Abstract
A major issue in organ transplantation is the development of a protocol that can preserve organs under optimal conditions. Damage to organs is commonly a consequence of flow deprivation and oxygen starvation following the restoration of blood flow and reoxygenation. This is known as ischemia-reperfusion injury (IRI): a complex multifactorial process that causes cell damage. While the oxygen deprivation due to ischemia depletes cell energy, subsequent tissue oxygenation due to reperfusion induces many cascades, from reactive oxygen species production to apoptosis initiation. Autophagy has also been identified in the pathogenesis of IRI, although such alterations and their subsequent functional significance are controversial. Moreover, proteasome activation may be a relevant pathophysiological mechanism. Different strategies have been adopted to limit IRI damage, including the supplementation of commercial preservation media with pharmacological agents or additives. In this review, we focus on novel strategies related to the ubiquitin proteasome system and oxidative stress inhibition, which have been used to minimize damage in liver transplantation.
Keywords: Liver transplant, Ischemia-reperfusion injury, Oxidative stress, Proteasome, Redox regulation, Ubiquitin
Core tip: Ischemia-reperfusion injury is a complex multifactorial process that causes cell damage during liver transplantation. The role of the ubiquitin proteasome system during liver transplantation remains unclear. The use of proteasome inhibitors is a new strategy aimed at improving organ preservation.
INTRODUCTION
The definitive treatment option for many liver diseases is liver transplantation; and in these cases, one important issue is the optimization of organ preservation.
During liver transplantation, damage is initiated by vascular occlusion during hepatic resections. This is aggravated by cold storage of the liver graft (usually at 4 °C) in the preservation solution; and finally by warm reperfusion and subsequent implantation into the recipient. During ischemia, a long period of tissue hypoxia can result in tissue injury and organ dysfunction; as the organ is deprived of oxygen, ATP and energy becomes depleted. Paradoxically, the reintroduction of oxygen during reperfusion aggravates the damage. The whole process has a plethora of consequences that are collectively known as ischemia-reperfusion injury (IRI)[1-3]. While oxygen deprivation due to ischemia depletes cell energy, subsequent reoxygenation due to reperfusion induces many cascades, from induction of reactive oxygen species (ROS)[4] to initiation of apoptosis (Figure 1). Such events are, in part, responsible for organ failure. Variables related to the donor (age, steatosis) and surgery (prolonged ischemia times)[5] are the most commonly reported risk factors for graft dysfunction[6]. Other factors such as shear stress and small graft size[7] have been recognized as important factors associated with oxidative stress, lack of primary function, early dysfunction allograft and biliary complications after liver transplantation[8]. Together with the immunological mechanisms of graft rejection, IRI remains as one of the main clinical problems following organ transplantation.
Figure 1.
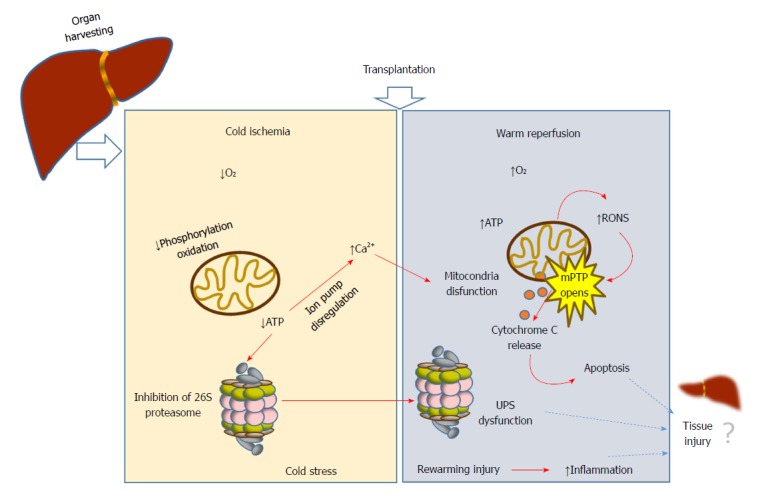
Ischemia-reperfusion injury: A complex multifactorial process that causes cell damage during liver transplantation. While the oxygen deprivation due to ischemia depletes cell energy, subsequent reoxygenation due to reperfusion induces many cascades, from production of reactive oxygen species to initiation of apoptosis. UPS: Ubiquitin-proteasome system; mPTP: mitochondrial permeability transition pore.
Current strategies to prevent and modulate IRI include: ischemic pre-[9-15] or post-conditioning[16] protocols ; static cold storage[17] or machine perfusion[18-20] and the use of pharmacological agents[21-23]. The most common methods applied in liver surgery have recently been reviewed[5,24]. Conventional hypothermic cold storage continues to be the main method for liver preservation, largely because of its cost-effectiveness, simplicity and logistics. However, hypothermia can have multiple side effects, including the induction of oxidative stress[25].
Because of the wide range of mechanisms that can contribute to cell damage in IRI (involving ROS, oxidized products, inflammatory mediators and cytokines), adoption of a specific therapeutic strategy often results in only limited improvements in organ transplantation. Given these circumstances, it is worthwhile to focus on the assessment of agents that can counteract the damage induced by oxidative stress. As oxidative stress is the result of an imbalance between the rate of ROS generation and the capacity to detoxify these reactive species[26-28], interventions that could result in ROS scavenging or the detoxifying of ROS products could protect against IRI. Three lines of defence against oxidative stress have been reported[29]. Antioxidant molecules (such as glutathione) represent the first line of antioxidant defence; the second line incorporates enzymatic antioxidants; and the third consists of repair system proteins, including the proteolytic pathways. In this third line, the ubiquitin-proteasome system (UPS) is widely recognized as the main system for degradation of cytosolic proteins[30,31]
The purpose of this review is to present an update of effects of oxidative stress while summarizing recent findings on the role of the UPS in organ preservation and liver transplantation. In this review we outline the current data of the literature, previous search in databases - PubMed, Web of Science and Scopus - that support the hypothesis on the potential involvement of UPS and oxidative stress in IRI. We will focus on the new strategies used to minimize damage in liver transplantation.
STATIC COLD STORAGE, MACHINE PERFUSION AND STORAGE SOLUTIONS
The strategy most commonly adopted to reduce ischemic injury is the cooling of organs and the use of a preservation solution to minimize enzymatic activity and depletion of the energy substrate. Cold storage slows down cellular metabolism[32,33], but this can be responsible for further ATP and energy depletion[34]. In addition, cold has adverse side effects due to the induction of cellular inflammation, alterations of the cytoskeleton[35] , and oxidative stress.
Protective strategies to reduce hepatic IRI include the use of the machine perfusion, which represents a new line of research opposed to static cold storage.
Perfusion machine involves a pulsatile perfusion of the liver with a cold (subnormothermic, hypothermic) perfusate[18,36-40]; with normothermic perfusate[41,42]; or with a gradual increase in of the perfusate temperature[43]. These references confirm that machine perfusion protects against IRI damage in animal models. Livers preserved by subnormothermic machine perfusion at 20 °C showed significantly less liver damage at the end of reperfusion compared to cold storage. The release of LDH was reduced while the production of bile, ATP levels, glycogen and glutathione content increased in preserved livers by subnormothermic machine perfusion than livers submitted to cold storage[44]. Normothermic machine perfusion has also been assessed in discarded human liver grafts[45]. The reported data demonstrate the viability of normothermic perfusion, which results in the continuous production of bile, the fall of lactate levels and the preservation of hepatic morphology. However, the safety and efficacy of machine perfusion is yet to be assessed by randomized controlled clinical trials.
Due to its cost-effectiveness and simplicity, cold storage with conventional hypothermia remains the main method for liver conservation. In fact, major advances in the field of organ preservation have included the development of new improved preservation solutions capable of reducing cold-induced cellular damage[46]. Euro-Collins solution was first developed in the 1970s; while more recently, University of Wisconsin (UW)[47] Celsior[48], Histidine-Ketoglutarate (HTK)[49] and Institute Georges Lopez (IGL-1)[50] solutions tend to be extensively used for liver transplantation. Commercial preservation solutions include oncotic agents, like hydroxy-ethyl starch (HES) in UW and polyethylenglycol-35 (PEG-35) in IGL-1, which confer high viscosity to the media. They also contain metabolic precursors (adenosine and ketoglutarate) and antioxidants (glutathione, allopurinol). Although the use of commercial preservation solutions has improved conditions for liver graft preservation, with the urgent need to expand the donor pool and the subsequent use of suboptimal grafts, new additives have been proposed to combat oxidant and apoptotic damage with the aim of prolonging graft quality during cold storage.
THE ROLE OF OXIDATIVE STRESS IN IRI
ROS are highly reactive and capable of oxidizing lipids, proteins and DNA[51], thereby leading to structural and cellular changes that may cause oxidative stress and cellular apoptosis. Abundant information has been published demonstrating that increased ROS production is involved in IRI pathology[28,50,52,53]. The involvement of ROS was initially observed based on the detection of enhanced production of chemical products generated by the reaction of ROS with cellular components. Lipid peroxidation products, such as malondialdehyde and hydroxynonenal[13,36,54-56] have been widely used as a biomarkers of oxidative stress in IRI. The accelerated ROS production in post-ischemic tissues has been attributed to enzymes capable of reducing molecular oxygen and forming superoxide: xantine oxidase[57-59], NADPH oxidase[60] and nitric oxide synthase[11]. The contribution of each of these enzymes in IRI is assessed in the excellent review by Granger and Kvietys[28]. All this indicates that radicals can be formed from different sources, and consequently several protective strategies to decrease liver IRI have targeted different sources of ROS: xanthine oxidase (using allopurinol[59,61]) or NADPH oxidase[62], for example. Other strategies have included pharmacological interventions with antioxidants resulting in the neutralization of ROS effects[63-66].
As mentioned above, Jung et al[29] describe three main lines of defence against oxidative stress. Thus, to those molecules widely recognized as antioxidants, such as glutathione, and enzymatic antioxidants, they add a third line of defence: repair system proteins. This system includes the proteolytic pathways, such as the UPS[30,31]. The activity of the UPS is necessary so that the cells can cope with oxidative stress, but in turn, the activity of the UPS are also modulated by the redox state[67].
THE UBIQUITIN-PROTEASOME SYSTEM
In order to eliminate damaged proteins, cells have highly regulated mechanisms, such as the autophagy-lysosome pathway and the UPS, which is recognized as the principal system for degrading oxidized cytosolic proteins. Proteasomes are protein complexes that, via proteolysis, degrade unnecessary or damaged proteins. It has always appeared that autophagy within lysosomes is involved in the pathogenesis of hepatic IRI, although the specific alterations it causes and their subsequent functional significance are highly controversial[68]. The use of proteasome inhibitors has been demonstrated to enhance myocardial viability[69,70] and protect liver against IRI[4,71-73], which suggests this may be a promising strategy to reduce the damage inherent to transplantation protocols.
Ubiquitin
Ubiquitin is a highly conserved and small 76-amino-acid protein that acts as a post-translational protein modifier and regulates protein lifespan. Ubiquitin was isolated in the 1970s by Goldstein et al[74] and since then has been identified in many cellular processes, including proteasomal proteolysis and also DNA damage repair. Ubiquitin can be attached covalently to a target protein in a process known as ubiquitination. Ubiquitin is conjugated to other proteins through a peptide bond between its C-terminal glycine and a primary amine on the substrate, most typically a lysine residue. Conjugation is dependent on the successive activities of enzymes named E1 (ubiquitin activating enzyme), E2 (ubiquitin conjugating enzyme), and E3 (ubiquitin ligase)[75]. In the 1980s, biochemical studies elucidated the chemical reactions catalysed by these enzymes[76,77]. E1 activates ubiquitin as a thioester of the active site cysteine residue at expenses of ATP[78]. The activated ubiquitin molecule is then transferred to an E2 enzyme via the formation of a new thioester with the catalytic cysteine residue of the E2 enzyme. E2 transfers the activated ubiquitin moieties to the protein substrate that is specifically bound to a unique ubiquitin ligase: E3. Finally, an isopeptide bond is formed between the C-terminal glycine residue of the ubiquitin (Gly76) and the e-NH2 group of an internal lysine (Lys119) of the protein substrate. Several protein families have been discovered that are evolutionarily related to ubiquitin, as they share the characteristics of the ubiquitin-fold proteins and the capacity to become conjugated to substrates through the action of related E1, E2, and E3 enzymes[79].
Target proteins can be modified by mono- or poly-ubiquitination. The seven lysines in ubiquitin contribute to the assembly of various poly-ubiquitin chains that may be involved in different cellular processes[80]. The K48-linked (Lys 48) poly-ubiquitin chain targets proteins for proteasomal degradation, while the K11-linked poly-ubiquitin chain is involved in endoplasmic reticulum-associated degradation[81] and the K63-linked poly-ubiquitin chain can act in non-proteolytic events, including protein trafficking, DNA repair, and inflammation[82]. Furthermore, ubiquitination can be reversed by the removal of ubiquitin from target proteins by de-ubiquitinating enzymes (DUBs).
Degradation of damaged proteins
The proteasome is a multicatalytic protease involved in the degradation of intracellular proteins. The processing of damaged proteins is mainly mediated by the 20S and 26S proteasomes[76,77].
The 20S proteasome is a large complex, formed of four stacked rings: two alpha and two beta rings, each containing seven different subunits (from α1 to α7 or from β1 to β7). Three of the beta subunits are responsible for the proteolytic activity. β1 exhibits caspase-like activity, while β2 exhibits trypsin-like activity, and β5, chymotrypsin-like activity. Whereas the beta rings are responsible for proteolytic degradation, the alpha rings perform substrate recognition and regulate substrate access to the proteolytic active centre.
The 26S proteasome is formed by the addition of one or two regulatory 19S subunits to an alpha ring in the 20S core proteasome in an ATP-dependent manner. The binding to this regulator increases the proteasomal activity and allows the proteasome to degrade the substrate proteins that have been tagged through the binding of a chain of ubiquitin, as explained above. The ubiquitin chain acts as a labelled sequence to direct proteins to the 26S proteasome where they are degraded. The 19S subunits first remove the poly-ubiquitin “tags” of the targeted proteins and unfold those proteins. Then, the unfolded proteins are degraded by the 20S core of the 26S proteasome. In this way, while the 20S core proteasome can degrade unfolded proteins in an ATP-independent manner, the 26S proteasome is only capable of degrading natively folded and functional proteins in an ATP- and ubiquitin-dependent manner[83].
ACTIVATION OF THE PROTEASOME UBIQUITIN SYSTEM (UPS) AND PROTEOLYSIS IN IRI
It has been well established that proteolysis is an important regulatory mechanism that helps maintain homeostasis[77]. The proteasome and the UPS are necessary for the control of protein concentrations, to prevent abnormal accumulations and to modulate regulatory proteins involved in cell metabolism. However, proteolysis can have detrimental effects on organs and tissues after transplantation[84]. Free amino acids in the effluent from human livers was used as a marker to predict postoperative graft function. In fact, the prevention of liver graft proteolysis and proteasome activation can be modulated by the use of additives to organ preservation solutions[85,86]. In this way, it has been demonstrated that lactobionate, a component of UW solution, prevents the release of metalloproteinases during cold preservation[85]. Moreover, the UPS is an energy-dependent system and ATP levels affects 26S proteasome assembly, stability, and function[87]. The following processes are dependent on ATP: The first step in ubiquitin conjugation by E1, which is required for the poly-ubiquitination of proteins; the assembly of alpha and beta rings and ATP-dependent proteases; and the degradation of poly-ubiquinated proteins by the 26S proteasome[79,87]. In addition, it is well known that oxygen deprivation leads to a significant decrease in ATP in liver grafts[34], which could affect liver outcomes.
Therefore, during cold storage of organs, and because of the ATP decrease, the formation of 26S proteasomes and poly-ubiquitin-dependent protein degradation are expected to be impaired. However, it has been well established that a subset of 26S proteasomes appears to be activated as ATP levels decline[70]. In that study, Geng et al[70] demonstrated that the activation of the 26S proteasome is a pathophysiologically relevant mechanism in cold ischemic myocardial injury. Such observations imply that a subset of 26S is acting as a harmful protease that is activated when the cellular energy supply decreases. The finding that ATP negatively regulates proteasome activity is consistent with previous results concerning increased proteolysis during cold preservation of human liver grafts[84]. Moreover, hypothermia also affects proteasome activity in isolated perfused rat liver, increasing chymotrypsin-like activity[36].
Degradation of protein substrates by 26S proteasomes requires the 19S regulatory cap to recognize ubiquitin protein conjugates and to regulate the entry of the substrate into the proteolytic cavity of the 20S core. Covalent regulation via phosphorylation of a subunit in the 19S regulatory cap of the proteasome allows for a fast increase in the 26S proteasome activity, which becomes an important regulatory mechanism for proteasome control[88-90]. In fact, we have previously reported that phosphorylation of the 19S subunit Rpt6 increases in cold perfused rat livers[36].
The combination of proteasome and oxidative stress in IRI
ROS are capable of oxidatively modifying cell structures. Due to the abundance of proteins, the presence of oxidatively modified proteins and aggregates of oxidized proteins has been reported in the cytosol. There is a certain degree of consensus that proteasomal activity degrades oxidized proteins, although the attribution to 20S or 26S is yet to be elucidated. The 26S proteasome was found to be less active in the degradation of oxidatively damaged proteins[91]. A growing body of evidence suggests that the degradation of oxidatively damaged proteins does not require ATP and polyubiquitination of the substrate[92]. Alternatively, oxidative proteins are removed independently of ubiquitin by the ATP-independent 20S proteasome[93].
The proteasome is responsible for the selective degradation of oxidatively damaged proteins. In this sense it has been shown that certain oxidized proteins degrade faster than their native counterparts[94], and, furthermore, it has been shown that inhibition of the proteasome stabilizes the oxidized proteins[95]. During oxidative stress, the ratio of oxidized to reduced glutathione increases[36]. and this redox status of the cells can also modulate protein ubiquitination by reversible S-thiolation[96,67]. This is concurrent with a decrease in the ubiquitin-activating enzyme, E-1 and ubiquitin conjugates. According to some models, glutathiolation of the E1 or E2 components of a ubiquitinated protein protects it from unnecessary degradation. Thus, S-glutathiolation can be regarded as a general mechanism of a redox signal controlling gene expression. In addition, direct oxidative modifications of the proteasome may also occur, including carbonylation, glycoxidation and modification with lipid peroxidation products. Although it is not clear to what extent these modifications affect the proteasome, they could modulate proteasomal activity. It should be noted that an inefficient proteasomal system would result in an accumulation of protein aggregates in the cytoplasm, as has been reported in brain due to aging or Alzheimer’s disease[97]; while excessive protein depletion activity may have deleterious effects by affecting detoxifying systems, membranes, or RNA stability[69,84].
PROTEASOME INHIBITION IN LIVER IRI
The use of proteasome inhibitors has been shown to offer protection and maintain the physiological ubiquitin-protein conjugate pool during cold organ preservation[98]. Table 1 summarizes the protective effects of different proteasome inhibitors against IRI during organ preservation. The potential pharmacological role of proteasome inhibitors was first reported by Campbell et al[99]. Those authors demonstrated that proteasome inhibition can prevent loss of cardiac contractile function in isolated perfused rat heart. The adherence of polymorphonuclear leukocytes to the endothelium was also reduced. However, controversy regarding whether inhibiting the proteasome is beneficial or detrimental to cardiac function continues[95]. Few studies have examined the role of UPS inhibitors as a strategy to reduce damage in liver IRI. In rat liver subjected to warm IRI, the administration of the proteasome inhibitor MG132 decreased LDH and AST levels during ischemia and reperfusion[4]. In addition, the same authors studied whether MG132 can modulate the prooxidant and antioxidant status of rat liver. The results showed that MG132 did not significantly affect liver lipid peroxidation. However, MG132 increased protein carbonyls and decreased the main antioxidant enzyme activities (catalase and superoxide dismutase).
Table 1.
Effect of different proteasome inhibitors on organ preservation
| Inhibitor | Organ and condition | Manifestations | Ref. |
| PS-519 | Heart after IRI (rat) | Improved cardiac contractility | Campbell et al[99] |
| Improved coronary flow | |||
| Reduced PMN infiltration | |||
| Epoxomicin | Heart after cold ischemia (rat) | Reduced edema formation | Geng et al[70] |
| Preserved ultrastructural integrity | |||
| MG132 | Liver, warm IRI (rat) | Decreased LDH and ALT | Alexandrova et al[4] |
| Increased protein oxidation | |||
| Decreased antioxidant activities | |||
| MG132 | Liver, cold IRI (rat) | Decreased AST and ALT | Zaouali et al[71] |
| Reduced inflammation (IL1β and TNFα) | |||
| Bortezomid | Liver, cold IRI (rat) | Decreased AST, ALT and mitochondrial damage | Zaouali et al[71] |
| Increased bile production | Bejaoui et al[72] | ||
| Decreased lipid peroxidation | |||
| Decreased apoptosis (Cyt C and Caspase 3) |
IRI: Ischemia-reperfusion injury.
In contrast to that modulation of oxidative stress, the proteasome inhibitor Bortezomib, used at a non-toxic low dose, up-regulates liver antioxidant enzymes in chronic ethanol-fed rats[86]. Exposure to the proteasome inhibitor increased antioxidant defences by enhancing the levels of mRNA and protein expression transcripts of glutathione reductase, glutathione synthetase and glutathione peroxidase, as well as superoxide dismutase in rat liver. The increase in antioxidant defences was concomitant with enhanced 26S proteasome activity. As mentioned by those authors, the beneficial effects of the proteasome inhibitor Bortezomib could be due to the low dose used and to the reversibility of the drug.
In accordance with the previous research, we have recently demonstrated that the addition of the reversible UPS inhibitors Bortezomib and MG132 to UW solution improved steatotic and non-steatotic rat liver preservation in the face of cold IRI[71]. Both inhibitors prevented liver injury, decreasing AST and ALT, and prevented the release of the inflammatory cytokines IL-1 beta and TNF-alpha. The protective effect of Bortezomib was superior to that of MG132. Bortezomib increased bile production, decreased vascular resistance in fatty rat liver through an increase in nitric oxide generation, prevented lipid peroxidation and mitochondrial damage, and increased AMP-activated protein kinase (AMPK) phosphorylation. It is well known that AMPK phosphorylation is a key process in fatty liver graft preservation, as it can reduce inflammation[9,44].
The supplementation of IGL-1 preservation solution with Bortezomib has also been shown to have protective effects[72], reducing steatotic liver injury and decreasing liver apoptosis (cytochrome c and caspase 3 release) in the face of cold IRI. These effects were partially mediated through the activation of the Akt/mTOR signalling pathway, a key regulator in cell growth and proliferation, and through the phosphorylation of AMPK.
Besides its role in preventing inflammation, AMPK activation also keeps the liver in an energy-conserving state during cold storage. AMPK triggers ATP-producing pathways, balancing the metabolic process towards increasing energy homeostasis in the cell. We have recently found a correlation between AMPK activation, ATP levels, lower proteasome activity and decreased damage in fatty liver grafts preserved in different solutions[73]. As AMPK is regulated and degraded by the UPS, the use of proteasome inhibitors in the preservation solution could avoid AMPK depletion and may contribute to maintaining the beneficial effects of proteasome inhibition after IRI.
CONCLUSION
Remarkable progress has been made over the past few decades regarding the role of the UPS in many cellular processes. However, the function and regulation of the proteasome in organ transplantation still remains unclear. We have proposed that UPS inhibitors reduce IRI in liver grafts via up-regulation of AMPK phosphorylation and the consequent preservation of the energy state. As some proteasome inhibitors have been approved for the treatment of different diseases, we propose to explore their use in liver. Well-designed randomized controlled trials will be needed to evaluate the use of proteasome inhibitors in liver transplantation. The supplementation of low and non-toxic doses of proteasome inhibitors offers a new opportunity for the improvement of organ preservation solutions.
ACKNOWLEDGMENTS
We are grateful to Christopher Evans for language assistance.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
Conflict-of-interest statement: The authors have no conflict of interest to declare.
Peer-review started: May 5, 2018
First decision: May 17, 2018
Article in press: June 30, 2018
P- Reviewer: Chedid MF, Hori T, Rubbini M, Smyrniotis V, Tao R S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
Contributor Information
Norma Alva, Department of Cell Biology, Physiology and Immunology, University of Barcelona, Barcelona 08028, Spain.
Arnau Panisello-Roselló, Experimental Pathology Department, Institute of Biomedical Research of Barcelona, Barcelona 08036, Spain.
Marta Flores, Department of Cell Biology, Physiology and Immunology, University of Barcelona, Barcelona 08028, Spain.
Joan Roselló-Catafau, Experimental Pathology Department, Institute of Biomedical Research of Barcelona, Barcelona 08036, Spain.
Teresa Carbonell, Department of Cell Biology, Physiology and Immunology, University of Barcelona, Barcelona 08028, Spain. tcarbonell@ub.edu.
References
- 1.Nagano K, Gelman S, Bradley EL Jr, Parks D. Hypothermia, hepatic oxygen supply-demand, and ischemia-reperfusion injury in pigs. Am J Physiol. 1990;258:G910–G918. doi: 10.1152/ajpgi.1990.258.6.G910. [DOI] [PubMed] [Google Scholar]
- 2.Iñiguez M, Dotor J, Feijoo E, Goñi S, Prieto J, Berasain C, Avila MA. Novel pharmacologic strategies to protect the liver from ischemia-reperfusion injury. Recent Pat Cardiovasc Drug Discov. 2008;3:9–18. doi: 10.2174/157489008783331643. [DOI] [PubMed] [Google Scholar]
- 3.Duval M, Plin C, Elimadi A, Vallerand D, Tillement JP, Morin D, Haddad PS. Implication of mitochondrial dysfunction and cell death in cold preservation--warm reperfusion-induced hepatocyte injury. Can J Physiol Pharmacol. 2006;84:547–554. doi: 10.1139/y06-014. [DOI] [PubMed] [Google Scholar]
- 4.Alexandrova A, Petrov L, Georgieva A, Kessiova M, Tzvetanova E, Kirkova M, Kukan M. Effect of MG132 on proteasome activity and prooxidant/antioxidant status of rat liver subjected to ischemia/reperfusion injury. Hepatol Res. 2008;38:393–401. doi: 10.1111/j.1872-034X.2007.00270.x. [DOI] [PubMed] [Google Scholar]
- 5.Gurusamy KS, Gonzalez HD, Davidson BR. Current protective strategies in liver surgery. World J Gastroenterol. 2010;16:6098–6103. doi: 10.3748/wjg.v16.i48.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neves DB, Rusi MB, Diaz LG, Salvalaggio P. Primary graft dysfunction of the liver: definitions, diagnostic criteria and risk factors. Einstein (Sao Paulo) 2016;14:567–572. doi: 10.1590/S1679-45082016RW3585. [DOI] [PubMed] [Google Scholar]
- 7.Hori T, Uemoto S, Walden LB, Chen F, Baine AM, Hata T, Nguyen JH. Pretreatment of Small-for-Size Grafts In Vivo by γ -Aminobutyric Acid Receptor Regulation against Oxidative Stress-Induced Injury in Rat Split Orthotopic Liver Transplantation. Int J Hepatol. 2013;2013:149123. doi: 10.1155/2013/149123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verhoeven CJ, Farid WR, de Jonge J, Metselaar HJ, Kazemier G, van der Laan LJ. Biomarkers to assess graft quality during conventional and machine preservation in liver transplantation. J Hepatol. 2014;61:672–684. doi: 10.1016/j.jhep.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 9.Serafín A, Roselló-Catafau J, Prats N, Gelpí E, Rodés J, Peralta C. Ischemic preconditioning affects interleukin release in fatty livers of rats undergoing ischemia/reperfusion. Hepatology. 2004;39:688–698. doi: 10.1002/hep.20089. [DOI] [PubMed] [Google Scholar]
- 10.Bejaoui M, Pantazi E, Calvo M, Folch-Puy E, Serafín A, Pasut G, Panisello A, Adam R, Roselló-Catafau J. Polyethylene Glycol Preconditioning: An Effective Strategy to Prevent Liver Ischemia Reperfusion Injury. Oxid Med Cell Longev. 2016;2016:9096549. doi: 10.1155/2016/9096549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peralta C, Closa D, Hotter G, Gelpí E, Prats N, Roselló-Catafau J. Liver ischemic preconditioning is mediated by the inhibitory action of nitric oxide on endothelin. Biochem Biophys Res Commun. 1996;229:264–270. doi: 10.1006/bbrc.1996.1790. [DOI] [PubMed] [Google Scholar]
- 12.Eipel C, Glanemann M, Nuessler AK, Menger MD, Neuhaus P, Vollmar B. Ischemic preconditioning impairs liver regeneration in extended reduced-size livers. Ann Surg. 2005;241:477–484. doi: 10.1097/01.sla.0000154264.41201.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li JY, Gu X, Yin HZ, Zhou Y, Zhang WH, Qin YM. Protective effect of ischemic preconditioning on hepatic ischemia-reperfusion injury by advancing the expressive phase of survivin in rats. Hepatobiliary Pancreat Dis Int. 2008;7:615–620. [PubMed] [Google Scholar]
- 14.Koti RS, Seifalian AM, Davidson BR. Protection of the liver by ischemic preconditioning: a review of mechanisms and clinical applications. Dig Surg. 2003;20:383–396. doi: 10.1159/000072064. [DOI] [PubMed] [Google Scholar]
- 15.Amador A, Grande L, Martí J, Deulofeu R, Miquel R, Solá A, Rodriguez-Laiz G, Ferrer J, Fondevila C, Charco R, et al. Ischemic pre-conditioning in deceased donor liver transplantation: a prospective randomized clinical trial. Am J Transplant. 2007;7:2180–2189. doi: 10.1111/j.1600-6143.2007.01914.x. [DOI] [PubMed] [Google Scholar]
- 16.Doeppner TR, Doehring M, Kaltwasser B, Majid A, Lin F, Bähr M, Kilic E, Hermann DM. Ischemic Post-Conditioning Induces Post-Stroke Neuroprotection via Hsp70-Mediated Proteasome Inhibition and Facilitates Neural Progenitor Cell Transplantation. Mol Neurobiol. 2017;54:6061–6073. doi: 10.1007/s12035-016-0137-3. [DOI] [PubMed] [Google Scholar]
- 17.Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation. 1988;45:673–676. doi: 10.1097/00007890-198804000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Ferrigno A, Rizzo V, Boncompagni E, Bianchi A, Gringeri E, Neri D, Richelmi P, Freitas I, Cillo U, Vairetti M. Machine perfusion at 20°C reduces preservation damage to livers from non-heart beating donors. Cryobiology. 2011;62:152–158. doi: 10.1016/j.cryobiol.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Schlegel A, Dutkowski P. Role of hypothermic machine perfusion in liver transplantation. Transpl Int. 2015;28:677–689. doi: 10.1111/tri.12354. [DOI] [PubMed] [Google Scholar]
- 20.Hessheimer AJ, Fondevila C, García-Valdecasas JC. Extracorporeal machine liver perfusion: are we warming up? Curr Opin Organ Transplant. 2012;17:143–147. doi: 10.1097/MOT.0b013e328351082a. [DOI] [PubMed] [Google Scholar]
- 21.Sola A, Roselló-Catafau J, Gelpí E, Hotter G. Fructose-1,6-biphosphate in rat intestinal preconditioning: involvement of nitric oxide. Gut. 2001;48:168–175. doi: 10.1136/gut.48.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Padrissa-Altés S, Franco-Gou R, Boillot O, Serafín A, Rimola A, Arroyo V, Rodés J, Peralta C, Roselló-Catafau J. Effect of angiotensin II and bradykinin inhibition in rat reduced-size liver transplantation. Liver Transpl. 2009;15:313–320. doi: 10.1002/lt.21693. [DOI] [PubMed] [Google Scholar]
- 23.Zaouali MA, Ben Abdennebi H, Padrissa-Altés S, Mahfoudh-Boussaid A, Roselló-Catafau J. Pharmacological strategies against cold ischemia reperfusion injury. Expert Opin Pharmacother. 2010;11:537–555. doi: 10.1517/14656560903547836. [DOI] [PubMed] [Google Scholar]
- 24.Bejaoui M, Pantazi E, Folch-Puy E, Baptista PM, García-Gil A, Adam R, Roselló-Catafau J. Emerging concepts in liver graft preservation. World J Gastroenterol. 2015;21:396–407. doi: 10.3748/wjg.v21.i2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carbonell T, Alva N, Palomeque J. Mechanisms activated by induced hypothermia: positive and adverse effects. In: Delgado JIV, Garza VGF, editors. Hypothermia: Prevention, Recognition and Treatment. New York: Nova Publishers;; 2012. pp. 109–122. [Google Scholar]
- 26.Halliwell B, Gutteridge JM. Free radicals and antioxidant protection: mechanisms and significance in toxicology and disease. Hum Toxicol. 1988;7:7–13. doi: 10.1177/096032718800700102. [DOI] [PubMed] [Google Scholar]
- 27.Gutteridge JM, Halliwell B. Reoxygenation injury and antioxidant protection: a tale of two paradoxes. Arch Biochem Biophys. 1990;283:223–226. doi: 10.1016/0003-9861(90)90635-c. [DOI] [PubMed] [Google Scholar]
- 28.Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015;6:524–551. doi: 10.1016/j.redox.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung T, Catalgol B, Grune T. The proteasomal system. Mol Aspects Med. 2009;30:191–296. doi: 10.1016/j.mam.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Ciechanover A. Intracellular protein degradation: from a vague idea thru the lysosome and the ubiquitin-proteasome system and onto human diseases and drug targeting. Biochim Biophys Acta. 2012;1824:3–13. doi: 10.1016/j.bbapap.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 31.Shang F, Taylor A. Ubiquitin-proteasome pathway and cellular responses to oxidative stress. Free Radic Biol Med. 2011;51:5–16. doi: 10.1016/j.freeradbiomed.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guibert EE, Petrenko AY, Balaban CL, Somov AY, Rodriguez JV, Fuller BJ. Organ Preservation: Current Concepts and New Strategies for the Next Decade. Transfus Med Hemother. 2011;38:125–142. doi: 10.1159/000327033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olschewski P, Gass P, Ariyakhagorn V, Jasse K, Hunold G, Menzel M, Schöning W, Schmitz V, Neuhaus P, Puhl G. The influence of storage temperature during machine perfusion on preservation quality of marginal donor livers. Cryobiology. 2010;60:337–343. doi: 10.1016/j.cryobiol.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Vajdová K, Graf R, Clavien PA. ATP-supplies in the cold-preserved liver: A long-neglected factor of organ viability. Hepatology. 2002;36:1543–1552. doi: 10.1053/jhep.2002.37189. [DOI] [PubMed] [Google Scholar]
- 35.Stefanovich P, Ezzell RM, Sheehan SJ, Tompkins RG, Yarmush ML, Toner M. Effects of hypothermia on the function, membrane integrity, and cytoskeletal structure of hepatocytes. Cryobiology. 1995;32:389–403. doi: 10.1006/cryo.1995.1039. [DOI] [PubMed] [Google Scholar]
- 36.Carbonell T, Alva N, Sanchez-Nuño S, Dewey S, Gomes AV. Subnormothermic Perfusion in the Isolated Rat Liver Preserves the Antioxidant Glutathione and Enhances the Function of the Ubiquitin Proteasome System. Oxid Med Cell Longev. 2016;2016:9324692. doi: 10.1155/2016/9324692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruinsma BG, Yeh H, Ozer S, Martins PN, Farmer A, Wu W, Saeidi N, Op den Dries S, Berendsen TA, Smith RN, et al. Subnormothermic machine perfusion for ex vivo preservation and recovery of the human liver for transplantation. Am J Transplant. 2014;14:1400–1409. doi: 10.1111/ajt.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Op den Dries S, Sutton ME, Karimian N, de Boer MT, Wiersema-Buist J, Gouw AS, Leuvenink HG, Lisman T, Porte RJ. Hypothermic oxygenated machine perfusion prevents arteriolonecrosis of the peribiliary plexus in pig livers donated after circulatory death. PLoS One. 2014;9:e88521. doi: 10.1371/journal.pone.0088521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrigno A, Di Pasqua LG, Berardo C, Siciliano V, Rizzo V, Mannucci B, Richelmi P, Croce AC, Vairetti M. Liver Graft Susceptibility during Static Cold Storage and Dynamic Machine Perfusion: DCD versus Fatty Livers. Int J Mol Sci. 2017;19 doi: 10.3390/ijms19010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vairetti M, Ferrigno A, Carlucci F, Tabucchi A, Rizzo V, Boncompagni E, Neri D, Gringeri E, Freitas I, Cillo U. Subnormothermic machine perfusion protects steatotic livers against preservation injury: a potential for donor pool increase? Liver Transpl. 2009;15:20–29. doi: 10.1002/lt.21581. [DOI] [PubMed] [Google Scholar]
- 41.Brockmann J, Reddy S, Coussios C, Pigott D, Guirriero D, Hughes D, Morovat A, Roy D, Winter L, Friend PJ. Normothermic perfusion: a new paradigm for organ preservation. Ann Surg. 2009;250:1–6. doi: 10.1097/SLA.0b013e3181a63c10. [DOI] [PubMed] [Google Scholar]
- 42.Ravikumar R, Leuvenink H, Friend PJ. Normothermic liver preservation: a new paradigm? Transpl Int. 2015;28:690–699. doi: 10.1111/tri.12576. [DOI] [PubMed] [Google Scholar]
- 43.Hoyer DP, Paul A, Luer S, Reis H, Efferz P, Minor T. End-ischemic reconditioning of liver allografts: Controlling the rewarming. Liver Transpl. 2016;22:1223–1230. doi: 10.1002/lt.24515. [DOI] [PubMed] [Google Scholar]
- 44.Vairetti M, Ferrigno A, Rizzo V, Richelmi P, Boncompagni E, Neri D, Freitas I, Cillo U. Subnormothermic machine perfusion protects against rat liver preservation injury: a comparative evaluation with conventional cold storage. Transplant Proc. 2007;39:1765–1767. doi: 10.1016/j.transproceed.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 45.op den Dries S, Karimian N, Sutton ME, Westerkamp AC, Nijsten MW, Gouw AS, Wiersema-Buist J, Lisman T, Leuvenink HG, Porte RJ. Ex vivo normothermic machine perfusion and viability testing of discarded human donor livers. Am J Transplant. 2013;13:1327–1335. doi: 10.1111/ajt.12187. [DOI] [PubMed] [Google Scholar]
- 46.Duret C, Moreno D, Balasiddaiah A, Roux S, Briolotti P, Raulet E, Herrero A, Ramet H, Biron-Andreani C, Gerbal-Chaloin S, et al. Cold Preservation of Human Adult Hepatocytes for Liver Cell Therapy. Cell Transplant. 2015;24:2541–2555. doi: 10.3727/096368915X687020. [DOI] [PubMed] [Google Scholar]
- 47.Ben Abdennebi H, Steghens JP, Margonari J, Ramella-Virieux S, Barbieux A, Boillot O. High-Na+ low-K+ UW cold storage solution reduces reperfusion injuries of the rat liver graft. Transpl Int. 1998;11:223–230. doi: 10.1007/s001470050132. [DOI] [PubMed] [Google Scholar]
- 48.Boudjema K, Grandadam S, Compagnon P, Salamé E, Wolf P, Ducerf C, Le Treut P, Soubrane O, Cherqui D, Mouchel C, et al. Efficacy and safety of Celsior preservation fluid in liver transplantation: one-year follow up of a prospective, multicenter, non-randomized study. Clin Transplant. 2012;26:199–207. doi: 10.1111/j.1399-0012.2011.01447.x. [DOI] [PubMed] [Google Scholar]
- 49.Stewart ZA, Cameron AM, Singer AL, Montgomery RA, Segev DL. Histidine-Tryptophan-Ketoglutarate (HTK) is associated with reduced graft survival in deceased donor livers, especially those donated after cardiac death. Am J Transplant. 2009;9:286–293. doi: 10.1111/j.1600-6143.2008.02478.x. [DOI] [PubMed] [Google Scholar]
- 50.Zaouali MA, Ben Abdennebi H, Padrissa-Altés S, Alfany-Fernandez I, Rimola A, Roselló-Catafau J. How Institut Georges Lopez preservation solution protects nonsteatotic and steatotic livers against ischemia-reperfusion injury. Transplant Proc. 2011;43:77–79. doi: 10.1016/j.transproceed.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 51.Halliwell B, Gutteridge JMC. Free radicals in biology and medicin. Oxford: Oxford University Press; 1999. ISBN: 0198500459 [Google Scholar]
- 52.Bessems M, ‘t Hart NA, Tolba R, Doorschodt BM, Leuvenink HG, Ploeg RJ, Minor T, van Gulik TM. The isolated perfused rat liver: standardization of a time-honoured model. Lab Anim. 2006;40:236–246. doi: 10.1258/002367706777611460. [DOI] [PubMed] [Google Scholar]
- 53.Kron P, Schlegel A, Mancina L, Clavien PA, Dutkowski P. Hypothermic oxygenated perfusion (HOPE) for fatty liver grafts in rats and humans. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.08.028. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 54.Polat KY, Aydinli B, Polat O, Aydin U, Yazici P, Ozturk G, Gundogdu C, Kiziltunc A. The protective effect of aprotinin and alpha-tocopherol on ischemia-reperfusion injury of the rat liver. Transplant Proc. 2008;40:63–68. doi: 10.1016/j.transproceed.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 55.Gulec B, Coskun K, Yigitler C, Yigit T, Aydin A, Oner K. Ischemia-reperfusion injury in the liver during renal transplantation: does perfusion solution play any role? Transplant Proc. 2008;40:59–62. doi: 10.1016/j.transproceed.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 56.Qi Q, Bie P. Different roles of hepatic hypothermic ischemia and ischemic preconditioning in chemically induced hepatocarcinogenesis in rats. J Surg Res. 2014;189:213–221. doi: 10.1016/j.jss.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 57.Thompson-Gorman SL, Zweier JL. Evaluation of the role of xanthine oxidase in myocardial reperfusion injury. J Biol Chem. 1990;265:6656–6663. [PubMed] [Google Scholar]
- 58.Peralta C, Closa D, Xaus C, Gelpí E, Roselló-Catafau J, Hotter G. Hepatic preconditioning in rats is defined by a balance of adenosine and xanthine. Hepatology. 1998;28:768–773. doi: 10.1002/hep.510280325. [DOI] [PubMed] [Google Scholar]
- 59.Martí R, Múrio E, Varela E, Bilbao I, Pascual C, Margarit C, Segura RM. Xanthine oxidoreductase and preservation injury in human liver transplantation. Transplantation. 2004;77:1239–1245. doi: 10.1097/01.tp.0000120384.52033.bc. [DOI] [PubMed] [Google Scholar]
- 60.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15–G26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 61.Lee WY, Koh EJ, Lee SM. A combination of ischemic preconditioning and allopurinol protects against ischemic injury through a nitric oxide-dependent mechanism. Nitric Oxide. 2012;26:1–8. doi: 10.1016/j.niox.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 62.Marden JJ, Zhang Y, Oakley FD, Zhou W, Luo M, Jia HP, McCray PB Jr, Yaniv M, Weitzman JB, Engelhardt JF. JunD protects the liver from ischemia/reperfusion injury by dampening AP-1 transcriptional activation. J Biol Chem. 2008;283:6687–6695. doi: 10.1074/jbc.M705606200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi S, Xue F. Current Antioxidant Treatments in Organ Transplantation. Oxid Med Cell Longev. 2016;2016:8678510. doi: 10.1155/2016/8678510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Junnarkar SP, Tapuria N, Dutt N, Fuller B, Seifalian AM, Davidson BR. Bucillamine improves hepatic microcirculation and reduces hepatocellular injury after liver warm ischaemia-reperfusion injury. HPB (Oxford) 2009;11:264–273. doi: 10.1111/j.1477-2574.2009.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Oca J, Cuadrado S, Vallet J, Benasco C, Martín E, Ardanuy C, Closa D, Hotter G, Jaurrieta E. Protective effects of lazaroid U74389G on intestinal graft after heterotopic small bowel transplantation in rats. J Surg Res. 1998;75:18–23. doi: 10.1006/jsre.1997.5244. [DOI] [PubMed] [Google Scholar]
- 66.Yilmaz HR, Uz E, Yucel N, Altuntas I, Ozcelik N. Protective effect of caffeic acid phenethyl ester (CAPE) on lipid peroxidation and antioxidant enzymes in diabetic rat liver. J Biochem Mol Toxicol. 2004;18:234–238. doi: 10.1002/jbt.20028. [DOI] [PubMed] [Google Scholar]
- 67.Demasi M, Netto LE, Silva GM, Hand A, de Oliveira CL, Bicev RN, Gozzo F, Barros MH, Leme JM, Ohara E. Redox regulation of the proteasome via S-glutathionylation. Redox Biol. 2013;2:44–51. doi: 10.1016/j.redox.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou H, Zhu J, Yue S, Lu L, Busuttil RW, Kupiec-Weglinski JW, Wang X, Zhai Y. The Dichotomy of Endoplasmic Reticulum Stress Response in Liver Ischemia-Reperfusion Injury. Transplantation. 2016;100:365–372. doi: 10.1097/TP.0000000000001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baker TA, Geng Q, Romero J, Picken MM, Gamelli RL, Majetschak M. Prolongation of myocardial viability by proteasome inhibition during hypothermic organ preservation. Biochem Biophys Res Commun. 2010;401:548–553. doi: 10.1016/j.bbrc.2010.09.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Geng Q, Romero J, Saini V, Baker TA, Picken MM, Gamelli RL, Majetschak M. A subset of 26S proteasomes is activated at critically low ATP concentrations and contributes to myocardial injury during cold ischemia. Biochem Biophys Res Commun. 2009;390:1136–1141. doi: 10.1016/j.bbrc.2009.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zaouali MA, Bardag-Gorce F, Carbonell T, Oliva J, Pantazi E, Bejaoui M, Ben Abdennebi H, Rimola A, Roselló-Catafau J. Proteasome inhibitors protect the steatotic and non-steatotic liver graft against cold ischemia reperfusion injury. Exp Mol Pathol. 2013;94:352–359. doi: 10.1016/j.yexmp.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 72.Bejaoui M, Zaouali MA, Folch-Puy E, Pantazi E, Bardag-Gorce F, Carbonell T, Oliva J, Rimola A, Abdennebi HB, Roselló-Catafau J. Bortezomib enhances fatty liver preservation in Institut George Lopez-1 solution through adenosine monophosphate activated protein kinase and Akt/mTOR pathways. J Pharm Pharmacol. 2014;66:62–72. doi: 10.1111/jphp.12154. [DOI] [PubMed] [Google Scholar]
- 73.Panisello-Roselló A, Verde E, Amine Zaouali M, Flores M, Alva N, Lopez A, Folch-Puy E, Carbonell T, Hotter G, Adam R, et al. The Relevance of the UPS in Fatty Liver Graft Preservation: A New Approach for IGL-1 and HTK Solutions. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18112287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goldstein G, Scheid M, Hammerling U, Schlesinger DH, Niall HD, Boyse EA. Isolation of a polypeptide that has lymphocyte-differentiating properties and is probably represented universally in living cells. Proc Natl Acad Sci USA. 1975;72:11–15. doi: 10.1073/pnas.72.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ciechanover A, Elias S, Heller H, Hershko A. “Covalent affinity” purification of ubiquitin-activating enzyme. J Biol Chem. 1982;257:2537–2542. [PubMed] [Google Scholar]
- 76.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 77.Ciechanover A. Intracellular protein degradation: from a vague idea through the lysosome and the ubiquitin-proteasome system and onto human diseases and drug targeting. Bioorg Med Chem. 2013;21:3400–3410. doi: 10.1016/j.bmc.2013.01.056. [DOI] [PubMed] [Google Scholar]
- 78.Hershko A, Heller H, Elias S, Ciechanover A. Components of ubiquitin-protein ligase system. Resolution, affinity purification, and role in protein breakdown. J Biol Chem. 1983;258:8206–8214. [PubMed] [Google Scholar]
- 79.Hameed DS, Sapmaz A, Ovaa H. How Chemical Synthesis of Ubiquitin Conjugates Helps To Understand Ubiquitin Signal Transduction. Bioconjug Chem. 2017;28:805–815. doi: 10.1021/acs.bioconjchem.6b00140. [DOI] [PubMed] [Google Scholar]
- 80.Kim W, Bennett EJ, Huttlin EL, Guo A, Li J, Possemato A, Sowa ME, Rad R, Rush J, Comb MJ, et al. Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol Cell. 2011;44:325–340. doi: 10.1016/j.molcel.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu P, Duong DM, Seyfried NT, Cheng D, Xie Y, Robert J, Rush J, Hochstrasser M, Finley D, Peng J. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell. 2009;137:133–145. doi: 10.1016/j.cell.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen ZJ, Sun LJ. Nonproteolytic functions of ubiquitin in cell signaling. Mol Cell. 2009;33:275–286. doi: 10.1016/j.molcel.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 83.Jung T, Grune T. The proteasome and the degradation of oxidized proteins: Part I-structure of proteasomes. Redox Biol. 2013;1:178–182. doi: 10.1016/j.redox.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Calmus Y, Cynober L, Dousset B, Lim SK, Soubrane O, Conti F, Houssin D, Giboudeau J. Evidence for the detrimental role of proteolysis during liver preservation in humans. Gastroenterology. 1995;108:1510–1516. doi: 10.1016/0016-5085(95)90701-7. [DOI] [PubMed] [Google Scholar]
- 85.Adam R, Delvart V, Karam V, Ducerf C, Navarro F, Letoublon C, Belghiti J, Pezet D, Castaing D, Le Treut YP, Gugenheim J, Bachellier P, Pirenne J, Muiesan P; ELTR contributing centres, the European Liver, Intestine Transplant Association (ELITA) Compared efficacy of preservation solutions in liver transplantation: a long-term graft outcome study from the European Liver Transplant Registry. Am J Transplant. 2015;15:395–406. doi: 10.1111/ajt.13060. [DOI] [PubMed] [Google Scholar]
- 86.Zaouali MA, Panisello-Roselló A, Lopez A, Castro Benítez C, Folch-Puy E, García-Gil A, Carbonell T, Adam R, Roselló-Catafau J. Relevance of proteolysis and proteasome activation in fatty liver graft preservation: An Institut Georges Lopez-1 vs University of Wisconsin appraisal. World J Gastroenterol. 2017;23:4211–4221. doi: 10.3748/wjg.v23.i23.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Majetschak M. Regulation of the proteasome by ATP: implications for ischemic myocardial injury and donor heart preservation. Am J Physiol Heart Circ Physiol. 2013;305:H267–H278. doi: 10.1152/ajpheart.00206.2012. [DOI] [PubMed] [Google Scholar]
- 88.Zhang F, Hu Y, Huang P, Toleman CA, Paterson AJ, Kudlow JE. Proteasome function is regulated by cyclic AMP-dependent protein kinase through phosphorylation of Rpt6. J Biol Chem. 2007;282:22460–22471. doi: 10.1074/jbc.M702439200. [DOI] [PubMed] [Google Scholar]
- 89.Djakovic SN, Marquez-Lona EM, Jakawich SK, Wright R, Chu C, Sutton MA, Patrick GN. Phosphorylation of Rpt6 regulates synaptic strength in hippocampal neurons. J Neurosci. 2012;32:5126–5131. doi: 10.1523/JNEUROSCI.4427-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jarome TJ, Kwapis JL, Ruenzel WL, Helmstetter FJ. CaMKII, but not protein kinase A, regulates Rpt6 phosphorylation and proteasome activity during the formation of long-term memories. Front Behav Neurosci. 2013;7:115. doi: 10.3389/fnbeh.2013.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grune T, Reinheckel T, Davies KJ. Degradation of oxidized proteins in K562 human hematopoietic cells by proteasome. J Biol Chem. 1996;271:15504–15509. doi: 10.1074/jbc.271.26.15504. [DOI] [PubMed] [Google Scholar]
- 92.Höhn TJ, Grune T. The proteasome and the degradation of oxidized proteins: part III-Redox regulation of the proteasomal system. Redox Biol. 2014;2:388–394. doi: 10.1016/j.redox.2013.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Davies KJ. Degradation of oxidized proteins by the 20S proteasome. Biochimie. 2001;83:301–310. doi: 10.1016/s0300-9084(01)01250-0. [DOI] [PubMed] [Google Scholar]
- 94.Shang F, Nowell TR Jr, Taylor A. Removal of oxidatively damaged proteins from lens cells by the ubiquitin-proteasome pathway. Exp Eye Res. 2001;73:229–238. doi: 10.1006/exer.2001.1029. [DOI] [PubMed] [Google Scholar]
- 95.Gomes AV, Zong C, Ping P. Protein degradation by the 26S proteasome system in the normal and stressed myocardium. Antioxid Redox Signal. 2006;8:1677–1691. doi: 10.1089/ars.2006.8.1677. [DOI] [PubMed] [Google Scholar]
- 96.Jahngen-Hodge J, Obin MS, Gong X, Shang F, Nowell TR Jr, Gong J, Abasi H, Blumberg J, Taylor A. Regulation of ubiquitin-conjugating enzymes by glutathione following oxidative stress. J Biol Chem. 1997;272:28218–28226. doi: 10.1074/jbc.272.45.28218. [DOI] [PubMed] [Google Scholar]
- 97.Bonet-Costa V, Pomatto LC, Davies KJ. The Proteasome and Oxidative Stress in Alzheimer’s Disease. Antioxid Redox Signal. 2016;25:886–901. doi: 10.1089/ars.2016.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Majetschak M, Patel MB, Sorell LT, Liotta C, Li S, Pham SM. Cardiac proteasome dysfunction during cold ischemic storage and reperfusion in a murine heart transplantation model. Biochem Biophys Res Commun. 2008;365:882–888. doi: 10.1016/j.bbrc.2007.11.092. [DOI] [PubMed] [Google Scholar]
- 99.Campbell B, Adams J, Shin YK, Lefer AM. Cardioprotective effects of a novel proteasome inhibitor following ischemia and reperfusion in the isolated perfused rat heart. J Mol Cell Cardiol. 1999;31:467–476. doi: 10.1006/jmcc.1998.0880. [DOI] [PubMed] [Google Scholar]


