Abstract
Lung cancer affects over 1. 8 million people worldwide and is the leading cause of cancer related mortality globally. Currently, diagnosis of lung cancer involves a combination of imaging and invasive biopsies to confirm histopathology. Non-invasive diagnostic techniques under investigation include “liquid biopsies” through a simple blood draw to develop predictive and prognostic biomarkers. A better understanding of circulating tumor cell (CTC) dissemination mechanisms offers promising potential for the development of techniques to assist in the diagnosis of lung cancer. Enumeration and characterization of CTCs has the potential to act as a prognostic biomarker and to identify novel drug targets for a precision medicine approach to lung cancer care. This review will focus on the current status of CTCs and their potential diagnostic and prognostic utility in this setting.
Keywords: lung cancer, NSCLC, SCLC, Circulating tumor cells, liquid biopsy
Introduction
Lung cancer is the leading cause of cancer-related mortality among men and women worldwide (1). In 2012, the incidence was estimated at 1.8 million new cases, accounting for 12.9% of all new cancers diagnosed globally (2). There is an estimated 18% survival rate beyond 5 years for all stages combined, with poor outcomes largely due to late diagnosis (1, 3). The majority of patients present with locally advanced or metastatic disease, with ~20–30% of patients presenting with early stage disease (3, 4). Late diagnosis is a major underlying cause for this advanced disease presentation (5). The annual mortality rate for lung cancer is higher than for colon, breast, and prostate cancers combined (6). The majority of patients presenting with advanced stage at diagnosis contributes to this poor outcome (4).
There are two main types of lung cancers, small cell lung carcinoma (SCLC) and non-small cell carcinoma (NSCLC). NSCLC is the most common, accounting for 80% of cases (7). NSCLC has three main histological subtypes: adenocarcinoma, squamous cell (epidermoid) carcinoma, and large cell undifferentiated carcinoma. Adenocarcinoma accounts for ~40% of cases although is increasing in relative incidence, and usually starts in mucus secreting epithelial cells (167). The prognosis of NSCLC subtypes depends on the stage of the tumor and the treatment availability.
Small cell lung cancer (SCLC) accounts for about 15% of all lung cancers diagnosed annually and up to 25% of lung cancer deaths. SCLC is characterized by a more aggressive clinical phenotype than NSCLC with progression to metastatic disease earlier in the disease course (8).
SCLC and NSCLC arise from different cell types and demonstrate varying clinical features as shown in Table 1.
Table 1.
Lung cancer classification.
| Location in the lung | Common features | Common mutations | ||
|---|---|---|---|---|
| NSCLC (80–85%) | Adenocarcinoma (40%) | Peripheral |
|
|
| Squamous cell carcinoma (25-30%) | Central and Peripheral |
|
||
| Large cell carcinoma (10-15%) | Peripheral |
|
||
| SCLC (15–20%) | Central |
|
|
|
Lung cancer may be initiated through exposure to carcinogens. The main risk factor for lung cancer is the use of tobacco. Tobacco is known to initiate and promote carcinogenesis and accounts for 85% of lung cancer cases (9). Additional known risks include exposure to pollutants such as asbestos, tar and metals including arsenic, and chromium. Common symptoms include persistent cough, worsening breathing, pneumonia that fails to resolve, chest discomfort, wheezing, blood in the sputum, and hoarseness (3, 10). A minority are asymptomatic, detected by chance through investigation of other illnesses or in screening programs (11).
Treatment options depend on the intent of treatment and may include loco-regional treatment such as surgery, image guided ablation including radical chemo-radiotherapy, stereotactic ablative radiation treatment, thermal ablation or cryotherapy, or systemic treatment such as chemotherapy, targeted agents, and immunotherapy, alongside novel agents under current investigation in clinical trials (11). An example of the power of targeted therapies in a precision medicine approach was demonstrated in 2004 by Lynch et al. (12) and Paez et al. (13) who demonstrated that patients with EGFR mutations present in the tumors of patients with non-small cell lung cancer exhibited a dramatic response to getfitinib, the epidermal growth factor (EGFR) tyrosine kinase inhibitor (TKI), bringing personalized medicine to reality for a subset of NSCLC patients (12, 13).
Utilization of expensive systemic targeted therapies, however, has traditionally required invasive biopsies in order to assess for targetable tumoral aberrations. This presents a challenge for the monitoring of lung cancers due to the requirement for longitudinal sampling of tumors (14).
Metastasis and epithelial-mesenchymal transition
Metastasis is an extremely complex, multistep process. Cells must gain the ability to intravasate into the blood from the bulk tumor, travel through the blood undergoing sheer stressors and immune evasion, and extravasate to favorable metastatic sites such as bone, brain and liver (15–17). In order to detach from the primary tumor and disseminate into the blood, cells must undergo a cellular process known as epithelial-mesenchymal transition (EMT) (18). EMT enables tumor cells to become motile and enhances migratory capabilities which in effect allows cells to penetrate into the lymph vasculature and circulate as single or clusters of circulating tumor cells (CTCs) (19). Whilst in blood, CTCs exist in a dynamic EMT state (20). CTCs extravasate having undergone the reverse process known as mesenchymal to epithelial transition (MET) and colonize at distant organs, (21). EMT is thought to support cell invasiveness but restrict proliferation, thereby maintaining cancer cell survival in metastatic sites whereas MET re-activates proliferative potential (22). The famous “seed and soil” hypothesis proposed by Stephen Pagent in the Nineteenth century suggesting that tumor cells (the “seed”) have a preference to metastasize in certain organs (the ‘soil) (23). This hypothesis has since been revisited by Fidler and Langly, still holding significance in cancer research today (24, 25).
Circulating tumor cells in lung cancer
CTCs were first described by an Australian physician, Thomas Ashworth in 1869, where cancer cells in the blood were observed which resembled the cells of the primary tumor (26). CTCs play a central role in the metastatic spread of lung cancer, that is ultimately responsible for patient morbidity and mortality from the disease (27). While the concept of CTCs were described over one hundred years ago, it is only recently that they have been utilized in cancer diagnosis and prognosis (28).
Evidence has shown that the presence of CTCs in the blood correlates with poor overall survival in patients with metastatic prostate, breast and colon cancers (29–31). Patients with SCLC have on average 10 times more CTCs than patients with any other tumor type (32–34).
Molecular targeted therapies such as tyrosine kinase inhibitors (TKIs) in epidermal growth factor receptor (EGFR) mutants and anaplastic lymphoma kinase (ALK) inhibitors in ALK rearranged NSCLC patients have recently advanced the management of lung cancer for a limited proportion of patients (35–39). To determine eligibility for such targeted therapies, tumor biopsies have traditionally been necessary, increasing the likelihood of biopsy-related complications (40). Even in patients developing resistance to first line EGFR TKIs, liquid biopsies using circulating tumor DNA plasma only detect T790M mutations in ~80% of cases, particularly in low volume disease, making a repeat biopsy necessary. Tumor heterogeneity within the primary site or between primary and metastatic sites, can also create potential sampling bias, which may mask the true genetic profile of the cancer. The prospect of longitudinal sampling in order to monitor for the development of therapeutic resistance to treatments is likewise limited if invasive biopsies are essential (41, 42).
Use of CTCs as a liquid biopsy is promising for serial assessment of tumor evolution during the course of the disease and during systemic treatment in a less invasive, real-time manner, by a simple blood draw (19, 43). This liquid biopsy also provides potential for the early diagnosis of cancer and valuable insights into tumor heterogeneity and genomic diversity for the early diagnosis of cancer and guidance of clinical treatment (44, 45). A sensitive and unbiased isolation method to capture CTCs is therefore essential to provide tumoral material for analysis and potentially drive treatment decisions (46, 47).
Circulating tumor cell detection methods in lung cancer
CTCs have the potential to accompany standard screening tests and be used for molecular characterization of a tumor (48). Detection of CTCs in NSCLC has been challenging due to the rarity in circulation (a few CTCs per billion normal blood cells) and the presence of non-epithelial characteristics (49). It is therefore imperative that sensitive and specific CTC detection methods are developed and optimized to assist in better patient monitoring and management (50–54). The advantages and disadvantages of the isolation methods in lung cancer are discussed and summarized in the Table 2. A summary of the CTC lung cancer studies are highlighted in Table 3.
Table 2.
The Summary of different Circulating Tumor Cell isolation methods currently used in research.
| Isolation method | Mode of action | Advantages | Disadvantages | Examples |
|---|---|---|---|---|
| FDA approved (clinical trials) | EpCAM positive based selection | Has become the “gold standard” for validation of CTCs with an epithelial phenotype. High reproducibility. High specificity. FDA-approved method. | CTCs can undergo EMT which may result in reduced expression of epithelial markers, leading to loss of effective capturing of cells with mesenchymal characteristics following EMT. | Cellsearch (Menarini Silicon Biosystems, Italy) |
| Positive Immunoselection | EpCAM positive based selection | Ability to process larger volumes of blood for the capture of higher numbers of CTCs. | As above | GILUPI CellCollector (GILUPI Nanomedizin) (55) Ephesia CTC-chip (56) |
| Negative Immunoselection | Depletion of Leukocytes by CD45 Antibodies | Has the ability to avoid false-negative results or loss of CTCs due to phenotypic heterogeneity. | CTCs are often contaminated with remaining blood cells resulting in low purity. | RosetteSep (StemCell Technologies, Canada) (57) EPISPOT (Epithelial Immunospot Assay; France) (58) |
| Size-Based Filtration | Cells are separated using filtration to remove smaller cells in the blood (e.g., White blood cells) | Simple process. | Will exclude small sized CTCs, filter clogging and limited blood processing/filter are potential problems. | Screen Cell (France) MetaCell (Ostrava, Czech Republic) Isolation by Size of Epithelial Tumor cells (ISET) (Rarecells Diagnostics, Paris, France) (59) Microdevice- Cote's group (60) Parsortix (61) Microcavity array System (MCA) (62) |
| Density-based Filtration | Cells are separated based on different densities after centrifugation. | Cells separated into distinct layers | CTC size and density not uniform CTCs may get lost in plasma or by formation of CTC aggregates Poor sensitivity |
Ficoll Lymphoprep (Stem Cell Technologies, Vancouver, Canada) (63) OncoQuick (VWR, Radnor, PA) Accucyte (64) |
| Microfluidics | Cells are separated based on their biological or physical properties | Higher sensitivity, purity, lower cost, reduced sample size, short processing time, compatibility with downstream assays | Small CTCs of comparable size to WBCs would typically be missed Cell morphology may be altered due to high shear stress during microfiltration |
Isoflux (Fluxion Biosciences) (65) CTC iChip (Nagrath) (66, 67) ClearCell FX/Spiral Microfluidics (ClearbridgeBiomedics, Singapore) (54) Herringbone Chip (Nagrath) (31) |
| Immunomagnetic | Enriches target cells and eliminates cells that are not bound to magnetic particles | Isolate cells easily accessible | Nonspecific contamination can be from adsorption of background cells to the capturing device | MagSweeper (Jeffrey Lab, Stanford, CA) (68) AdnaTest (Qiagen, Hannover, Germany) (69) Magnetic Activated Cell Sorting System (Miltenyi Biotec, Germany) (70, 71) MagSifter (72) |
| Electrophoresis | Cells are separated based on their electrical signature using an electric field | Single-cell-level precision High accuracy and precision |
Process can be slow resulting in low sample throughput | DEPArray (Silicon Biosystems) |
| Enrichment free platforms | Cells are detected through imaging platforms with no need for enrichment due to advancements in fluorescence imaging | Multiple analysis parameters can be used to identify and characterize specific populations of interest High specificity and sensitivity. No need for enrichment. |
Potential for high speed imaging to reduce resolution thereby worsening accuracy. | HD-CTC (EPIC Sciences, California) (44, 73, 74) FastCell (SRI Biosciences) (75) CytoTrack (Denmark) (76, 77) |
Table 3.
Summary of a number of Circulating Tumor Cell studies in lung cancer.
| Study | Histology | Sample number | Isolation method | Major findings | References |
|---|---|---|---|---|---|
| Das et al., 2012 | NSCLC | 57 | FastCell | CTCs were detected in 42% of patients. | (78) |
| Devriese et al., 2012 | NSCLC | 46 | Cellsearch | CTCs were detected in 62% of patients. 30% of patients positive for CK7 and 9% positive for CK19. | (79) |
| Hiltermann et al., 2012 | SCLC | 59 | Cellsearch | Lower number of CTCs in patients with early stage SCLC. CTC decrease after one cycle of chemotherapy- no change after four cycles | (80) |
| Hirose et al., 2012 | NSCLC | 33 | Cellsearch | CTCs were detected in 36.4% of patients and 15.2% had five or more CTCs before chemotherapy. No difference in response to chemotherapy between CTC-positive and CTC-negative patients. Progressive disease higher in CTC-positive patients. | (81) |
| Hofman et al., 2012 | NSCLC | 250 | ISET | CNHC's were detected in 49% of patients corresponding to malignant (41%), uncertain malignant (6% and benign cells (2%) respectively. | (82) |
| Hou et al. 2012 | SCLC | 97 | Cellsearch | CTCs present in 85% of patients. OS of 5.4mths for ≥50 CTCs/7.5ml blood | (83) |
| Illie et al., 2012 | NSCLC | 87 | ISET | CTCs positive for ALK from 5 patients corresponded to patients having ALK-rearrangement in tumors. | (84) |
| Isobe et al., 2012 | NSCLC | 24 | Cellsearch | CTCs detected in 33.3% of patients | (85) |
| Krebs et al., 2012 | NSCLC | 45 | Cellsearch/ISET | CTCs detected in 80% of patients using ISET compared to 23% of patients using Cellsearch. Subpopulation of cells detected by ISET did not express epithelial markers | (86) |
| Naito et al., 2012 | SCLC | 51 | Cellsearch | Patients with ≥8 had worse survival than those with < 8 CTCs. | (87) |
| Punnoose et al., 2012 | NSCLC | 41 | Cellsearch | CTCs were detected in 78% of patients at baseline. High baseline CTC counts associated with response to treatment. Decreased CTCs associated with PFS. | (88) |
| Saucedo-Zeni et al., 2012 | NSCLC | 24 | GILUPI CellCollector | CTCs were successfully enriched from over 90% of patients with breast cancer or non-small cell lung cancer. | (55) |
| Wendel et al., 2012 | NSCLC | 78 | HD-CTC assay | CTCs were detected in 73% of patients. No significant difference between stages. | (89) |
| Funaki et al., 2013 | NSCLC | 130 | Rosette Sep | ITCs were detected in 74% of patients. | (90) |
| Hosokawa et al., 2013 | NSCLC | 22 | MCA | CTCs were detected in 77% of patients using the MCA system versus 32% using the Cellsearch system. MCA system also isolated CTC clusters from patients identified as CTC-negative using Cellsearch. | (91) |
| Ni et al., 2013 | NSCLC and SCLC | 11 | Cellsearch | Copy number variations reported from single CTCs similar to that of the metastatic tumor of the same patient. | (92) |
| Pailler et al., 2013 | NSCLC | 18 | Cellsearch/ISET | ALK rearrangements detected in CTCs of patients with ALK positive NSCLC enabling monitoring and testing of crizotinib. | (93) |
| Swennenhuis et al., 2013 | NSCLC and SCLC | 10 | Cellsearch | CTCs from 25% of patients were identified and single CTCs were isolated and amplified. | (94) |
| Carlsson et al., 2014 | NSCLC | 129 | HD-CTC assay | Presence of CTM combined with clinical and imaging data assisted in discriminating for diagnostic accuracy in all NSCLC patients. | (95) |
| Earhart et al., 2014 | NSCLC | 6 | Magnetic Sifter | CTCs detected in 100% of patients. | (96) |
| Illie et al., 2014 | 168 | ISET | CTCs were detected in 3% of COPD patients | (97) | |
| Juan et al., 2014 | NSCLC | 37 | Cellsearch/ISET | ALK rearrangements detected in CTCs of patients with ALK positive NSCLC enabling monitoring and testing of crizotinib. | (98) |
| Marchetti et al., 2014 | NSCLC | 37 | Cellsearch | CTCs were detected in 41% of patients. EGFR mutations identified by NGS in 84% of patients. | (99) |
| Muinelo –Romay et al., 2014 | NSCLC | 43 | Cellsearch | At baseline 41.9% of patients were positive for CTCs. Patients with ≥5 baseline had worse PFS and OS. Patients with increased levels of CTCs has worse PFS and OS. | (100) |
| Nel et al., 2014 | NSCLC | 43 | Negative depletion | Increased CD133-positive to pan-CK-positive cell type ratio (stem like to epithelial ratio) and presence of mesenchymal N-cad-positive cells, associated with shorter PFS. | (101) |
| Normanno et al., 2014 | SCLC | 60 | Cellsearch | At baseline 90% of patients were positive for CTCs and strongly associated with organs involved. CTC reduction as high as 89% following chemotherapy. | (102) |
| Chudsama et al., 2015 | NSCLC | 20 | Screen Cell | An increase in CTCs following EC observed in 75% of patients. Could have implications for tumor dissemination and metastatic spread. | (103) |
| Dorsey et al., 2015 | NSCLC | 23 | Density gradient centrifugation | CTCs positive in 65% of patients. CTC count reflect clinical course and response to treatment. | (104) |
| Tu et al., 2015 | NSCLC and SCLC | 18 | Cellsearch | CSFTC were positive in 78% of MRI confirmed LM samples. CSFTC clusters were observed in 67% of patients. | (105) |
| Aieta et al., 2016 | NSCLC | 1 | Cellsearch | Presense of EML4-ALK+ CTCs at baseline. EML4-ALK+ CTCs could be interpreted as resistance sign to crizotinib treatment leading to progressive disease. | (106) |
| Cheng et al., 2016 | SCLC | 89 | Cellsearch | CTCs positive in 87.6% of patients. CTC count independent indicator for PFS and OS. | (107) |
| Crosbie et al., 2016 | NSCLC | 27 | Cellsearch | CTCs positive in 22% of patients at baseline. CTC detection at baseline associated with reduced DFS and 3-year survival. | (108) |
| Hanssen et al., 2016 | NSCLC | 48 | Cellsearch | CTCs positive in 15% of patients. CTC positivity was associated with patient disease state. | (109) |
| He et al., 2016 | NSCLC | 66 | Cellsearch | Presence of CTCs at baseline associated with significantly shorter PFS. | (110) |
| Morrow et al., 2016 | NSCLC | 1 | Cellsearch | CDX derived from CTCs enriched from NSCLC patient. | (111) |
| Nicolazzo et al., 2016 | NSCLC | 24 | Cellsearch | Patients with PD-L1 negative CTCs all had clinical benefit, while patients with PD-L1 (+) CTCs all experienced progressive disease. | (112) |
| Tan et al., 2016 | NSCLC | 27 | ClearCell FX | CTCs positive in 100% of patients, 14 were ALK-positive. | (113) |
| Zhang et al., 2016 | NSCLC | 46 | Negative immunoselection | CTCs positive in 87% of patients. CTC count of more than eight prior to chemotherapy was a strong predictor of PFS. | (114) |
| Chudsama et al., 2017 | NSCLC | 10 | ScreenCell | A significant increase in CTCs was observed from baseline levels following lung manipulation. | (115) |
| Chudsama et al., 2017 | NSCLC | 23 | ScreenCell | CTCs positive in 78.3% and 73.9% reviewed by 2 pathologists. | (116) |
| Coco et al., 2017 | NSCLC | 73 | ScreenCell | Baseline CTC count had no significant association with OS or PFS. | (117) |
| Illie et al., 2017 | NSCLC | Cellsearch/ISET | CTCs positive in 32% of patients evaluated on Cellsearch. CTCs positive in 75% of patients evaluated on ISET. Expression of MET was positive in 72% of cases. | (118) | |
| Lindsay et al., 2017 | NSCLC | 125 | Cellsearch | CTCs positive in 40.8% of patients. Patients with ≥2 CTCs at baseline had poorer prognosis. | (119) |
| Messaritakis et al., 2017 | SCLC | 64 | Cellsearch | CTCs positive in 50% of patients before treatment. Pazopanib treatment significantly reduced proportion of patients with increased CTC numbers. High CTC number at baseline correlated with reduced PFS and OS. Detection of VEGFR2+ CTCs during treatment could be associated with resistance to pazopanib. | (120) |
| Messaritakis et al., 2017 | SCLC | 108 | Cellsearch | CTCs positive in 60.2% of patients at baseline. Presence of proliferative (CK67+) and non-proliferative (Ki67-), apoptotic (M30+) and non-apoptotic (M30-) as well as EMT (Vim+) CTCs were present in the same patient. | (121) |
| Pailler et al., 2017 | NSCLC | 39 | Cellsearch/ISET | Significant association between the decrease in CTC number with ALK-CNG on crizotinib and longer PFS. ALK-CNG may be a predictive biomarker for crizotinib efficacy in ALK-rearranged NSCLC patients. | (122) |
| Salgia et al., 2017 | SCLC | 42 | Cellsearch | CTCs positive in 83% of patients at baseline. Presence of CTCs at baseline were prognostic of shorter PFS and OS. | (123) |
| Tong et al., 2017 | NSCLC | 127 | Negative immunoselection | CTCs positive in 80.31% of patients at baseline. Patients with post-treatment increases in CTC count had poorer OS and PFS than those without increases. Baseline CTC count and change in CTC count during treatment were valuable prognostic indicators for NSCLC. | (124) |
| Wang et al., 2017 | SCLC | 42 | Negative immunomagnetic enrichment | CTCs positive in 76.19% of patients with SCLC and negative in controls. PFS correlates with CTC numbers and the change in CTC numbers after 1 cycle of chemotherapy. | (125) |
| Yang et al., 2017 | NSCLC | 107 | Cellsearch | CTCs positive in 44% of patients at baseline. CTC >5 at baseline was a strong negative predictor of PFS and TTF. Five or more CTCs on day 28 were strongly associated with a poor PFS. | (126) |
| Yuanling et al., 2017 | NSCLC | 105 | Cellsearch | CTCs positive (≥2) in 29% of patients at baseline and 9% had ≥5 CTCs. CTC count of ≥5 CTCs correlated with poor PFS and OS. | (127) |
| Alamgeer et al., 2018 | SCLC | 28 | Cellsearch | At baseline, two or more CTCs were detected in 86.6% of patients. | (128) |
| Guibert et al., 2018 | NSCLC | 96 | ISET | CTCs positive in 93% of patients at baseline. CTCs more frequently PD-L1+ than tissue (83 vs. 41%). Pre-treatment high CTC counts associated with increased risk of death and progression. Pre-treatment PD-L1+CTCs associated with bad prognosis in patients treated with PD-1 inhibitors. | (129) |
| Milano et al., 2018 | NSCLC | 10 | Density gradient centrifugation | CTCs undergoing EMT (CTCsEMT) positive in 30% of patients. CTCsEMT detection related to poor therapeutic response. | (130) |
| Tong et al., 2018 | NSCLC | 43 | Negative immunoselection | CTCs positive in 76.7% of patients at baseline. CTC count was a strong predictor of PFS and OS. | (131) |
NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; CTC, circulating tumor cells; CNHCs, circulating non-hematological cells; ITC, isolated tumor cells; CTM, circulating tumor microemboli; OS, overall survival; PFS, progression-free survival; TTF, time-to-treatment failure; COPD, chronic obstructive pulmonary disease; EC, endobronchial cryotherapy; CSFTC, cerebrospinal fluid tumor cell; MRI, Magnetic Resonance Imaging; LM, leptomeningeal metastasis; NGS, next-generation sequencing; CDX, cell line-derived xenograft.
Ex-vivo expansion of circulating tumor cells
Despite limitations of current CTC isolation techniques, these cells have been detected in a number of cancers, including breast, head, and neck cancer, lung, prostate, colon and gastric cancer (21, 50, 53, 109, 132, 133–135). Successful ex-vivo culture of CTCs represents a “Holy Grail” in the study of cancer metastasis as it allows for in depth characterization of metastasis initiating cells as well as the testing of functional assays (136).
Short-term CTC culture (3–14 days) has been achieved in a number of cancer types, even from early stage cancers (137–139). This allows for the recapitulation of the disease in an ex vivo/in vivo setting for the testing of therapies and functional analysis (140). A summary of this is in Table 4. In comparison, long-term cultures have only been established in advanced metastatic cases where a large number of CTCs have been isolated (111, 142, 143) (Table 5). Long-term culture studies have shown that some CTCs in patient blood are immortalized and can be cultured ex vivo into stable cell lines (Figure 1) (139). There are only a few reports of successful long-term culture, notably, in patients with advanced stages of disease (136, 145, 146). CTC-expansion has been limited due to the influence of CTC enrichment. Certain cancers also require specific culture conditions for primary and metastatic samples (136). The successful culture of CTCs long-term holds great promise in developing personalized cancer treatment for testing of therapeutic efficacy using drug screening (140). This approach could assist in determining the choice of therapeutic regimen beneficial for patients and hence holds significance in advancement of precision medicine and personalized oncology (139).
Table 4.
Summary of short-term Circulating Tumor Cell culture in Lung cancer.
| Study | Method of CTC isolation | CTC culture conditions | Group size | Morphology | Reference |
|---|---|---|---|---|---|
| Zhang et al., 2014 | In situ capture and culture | 4 Culture Conditions: 1. 3D co: Collagen, matrigel and cancer associated fibroblasts (from pancreatic tumor) 2. 3D mono: Only gel culture 3. 2D co: Only cancer associated fibroblasts 4. 2D mono: No gel or fibroblasts |
14 | Spheroids | (141) |
Table 5.
Summary of long-term Circulating Tumor Cell culture in Lung cancer.
| Study | Histology | Method of CTC isolation | CTC culture conditions | Group size | CTC lines established | Morphology/Histology | References |
|---|---|---|---|---|---|---|---|
| Hodgkinson et al., 2014 | SCLC | RosetteSep/ Ficoll/ xenotransplantation | Xenotransplantation | 6 | Morphology of CDX macrometastases: 1. Clusters 2. Sheets of densely packed small round or oval cells 3. Scant cytoplasm 4. Enlarged/inconspicuous nuclei 5. Speckled chromatin 6. Focal nuclear molding |
(142) | |
| Hamilton et al., 2015 | SCLC | Ficoll-Hypaque | RPMI 1640 medium, serum-free (insulin, IGF-1, selenite) | 30 | 3 | Spheroids or attached | (143) |
| Morrow et al., 2016 | NSCLC | RosetteSep/Ficoll/ xenotransplantation | Xenotransplantation | 1 | Morphology of CDX macrometastases: 1. Diffuse sheets of large polygonal cells 2. Abundant eosinophilic cytoplasm 3. Vesicular chromatin 4. Enlarged nucleoli |
(111) | |
| Drapkin et al., 2018 | SCLC | RosetteSep/Ficoll/ xenotransplantation | Xenotransplantation | 46 | Cytoplasmic expressions of chromogranin, synaptophysin and/or CD56 as well as the lack of CD45 expression confirmed diagnosis | (144) |
Figure 1.
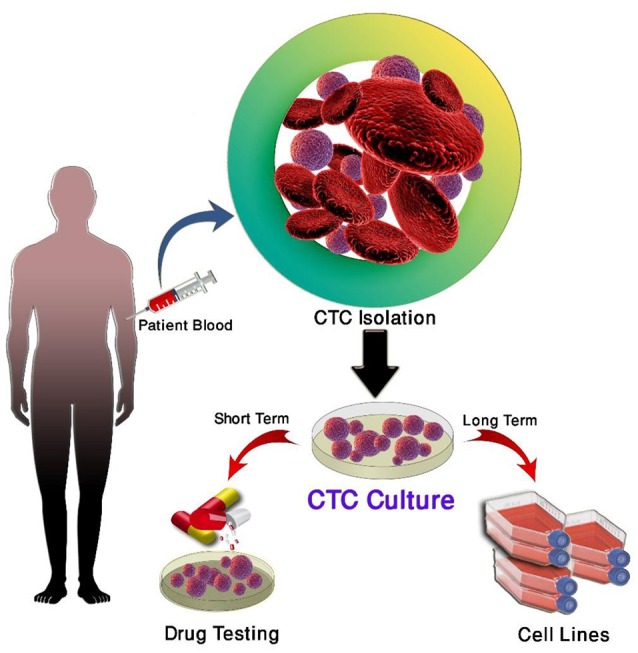
Culture of circulating tumor cells.
Three main strategies are used for the propagation of CTCs in culture; two-dimensional (2D) culture, very commonly used for expansion of CTCs short-term, three-dimensional (3D) culture used for long-term expansion and xenotransplantation and four dimensional (4D) shown to mimic the process of metastasis (137, 147–150).
The expansion of CTCs in-vivo to generate patient derived xenografts (PDXs) may also be used to comprehensively analyse advanced disease biology and present a valuable model to understand cancer metastasis. The use of PDX's have been shown to mimic patient's disease and mirror response to chemotherapy (e.g., Platinum agents) (142, 151). However, PDXs have been challenging due to CTC heterogeneity causing unreliability of these models to translate clinically. PDX model development also takes 4–8 months and therefore are not optimal for rapid studies necessary for patients with advanced disease (151). In an ideal world cancer cell lines would be routinely generated from each cancer patient but this is not realistic at present (136, 139, 152).
Clinical significance
The immediate need for early detection of lung cancer recurrence and monitoring treatment response is essential to facilitate improved survival of patients. Previous studies have shown computerized tomography (CT) screening has helped to reduce mortality, however CT has risks such as radiation exposure, leading to an increased risk of long-term cancer (153). This signifies the need for less invasive techniques for the early detection of metastasis and aid the personalized treatment of lung cancer. The use of CTCs as a liquid biopsy has the potential to accompany standard screening tests and also allow for molecular and genetic characterization of the tumor (48).
Enumeration of CTCs could provide a biomarker for cancer surveillance following treatment of early, locally advanced and advanced lung cancer and provided a better understanding on the mechanisms of metastasis (33). Although chemotherapy, targeted small molecules and immune checkpoint inhibitor therapies have shown significant benefits, the occurrence of acquired drug resistance and disease relapse are very common. Through serial sampling a longitudinal analysis of CTCs for identification of tumor evolution could provide valuable insights into mechanisms underlying resistance (154).
Detection of CTCs in lung cancer has been challenging, as CTCs usually present with non-epithelial characteristics (49). This emphasizes the need for more sensitive technologies to better capture CTCs for in-depth characterization and functional studies using cell culture and xenograft models. This will then ultimately assist in optimizing personalized therapies for lung cancer patients, with CTCs potentially being a prognostic biomarker.
Conclusion
The clinical significance of CTCs is yet to be established, however, advances in CTC detection and single-cell profiling have significantly improved our knowledge of underlying mechanisms of the evolution and dissemination of cancer and is progressively being translated to clinical studies. With lung cancer being the largest cause of cancer mortality worldwide, one of the biggest challenges for managing and treating patients is the lack of early screening/diagnostic methods (4). The isolation of CTCs from cerebrospinal fluid (CSF), may represent a unique subpopulation CTCs with ability to survive the journey in blood circulation and subsequent invasion of the CNS (105, 155). CTCs hold great promise as biomarkers for the early diagnosis and treatment selection of patients as well as broadening the current knowledge of metastasis (154).
Recurrence and progress of the disease, severity of symptoms and side-effects dramatically decrease patient's quality of life (QoL) (156). Therefore there is a vital need to monitor tumor evolution and understand mechanisms underlying development of therapeutic resistance.
Challenges for the field to address include the low sensitivity and specificity of current technologies prohibiting their use in current clinical settings, the large number of CTCs required for the development of CTC lines and patient xenografts for downstream functional analyses and the limited number of CTCs frequently found in patients with early stage disease (157). CTCs have demonstrated prognostic clinical utility is breast, lung and prostate cancers using the CellSearch technology (158, 159). Recent studies have demonstrated renewed interest in the FDA-approved Cellsearch platform for CTC PD-L1 analysis (160–162). These studies demonstrate how CTCs could be used to identify patients for anti PD-1/PD-L1 therapy (immunotherapy). Cellsearch relies on CTC enrichment using EpCAM (when CTCs undergo EMT, EpCAM is downregulated). As such the field is moving toward label-free technologies for CTC isolation. Currently, there are a number of technologies to enrich CTCs (i.e., Rarecyte, iChip, ISET, DEPArray, EPISPOT etc). The current label-free technologies are being validated for a number of cancers in larger clinical trials (163, 164). This is highlighted by the Cancer-ID network consortium in standardizing CTC/ctDNA and exosome isolation, analysis and reporting (165). The current gold standard in isolating CTCs from patient blood relies on the EpCAM status of these cells, thereby excluding a large majority of CTCs present in the blood of metastatic patients. Furthermore, Cellsearch does not allow for subsequent culture as the cells are fixed (166). CTCs as a liquid biopsy have valuable potential to improve early diagnosis, monitoring of disease, and direct treatment of lung cancer, however a better understanding of CTC biology is crucial for the field to move forward.
Author contributions
JK, AK, KO, CP: Idea. JK, AK, MW: Preparation of figures and tables. All authors were involved in the preparation, review and editing of the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Mr. Sadegh Ghorbani for assisting in the schematic. This study was supported by the Queensland Centre for Head and Neck funded by Atlantic Philanthropies, the Queensland Government and the Translational Research Institute (TRI) Spore grant. QUT VC Fellowship for CP. QUT postgraduate research scholarship for JK.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. (2017) 67:7–30. 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.Wong MCS, Lao XQ, Ho K-F, Goggins WB, Tse SLA. Incidence and mortality of lung cancer: global trends and association with socioeconomic status. Sci Rep. (2017) 7:14300. 10.1038/s41598-017-14513-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Insitute NNC. Cancer Stat Facts: Lung and Bronchus Cancer [Online]. NIH- National Cancer Institute (2017). Available online at: https://seer.cancer.gov/statfacts/html/lungb.html (Accessed July 17, 2017).
- 4.Ellis PM, Vandermeer R. Delays in the diagnosis of lung cancer. J Thorac Dis. (2011) 3:183–8. 10.3978/j.issn.2072-1439.2011.01.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birring SS, Peake MD. Symptoms and the early diagnosis of lung cancer. Thorax (2005) 60:268–9. 10.1136/thx.2004.032698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malik PS, Raina V. Lung cancer: prevalent trends & emerging concepts. Indian J Med Res. (2015) 141:5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsen JE, Minna JD. Molecular biology of lung cancer: clinical implications. Clin Chest Med. (2011) 32:703–40. 10.1016/j.ccm.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Australia CC. Lung Cancer [Online] (2017). Available online at: http://www.cancer.org.au/about-cancer/types-of-cancer/lung-cancer.html#jump_9 (Accessed June 27, 2017).
- 9.Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med. (2011). 32:605–44. 10.1016/j.ccm.2011.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooley ME. Symptoms in adults with lung cancer: a systematic research review. J Pain Symptom Manage (2000) 19:137–53. 10.1016/S0885-3924(99)00150-5 [DOI] [PubMed] [Google Scholar]
- 11.Institute NNC. Lung Cancer [Online]. (2017). Available online at: https://www.cancer.gov/types/lung/patient/non-small-cell-lung-treatment-pdq (Accessed July 17, 2017).
- 12.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small-cell lung cancer to gefitinib. N Engl J Med. (2004) 350:2129–39. 10.1056/NEJMoa040938 [DOI] [PubMed] [Google Scholar]
- 13.Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. Mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science (2004) 304:1497. 10.1126/science.1099314 [DOI] [PubMed] [Google Scholar]
- 14.Hanssen A, Loges S, Pantel K, Wikman H. Detection of circulating tumor cells in non-small cell lung cancer. Front Oncol. (2015) 5:207. 10.3389/fonc.2015.00207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss L. Metastasis of cancer: a conceptual history from antiquity to the 1990s. Cancer Metastasis Rev. (2000) 19:I–XI, 193–383. [PubMed] [Google Scholar]
- 16.Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R, et al. Metastatic patterns in adenocarcinoma. Cancer (2006) 106:1624–33. 10.1002/cncr.21778 [DOI] [PubMed] [Google Scholar]
- 17.Maheswaran S, Haber DA. Circulating tumor cells: a window into cancer biology and metastasis. Curr Opin Genet Dev. (2010) 20:96–9. 10.1016/j.gde.2009.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer (2002) 2:442–54. 10.1038/nrc822 [DOI] [PubMed] [Google Scholar]
- 19.Nurwidya F, Zaini J, Putra AC, Andarini S, Hudoyo A, Syahruddin E, et al. Circulating tumor cell and cell-free circulating tumor DNA in lung cancer. Chonnam Med J. (2016) 52:151–8. 10.4068/cmj.2016.52.3.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science (2013) 339:580–4. 10.1126/science.1228522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulasinghe A, Kenny L, Perry C, Thiery J-P, Jovanovic L, Vela I, et al. Impact of label-free technologies in head and neck cancer circulating tumour cells. Oncotarget (2016) 7:71223–34. 10.18632/oncotarget.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ocaña OH, Córcoles R, Fabra Á, Moreno-Bueno G, Acloque H, Vega S, et al. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell (2012) 22, 709–724. 10.1016/j.ccr.2012.10.012 [DOI] [PubMed] [Google Scholar]
- 23.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. (1989) 8:98–101. [PubMed] [Google Scholar]
- 24.Fidler IJ. The pathogenesis of cancer metastasis: theseed and soil hypothesis revisited. Nat Rev Cancer (2003) 3:453. 10.1038/nrc1098 [DOI] [PubMed] [Google Scholar]
- 25.Langley RR, Fidler IJ. The seed and soil hypothesis revisited–the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer (2011) 128:2527–35. 10.1002/ijc.26031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashworth TR. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Med J Aust. (1869) 14:146–7. [Google Scholar]
- 27.Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer (2006) 6:449–58. 10.1038/nrc1886 [DOI] [PubMed] [Google Scholar]
- 28.Hong Y, Fang F, Zhang Q. Circulating tumor cell clusters: what we know and what we expect (Review). Int J Oncol. (2016) 49:2206–16. 10.3892/ijo.2016.3747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. (2004) 351:781–91. 10.1056/NEJMoa040766 [DOI] [PubMed] [Google Scholar]
- 30.Scher HI, Jia X, de Bono JS, Fleisher M, Pienta KJ, Raghavan D, et al. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol. (2009) 10:233–9. 10.1016/s1470-2045(08)70340-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stott SL, Hsu CH, Tsukrov DI, Yu M, Miyamoto DT, Waltman BA, et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc Natl Acad Sci USA. (2010) 107:18392–7. 10.1073/pnas.1012539107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka F, Yoneda K, Hasegawa S. Circulating tumor cells (CTCs) in lung cancer: current status and future perspectives. Lung Cancer (2010) 1:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krebs MG, Sloane R, Priest L, Lancashire L, Hou JM, Greystoke A, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol. (2011) 29:1556–63. 10.1200/JCO.2010.28.7045 [DOI] [PubMed] [Google Scholar]
- 34.Alix-Panabieres C, Pantel K. Characterization of single circulating tumor cells. FEBS Lett. (2017) 591:2241–50. 10.1002/1873-3468.12662 [DOI] [PubMed] [Google Scholar]
- 35.Crinò L, Kim D, Riely GJ, Janne PA, Blackhall FH, Camidge DR, et al. Initial phase II results with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC): PROFILE 1005. J Clin Oncol. (2011) 29(15_suppl.):7514 10.1200/jco.2011.29.15_suppl.7514 [DOI] [Google Scholar]
- 36.Camidge DR, Bang Y-J, Kwak EL, Iafrate AJ, Varella-Garcia M, Fox SB, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. (2012) 13:1011–9. 10.1016/S1470-2045(12)70344-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. (2012) 13:239–46. 10.1016/s1470-2045(11)70393-x [DOI] [PubMed] [Google Scholar]
- 38.Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó L, Ahn MJ, et al. Crizotinib versus Chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. (2013) 368:2385–94. 10.1056/NEJMoa1214886 [DOI] [PubMed] [Google Scholar]
- 39.Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. (2014) 371:2167–77. 10.1056/NEJMoa1408440 [DOI] [PubMed] [Google Scholar]
- 40.Lindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, Giaccone G, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. (2013) 8:823–59. 10.1097/JTO.0b013e318290868f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. (2012) 366:883–92. 10.1056/NEJMoa1113205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moreira AL, Thornton RH. Personalized medicine for non–small-cell lung cancer: implications of recent advances in tissue acquisition for molecular and histologic testing. Clin Lung Cancer (2012) 13:334–9. 10.1016/j.cllc.2012.01.004 [DOI] [PubMed] [Google Scholar]
- 43.Alix-Panabieres C, Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. (2016) 6:479–91. 10.1158/2159-8290.CD-15-1483 [DOI] [PubMed] [Google Scholar]
- 44.Kuhn P, Bethel K. A fluid biopsy as investigating technology for the fluid phase of solid tumors. Phys Biol. (2012) 9:010301. 10.1088/1478-3975/9/1/010301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alberter B, Klein CA, Polzer B. Single-cell analysis of CTCs with diagnostic precision: opportunities and challenges for personalized medicine. Expert Rev Mol Diagn. (2016) 16:25–38. 10.1586/14737159.2016.1121099 [DOI] [PubMed] [Google Scholar]
- 46.Alix-Panabieres C, Pantel K. Circulating tumor cells: liquid biopsy of cancer. Clin Chem. (2013) 59:110–8. 10.1373/clinchem.2012.194258 [DOI] [PubMed] [Google Scholar]
- 47.Alix-Panabieres C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer (2014) 14:623–31. 10.1038/nrc3820 [DOI] [PubMed] [Google Scholar]
- 48.Murlidhar V, Ramnath N, Nagrath S, Reddy RM. Optimizing the detection of circulating markers to aid in early lung cancer detection. Cancers (2016) 8:61. 10.3390/cancers8070061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lianidou ES, Markou A, Strati A. The role of CTCs as tumor biomarkers. Adv Exp Med Biol. (2015) 867:341–67. 10.1007/978-94-017-7215-0_21 [DOI] [PubMed] [Google Scholar]
- 50.Yu M, Stott S, Toner M, Maheswaran S, Haber DA. Circulating tumor cells: approaches to isolation and characterization. J Cell Biol. (2011) 192:373–82. 10.1083/jcb.201010021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harouaka R, Kang Z, Zheng SY, Cao L. Circulating tumor cells: advances in isolation and analysis, and challenges for clinical applications. Pharmacol Ther. (2014) 141:209–21. 10.1016/j.pharmthera.2013.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caceres G, Puskas JA, Magliocco AM. Circulating tumor cells: a window into tumor development and therapeutic effectiveness. Cancer Control. (2015) 22:167–76. 10.1177/107327481502200207 [DOI] [PubMed] [Google Scholar]
- 53.Yang MH, Imrali A, Heeschen C. Circulating cancer stem cells: the importance to select. Chin J Cancer Res. (2015) 27:437–49. 10.3978/j.issn.1000-9604.2015.04.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Warkiani ME, Khoo BL, Wu L, Tay AK, Bhagat AA, Han J, et al. Ultra-fast, label-free isolation of circulating tumor cells from blood using spiral microfluidics. Nat Protoc. (2016) 11:134–48. 10.1038/nprot.2016.003 [DOI] [PubMed] [Google Scholar]
- 55.Saucedo-Zeni N, Mewes S, Niestroj R, Gasiorowski L, Murawa D, Nowaczyk P, et al. A novel method for the in vivo isolation of circulating tumor cells from peripheral blood of cancer patients using a functionalized and structured medical wire. Int J Oncol. (2012) 41:1241–50. 10.3892/ijo.2012.1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saliba A-E, Saias L, Psychari E, Minc N, Simon D, Bidard F-C, et al. Microfluidic sorting and multimodal typing of cancer cells in self-assembled magnetic arrays. Proc Natl Acad Sci USA. (2010) 107:14524–9. 10.1073/pnas.1001515107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He W, Kularatne SA, Kalli KR, Prendergast FG, Amato RJ, Klee GG, et al. Quantitation of circulating tumor cells in blood samples from ovarian and prostate cancer patients using tumor-specific fluorescent ligands. Int J Cancer (2008) 123:1968–73. 10.1002/ijc.23717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alix-Panabieres C. EPISPOT assay: detection of viable DTCs/CTCs in solid tumor patients. Recent Results Cancer Res. (2012) 195:69–76. 10.1007/978-3-642-28160-0_6 [DOI] [PubMed] [Google Scholar]
- 59.Hofman V, Bonnetaud C, Ilie MI, Vielh P, Vignaud JM, Flejou JF, et al. Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin Cancer Res. (2011) 17:827–35. 10.1158/1078-0432.CCR-10-0445 [DOI] [PubMed] [Google Scholar]
- 60.Lin HK, Zheng S, Williams AJ, Balic M, Groshen S, Scher HI, et al. Portable filter-based microdevice for detection and characterization of circulating tumor cells. Clin Cancer Res. (2010) 16:5011–8. 10.1158/1078-0432.ccr-10-1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Obermayr E, Maritschnegg E, Speiser P, Singer C, Schuster EM, Danzinger S, et al. Circulating rare cells enable highly efficient cancer detection. Cancer Res. (2015) 75(15 Suppl.). 10.1158/1538-7445.am2015-lb-197. [DOI] [Google Scholar]
- 62.Hosokawa M, Yoshikawa T, Negishi R, Yoshino T, Koh Y, Kenmotsu H, et al. Microcavity array system for size-based enrichment of circulating tumor cells from the blood of patients with small-cell lung cancer. Anal Chem. (2013) 85:5692–8. 10.1021/ac400167x [DOI] [PubMed] [Google Scholar]
- 63.Weitz J, Kienle P, Lacroix J, Willeke F, Benner A, Lehnert T, et al. Dissemination of tumor cells in patients undergoing surgery for colorectal cancer. Clin Cancer Res. (1998) 4:343–8. [PubMed] [Google Scholar]
- 64.Campton DE, Ramirez AB, Nordberg JJ, Drovetto N, Clein AC, Varshavskaya P, et al. High-recovery visual identification and single-cell retrieval of circulating tumor cells for genomic analysis using a dual-technology platform integrated with automated immunofluorescence staining. BMC Cancer (2015) 15:360. 10.1186/s12885-015-1383-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harb W, Fan A, Tran T, Danila DC, Keys D, Schwartz M, et al. Mutational analysis of circulating tumor cells using a novel microfluidic collection device and qPCR assay. Transl Oncol. (2013) 6:528–38. 10.1593/tlo.13367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature (2007) 450:1235–9. 10.1038/nature06385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sequist LV, Nagrath S, Toner M, Haber DA, Lynch TJ. The CTC-chip: an exciting new tool to detect circulating tumor cells in lung cancer patients. J Thorac Oncol. (2009) 4:281–3. 10.1097/JTO.0b013e3181989565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Talasaz AH, Powell AA, Huber DE, Berbee JG, Roh K-H, Yu W, et al. Isolating highly enriched populations of circulating epithelial cells and other rare cells from blood using a magnetic sweeper device. Proc Natl Acad Sci USA. (2009) 106:3970–5. 10.1073/pnas.0813188106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zieglschmid V, Hollmann C, Gutierrez B, Albert W, Strothoff D, Gross E, et al. Combination of immunomagnetic enrichment with multiplex RT-PCR analysis for the detection of disseminated tumor cells. Anticancer Res. (2005) 25:1803–10. [PubMed] [Google Scholar]
- 70.Miltenyi S, Muller W, Weichel W, Radbruch A. High gradient magnetic cell separation with MACS. Cytometry (1990) 11:231–8. 10.1002/cyto.990110203 [DOI] [PubMed] [Google Scholar]
- 71.Mayo C, Ortega FG, Giménez-Capitán A, Molina-Vila MA, Serrano MJ, Viteri S, et al. CK-coated magnetic-based beads as a tool to isolate circulating tumor cells (CTCs) in human tumors. Transl Lung Cancer Res. (2013) 2:65–71. 10.3978/j.issn.2218-6751.2013.02.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Earhart CM, Hughes CE, Gaster RS, Ooi CC, Wilson RJ, Zhou LY, et al. Isolation and mutational analysis of circulating tumor cells from lung cancer patients with magnetic sifters and biochips. Lab Chip. (2014) 14:78–88. 10.1039/c3lc50580d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bazhenova L, Nieva JJ, Kolatkar A, Luttgen M, Marinucci D, Bethel K, et al. Performance of the high-definition circulating tumor cells (HD-CTC) assay in patients with non-small cell lung cancer (NSCLC). J Clin Oncol. (2012) 30(15_suppl):e21074 10.1200/jco.2012.30.15_suppl.e21074 [DOI] [Google Scholar]
- 74.Marrinucci D, Bethel K, Kolatkar A, Luttgen MS, Malchiodi M, Baehring F, et al. Fluid biopsy in patients with metastatic prostate, pancreatic and breast cancers. Phys Biol. (2012) 9:016003. 10.1088/1478-3975/9/1/016003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Krivacic RT, Ladanyi A, Curry DN, Hsieh HB, Kuhn P, Bergsrud DE, et al. A rare-cell detector for cancer. Proc Natl Acad Sci USA. (2004) 101:10501–4. 10.1073/pnas.0404036101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hillig T, Horn P, Nygaard AB, Haugaard AS, Nejlund S, Brandslund I, et al. In vitro detection of circulating tumor cells compared by the CytoTrack and CellSearch methods. Tumour Biol. (2015) 36:4597–601. 10.1007/s13277-015-3105-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ferreira MM, Ramani VC, Jeffrey SS. Circulating tumor cell technologies. Mol Oncol. (2016) 10:374–94. 10.1016/j.molonc.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Das M, Riess JW, Frankel P, Schwartz E, Bennis R, Hsieh HB, et al. ERCC1 expression in circulating tumor cells (CTCs) using a novel detection platform correlates with progression-free survival (PFS) in patients with metastatic non-small-cell lung cancer (NSCLC) receiving platinum chemotherapy. Lung Cancer (2012) 77:421–6. 10.1016/j.lungcan.2012.04.005 [DOI] [PubMed] [Google Scholar]
- 79.Devriese LA, Bosma AJ, van de Heuvel MM, Heemsbergen W, Voest EE, Schellens JHM. Circulating tumor cell detection in advanced non-small cell lung cancer patients by multi-marker QPCR analysis. Lung Cancer (2012) 75:242–7. 10.1016/j.lungcan.2011.07.003 [DOI] [PubMed] [Google Scholar]
- 80.Hiltermann TJ, Pore MM, van den Berg A, Timens W, Boezen HM, Liesker JJ, et al. Circulating tumor cells in small-cell lung cancer: a predictive and prognostic factor. Ann Oncol. (2012) 23:2937–42. 10.1093/annonc/mds138 [DOI] [PubMed] [Google Scholar]
- 81.Hirose T, Murata Y, Oki Y, Sugiyama T, Kusumoto S, Ishida H, et al. Relationship of circulating tumor cells to the effectiveness of cytotoxic chemotherapy in patients with metastatic non-small-cell lung cancer. Oncol Res. (2012) 20:131–7. 10.3727/096504012X13473664562583 [DOI] [PubMed] [Google Scholar]
- 82.Hofman V, Long E, Ilie M, Bonnetaud C, Vignaud JM, Flejou JF, et al. Morphological analysis of circulating tumour cells in patients undergoing surgery for non-small cell lung carcinoma using the isolation by size of epithelial tumour cell (ISET) method. Cytopathology (2012) 23:30–8. 10.1111/j.1365-2303.2010.00835.x [DOI] [PubMed] [Google Scholar]
- 83.Hou JM, Krebs MG, Lancashire L, Sloane R, Backen A, Swain RK, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol. (2012) 30:525–32. 10.1200/JCO.2010.33.3716 [DOI] [PubMed] [Google Scholar]
- 84.Ilie M, Long E, Butori C, Hofman V, Coelle C, Mauro V, et al. ALK-gene rearrangement: a comparative analysis on circulating tumour cells and tumour tissue from patients with lung adenocarcinoma. Ann Oncol. (2012) 23:2907–13. 10.1093/annonc/mds137 [DOI] [PubMed] [Google Scholar]
- 85.Isobe K, Hata Y, Kobayashi K, Hirota N, Sato K, Sano G, et al. Clinical significance of circulating tumor cells and free DNA in non-small cell lung cancer. Anticancer Res. (2012) 32:3339–44. [PubMed] [Google Scholar]
- 86.Krebs MG, Hou J-M, Sloane R, Lancashire L, Priest L, Nonaka D, et al. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol. (2012) 7:306–15. 10.1097/JTO.0b013e31823c5c16 [DOI] [PubMed] [Google Scholar]
- 87.Naito T, Tanaka F, Ono A, Yoneda K, Takahashi T, Murakami H, et al. Prognostic impact of circulating tumor cells in patients with small cell lung cancer. J Thorac Oncol. (2012) 7:512–9. 10.1097/JTO.0b013e31823f125d [DOI] [PubMed] [Google Scholar]
- 88.Punnoose EA, Atwal S, Liu W, Raja R, Fine BM, Hughes BG, et al. Evaluation of circulating tumor cells and circulating tumor DNA in non-small cell lung cancer: association with clinical endpoints in a phase II clinical trial of pertuzumab and erlotinib. Clin Cancer Res. (2012) 18:2391–401. 10.1158/1078-0432.CCR-11-3148 [DOI] [PubMed] [Google Scholar]
- 89.Wendel M, Bazhenova L, Boshuizen R, Kolatkar A, Honnatti M, Cho EH, et al. Fluid biopsy for circulating tumor cell identification in patients with early and late stage non-small cell lung cancer; a glimpse into lung cancer biology. Phys Biol. (2012) 9:016005. 10.1088/1478-3967/9/1/016005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Funaki S, Sawabata N, Abulaiti A, Nakagiri T, Shintani Y, Inoue M, et al. Significance of tumour vessel invasion in determining the morphology of isolated tumour cells in the pulmonary vein in non-small-cell lung cancer. Eur J Cardiothorac Surg. (2013) 43:1126–30. 10.1093/ejcts/ezs553 [DOI] [PubMed] [Google Scholar]
- 91.Hosokawa M, Kenmotsu H, Koh Y, Yoshino T, Yoshikawa T, Naito T, et al. Size-based isolation of circulating tumor cells in lung cancer patients using a microcavity array system. PLoS ONE (2013) 8:e67466. 10.1371/journal.pone.0067466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ni X, Zhuo M, Su Z, Duan J, Gao Y, Wang Z, et al. Reproducible copy number variation patterns among single circulating tumor cells of lung cancer patients. Proc Natl Acad Sci USA. (2013) 110:21083–8. 10.1073/pnas.1320659110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pailler E, Adam J, Barthelemy A, Oulhen M, Auger N, Valent A, et al. Detection of circulating tumor cells harboring a unique ALK rearrangement in ALK-positive non-small-cell lung cancer. J Clin Oncol. (2013) 31:2273–81. 10.1200/JCO.2012.44.5932 [DOI] [PubMed] [Google Scholar]
- 94.Swennenhuis JF, Reumers J, Thys K, Aerssens J, Terstappen LW. Efficiency of whole genome amplification of single circulating tumor cells enriched by CellSearch and sorted by FACS. Genome Med. (2013) 5:106. 10.1186/gm510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Carlsson A, Nair VS, Luttgen MS, Keu KV, Horng G, Vasanawala M, et al. Circulating tumor microemboli diagnostics for patients with non-small-cell lung cancer. J Thorac Oncol. (2014) 9:1111–9. 10.1097/JTO.0000000000000235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Earhart CM, Hughes CE, Gaster RS, Ooi CC, Wilson RJ, Zhou LY, et al. Isolation and mutational analysis of circulating tumor cells from lung cancer patients with magnetic sifters and biochips. Lab Chip (2014) 14:78–88. 10.1039/C3LC50580D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ilie M, Hofman V, Long-Mira E, Selva E, Vignaud JM, Padovani B, et al. “Sentinel” circulating tumor cells allow early diagnosis of lung cancer in patients with chronic obstructive pulmonary disease. PLoS ONE (2014) 9:e111597. 10.1371/journal.pone.0111597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Juan O, Vidal J, Gisbert R, Munoz J, Macia S, Gomez-Codina J. Prognostic significance of circulating tumor cells in advanced non-small cell lung cancer patients treated with docetaxel and gemcitabine. Clin Transl Oncol. (2014) 16:637–43. 10.1007/s12094-013-1128-8 [DOI] [PubMed] [Google Scholar]
- 99.Marchetti A, Del Grammastro M, Felicioni L, Malatesta S, Filice G, Centi I, et al. Assessment of EGFR mutations in circulating tumor cell preparations from NSCLC patients by next generation sequencing: toward a real-time liquid biopsy for treatment. PLoS ONE (2014) 9:e103883. 10.1371/journal.pone.0103883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Muinelo-Romay L, Vieito M, Abalo A, Nocelo MA, Baron F, Anido U, et al. Evaluation of circulating tumor cells and related events as prognostic factors and surrogate biomarkers in advanced NSCLC patients receiving first-line systemic treatment. Cancers (2014) 6:153–65. 10.3390/cancers6010153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nel I, Jehn U, Gauler T, Hoffmann AC. Individual profiling of circulating tumor cell composition in patients with non-small cell lung cancer receiving platinum based treatment. Transl Lung Cancer Res. (2014) 3:100–6. 10.3978/j.issn.2218-6751.2014.03.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Normanno N, Rossi A, Morabito A, Signoriello S, Bevilacqua S, Di Maio M, et al. Prognostic value of circulating tumor cells' reduction in patients with extensive small-cell lung cancer. Lung Cancer (2014) 85:314–9. 10.1016/j.lungcan.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 103.Chudasama D, Rice A, Soppa G, Anikin V. Circulating tumour cells in patients with lung cancer undergoing endobronchial cryotherapy. Cryobiology (2015) 71:161–3. 10.1016/j.cryobiol.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 104.Dorsey JF, Kao GD, MacArthur KM, Ju M, Steinmetz D, Wileyto EP, et al. Tracking viable circulating tumor cells (CTCs) in the peripheral blood of non-small cell lung cancer patients undergoing definitive radiation therapy: pilot study results. Cancer (2015) 121:139–49. 10.1002/cncr.28975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tu Q, Wu X, Le Rhun E, Blonski M, Wittwer B, Taillandier L, et al. CellSearch technology applied to the detection and quantification of tumor cells in CSF of patients with lung cancer leptomeningeal metastasis. Lung Cancer (2015) 90:352–7. 10.1016/j.lungcan.2015.09.008 [DOI] [PubMed] [Google Scholar]
- 106.Aieta M, Facchinetti A, De Faveri S, Manicone M, Tartarone A, Possidente L, et al. Monitoring and characterization of circulating tumor cells (ctCs) in a patient with EML4-ALK–positive non–small cell lung cancer (NSCLC). Clin Lung Cancer (2016) 17:e173–7. 10.1016/j.cllc.2016.05.002 [DOI] [PubMed] [Google Scholar]
- 107.Cheng Y, Liu XQ, Fan Y, Liu YP, Liu Y, Liu Y, et al. Circulating tumor cell counts/change for outcome prediction in patients with extensive-stage small-cell lung cancer. Future Oncol. (2016) 12:789–99. 10.2217/fon.15.346 [DOI] [PubMed] [Google Scholar]
- 108.Crosbie PAJ, Shah R, Krysiak P, Zhou C, Morris K, Tugwood J, et al. Circulating tumor cells detected in the tumor-draining pulmonary vein are associated with disease recurrence after surgical resection of NSCLC. J Thorac Oncol. (2016) 11:1793–7. 10.1016/j.jtho.2016.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hanssen A, Wagner J, Gorges TM, Taenzer A, Uzunoglu FG, Driemel C, et al. Characterization of different CTC subpopulations in non-small cell lung cancer. Sci Rep. (2016) 6:28010. 10.1038/srep28010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhou J, Dong F, Cui F, Xu R, Tang X. The role of circulating tumor cells in evaluation of prognosis and treatment response in advanced non-small-cell lung cancer. Cancer Chemother Pharmacol. (2017) 79:825–33. 10.1007/s00280-017-3269-x [DOI] [PubMed] [Google Scholar]
- 111.Morrow CJ, Trapani F, Metcalf RL, Bertolini G, Hodgkinson CL, Khandelwal G, et al. Tumourigenic non-small-cell lung cancer mesenchymal circulating tumour cells: a clinical case study. Ann Oncol. (2016) 27:1155–60. 10.1093/annonc/mdw122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nicolazzo C, Raimondi C, Mancini M, Caponnetto S, Gradilone A, Gandini O, et al. Monitoring PD-L1 positive circulating tumor cells in non-small cell lung cancer patients treated with the PD-1 inhibitor Nivolumab. Sci Rep. (2016) 6:31726. 10.1038/srep31726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tan CL, Lim TH, Lim TKH, Tan DS-W, Chua YW, Ang MK, et al. Concordance of anaplastic lymphoma kinase (ALK) gene rearrangements between circulating tumor cells and tumor in non-small cell lung cancer. Oncotarget (2016) 7:23251–62. 10.18632/oncotarget.8136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang Z, Xiao Y, Zhao J, Chen M, Xu Y, Zhong W, et al. Relationship between circulating tumour cell count and prognosis following chemotherapy in patients with advanced non-small-cell lung cancer. Respirology (2016) 21:519–25. 10.1111/resp.12696 [DOI] [PubMed] [Google Scholar]
- 115.Chudasama D, Burnside N, Beeson J, Karteris E, Rice A, Anikin V. Perioperative detection of circulating tumour cells in patients with lung cancer. Oncol Lett. (2017) 14:1281–6. 10.3892/ol.2017.6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chudasama D, Barr J, Beeson J, Beddow E, Mcgonigle N, Rice A, et al. Detection of circulating tumour cells and survival of patients with non-small cell lung cancer. Anticancer Res. (2017) 37:169–73. 10.21873/anticanres.11302 [DOI] [PubMed] [Google Scholar]
- 117.Coco S, Alama A, Vanni I, Fontana V, Genova C, Dal Bello MG, et al. Circulating cell-free DNA and circulating tumor cells as prognostic and predictive biomarkers in advanced non-small cell lung cancer patients treated with first-line chemotherapy. Int J Mol Sci. (2017) 18:1035 10.3390/ijms18051035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ilie M, Szafer-Glusman E, Hofman V, Long-Mira E, Suttmann R, Darbonne W, et al. Expression of MET in circulating tumor cells correlates with expression in tumor tissue from advanced-stage lung cancer patients. Oncotarget (2017) 8:26112–21. 10.18632/oncotarget.15345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lindsay CR, Faugeroux V, Michiels S, Pailler E, Facchinetti F, Ou D, et al. A prospective examination of circulating tumor cell profiles in non-small-cell lung cancer molecular subgroups. Ann Oncol. (2017) 28:1523–31. 10.1093/annonc/mdx156 [DOI] [PubMed] [Google Scholar]
- 120.Messaritakis I, Politaki E, Plataki M, Karavassilis V, Kentepozidis N, Koinis F, et al. Heterogeneity of circulating tumor cells (CTCs) in patients with recurrent small cell lung cancer (SCLC) treated with pazopanib. Lung Cancer (2017) 104:16–23. 10.1016/j.lungcan.2016.12.008 [DOI] [PubMed] [Google Scholar]
- 121.Messaritakis I, Politaki E, Kotsakis A, Dermitzaki E-K, Koinis F, Lagoudaki E, et al. Phenotypic characterization of circulating tumor cells in the peripheral blood of patients with small cell lung cancer. PLoS ONE (2017) 12:e0181211. 10.1371/journal.pone.0181211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pailler E, Oulhen M, Borget I, Remon J, Ross K, Auger N, et al. Circulating tumor cells with aberrant ALK copy number predict progression-free survival during crizotinib treatment in ALK-rearranged non-small cell lung cancer patients. Cancer Res. (2017) 77:2222–30. 10.1158/0008-5472.can-16-3072 [DOI] [PubMed] [Google Scholar]
- 123.Salgia R, Weaver RW, McCleod M, Stille JR, Yan SB, Roberson S, et al. Prognostic and predictive value of circulating tumor cells and CXCR4 expression as biomarkers for a CXCR4 peptide antagonist in combination with carboplatin-etoposide in small cell lung cancer: exploratory analysis of a phase II study. Invest New Drugs (2017) 35:334–44. 10.1007/s10637-017-0446-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tong B, Xu Y, Zhao J, Chen M, Xing J, Zhong W, et al. Prognostic significance of circulating tumor cells in non-small cell lung cancer patients undergoing chemotherapy. Oncotarget (2017) 8:86615–24. 10.18632/oncotarget.21255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang YL, Liu CH, Li J, Ma XP, Gong P. Clinical significance of circulating tumor cells in patients with small-cell lung cancer. Tumori J. (2017) 103:242–8. 10.5301/tj.5000601 [DOI] [PubMed] [Google Scholar]
- 126.Yang B, Qin A, Zhang K, Ren H, Liu S, Liu X, et al. Circulating tumor cells predict prognosis following tyrosine kinase inhibitor treatment in EGFR-mutant non-small cell lung cancer patients. Oncol Res. (2017) 25:1601–6. 10.3727/096504017X14928634401178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Qi Y, Wang W. Clinical significance of circulating tumor cells in squamous cell lung cancer patients. Cancer Biomark (2017) 18:161–7. 10.3233/cbm-160090 [DOI] [PubMed] [Google Scholar]
- 128.Alamgeer M, Neil Watkins D, Banakh I, Kumar B, Gough DJ, Markman B, et al. A phase IIa study of HA-irinotecan, formulation of hyaluronic acid and irinotecan targeting CD44 in extensive-stage small cell lung cancer. Investig New Drugs (2018) 36:288–98. 10.1007/s10637-017-0555-8 [DOI] [PubMed] [Google Scholar]
- 129.Guibert N, Delaunay M, Lusque A, Boubekeur N, Rouquette I, Clermont E, et al. PD-L1 expression in circulating tumor cells of advanced non-small cell lung cancer patients treated with nivolumab. Lung Cancer (2018) 120:108–12. 10.1016/j.lungcan.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 130.Milano A, Mazzetta F, Valente S, Ranieri D, Leone L, Botticelli A, et al. Molecular detection of EMT markers in circulating tumor cells from metastatic non-small cell lung cancer patients: potential role in clinical practice. Anal Cell Pathol. (2018) 2018:3506874. 10.1155/2018/3506874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tong B, Xu Y, Zhao J, Chen M, Zhong W, Xing J, et al. Prognostic role of circulating tumor cells in patients with EGFR-mutated or ALK-rearranged non-small cell lung cancer. Thorac Cancer (2018) 9:640–5. 10.1111/1759-7714.12631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hristozova T, Konschak R, Stromberger C, Fusi A, Liu Z, Weichert W, et al. The presence of circulating tumor cells (CTCs) correlates with lymph node metastasis in nonresectable squamous cell carcinoma of the head and neck region (SCCHN). Ann Oncol. (2011) 22:1878–85. 10.1093/annonc/mdr130 [DOI] [PubMed] [Google Scholar]
- 133.Baccelli I, Schneeweiss A, Riethdorf S, Stenzinger A, Schillert A, Vogel V, et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol. (2013) 31:539–44. 10.1038/nbt.2576 [DOI] [PubMed] [Google Scholar]
- 134.Kulasinghe A, Perry C, Warkiani ME, Blick T, Davies A, O'Byrne K, et al. Short term ex-vivo expansion of circulating head and neck tumour cells. Oncotarget (2016) 7:60101–9. 10.18632/oncotarget.11159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kulasinghe A, Tran TH, Blick T, O'Byrne K, Thompson EW, Warkiani ME, et al. Enrichment of circulating head and neck tumour cells using spiral microfluidic technology. Sci Rep. (2017) 7:42517. 10.1038/srep42517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Maheswaran S, Haber DA. Ex Vivo Culture of CTCs: an emerging resource to guide cancer therapy. Cancer Res. (2015) 75:2411–5. 10.1158/0008-5472.CAN-15-0145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gao D, Vela I, Sboner A, Iaquinta PJ, Karthaus WR, Gopalan A, et al. Organoid cultures derived from patients with advanced prostate cancer. Cell (2014) 159:176–87. 10.1016/j.cell.2014.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Alix-Panabieres C, Bartkowiak K, Pantel K. Functional studies on circulating and disseminated tumor cells in carcinoma patients. Mol Oncol. (2016) 10:443–9. 10.1016/j.molonc.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang R, Chu GCY, Mrdenovic S, Annamalai AA, Hendifar AE, Nissen NN, et al. Cultured circulating tumor cells and their derived xenografts for personalized oncology. Asian J Urol. (2016) 3:240–53. 10.1016/j.ajur.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Khoo BL, Grenci G, Jing T, Lim YB, Lee SC, Thiery JP, et al. Liquid biopsy and therapeutic response: Circulating tumor cell cultures for evaluation of anticancer treatment. Sci Adv. (2016) 2:e1600274. 10.1126/sciadv.1600274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang Z, Shiratsuchi H, Lin J, Chen G, Reddy RM, Azizi E, et al. Expansion of CTCs from early stage lung cancer patients using a microfluidic co-culture model. Oncotarget (2014) 5:12383–97. 10.18632/oncotarget.2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hodgkinson CL, Morrow CJ, Li Y, Metcalf RL, Rothwell DG, Trapani F, et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat Med. (2014) 20:897–903. 10.1038/nm.3600 [DOI] [PubMed] [Google Scholar]
- 143.Hamilton G, Burghuber O, Zeillinger R. Circulating tumor cells in small cell lung cancer: ex vivo expansion. Lung (2015) 193:451–2. 10.1007/s00408-015-9725-7 [DOI] [PubMed] [Google Scholar]
- 144.Drapkin BJ, George J, Christensen CL, Mino-Kenudson M, Dries R, Sundaresan T, et al. Genomic and functional fidelity of small cell lung cancer patient-derived xenografts. Cancer Discov. 8:600–15. 10.1158/2159-8290.CD-17-0935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pantel K, Alix-Panabières C. Cell lines from circulating tumor cells. Oncoscience (2015) 2:815–6. 10.18632/oncoscience.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pantel K, Alix-Panabieres C. Functional studies on viable circulating tumor cells. Clin Chem. (2016) 62:328–34. 10.1373/clinchem.2015.242537 [DOI] [PubMed] [Google Scholar]
- 147.Khoo BL, Lee SC, Kumar P, Tan TZ, Warkiani ME, Ow SG, et al. Short-term expansion of breast circulating cancer cells predicts response to anti-cancer therapy. Oncotarget (2015) 6:15578–93. 10.18632/oncotarget.3903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kolostova K, Spicka J, Matkowski R, Bobek V. Isolation, primary culture, morphological and molecular characterization of circulating tumor cells in gynecological cancers. Am J Transl Res. (2015) 7:1203–13. [PMC free article] [PubMed] [Google Scholar]
- 149.Mishra DK, Creighton CJ, Zhang Y, Chen F, Thrall MJ, Kim MP. Ex vivo four-dimensional lung cancer model mimics metastasis. Ann Thorac Surg. (2015) 99:1149–56. 10.1016/j.athoracsur.2014.08.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mishra DK, Miller RA, Pence KA, Kim MP. Small cell and non small cell lung cancer form metastasis on cellular 4D lung model. BMC Cancer (2018) 18:441. 10.1186/s12885-018-4358-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hidalgo M, Amant F, Biankin AV, Budinska E, Byrne AT, Caldas C, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. (2014) 4:998–1013. 10.1158/2159-8290.CD-14-0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dive C, Brady G. Snapshot: circulating tumor cells. Cell (2017) 168:742.e741. 10.1016/j.cell.2017.01.026 [DOI] [PubMed] [Google Scholar]
- 153.Albert JM. Radiation risk from CT: implications for cancer screening. Am J Roentgenol. (2013) 201:W81–7. 10.2214/AJR.12.9226 [DOI] [PubMed] [Google Scholar]
- 154.Krebs MG, Metcalf RL, Carter L, Brady G, Blackhall FH, Dive C. Molecular analysis of circulating tumour cells-biology and biomarkers. Nat Rev Clin Oncol. (2014) 11:129–44. 10.1038/nrclinonc.2013.253 [DOI] [PubMed] [Google Scholar]
- 155.Faltas B. Circulating tumor cells in the cerebrospinal fluid: “tapping” into diagnostic and predictive potential. Oncotarget (2011) 2:822. 10.18632/oncotarget.349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Polanski J, Jankowska-Polanska B, Rosinczuk J, Chabowski M, Szymanska-Chabowska A. Quality of life of patients with lung cancer. Onco Targets Ther. (2016) 9:1023–8. 10.2147/OTT.S100685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Man Y, Wang Q, Kemmner W. Currently used markers for CTC isolation - advantages, limitations and impact on cancer prognosis. J Clin Exp Path. (2011) 1:102 10.4172/2161-0681.1000102 [DOI] [Google Scholar]
- 158.Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. (2004) 10:6897–904. 10.1158/1078-0432.ccr-04-0378 [DOI] [PubMed] [Google Scholar]
- 159.Bidard FC, Michiels S, Riethdorf S, Mueller V, Esserman LJ, Lucci A, et al. Circulating tumor cells in breast cancer patients treated by neoadjuvant chemotherapy: a meta-analysis. J Natl Cancer Inst. (2018) 110:560–7. 10.1093/jnci/djy018 [DOI] [PubMed] [Google Scholar]
- 160.Mazel M, Jacot W, Pantel K, Bartkowiak K, Topart D, Cayrefourcq L, et al. Frequent expression of PD-L1 on circulating breast cancer cells. Mol Oncol. (2015) 9:1773–82. 10.1016/j.molonc.2015.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kulasinghe A, Perry C, Kenny L, Warkiani ME, Nelson C, Punyadeera C. PD-L1 expressing circulating tumour cells in head and neck cancers. BMC Cancer (2017) 17:333. 10.1186/s12885-017-3316-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Strati A, Koutsodontis G, Papaxoinis G, Angelidis I, Zavridou M, Economopoulou P, et al. Prognostic significance of PD-L1 expression on circulating tumor cells in patients with head and neck squamous cell carcinoma. Ann Oncol. (2017) 28:1923–33. 10.1093/annonc/mdx206 [DOI] [PubMed] [Google Scholar]
- 163.Pinzani P, Salvadori B, Simi L, Bianchi S, Distante V, Cataliotti L, et al. Isolation by size of epithelial tumor cells in peripheral blood of patients with breast cancer: correlation with real-time reverse transcriptase–polymerase chain reaction results and feasibility of molecular analysis by laser microdissection. Hum Pathol. (2006) 37:711–8. 10.1016/j.humpath.2006.01.026 [DOI] [PubMed] [Google Scholar]
- 164.Kaifi JT, Kunkel M, Das A, Harouaka RA, Dicker DT, Li G, et al. Circulating tumor cell isolation during resection of colorectal cancer lung and liver metastases: a prospective trial with different detection techniques. Cancer Biol Ther. (2015) 16:699–708. 10.1080/15384047.2015.1030556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Schlange T, Stoecklein N, Neves RP, Pleier S, Bender S, Brychta N, et al. Abstract 513: Standardization of technologies for CTC, ctDNA and miRNA enrichment, isolation and analysis for liquid biopsies during the first year of IMI's CANCER-ID. Cancer Res. (2016) 76:513 10.1158/1538-7445.AM2016-51326772756 [DOI] [Google Scholar]
- 166.Neumann MH, Schneck H, Decker Y, Schomer S, Franken A, Endris V, et al. Isolation and characterization of circulating tumor cells using a novel workflow combining the CellSearch((R)) system and the CellCelector(). Biotechnol Prog. (2017) 33:125–32. 10.1002/btpr.2294 [DOI] [PubMed] [Google Scholar]
- 167.American, Cancer Society [Online]. Available online at: https://www.cancer.org/cancer/non-small-cell-lung-cancer.html (Accessed October 11, 2017).


