate the total distance traveled by the mite on leaflets using Weston and Snyder’s (1990) methodology. Leaflets (replications) from each genotype were sampled and fixed with abaxial side up on polystyrene sheets, using a metallic thumbtack (diameter 10 mm). Seven tomato genotypes (treatments) with 20 replications each were tested in a completely randomized design. Ten 6-day-old females were transferred onto each thumbtack, using a fine paint brush. After 10, 20, 40, and 60 min, the distances traveled by the females on the leaflet surface were recorded. The mean distances traveled by the mites after 60 min were determined for each genotype. This test is based on the assumption that lower distances covered by the mites indicate higher levels of mite repellence (e.g., negative effects on movement behavior).
Biological Parameters of Tetranychus urticae on Tomato Genotypes
This study comprised two experiments. In the first, the incubation period and viability of the eggs, the duration and viability of young stages (larvae, protonymph, and deutonymph) and the longevity of adults were measured for the seven genotypes tested. Six leaf disks (diameter 2 cm) of each genotype, with abaxial side up, were equidistantly distributed into arenas (petri dishes, diameter 10 cm) coated inside with a layer of household sponge topped with a layer of cotton-wool, both moistened in distilled water. A 10-day-old T. urticae female + male pair was transferred and kept for 24 h onto each leaf disk; after that, the mites and eggs (except one), were carefully removed with a fine artist’s brush under a stereomicroscope, leaving one single egg per disk. Eggs, young stages and adults were daily observed to determine the parameters previously cited. Each set of six leaf disks within an arena was considered one replication. Altogether, 10 replications per treatment were used, in a randomized complete design.
In a second experiment, mite fecundity rate was evaluated. Ten leaf disks (diameter 2 cm) of a same tomato genotype, with abaxial side up, were equidistantly placed into plastic boxes (11 × 11 × 3.5 cm) coated inside with a layer of household sponge topped with a layer of cotton-wool, both moistened in distilled water. A 10-day-old T. urticae female was transferred onto each leaf disk, and was observed daily for 10 days to record the total number of eggs laid. During this period, the leaf disks were replaced by new disks of the same genotype every 2 days keeping the same female on the new disk.
Each plastic box with ten leaf disks of the same genotype, was considered a replication. Ten replications distributed in a completely randomized design were used for each of the seven genotypes tested.
Altogether, 60 and 100 observations (leaf disks) were taken per genotype respectively in the first and second experiments.
Both bioassays were carried out in a BOD incubator chamber at 25 ± 2°C; 70 ± 10% relative humidity, and photoperiod of 12L:12D.
Statistical Analyses
Data were previously tested using Bartlett’s test to check for homogeneity of variance (p < 0.05), and then transformed to (x + 0.5)1/2 when necessary to fulfill the pre-requisites of analysis of variance (ANOVA). The mean number of trichomes and data related to behavioral and biological parameters of the mites were submitted to ANOVA, and treatment means were compared by Tukey test (p < 0.05). Data of T. urticae preferences (%) from the free-choice test were compared using Pearson’s Chi-Square test (χ2). The types and densities of trichomes and ZGB contents were submitted to Pearson’s correlation analysis and compared by the Student’s t-test using Microsoft Excel® program. The biological and behavioral parameters evaluated were submitted to similarity grouping using the cluster analysis according to Linkage’s method. Statistical analyses were performed using the Sisvar® program (Ferreira, 2011).
Results
Trichomes and Correlations With Zingiberene Content
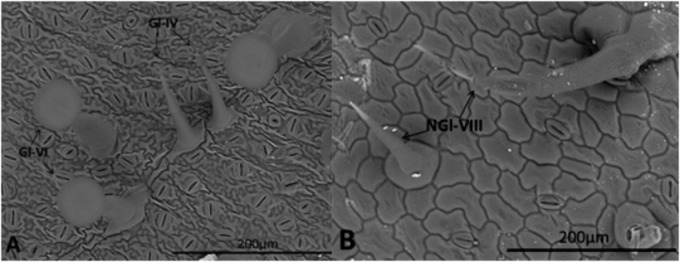
Densities of type VIII non-glandular trichomes and types IV/VI glandular trichomes varied among the genotypes in both abaxial and adaxial leaflet surfaces (Table 1). The highest non-glandular trichome density was observed in S. lycopersicum cv. Redenção, followed by the genotype RVTZ-09 (low ZGB content); in contrast, no non-glandular trichomes were found on wild accession (S. habrochaites var. hirsutum PI-127826) (Table 1 and Figures 1A,B), and very low densities in the high ZGB genotypes.
Table 1.
Zingiberene content (ZGB) and mean number (± SE) of glandular (types IV and VI) and non-glandular (type VIII) trichomes per mm2 present on abaxial (Ab) and on adaxial (Ad) surfaces of leaflets obtained from different tomato genotypes.
| Genotype | ZGB content1 | Glandular trichome type IV2 | Glandular trichome type VI2 | Non-glandular trichome type VIII2 | Glandular types IV and VI (Ab + Ad)2 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Ab | Ad | Total | Ab | Ad | Total | Total | |||
| S. lycopersicum (cv. Redenção) | 0.084 | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.0 ± 0.0d | 84.5 ± 1.0a | 0.0 |
| S. habrochaites var. hirsutum (PI-127826) | 1.099 | 20.7 ± 1.1bc | 39 ± 2.7a | 59.7 ± 1.4ab | 23.2 ± 2.7a | 4.0 ± 1.2ab | 27.2 ± 3.0a | 0.0 | 87.0 ± 4.0a |
| F1 plant (Redenção × PI-127826) | 0.328 | 24.0 ± 1.3b | 12.2 ± 0.9bc | 36.2 ± 1.1c | 6.0 ± 0.9b | 4.5 ± 0.6a | 10.5 ± 1.2bc | 0.2 ± 0.2cd | 46.7 ± 1.4b |
| RVTZ-09 (= Low) | 0.247 | 8.0 ± 2.7c | 4.7 ± 0.0c | 12.7 ± 2.5d | 0.0 ± 0.0d | 1.5 ± 1.0ab | 1.5 ± 0.9cd | 13.5 ± 2.1b | 14.2 ± 2.0c |
| RVTZ-79 (= High) | 0.715 | 35.7 ± 6.2ab | 8.2 ± 1.1b | 44.0 ± 7.1bc | 7.7 ± 1.1b | 0.7 ± 0.5ab | 8.5 ± 1.0bcd | 5.7 ± 0.2c | 52.5 ± 7.9b |
| RVTZ-142 (= High) | 0.813 | 33.5 ± 6.5ab | 16.5 ± 1.3b | 50.0 ± 7.3bc | 7.5 ± 1.3b | 4.5 ± 2.9ab | 12.0 ± 4.1b | 3.5 ± 0.6cd | 62.0 ± 4.7b |
| RVTZ-331 (= High) | 0.746 | 51 ± 7.4a | 35.2 ± 0.9a | 86.2 ± 7.8a | 1.5 ± 0.9c | 2.7 ± 1.0ab | 4.2 ± 1.6bcd | 0.2 ± 0.2cd | 90.5 ± 7.4a |
| Correlation coefficient with ZGB (r) | – | – | 0.81∗∗ | – | – | 0.80∗ | –0.66ns | 0.90∗∗ | |
| CV (%) | 17.5 | 16.9 | 11.8 | 16.6 | 35.5 | 20.6 | 9.1 | 9.4 | |
Means followed by the same lowercase letter within a column do not differ significantly by the Tukey test (p < 0.05).
nsNon-significant,∗and ∗∗Significant different by Student’s t-test (p < 0.05 and p < 0.01, respectively).
1Zingiberene content determined at 270 nm (see section Materials and Methods).
2Original data presented [for analysis, data were transformed in (x + 0.5)1/2].
FIGURE 1.
Leaflet surface of S. habrochaites var. hirsutum (A) and S. lycopersicum cv. Redenção (B) obtained in a scanning electron microscope (SEM) showing different trichome types. Type IV (Gl-IV) and VI (Gl-VI) glandular trichomes (A), and type VIII (NGI-VIII) non-glandular trichome (B) according to Luckwill (1943) and Toscano et al. (2001) classification.
On the other hand, the wild accession PI-127826 and genotypes selected for high ZBG content (RVTZ-79, RVTZ-142, and RVTZ-331) presented high densities of types IV and VI glandular trichomes, on both surfaces, when compared to S. lycopersicum cv. Redenção (in which no glandular trichomes were observed) and the low ZGB genotype RVTZ-09 (Table 1). Densities of both types IV and VI glandular trichomes were significantly and positively correlated with ZGB content (r = 0.81, p < 0.01; and r = 0.80, p < 0.05, respectively) (Table 1).
Free- and No-Choice Tests
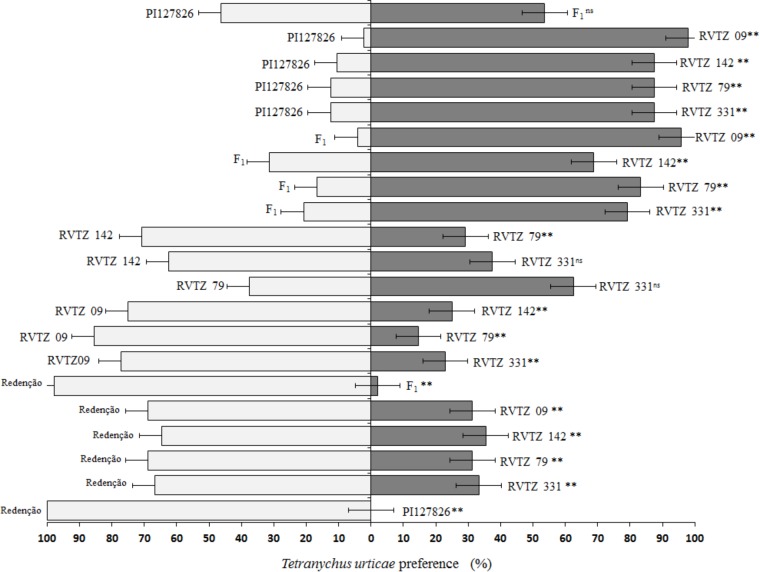
In the free-choice test, behavior of T. urticae females differed among the genotypes tested (Figure 2). Mites had significantly higher preference toward S. lycopersicum cv. Redenção, when paired with all other genotypes evaluated: over 60% of preference, reaching 100% when paired to the wild accession PI-177826. PI-177826 and F1 plant were significantly the least preferred (p < 0.01) by the mites at all genotype pairs assessed, even when compared to genotypes selected for high ZGB content. In addition, no statistical difference was observed between wild accession and F1 plant, indicating that F1 plant mite resistance level is similar to that of the resistant check treatment (Figure 2).
FIGURE 2.
Preference ratios (%) of Tetranychus urticae on the 21 different pairwise combinations of tomato genotypes during the free-choice test. Redenção = S. lycopersicum cv. Redenção, PI127826 = S. habrochaites var. hirsutum accession PI-127826, F1 = F1 plant (PI-127826 × cv. Redenção), RVTZ-09 = RVTZ 2011-09 (low ZGB content), and RVTZ-79, RVTZ-142, and RVTZ-331 = RVTZ2011-79, RVTZ2011-142, and RVTZ2011-331, respectively (genotypes with high ZGB content). ns non-significant different, and ∗∗ significant different (p < 0.01) using Pearson’s Chi-Square test (χ2).
Genotypes selected for high ZGB content (RVTZ-79, RVTZ-142, and RVTZ-331) remained in an intermediary position between the least preferred genotypes PI-127826/F1 and the most preferred S. lycopersicum cv. Redenção/RVTZ-09 (low ZGB content). This latter genotype, showed similar responses to S. lycopersicum cv. Redenção when paired with genotypes rich in ZGB; therefore, it was also considered susceptible to the mite (Figure 2).
The no-choice test showed that the fecundity (eggs laid during 24 h) was reduced on genotypes with high ZGB content: (PI-127826, RVTZ-79, RVTZ-142, RVTZ-331, and F1) when compared to low-ZGB genotypes S. lycopersicum cv. Redenção and RVTZ-09. Fecundity in the high ZGB genotypes was ca. ≥ 4 times lower than in low ZGB genotypes (Table 2).
Table 2.
Mean number (± SE) of eggs laid on leaf disks from different tomato genotypes during first 24 h of the no-choice test, and total distance traveled (mm) by the mites on the abaxial leaflet surface of the genotypes after 60 min.
| Genotypes | Mean (± SE) of eggs laid1 | Total distance traveled (mm) |
|---|---|---|
| S. lycopersicum (cv. Redenção) | 19.1 ± 2.2a | 35.0 ± 4.2a |
| S. habrochaites var. hirsutum | 2.2 ± 1b | 10.0 ± 2.1b |
| F1 (Redenção × PI-127826) | 2.9 ± 0.6b | 9.9 ± 2.3b |
| RVTZ-09 (= Low) | 17.5 ± 1.7a | 21.0 ± 5.3ab |
| RVTZ-79 (= High) | 3.7 ± 0.9b | 16.2 ± 2.5b |
| RVTZ-142 (= High) | 3.4 ± 1.0b | 18.2 ± 3.2b |
| RVTZ-331 (= High) | 4.4 ± 1.3b | 13.7 ± 1.9b |
| CV (%) | 29.2 | 38.0 |
| F | 23.9∗∗ | 7.9∗∗ |
Means followed by the same lowercase letter within a column do not differ significantly by the Tukey test (p < 0.05).
∗∗Significant different by F-test (p < 0.01).
1Original data presented [for analysis, data were transformed in (x + 0.5)1/2].
Significant effects were observed on the movement behavior of the T. urticae released on the different tomato genotypes. Females released on leaflets of S. lycopersicum cv. Redenção were able to travel a significant higher distance (35 mm) after 60 min of evaluation than all other genotypes (<18 mm), except the low ZGB genotype RVTZ-09, which did not differ significantly from cv. Redenção (Table 2). The lower distances covered by the mite on genotypes with high ZGB content, was associated the presence of glandular trichomes (IV and VI), which were absent on S. lycopersicum cv. Redenção, and present at low density on RVTZ-09 (Table 1).
Biological Parameters of Tetranychus urticae on Tomato Genotypes
The duration of egg incubation on genotypes with high ZGB content, RVTZ-142 (4.8 days), wild accession PI-127826 (4.5 days) and RVTZ-79 (4.3 days) tended to be significantly longer compared to genotypes with low ZGB content, (cv. Redenção and RVTZ-09). However, the viability of eggs in all genotypes evaluated was high (≥98%) (Table 3).
Table 3.
Biological parameters [duration (days ± SE) of egg incubation and nymphal development time, egg and nymph viability (%), longevity of adults, and fecundity rate] of Tetranychus urticae kept on leaf disks of different tomato genotypes.
| Genotype | Egg |
Nymph |
Adult |
|||
|---|---|---|---|---|---|---|
| Incubation (days)1 | Viability (%) | Duration (days)1 | Viability (%) | Longevity (days) | No. eggs/female/day1 | |
| S. lycopersicum (cv. Redenção) | 3.4 ± 0.1c | 100 | 11.9 ± 0.2a | 100 | 12.7 ± 0.5a | 3.8 ± 0.2a |
| S. habrochaites var. Hirsutum (PI-127826) | 4.5 ± 0.3ab | 100 | 5.8 ± 0.32b | 0.03 | – | 0.2 ± 0.1c |
| F1 plant (Redenção × PI-127826) | 3.8 ± 0.2bc | 100 | 6.1 ± 0.22b | 0.03 | – | 0.2 ± 0.1c |
| RVTZ-09 (= Low) | 3.4 ± 0.1c | 100 | 11.8 ± 0.1a | 100 | 12.0 ± 0.5a | 3.7 ± 0.2a |
| RVTZ-79 (= High) | 4.3 ± 0.2ab | 100 | 5.5 ± 0.22b | 0.03 | – | 0.6 ± 0.1b |
| RVTZ-142 (= High) | 4.8 ± 0.2a | 100 | 5.4 ± 0.32b | 0.03 | – | 0.8 ± 0.2b |
| RVTZ-331 (= High) | 3.8 ± 0.2bc | 98.3 | 5.8 ± 0.22b | 0.03 | – | 0.4 ± 0.1bc |
| CV (%) | 32.6 | 17.6 | 31.1 | 14.9 | ||
| F | 9.9∗∗ | 298.7∗∗ | – | 99.0∗∗ | ||
Means followed by the same lowercase letter within a column do not differ significantly by the Tukey test (p < 0.05), except for longevity of adults, in which was applied Student’s t-test (p < 0.05) to compare between S. lycopersicum cv. Redenção and RVTZ-09.
∗∗Significant different by F-test (p < 0.01).
1Original data presented [for analysis, data were transformed in (x + 0.5)1/2].
2Nymph development time up to 2nd stadium.
3Total mortality of nymphs in the 3rd stadium.
The nymph stage was only completed in mites placed either on S. lycopersicum cv. Redenção or RVTZ-09; on both, all nymphs evaluated reach adulthood in a similar time (11.9 and 11.8 days, respectively), and adult longevities were also similar. In genotypes with high ZGB content (PI-127826, RVTZ-79, RVTZ-142, and RVTZ-331) and in F1 plants mites did not reach adulthood, because the mortality of the nymphs was total in the third instar. On those genotypes, nymphal stage had a short time span until death, ranging from 5.4 to 6.1 days – a span corresponding to the second nymphal stage (Table 3).
The fecundity rate was also adversely affected by the tomato genotypes with high ZGB content (fecundity rate from 0.2 to 0.8 eggs/female/day), whereas on S. lycopersicum cv. Redenção and RVTZ-09 the mites laid significantly more eggs (3.8 and 3.7 eggs/female/day, respectively) (Table 3).
Cluster Analysis
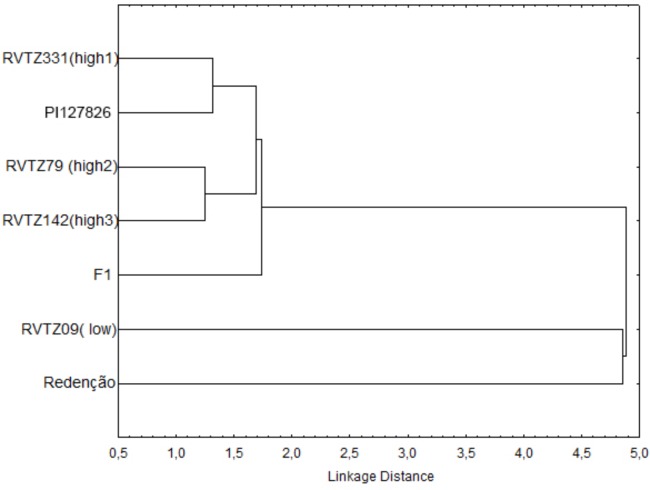
Cluster analysis of the tomato genotypes showed a clear contrasting relationship between the genotypes evaluated (Figure 3), and two clear groups were formed: one group comprised by S. lycopersicum cv. Redenção and RVTZ-09 (low ZGB content), and a second group formed by the genotypes with high ZGB content (RVTZ-79, RVTZ-142, RVTZ-331, and PI-127826) and the F1. Within-group differences between Redenção and RVTZ-09 could be at least partly explained by the slightly higher ZGB concentration in the latter (Table 1). However, ZGB content in RVTZ-09 is only slightly lower than in the F1 (Table 1), indicating that factors other than ZGB content in RVTZ-09 may account for its dissimilarity with Redenção.
FIGURE 3.
Relationships (dendrogram) between seven tomato genotypes with different contents of zingiberene using hierarchical cluster analysis (Linkage’s method). The cladogram has been based on number and type of trichomes, zingiberene content, and behavioral and biological parameters of Tetranychus urticae.
All genotypes selected for high ZGB content might be considered close to the resistant check (wild accession PI-127826), as shown in the cluster analysis (Figure 3). The genotypes selected for high ZGB content formed two distinctive pairs strongly related each other __ first pair composed by RVTZ-331 and PI-127826, and the second one by RVTZ-79 + RVTZ-142.
The presence of glandular trichomes on genotypes selected for high ZGB content (Table 1) was strongly and negatively associated with the behavioral and biological parameters of the mite, except with egg incubation period (Table 3), which was the parameter less affected by the genotypes rich in ZGB. Thus, the results indicate that the presence of glandular trichomes lead to negative effects on mite behavior and biology.
Discussion
Plants that produce and release chemical compounds (allelochemicals) affecting arthropod behavior and biology, may express resistance through both antixenosis (non-preference) and/or antibiosis (War et al., 2012). In tomato, resistance has been extensively reported in the literature as mediated by different allelochemicals against arthropod pests such as mites (Gonçalves et al., 2006; Resende et al., 2008; Lucini et al., 2015; Lima et al., 2016), whiteflies (Freitas et al., 2002; Silva et al., 2009; Lima et al., 2016), and lepidopterans (Maluf et al., 2010; Dias et al., 2013; Lima et al., 2015).
In the behavioral test (free-choice test), our results indicated lower preference of T. urticae toward the wild ZGB-rich genotype (PI-127826), followed by the F1 plant and genotypes selected for high ZGB content (RVTZ-79, RVTZ-142, and RVTZ-331). In contrast, S. lycopersicum cv. Redenção and RVTZ-09 (low ZGB content) were the most preferred genotypes by T. urticae. Lima et al. (2016) evaluating these same ZGB-rich genotypes observed a strong repellency effect against T. urticae and whitefly Bemisia tabaci (Genn.). The results indicated that non-preference is a mechanism by which ZGB-rich genotypes express resistance to the two-spotted spider mite.
Nonetheless, the genotypes rich in ZGB also presented the antibiosis type of resistance, indicated by their deleterious effects on biological parameters of T. urticae. In general, with ZGB-rich genotypes there was an increase of the egg incubation period, total mortality of nymphs, and a strong decrease in the fecundity rate. The adverse effect of tomato genotypes with high allelochemical contents on arthropod biology parameters is also documented in the literature for several species (Eigenbrode and Trumble, 1993; Azevedo et al., 2003; Fancelli et al., 2005; Moreira et al., 2009; Silva et al., 2013). Evaluating tomato genotypes with high content of the allelochemical acylsugar, Lucini et al. (2015) observed that they also presented both mechanisms non-preference and antibiosis against T. urticae, a situation analogous to our current findings for ZGB-rich genotypes. No similar reports on the deleterious effect of high-ZGB tomato genotypes on T. urticae mite survival, fecundity and longevity could be found in the literature.
The high density of glandular trichomes and its associated high ZGB content were therefore responsible for adverse effects upon both the behavior and the biology of T. urticae mites, indicating that both resistance mechanisms, non-preference and antibiosis, are present. In addition, S. lycopersicum cv. Redenção presented a high number of non-glandular trichomes, which did not cause negative effects on mite behavior and biology.
Other studies have demonstrated the effect of glandular trichomes present on S. habrochaites var. hirsutum on Tetranychus spp. (Carter and Snyder, 1985; Maluf et al., 2001; Freitas et al., 2002). Several other studies have also reported negative effects on behavior and biology of arthropod pests associated with glandular trichomes present in wild tomato accessions and in genotypes selected for high allelochemical contents (Freitas et al., 2002; Maluf et al., 2007; Alba et al., 2009; Lucini et al., 2015).
In this study, we demonstrated that ZGB content was positively correlated with both types of glandular trichomes (IV and VI), although the density of type IV was much higher than that type VI trichomes. These results agree with reports in the literature (Freitas et al., 2002; Gonçalves et al., 2006). Allelochemicals other than ZGB have been associated with glandular trichomes; e.g., acylsugar stored in type IV glandular trichomes of Solanum pennellii Correll accession LA-716 (Lucini et al., 2015) and Solanum pimpinellifolium accession TO-937 (Alba et al., 2009).
The results support that genotypes selected for high ZGB content (RVTZ-79, RVTZ-142, and RVTZ-331) are potential sources of resistant genes against T. urticae mites and possibly against other pests, in tomato breeding programs.
Author Contributions
JdO designed and performed the experiments, carried out the statistical analyses, and wrote the project and the manuscript with support of TL and CN. JdR and WM devised the project, the main conceptual ideas and provided the plants and laboratory apparatus. TL and CN contributed to the design and implementation of the research, to the analysis of the results, and to the writing of the manuscript. CN were involved in planning and supervised the work. RdLF and IdL performed the plants and acari experiments with support of JdO and contributed in discussion of the manuscript in consultation with JdO. All authors provided critical feedback, helped in discussion, and contributed to the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer GDS and handling Editor declared their shared affiliation at time of review.
Acknowledgments
We acknowledge Dr. André Mattioli, Institute of Biology, Campinas, São Paulo, Brazil, for identification of the mite species.
Footnotes
Funding. The authors acknowledge to CAPES/Ministry of Education for scholarship to JdO, and to CNPq (National Council for Scientific and Technological Development) for scholarships to JdR and WM, and for their financial support of this research. They also acknowledge the financial support from National Institute of Science and Technology – Semiochemicals in Agriculture (FAPESP and CNPq – grants #2014/50871-0 and #465511/2014-7, respectively).
References
- Alba J. M., Montserrat M., Fernández-Muñoz R. (2009). Resistance to the two-spotted spider mite (Tetranychus urticae) by acylsucroses of wild tomato (Solanum pimpinellifolium) trichomes studied in a recombinant inbred line population. Exp. Appl. Acarol. 47 35–47. 10.1007/s10493-008-9192-4 [DOI] [PubMed] [Google Scholar]
- Azevedo S. M., Faria M. V., Maluf W. R., Oliveira A. C. B., Freitas J. A. (2003). Zingiberene-mediated resistance to the South American tomato pinworm derived from Lycopersicon hirsutum var. hirsutum. Euphytica 134 347–351. 10.1023/B:EUPH.0000005007.14924.d2 [DOI] [Google Scholar]
- Carter C. D., Sacalis J. N., Gianfagna T. J. (1989). Zingiberene and resistance to Colorado potato beetle in Lycopersicon hirsutum f. hirsutum. J. Agric. Food Chem. 37 206–210. 10.1021/jf00085a047 16653137 [DOI] [Google Scholar]
- Carter C. D., Snyder J. C. (1985). Mite responses in relation to trichomes of Lycopersicon esculentum x Lycopersicon hirsutum F2 hybrids. Euphytica 34 177–185. 10.1007/BF00022877 [DOI] [Google Scholar]
- Clotuche G., Mailleux A. C., Fernández A. A., Deneubourg J. L., Detrain C., Hance T. (2011). The formation of collective silk balls in the spider mite Tetranychus urticae Koch. PLoS One 6:e18854. 10.1371/s0018854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias D. M., Resende J. T. V., Faria M. V., Camargo L. K. P., Lima I. P. (2013). Selection of processing tomato genotypes with high acyl sugar content that are resistant to the tomato pinworm. Genet. Mol. Res. 12 381–389. 10.4238/2013.February.8.2 [DOI] [PubMed] [Google Scholar]
- Eigenbrode S. D., Trumble J. T. (1993). Antibiosis to beet armyworm (Spodoptera exigua) in Lycopersicon accessions. HortScience 28 932–934. [Google Scholar]
- Fancelli M., Vendramim J., Frighetto R. T. S., Lourenção A. L. (2005). Exsudato glandular de genótipos de tomateiro e desenvolvimento de Bemisia tabaci (Genn.) (Sternorrhyncha: Aleyrodidae) biótipo B. Neotrop. Entomol. 34 59–665. 10.1590/S1519-566X2005000400017 [DOI] [Google Scholar]
- Ferreira D. F. (2011). Sisvar: a computer statistical analysis system. Ciênc. Agrotec. 35 1039–1042. 10.1590/S1413-70542011000600001 [DOI] [Google Scholar]
- Freitas J. A., Maluf W. R., Cardoso M. G., Gomes L. A. A., Bearzotti E. (2002). Inheritance of foliar zingiberene contents and their relationship to trichome densities and whitefly resistance in tomatoes. Euphytica 127 275–287. 10.1023/A:1020239512598 [DOI] [Google Scholar]
- Freitas J. A., Maluf W. R., Cardoso M. G., Oliveira A. C. B. (2000). Seleção de plantas de tomateiro visando à resistência à artrópodes-praga mediada por zingibereno. Acta Sci. 22 919–923. [Google Scholar]
- Gonçalves L. D., Maluf W. R., Cardoso M. G., Resende J. T. V., Castro E. M., Santos N. M., et al. (2006). Relação entre zingibereno, tricomas foliares e repelência de tomateiros a Tetranychus evansi. Pesqui. Agropecu. Bras. 41 267–273. 10.1590/S0100-204X2006000200011 [DOI] [Google Scholar]
- Gurr G. M., McGrath D. (2001). Effect of plant variety, plant age and photoperiod on glandular pubescence and host-plant resistance to potato moth (Phthorimaea operculella) in Lycopersicon spp. Ann. Appl. Biol. 138 221–230. 10.1111/j.1744-7348.2001.tb00106.x [DOI] [Google Scholar]
- Lima I. P., Resende J. T. V., Oliveira J. R. F., Faria M. V., Dias D. M., Resende N. C. V. (2016). Selection of tomato genotypes for processing with high zingiberene content, resistant to pests. Hortic. Bras. 34 387–391. 10.1590/S0102-05362016003013 [DOI] [Google Scholar]
- Lima I. P., Resende J. T. V., Oliveira J. R. F., Faria M. V., Resende N. C. V., Lima-Filho R. B. (2015). Indirect selection of industrial tomato genotypes rich in zingiberene and resistant to Tuta absoluta Meyrick. Genet. Mol. Res. 14 15081–15089. 10.4238/2015.November.24.16 [DOI] [PubMed] [Google Scholar]
- Lucini T., Faria M. V., Rohde C., Resende J. T. V., Oliveira J. R. F. (2015). Acylsugar and the role of trichomes in tomato genotypes resistance to Tetranychus urticae. Arthropod Plant Interact. 9 45–53. 10.1007/s11829-014-9347-7 [DOI] [Google Scholar]
- Lucini T., Resende J. T. V., Oliveira J. R. F., Scabeni C. J., Zeist A. R., Resende N. C. V. (2016). Repellent effects of various cherry tomato accessions on the two-spotted spider mite Tetranychus urticae Koch (Acari: Tetranychidae). Genet. Mol. Res. 15:15017736. 10.4238/s15017736 [DOI] [PubMed] [Google Scholar]
- Luckwill L. C. (1943). The Genus Lycopersicon: an Historical, Biological, and Taxonomic Survey of the Wild and Cultivated Tomatoes. Aberdeen: The University Press. [Google Scholar]
- Maluf W. R., Campos G. A., Cardoso M. G. (2001). Relationships between trichome types and spider mite (Tetranychus evansi) repellence in tomatoes with respect to foliar zingiberene contents. Euphytica 121 73–80. 10.1023/A:1012067505361 [DOI] [Google Scholar]
- Maluf W. R., Inoue I. F., Ferreira R. P. D., Gomes L. A. A., Castro E. M., Cardoso M. G. (2007). Higher glandular trichome density in tomato leaflets and repellence to spider mites. Pesqui. Agropecu. Bras. 42 1227–1235. 10.1590/S0100-204X2007000900003 [DOI] [Google Scholar]
- Maluf W. R., Silva V. F., Cardoso M. G., Gomes L. A. A., Gonçalves-Neto A. C., Maciel G. M., et al. (2010). Resistance to the South American tomato pinworm Tuta absoluta in high acylsugar and/or high zingiberene tomato genotypes. Euphytica 176 113–123. 10.4238/2015.November.24.16 [DOI] [PubMed] [Google Scholar]
- Meck E. D., Kennedy G. G., Walgenbach J. F. (2013). Effect of Tetranychus urticae (Acari: Tetranychidae) on yield, quality, and economics of tomato production. Crop Prot. 52 84–90. 10.1016/j.cropro.2013.05.011 [DOI] [Google Scholar]
- Moreira L. A., Picanço M. C., Silva G. A., Semeão A. A., Casali V. W. D., Campos M. R., et al. (2009). Antibiosis of eight Lycopersicon genotypes to Tuta absoluta (Lepidoptera: Gelechiidae). Rev. Ceres 56 283–287. [Google Scholar]
- Oliveira C. M., Andrade V. C., Jr., Maluf W. R., Neiva P. I, Maciel G. M. (2012). Resistance of tomato strains to the moth Tuta absoluta imparted by allelochemicals and trichome density. Cienc. Agrotec. 36 45–52. 10.1590/S1413-70542012000100006 [DOI] [Google Scholar]
- Resende J. T. V., Maluf W. R., Cardoso M. G., Faria M. V., Gonçalves L. D., Nascimento I. R. (2008). Resistance of tomato genotypes with high level of acylsugars to Tetranychus evansi baker & pritchard. Sci. Agric. 65 31–35. 10.1590/S0103-90162008000100005 [DOI] [Google Scholar]
- Resende J. T. V., Maluf W. R., Cardoso M. G., Gonçalves L. D., Faria M. V., Nascimento I. R. (2009). Resistance of tomato genotypes to the silverleaf whitefly mediated by acylsugars. Hortic. Bras. 27 345–348. 10.1590/S0102-05362009000300015 [DOI] [Google Scholar]
- Sánchez-Peña P., Oyama K., Núñez-Farfán J., Fornoni J., Hernández-Verdugo S., Márquez-Guzmán J., et al. (2006). Sources of resistance to whitefly (Bemisia spp.) in wild populations of Solanum lycopersicon var. cerasiforme (Dunal) Spooner G.J. Anderson et R.K. Jansen in Northwestern Mexico. Genet. Resour. Crop Evol. 53 711–719. 10.1007/s10722-004-3943-9 [DOI] [Google Scholar]
- Silva A. A., Maluf W. R., Moraes J. C., Alvarenga R., Costa E. M. R. (2013). Resistência a Myzus persicae em genótipos de tomateiro com altos teores foliares de aleloquímicos. Bragantia 72 173–179. 10.1590/S0006-87052013005000022 [DOI] [Google Scholar]
- Silva V. F., Maluf W. R., Cardoso M. G., Gonçalves-Neto A. C., Maciel G. M., Nízio D. A. C., et al. (2009). Resistência mediada por aleloquímicos de genótipos de tomateiro à mosca-branca e ao ácaro-rajado. Pesqui. Agropecu. Bras. 44 1262–1269. 10.1590/S0100-204X2009001000008 [DOI] [Google Scholar]
- Simmons A. T., Gurr G. M. (2005). Trichomes of Lycopersicon species and their hybrids: effects on pests and natural enemies. Agric. For. Entomol. 7 265–276. 10.1111/j.1461-9555.2005.00271.x [DOI] [Google Scholar]
- Simmons A. T., Gurr G. M., McGrath D., Nicol H. I., Martin P. M. (2003). Trichomes of Lycopersicon spp. and their effect on Myzus persicae (Sulzer) (Hemiptera: Aphididae). Aust. J. Entomol. 42 373–378. 10.1046/j.1440-6055.2003.00376.x [DOI] [Google Scholar]
- Toscano L. C., Boiça A. L., Jr., Santos J. M., Almeida J. B. S. A. (2001). Tipos de tricomas em genótipos de Lycopersicon. Hortic. Bras. 19 204–206. 10.1590/S0102-05362001000300009 [DOI] [Google Scholar]
- War A. R., Paulraj M. G., Ahmad T., Buhroo A. A., Hussain B., Ignacimuthu S., et al. (2012). Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 7 1306–1320. 10.4161/psb.21663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston P. A., Johnson D. A., Burton H. T., Snyder J. C. (1989). Trichome secretion composition, trichome densities, and spider mite resistance of ten accessions of Lycopersicum hirsutum. J. Am. Soc. Hortic. Sci. 114 492–498. [Google Scholar]
- Weston P. A., Snyder J. C. (1990). Thumbtack bioassay: a quick method of measuring plant resistance to two-spotted spider mites (Acari: Tetranychidae). J. Econ. Entomol. 83 501–504. 10.1093/jee/83.2.500 [DOI] [Google Scholar]