Abstract
The leaves of Morus alba L. are an important herbal medicine in Asia. The systematic isolation of the metabolites of the leaves of Morus alba L. was achieved using a combination of liquid chromatography techniques. The structures were elucidated by spectroscopic data analysis and the absolute configuration was determined based on electronic circular dichroism (ECD) spectroscopic data and hydrolysis experiments. Their biological activity was evaluated using different biological assays, such as the assessment of their capacity to inhibit the aldose reductase enzyme; the determination of their cytotoxic activity and the evaluation of their neuroprotective effects against the deprivation of serum or against the presence of nicouline. Chemical investigation of the leaves of Morus alba L. resulted in four new structures 1–4 and a known molecule 5. Compounds 2 and 5 inhibited aldose reductase with IC50 values of 4.33 μM and 6.0 μM compared with the potent AR inhibitor epalrestat (IC50 1.88 × 10−3 μM). Pretreatment with compound 3 decreased PC12 cell apoptosis subsequent serum deprivation condition and pretreatment with compound 5 decreased nicouline-induced PC12 cell apoptosis as compared with control cells (p < 0.001).
Keywords: Morus alba L., aldose reductase inhibitor, neuroprotective agent, natural products
1. Introduction
The species Morus alba L., known as white mulberry, belongs to the genus Morus of the family Moraceae, is native to China and now is cultivated throughout the world [1,2]. All parts of this plant have been used medicinally in Traditional Chinese Medicine including the leaves, root bark, stem and fruits [3,4]. In East Asia the leaves have been an important herbal medicine for treatment of cold, fever, headache, cough and rheumatic diseases for thousands of years [3,5]. Extracts or constituents of M. alba L. leaves were reported to possess anti-inflammatory, antioxidant, antiobesity, antidiabetic, and hypolipidemic properties [5,6]. Phytochemical investigations of the leaves of M. alba reported the presence of flavonoids, lignans, pyrrole alkaloids, polyphenols, fatty acids, and anthocyanin [6,7]. Previous phytochemical studies and the pharmacological potentials of constituents of the genus Morus have been reviewed by Yang et al. [8].
Aldose reductase is the first and rate-controlling enzyme in the polyol pathway that reduces glucose into sorbitol and then fructose. Intracellular excess sorbitol is thought to lead to diabetic complications, including neuropathy, nephropathy, retinopathy, and cataract [9]. Fructose can lead to the formation of 3-deoxyglucosone, a key intermediate known to accelerate the formation of Advanced Glycation End products (AGEs) [10]. The presence and accumulation of AGEs contribute to the development of atherosclerosis and promotes renal damage, diabetic nephropathy, and a series of cancers [11,12,13,14]. Besides reducing glucose, aldose reductase is also involved in the reduction of oxidative stress-generated lipid aldehydes and their conjugate with GSH, which can alter cellular signals by mediating transcription factors such as NF-Kb and AP1 [15]. Aldose reductase inhibitors have been shown to be an effective multi-disease target to prevent diabetic complications, cancers, cardiovascular diseases, and inflammatory complications [16]. This study investigated the acetone and chloroform fractions of the ethanol extract of the leaves of M. alba L., which showed effects against diabetes and human cancer cell lines in our previous study, leading to the identification of four new structures, namely a sesquiterpenoid glucoside 1, an aromatic glucoside 2, a farnesylacetone derivative 3, a flavan 4, and a known compound, (9R)-hydroxyl-(10E,12Z,15Z)-octadecatrienoic acid (5). In addition, we report the results of aldose reductase inhibitory and neuroprotective activity evaluations.
2. Results and Discussion
2.1. Characterization
The crude extract of the leaves of Morus alba. L was divided into four fractions by flash silica gel column chromatography. The generated acetone and chloroform fractions were further isolated by the combination of resin column chromatography, silica gel column chromatography, medium pressure liquid chromatography (MPLC), and high performance liquid chromatography (HPLC), generating four new compounds and a known one (Figure 1).
Figure 1.
Structures of Compounds 1–5.
Moralsin (1) was obtained as a white powder, −72 (c 0.19, MeOH). The IR spectrum of 1 showed the presence of hydroxy (2957 cm−1), alkyl (3402 cm−1) and ester carbonyl (1760 cm−1) functional groups. The molecular formula, C21H30O8, was determined from its sodium adduct ion in the HRESIMS (433.1826 [M + Na]+, calcd. 433.1833), corresponding to seven indices of hydrogen deficiency. The 1H-NMR and 13C-NMR spectra (Table 1 and Table 2) revealed the presence of two trisubstituted double bonds [δH 6.82 (t, J = 3.0 Hz, H-4), 5.66 (d, J = 9.3 Hz, H-8); δC 132.4 (C-4), 130.7 (C-5), 129.8 (C-8), 138.8 (C-9)], one terminal double bond [δH 6.44 (dd, J = 17.5, 10.5 Hz, H-10), 5.37 (d, J = 17.5 Hz, H-11a), 5.20 (d, J = 10.5 Hz, H-11b)], one ester carbonyl (δC 168.8), two oxygenated methines [δH 4.41 (m, H-3), 5.12 (t, J = 9.3 Hz, H-7); δC 70.4 (C-3), 76.8 (C-7)], three methyls [δH 0.90 (3H, s), 0.87 (3H, s), 1.88 (3H, s)], one methylene [δH 1.86 (m, H-2α), 1.60 (dd, J = 15.0, 6.5 Hz, H-2β)], two methines [δH 2.57 (m, H-6), 5.12 (t, J = 9.3 Hz, H-7)], one tertiary carbon group [δC 29.5 (C-1)] and a glucopyranosyl unit [δH 4.35 (d, J = 8.0 Hz, H-1′), 2.9-3.7 (6H, H-2′-6′)]. In combination with analysis of 1H-1H COSY spectrum, the NMR date displayed there were two spin systems C2-C3-C4 and C6-C7-C8 in 1. In the HMBC spectrum, the correlations of H-12, H-13/C-1, C-2, C-6; H-4/C-5, C-14; H-6/C-4; H-11/C-9; and H-15/C-8, C-10 determined the monocyclofarnesane carbon skeleton containing a 14,7-olide ring. In addition, the correlations of H-1′/C-3 indicated the glucopyranosyl unit was connected to C-3 of the monocyclofarnesane-type sesquiterpenoid aglycone.
Table 1.
1H-NMR Spectroscopic Data of Compounds 1–4a.
| 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|
| Position | δH (J in Hz) | Position | δH (J in Hz) | Position | δH (J in Hz) | Position | δH (J in Hz) |
| 2α | 1.86, (overlapped) | 1 | 2.25, s | 2 | 5.27, dd, (9.6, 1.8) | 3 | 6.65, d, (8.4) |
| 2β | 1.60, dd, (15.0, 6.5) | 3 | 6.09, d, (16.5) | 3a | 2.20, m | 4 | 7.18, dd, (8.4) |
| 3 | 4.41, m | 4 | 7.45, dd, (16.5, 11.5) | 3b | 1.85, m | 5 | 6.55, d, (8.4) |
| 4 | 6.82, t, (3.0) | 5 | 6.23, d, (11.5) | 4a | 2.86, m | 2′, 6′ | 7.47, m |
| 6 | 2.57, m | 7 | 3.93, t, (6.5) | 4b | 2.63, m | 3′, 5′ | 7.36, m |
| 7 | 5.12, t, (9.3) | 8 | 1.47, m | 5 | 6.79, d, (8.4) | 4′ | 7.36, m |
| 8 | 5.66, d, (9.3) | 9 | 1.97, t, (7.5) | 6 | 6.30, d, (8.4) | 7′a | 5.33, d, (12.6) |
| 10 | 6.44, dd, (17.5, 9.0) | 11 | 5.03, t, (7.0) | 3′ | 6.40, d, (2.4) | 7′b | 5.22, d, (12.6) |
| 11a | 5.37, d, (17.5) | 12 | 2.12, dd, (7.5, 7.0) | 5′ | 6.42, dd, (8.4, 2.4) | 1″ | 4.84, d, (7.2) |
| 11b | 5.20, d, (10.5) | 13 | 2.42, t, (7.5) | 6′ | 7.25, d, (8.4) | 2″ | 3.21, m |
| 12 | 0.90, s | 15 | 1.06, s | 3′′ | 3.73, dd, (7.2, 6.0) | 3″ | 3.49, m |
| 13 | 0.87, 3H, s | 16 | 1.85 (3H, s) | 4′′a | 2.92, dd, (17.4, 7.8) | 4″ | 3.09, t, (8.7) |
| 15 | 1.88, s | 17 | 1.62 (3H, s) | 4′′b | 2.54, dd, (17.4, 5.4) | 5″ | 3.25, m |
| 1′ | 4.35, d, (8.0) | 5′′ | 1.22, s b | 6″a | 3.86, d, (8.1) | ||
| 2′ | 2.91, t, (8.0) | 6′′ | 1.31, s b | 6″b | 3.44, m | ||
| 3′ | 3.15, m | -OMe | 3.75, s | 1′′′ | 4.83, d, (3.0) | ||
| 4′ | 3.03, t, (8.0) | 2′′′ | 3.75, d, (3.0) | ||||
| 5′ | 3.15, m | 4′′′a | 3.89 (1H, d, 9.0) | ||||
| 6′a | 3.68, dd, (10.5, 1.0) | 4′′′a | 3.59 (1H. d, 9.0) | ||||
| 6′b | 3.44, dd, (10.5, 6.0) | 5′′′ | 3.35 (1H, m) | ||||
a 1H–NMR data (δ) were measured at 500 MHz in DMSO-d6 for 1, 2, 4, and at 600 MHz in methanol-d4 for 3. The assignments were based on HSQC and HMBC experiments; b Interchangeable.
Table 2.
13C-NMR Spectroscopic Data of Compounds 1–4a.
| 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|
| Position | δc, Type | Position | δc, Type | Position | δc, Type | Position | δc, Type |
| 1 | 29.5, C | 1 | 27.1, CH3 | 2 | 74.2, CH | 1 | 120.0, C |
| 2 | 43.2, CH2 | 2 | 198.3, CH | 3 | 29.9, CH2 | 2 | 155.3, C |
| 3 | 70.4, CH | 3 | 129.9, CH | 4 | 25.8, CH2 | 3 | 105.5 b, CH |
| 4 | 132.4, CH | 4 | 139.4, CH | 5 | 128.5, CH | 4 | 131.0, CH |
| 5 | 130.7, C | 5 | 122.3, CH | 6 | 109.7, CH | 5 | 109.4 b, CH |
| 6 | 52.3, CH | 6 | 153.1, C | 7 | 153.0, C | 6 | 155.4, C |
| 7 | 76.8, CH | 7 | 74.7, CH | 8 | 109.3, C | 7 | 165.8, C |
| 8 | 129.8, CH | 8 | 33.3, CH2 | 4a | 114.4, C | 1′ | 136.2, C |
| 9 | 138.8, C | 9 | 27.5, CH2 | 8a | 154.5, C | 2′, 6′ | 127.8, CH |
| 10 | 140.0, CH | 10 | 135.3, C | 1′ | 122.4, C | 3′, 5′ | 128.3, CH |
| 11 | 115.6, CH2 | 11 | 124.0, CH | 2′ | 156.1, C | 4′ | 127.8, CH |
| 12 | 21.0, CH3 | 12 | 21.8, CH2 | 3′ | 102.1, CH | 7′ | 66.0, CH2 |
| 13 | 28.9, CH3 | 13 | 43.1, CH2 | 4′ | 161.4, C | 1” | 100.4, CH |
| 14 | 168.8, C | 14 | 208.1, C | 5′ | 105.7, CH | 2” | 73.27, CH |
| 15 | 12.3, CH3 | 15 | 29.8, CH3 | 6′ | 128.0, CH | 3” | 75.6, CH |
| 1′ | 102.7, CH | 16 | 13.4, CH3 | 2” | 77.4, C | 4” | 70.0, CH |
| 2′ | 73.5, CH | 17 | 23.2, CH3 | 3” | 70.5, CH | 5” | 76.8, CH |
| 3′ | 76.8, CH | 4” | 27.3, CH2 | 6” | 67.8, CH2 | ||
| 4′ | 70.1, CH | 5′′ | 20.8 | 1′′′ | 109.4, CH | ||
| 5′ | 76.9, CH | 6′′ | 25.8 | 2′” | 75.9, CH | ||
| 6′ | 61.2, CH2 b 4.29, dd, (11.5, 6) | -OMe | 55.7 | 3′′′ | 78.7, C | ||
| 4′′′ | 73.30, CH2 | ||||||
| 5′′′ | 63.2, CH2 | ||||||
a 1H NMR data (δ) were measured at 125 MHz in DMSO-d6 for 1, 2, 4, and at 150 MHz in methanol-d4 for 3. The assignments were based on HSQC and HMBC experiments; b interchangeable.
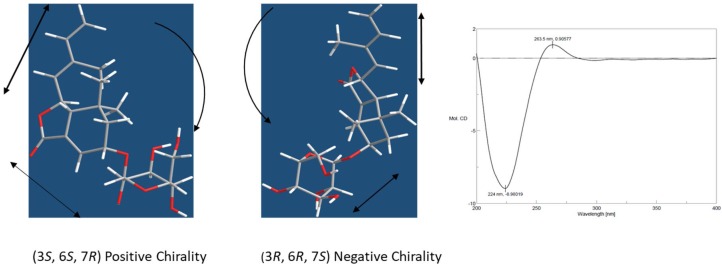
A β-anomeric configuration for the glucosyl unit was assigned via its large 3J1,2 coupling constant (8.0 Hz). The D-configuration of the glucose was determined by GC analysis of the trimethylsilyl l-cysteine derivatives after acid hydrolysis of 1. The E-configuration of the 8,9-double bond was demonstrated by the nuclear Overhauser effect (NOE) effect of H-7/H-15 and H-8/H-10 in the ROESY 1D experiment. NOE effect of H-3/H-2β, H-7/H-12, and H-6/H-8, H-2β, and H-13 showed that H-2β, H-3, H-6, C-13, and C-8 were cofacial, assigned as the β-orientation, and H-7 and C-12 were α-oriented. The absolute configuration of aglycone moiety was assigned by analysis of the electronic circular dichroism (ECD) spectroscopy using excitation chirality method [17]. The ECD spectrum showed positive Cotton effect at 263 nm and negative Cotton effect at 224 nm arising from coupling between conjugated diene and α,β-unsaturated ester chromophores (Supplementary Materials). Such a pattern was in agreement of a negative chirality of 1 as depicted in Figure 2. Thus the absolute configuration of 1 was unequivocally assigned as (3S, 6S, 7R) and the structure of moralsin was determined as 1.
Figure 2.
ECD Spectrum of compound 1. The positive Cotton effect of ECD spectrum is in agreement with the negative chirality of (3S, 6S, 7R) diastereoisomer of compound 1.
Compound 2 was obtained as a yellow oil. The IR spectrum of 2 showed the presence of hydroxy (3419 cm−1), alkyl (2931 cm−1), and carbonyl (1712 cm−1) functional groups. The molecular formula, C17H26O3, was determined from its sodium adduct ion in the HRESIMS (301.1785 [M + Na]+, calcd. 301.1774), corresponding to five indices of hydrogen deficiency. The 1H-NMR (Table 1), 13C-NMR (Table 2), and DEPT (Supplementary Information) spectra revealed the presence of three double bonds at δH 6.09 (d, J = 16.5 Hz, H-3), 7.45 (dd, J = 16.5, 11.5 Hz, H-4), 6.23 (d, J = 11.5 Hz, H-5), 5.03 (t, J = 7.0 Hz, H-11) and δC 129.9 (C-3), 139.4 (C-4), 122.3 (C-5), 153.1 (C-6), 135.3 (C-10), 124.0 (C-11), a oxymethine group at δH 3.93 (t, J = 6.5 Hz, H-7), four methylene groups at δH 1.47 (m, H-8), 1.97 (t, J = 7.5 Hz, H-9), 2.12 (m, H-12), 2.42 (t, J = 7.5 Hz, H-13), four terminal methyl groups at δH 2.25 (s, H-1), 2.06 (s, H-15), 1.85 (s, H-16), 1.62 (s, H-17), and two carbonyl groups at δC 198.3 (C-2), 208.1 (C-14). The coupling patterns in 1H-NMR spectrum and the correlations in 1H-1H COSY spectrum showed the presence of three spin systems of C-3-C-4-C-5, C-7-C-8-C-9, and C-11-C-12-C-13. In the HMBC spectrum the correlations of H-1, H-3, H-4/C-1, H-4, H-8/C-6, H-5/C-7, H-8, H-12/C-10, H-11/C-9, and H-12, H-14, H-15/C-14 clarified the connections of the two terminal methyl groups (C-1, 15), the two carbonyl groups and the three spin systems, suggesting a linear structure of pentadecatrien-2,14-dione. The HMBC correlations of H-5, H-7/C-16, H-16/C-5, C-7, H-9, H-11/C-17, and H-17/C-9, C-10, C-11 allowed the attachments of C-16 to C-6 and C-17 to C-10, which also further supported the presence of a pentadecatrien-2,14-dione moiety.
A 3E geometry was assigned via its large 3J3,4 coupling constant (16.5 Hz). The NOESY correlations of H-5/H-7 and H-11/H-17 revealed that the geometries of the C-5 and C-10 olefins in 2 were 5E and 10Z. Compound 2 was characterized as (3E,5E,10Z)-7-hydroxy-6,10-dimethyl-pentadecatrien-2,14-dione. The optical rotation of 2 that is close to 0 suggested 2 is a pair of enantiomers, which was proved by its separation on HPLC using a chiral chromatography column.
Compound 3 was obtained as a yellow oil. The molecular formula, C21H24O5, was established from its proton adduct ion in the HRESIMS (357.16873 [M + H]+, calcd. 357.1702), which was also supported by NMR data. Analysis of the 1H-NMR spectrum (Table 1) of 3 revealed the presence of a set of ABX system aromatic protons at δH 6.40 (d, J = 2.4 Hz, H-3′), 6.42 (dd, J = 8.4, 2.4 Hz, H-5′), and 7.25 (d, J = 8.4 Hz, H-6′), two ortho-coupled doublet aromatic protons at δH 6.79 (d, J = 8.4 Hz, H-5) and 6.30 (d, J = 8.4 Hz, H-6), and a set of aliphatic proton signals at δH 5.27 (dd, J = 9.6, 1.8 Hz, H-2), 2.20 (m, H-3a), 1.85 (m, H-3b), 2.86 (m, H-4a), 2.63 (m,H-4b), suggesting a flavan skeleton for 3, which was consistent with the 13C-NMR data. The proton signals at δH 3.73 (dd, J = 7.2, 6.0 Hz, H-3″), 2.92 (dd, J = 17.4, 7.8 Hz, H-4″a), 2.54 (dd, J = 17.4, 5.4 Hz, H-4″b), 1.31 (s, 3H) and 1.22 (s, 3H), in combination with 13C-NMR signals at δC at 77.4 (C-2′′′), 70.5 (C-3′′′), 27.3 (C-4′′′), and 25.8, 20.8 (-Me) showed the presence of a 3″-hydroxyprenyl residue forming a furan ring with a hydroxyl group. The correlations of H-3″, H-4″a, H-4″b/C-8 confirmed the isoprenyl substituent was located at C-8, cyclizing onto 7-hydroxyl group. Accordingly, the structure of 3 was assigned as shown in Figure 1.
The optical rotation of 3 that was close to 0, suggesting 3 is a pair of enantiomers, which was proved by the separation on HPLC using a chiral chromatography column.
Compound 4 was obtained as a yellow oil. The IR spectrum of 4 showed the presence of hydroxy (3347 cm−1), carbonyl (1726 cm−1) and aromatic (1606 and 1466 cm−1) functional groups. The molecular formula, C25H30O13, was determined from its sodium adduct ion in the HRESIMS (561.1583 [M + Na]+, calcd. 561.1579) and also supported by the NMR spectroscopic data. The 1H-NMR (Table 1) spectrum showed signals attributable to a monosubstituted aromatic ring at δH 7.47 (2H, m, H-2′, 6′) and 7.36 (3H, m, H-3′, 4′, 5′), and an oxymethylene group at δH 5.33 (d, J = 12.6 Hz, H-7′a) and 5.22 (d, J = 12.6 Hz, H-7′b), revealing the presence of a benzyl group in combination with the correlations of H-7′/C-2′,6′ in HMBC spectrum. An ABC spin system attributed to anomeric protons at δH 6.65 (d, J = 8.4 Hz, H-3), 7.18 (dd, J = 8.4 Hz, H-4), and 6.55 (d, J = 8.4 Hz, H-5). The correlations of H-3, H-5/C-7 (δC 165.8) in HMBC spectrum showed the presence of a 2,6-bisubstituted benzoyl moiety. Together with the coupling patterns of oxymethylene and oxymethine protons resonating between δH 3.09 and 4.84 indicated the presence of a glucopyranosyl and a apiofuranosyl units. The correlations of H-7′/C-7, H-1″/C-2, H-1′′′/C-6″ in HMBC spectrum determined a moiety of apiofuranosyl(1→6)-glucopyranose was attached to C-2 of benzoyl moiety and the benzyl group was connected to C-7of the benzoyl moiety. A β-anomeric configuration for the glucosyl unit was assigned via its large 3J1’’,2’’ coupling constant (7.2 Hz). The β-configuration for apiofuranosyl unit was assigned via its 3J1’’’,2’’’ coupling constant (3.0 Hz) and the chemical shift of anomeric carbon (δC 109.4) [6,18]. The D-configurations of glucopyranosyl and a apiofuranosyl units were determined by gas chromatography (GC) analysis of the trimethylsilyl l-cysteine derivatives after acid hydrolysis of 4. On the basis of the above data, 4 was characterized as benzyl 2-O-[β-d-apiofuranosyl(1→6)-β-d-glucopyranosyl]-2,6-dihydroxy-benzoate.
The known compound was identified as (9R)-hydroxyl-(10E,12Z,15Z)-octadecatrienoic acid (5) by NMR analysis and comparison with literature data [19].
2.2. Aldose Reductase Inhibitory Effects of 2 and 5 and Neuroprotective Effects of Compounds 1–5
The aldose reductase inhibitory and neuroprotective bioactivities of compounds 1–5 were assessed. Compounds 2 and 5 possessed inhibition activities against aldose reductase, with IC50 values of 4.33 μM and 6.0 μM compared with the potent AR inhibitor epalrestat (IC50 1.88 × 10−3 μM) [20]. Compound 3 exhibited neuroprotective activity against PC12 cell damage induced by serum deprivation and 5 appeared to protect against PC12 cell damage caused by nicouline (Table 3), an assay extensively used in screening active agents for Parkinson’s disease [21,22,23,24]. Compounds 1–5 were evaluated for their cytotoxic activities against eight human cancer cell lines (human colon carcinoma cell line HCT-8, hepatocellular carcinoma cell line Bel-7402, human renal cell carcinoma cell line KETR3, Human cervical carcinoma cell line HELA, human gastric cancer cell line BGC-823, human ovarian carcinoma cell line A2780, human breast cancer cell line MCF-7, and human lung carcinoma cell line A549) by means of the MTT assay [25], using paclitaxel and 5-fluouracil as positive controls. Nevertheless, all the isolated compounds resulted to be inactive.
Table 3.
Neuroprotective Effects of Compounds 1–5 at concentration of 10−5 M (means ± SD, n = 6).
| Sample | Serum Deprivation (%) | Nicouline 4 μM (%) |
|---|---|---|
| control | 100.0 ± 3.7 | 100.0 ± 1.4 |
| model | 41.4 ± 3.8 ### | 74.3 ± 1.4 ### |
| 1 | 50.2 ± 12.7 | 78.6 ± 2.9 |
| 2 | 66.2 ± 12.6 | 78.4 ± 2.0 * |
| 3 | 59.8 ± 2.7*** | 75.7 ± 3.0 |
| 4 | 50.9 ± 7.8 | 76.9 ± 3.6 |
| 5 | 70.2 ± 16.1 | 86.2 ± 7.6 *** |
###p < 0.001 vs. control,* p < 0.05,** p < 0.001, *** p < 0.0001 vs. model.
2.3. Discussion
We found it was helpful to subject the chloroform fraction of the ethanol extract to flash silica gel column chromatography repeatedly before isolation to remove pigments. The 80% MeOH-H2O mobile phase of Sephadex LH-20 column chromatography worked well for all kinds of structures in our study. We obtained two pairs of enantiomers, (2a,2b) and (3a,3b). Yang et al. reported an interesting phenomenon whereby the R and S configurations of C-2 of a similar flavan were interconvertible, which (3a,3b) may be subject to [5]. In addition, considering the wide range of examples that the isomers of enantiomers showed differences in pharmacological processes, further separation and research of the two pairs of enantiomers is needed.
Polyphenols from Morus plants have indicated extensive antioxidative activities, especially the kind of Diels-Alder type adducts [8,26]. Oxidative stress played a key role in neurodegenerative diseases, which implied the potential of polyphenols from Morus plants against neurodegenerative disorders [27]. Unfortunately, most of the previous studies on Morus polyphenols had been focused on their anti-oxidant properties. More efforts are suggested to explore the neuroprotective action of constituents of Morus plants.
3. Materials and Methods
3.1. Plant Material
The leaves of Morus alba L. were collected in the Anding Mulberry Garden (Beijing, China), in July 2011, and identified by Prof. Lin Ma (Institute of Materia Medica, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China). A voucher specimen (No. ID-S-2543) has been deposited at the Herbarium of Institude of Materia Medica, Chinese Academy of Medical Sciences & Peking Union Medical College.
3.2. General Experimental Procedures
Optical rotations were measured with a P-2000 polarimeter (Jasco, Tokyo, Japan) and UV spectra with a Jasco V-650 spectrophotometer. ECD spectra were measured on a Jasco J-815 spectrometer. IR spectra were recorded on a model 5700 spectrometer (Nicolet, Madison, SD, USA) by an FT-IR microscope transmission method. NMR measurements were performed using VNS-600 (Varian Medical Systems, Inc., Palo Alto, CA, USA), Mercury-300 (Varian Medical Systems, Inc., Palo Alto, CA, USA), Bruker-AV-III-500 (Bruker Corporation, Karlsruhe, Germany), and Inova-500 (Varian Medical Systems, Inc., Palo Alto, CA, USA) spectrometers. ESIMS was performed on Agilent 1100 Series LC/MSD Trap SL mass spectrometer and HRESIMS data were obtained using an Agilent 6520 Accurate-Mass Q-TOF LC/MS (Agilent Technologies, Ltd., Santa Clara, CA, USA). Gas chromatography (GC) was operated on Agilent 7890A system. HPLC was performed on a Lumtech instrument (Lumiere Tech Ltd. Beijing, China) equipped with a 500 ELSD detector (Alltech, Deerfield, IL, USA) and a YMC-Pack ODS-A column (250 × 20 mm, 5 μm, YMC, Tokyo, Japan). Silica gel (200−300 mesh, Qingdao Marine Chemical Factory, Qingdao, China), Sephadex LH-20 (GE), and ODS (50 μm, YMC) were used for column chromatography. TLC was carried out with GF254 plates (Qingdao Marine Chemical Factory). Spots were visualized by spraying with 10% H2SO4 in EtOH followed by heating.
3.3. Cell Lines, Chemicals and Biochemicals
PC12 cells (adrenal gland; pheochromocytoma) were purchased from the American Type Culture Collection (Manassas, VA, USA). Dimethyl sulphoxide (DMSO), nicouline, more commonly known as rotenone, and 3-(3,4-dimehylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) were obtained from Sigma (St. Louis, MO, USA). Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS), and horse serum were purchased from Gibco BRL (New York, NY, USA). Epelrestat was purchased from Dayin Marine Bio-Pharmaceutical Co., Ltd. (Rongcheng, Shandong, China). NADPH-Na4, paclitaxel and 5-fluorouracil were purchased from Sigma-Aldrich (Beijing, China). All other chemicals were of analytical grade and were commercially available.
3.4. Extraction and Isolation
Air-dried leaves of Morus alba L. (30 kg) were exhaustively extracted with 95% aqueous EtOH (3 × 100 L, 2 h) at reflux. The combined extracts were concentrated under reduced pressure to dryness. The residue (2.9 kg) was subjected to column chromatography on silica gel and eluted with petroleum ether, chloroform, acetone and methanol. The acetone residue (423 g) was subjected to D101 macroporous resins column chromatography by a gradient elution with EtOH/H2O (0:100, 30:70, 60:40, 95:5) to yield four fractions (fractions A–D). The separation of fraction B (120 g) was carried out on silica gel column chromatography eluted with CHCl3/MeOH (10:1–3:1) to provide three subfractions B1–B3. Subfraction B2 (80 g) was further purified by MPLC (ODS, 50 μm, YMC) and eluted with 15, 35, 55, 75 and 100% MeOH−H2O, to afford 40 subfractions. Fraction B2-18 (42 mg) was purified by preparative HPLC using 20% MeCN−H2O (8 mL/min) as the mobile phase to yield compound 1 (4 mg) and fraction B2-20 (20 mg) was purified by preparative HPLC using 25% MeCN−H2O (8 mL/min) to yield compound 4 (18 mg). The chloroform residue (355 g) was subjected to silica gel column chromatography by a gradient elution with petroleum ether/acetone (100:0, 95:5, 90:10, 80:20, 70:30, 60:40) to yield six fractions (fractions E–M). The separation of fraction M (38 g) was carried out by MPLC (ODS, 50 μm, YMC) and eluted with 5, 15, 35, 55, 75, and 100% MeOH−H2O, to afford 25 subfractions. Fraction M-7 (100 mg) was purified by preparative HPLC using 20% MeOH−H2O (8 mL/min) as the mobile phase to yield compound 5 (45 mg). The separation of fraction N (20 g) was carried out by MPLC (ODS, 50 μm, YMC) and eluted with 10, 30, 55, 75, and 100% MeOH−H2O, to afford 28 subfractions. Fraction N-6 (50 mg) was purified by preparative HPLC using 30% MeOH−H2O (8 mL/min) as the mobile phase to yield compound 2 (10 mg). The separation of fraction g (139 g) was carried out by MPLC (ODS, 50 μm, YMC) and eluted with 15, 35, 55, 75, 85, and 100% MeOH-H2O, to afford 38 subfractions. Fraction G-10 (385 mg) was subjected to fractionation using Sephadex LH-20 column chromatography (80% MeOH-H2O) to provide 40 subfractions. Fraction G-10-18 (42 mg) was purified by the preparative HPLC using 35% MeCN−H2O (8 mL/min) as the mobile phase to yield compound 3 (15 mg).
3.5. Characterization
Moralsin (1): white powder; −72 (c 0.19, MeOH); UV(MeOH): λmax (log ε) 228 (2.10) nm; IR νmax 3402, 2957, 1760, 1606, 1080 cm−1; 1H-NMR (DMSO-d6, 500 MHz) and 13C-NMR (DMSO-d6, 125 MHz) see Table 2; positive-ion HRESIMS m/z 433.1826 [M + Na]+ (calcd. 433.1833).
(3E,5E,10Z)-7-Hydroxy-6,10-dimethyl-pentadecatrien-2,14-dione (2): yellow oil; UV(MeOH): λmax (log ε) 207 (4.31) nm, 247 (0.11) nm; IR νmax 3347, 2926, 1726, 1606, 1466, 1051, 1025 cm−1; 1H-NMR (DMSO-d6, 500 MHz) and 13C-NMR (DMSO-d6, 125 MHz) see Table 1 and Table 2; positive-ion HRESIMS m/z 561.1583 [M + Na]+ (calcd. 561.1579).
2′-Hydroxy-4′-methoxyl-2H-(2′′′, 2′′′-dimethyl-3′′′-hydroxy)-pyran-(5′′′,6′′′:8,7)-flavane (3): yellow oil; UV(MeOH): λmax (logε) 206 (3.90) nm, 280 (2.80) nm; IR νmax 3372, 1718, 1602 cm−1; 1H-NMR (MeOH-d4, 600 MHz) Table 1; 13C-NMR (MeOH-d4, 150 MHz) Table 2; positive-ion HRESIMS m/z 357.16873 [M + H]+ (calcd. for C21H25O5, 357.1702).
Benzyl 2-O-[β-d-apiofuranosyl(1→6)-β-d-glucopyranosyl]-2,6-dihydroxybenzoate (4): yellow oil; UV(MeOH): λmax (log ε) 207 (4.32) nm, 247 (0.1) nm; IR νmax 3347, 2926, 1726, 1606, 1466, 1051, 1025 cm−1; 1H-NMR (DMSO-d6, 300 MHz) and 13C-NMR (DMSO-d6, 125 MHz) see Table 1 and Table 2; positive-ion HRESIMS m/z 561.1583 [M + Na]+ (calcd. 561.1579).
(9R)-Hydroxy-(10E,12Z,15Z)-octadecatrienoic acid (5): Yellow powder, −3.04° (0.58 CHCl3); ESIMS m/z 317.2 [M + Na]+, 1H-NMR (DMSO-d6, 500 MHz) δ: 2.18 (2H, t, 9.6), 1.47 (2H, m, H-3), 1.38 (2H, m, H-8), 2.04 (2H, m, H-17), 0.92 (3H, t, J = 9.6 Hz, H-18), 3.97 (1H, m, H-9), 5.66 (1H, dd, J = 15.5, 6.0 Hz, H-10), 6.44 (1H, dd, J = 15.5, 11.5 Hz, H-11), 5.26-5.40 (3H, m, H-12, 15, 16), 5.96 (1H, t, J = 11.0 Hz, H-13), 2.88 (2H, dd, J = 11.0, 7.5, Hz, H-14), 1.24 (6H, m, H-4-6). 13C-NMR (DMSO-d6, 125MHz) δ: 174.5 (C-1), 138.6 (C-10), 131.7 (C-16), 128.7 (C-13), 128.3 (C-12), 126.7 (C-15), 123. 5 (C-11), 70.44 (C-9), 37.2 (C-8), 33.7 (C-2), 28.5, 28.8, 28.9 (C-4-6), 25.5 (C-14), 24.9 (C-7), 24.5 (C- 3), 20.0 (C-17), 14.1 (C-18).
3.6. Acid Hydrolysis of the Saponins and Determination of the Absolute Configuration of the Monosaccharides
Compound 1 (2 mg) was hydrolyzed in 2 M HCl/H2O at 80 °C for 2 h. The residue was reacted sequentially with l-cysteine methyl ester hydrochloride and N-trimethylsilylimidazole. The resulting monosaccharide N-trimethylsilylimidazole derivatives were analyzed by GC. d-Glucose was confirmed by comparison of the retention time of the derivatives with those of authentic sugars derivatized in a similar way, which showed retention times of 27.93 min. The constituent sugars of compounds 4 were identified by the same method as 1. Retention times of authentic samples were detected at 17.87 min (d-apiofuranose) and at 27.93 (d-glucose). The reaction and GC conditions were as described in the literature [28].
3.7. Aldose Reductase Assay
The assay was operated in 96 well culture plate. A 100 μL mixture that contained 10 mM DL-glyceraldehyde, 0.16 mM NADPH-Na4 and aldose reductase in 0.1 M sodium phosphate buffer (pH 6.2), with or without test compounds was prepared at 0 °C. Appropriate blank were employed for corrections. The assay mixture was incubated at 25 °C. After 10 min of incubation, the plate was immediately cooled at −20 °C for 5 min to stop the reaction. The change in the absorbance at 340 nm due to NADPH oxidation was measured in a plate reader [29].
3.8. Neuroprotection Bioassays
The PC12 cells were cultured in DMEM medium supplemented with 5% horse serum and 5% fetal bovine serum. Then, 100 μL of cells with an initial density of 5 × 104 cells/mL was seeded in each well of a poly-L-lysine-coated, 96-well culture plate and precultured for 24 h. The medium was then replaced by different fresh medium including the control (complete medium), the model (complete medium with 4 μM rotenone or serum free medium), and the sample (the test compounds with different drug concentrations, 10, 1, and 0.1 μM, were added to the aforementioned model medium), and the cells were cultured for 48 h. Then, 10 μL of MTT (5 mg/mL) was added to each well. After incubation for 4 h, the medium was removed, and 150 μL of DMSO was added to dissolve the formazan crystals. The optical density (OD) of the PC12 cells was measured on a microplate reader at 570 nm [30].
4. Conclusions
The plants of genus Morus have been extensively investigated for their medicinal constituents and a series of unique structures were characterized [31,32,33,34,35,36,37]. This studied focused on the fractions that had been rarely researched before and generated four new structures with aldose reductase inhibitory or neuroprotective activities. The known molecule 5 was isolated for the first time from the genus Morus and its neuroprotective activity reported for the first time. The compounds reported here provide new potential aldose reductase inhibitory or neuroprotective agents for further research.
Acknowledgments
The author is grateful to Li Li who helped measure the CD spectra, Zhu-Fang Shen for the aldose reductase assay and Nai-Hong Chen for the neuroprotection bioassay. This work was supported by CAMS Innovation Fund for Medical Sciences (CIFMS) (2016-I2M-1-010).
Supplementary Materials
The following are available online.
Author Contributions
D.-q.Y. and R.-y.C. conceived and designed the experiments; X.-y.C. performed the experiments and wrote the paper; T.Z. and X.W. contributed data analysis, M.T.H. and J.K. contributed writing.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Sample of the compound 5 is available from the authors.
References
- 1.Wu Z.Y., Raven P.H., Hong D.Y. Flora of China. 2003 ed. Volume 5. Science Press; Beijing, China: Missouri Botanical Garden Press; St. Louis, MI, USA: 2003. 2003. pp. 22–26. [Google Scholar]
- 2.Gao L., Li Y.D., Zhu B.K., Li Z.Y., Huang L.B., Li X.Y., Wang F., Ren F.C., Liao T.G. Two new prenylflavonoids from Morus alba. J. Asian Nat. Prod. Res. 2017;23:117–121. doi: 10.1080/10286020.2017.1343303. [DOI] [PubMed] [Google Scholar]
- 3.Chinese Pharmacopoeia Commission . Pharmacopoeia of the People’s Republic of China. Volume 1. China Medical Science Press; Beijing, China: 2010. pp. 279–281. [Google Scholar]
- 4.Pel P., Chae H.S., Nhoek P., Kim Y.M., Chin Y.W. Chemical Constituents with Proprotein Convertase Subtilisin/Kexin Type 9 mRNA Expression Inhibitory Activity from Dried Immature Morus alba Fruits. J. Agric. Food Chem. 2017;65:5316–5321. doi: 10.1021/acs.jafc.7b02088. [DOI] [PubMed] [Google Scholar]
- 5.Yang Z.Z., Wang Y.C., Wang Y., Zhang Y. Bioassay-guided screening and isolation of α-glucosidase and tyrosinase inhibitors from leaves of Morus alba. F. Food Chem. 2012;131:617–625. doi: 10.1016/j.foodchem.2011.09.040. [DOI] [Google Scholar]
- 6.Zhao G.J., Xi Z.X., Chen W.X., Li X., Sun L., Sun L.N. Chemical constituents from Tithonia diversifolia and their chemotaxonomic significance. Biochem. Syst. Ecol. 2012;44:250–254. doi: 10.1016/j.bse.2012.06.019. [DOI] [Google Scholar]
- 7.Li H.X., Jo E., Myung C.S., Kim Y.H., Yang S.Y. Lipolytic effect of compounds isolated from leaves of mulberry (Morus alba L.) in 3T3-L1 adipocytes. Nat. Prod. Res. 2017 doi: 10.1080/14786419.2017.1354190. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y., Tan Y.X., Chen R.Y., Kang J. The latest review on the polyphenols and their bioactivities of Chinese Morus plants. J. Asian Nat. Prod. Res. 2014;16:690–702. doi: 10.1080/10286020.2014.923405. [DOI] [PubMed] [Google Scholar]
- 9.Sangshetti J., Chouthe R., Sakle N., Gonjari I., Shinde D. Aldose Reductase: A Multi-disease Target. Curr. Enzym. Inhib. 2014;10:2–12. doi: 10.2174/15734080113096660007. [DOI] [Google Scholar]
- 10.Shen B., Vetri F., Mao L., Xu H.L., Paisansathan C., Pelligrino DA. Aldose reductase inhibition ameliorates the detrimental effect of estrogen replacement therapy on neuropathology in diabetic rats subjected to transient forebrain ischemia. Brain Res. 2010;1342:118–126. doi: 10.1016/j.brainres.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldin A., Beckman J.A., Schmidt AM., Creager MA. Advanced Glycation End Products Sparking the Development of Diabetic Vascular Injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 12.Thomas M.C. Advanced glycation end products. Contrib. Nephrol. 2011;170:66–74. doi: 10.1159/000324945. [DOI] [PubMed] [Google Scholar]
- 13.Ishiguro H., Nakaigawa N., Miyoshi Y., Fujinami K., Kubota Y., Uemura H. Receptor for advanced glycation end products (RAGE) and its ligand, amphoterin are overexpressed and associated with prostate cancer development. Prostate. 2005;64:92–100. doi: 10.1002/pros.20219. [DOI] [PubMed] [Google Scholar]
- 14.Kuniyasu H., Oue N., Wakikawa A., Shigeishi H., Matsutani N., Kuraoka K., Ito R., Yokozaki H., Yasui W. Expression of receptors for advanced glycation end-products (RAGE) is closely associated with the invasive and metastatic activity of gastric cancer. J. Pathol. 2002;196:163–170. doi: 10.1002/path.1031. [DOI] [PubMed] [Google Scholar]
- 15.Ramana K.V. ALDOSE REDUCTASE: New Insights for an Old Enzyme. Biomol. Concepts. 2011;2:103–114. doi: 10.1515/bmc.2011.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srivastava K.S., Yadav U., Reddy A., Saxena A., Tammali R., Mohammad S., Ansari H.N., Bhatnagar A., Petrash J.M., Srivastava S., et al. Aldose reductase inhibition suppresses oxidative stress-induced inflammatory disorders. Chem. Biol. Interact. 2011;191:330–338. doi: 10.1016/j.cbi.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harada N., Nakanishi K. Exciton chirality method and its application to configurational and conformational studies of natural products. Acc. Chem. Res. 1972;5:257–263. doi: 10.1021/ar50056a001. [DOI] [Google Scholar]
- 18.Kitagawa I., Hori K., Sakagami M. Saponin and Sapogenol. XLIX. On the Constitutents of the Roots of Glycyrrhiza inflata BATALIN from Xinjiang, China. Characterization of Two Sweet Oleanane-Type Triterpene Oligoglycosides, Apioglycyrrhizin and Araboglycyrrhizin. Chem. Pharm. Bull. 1993;41:1350–1357. doi: 10.1248/cpb.41.1350. [DOI] [PubMed] [Google Scholar]
- 19.McLean S.F., Reynolds W., Tinto W.F., Chan W.R., Shepherd V. Complete 13C and 1H Spectral Assignments of Prenylated Flavonoids and a Hydroxy Fatty Acid from the Leaves of Caribbean Artocarpus communis. Magn. Reson. Chem. 1996;34:719–722. doi: 10.1002/(SICI)1097-458X(199609)34:9<719::AID-OMR948>3.0.CO;2-Q. [DOI] [Google Scholar]
- 20.Ramirez M.A., Borja N.L. Epalrestat: An aldose reductase inhibitor for the treatment of diabetic neuropathy. Pharmacotherapy. 2008;28:646–655. doi: 10.1592/phco.28.5.646. [DOI] [PubMed] [Google Scholar]
- 21.Yoon I.S., Au Q., Barber J.R., Ng S.C., Zhang B. Development of a high-throughput screening assay for cytoprotective agents in rotenone-induced cell death. Anal. Biochem. 2010;407:205–210. doi: 10.1016/j.ab.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Bansol P.K., Deshmukh R. Animal Models of Neurological Disordors. Springer Nature; Singapore: 2017. p. 30. [Google Scholar]
- 23.Javed H., Azimullah S., Abul Khair S.B., Ojha S., Haque M.E. Neuroprotective effect of nerolidol against neuroinflammation and oxidative stress induced by rotenone. BMC Neurosci. 2016;17:58. doi: 10.1186/s12868-016-0293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramkumar M., Rajasankar S., Gobi V.V., Dhanalakshmi C., Manivasagam T., Justin Thenmozhi A., Essa M.M., Kalandar A., Chidambaram R. Neuroprotective effect of Demethoxycurcumin, a natural derivative of Curcumin on rotenone induced neurotoxicity in SH-SY 5Y Neuroblastoma cells. BMC Complement. Altern. Med. 2017;17:217. doi: 10.1186/s12906-017-1720-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ni G., Zhang Q.J., Zheng Z.F., Chen R.Y., Yu D.Q. 2-Arylbenzofuran Derivatives from Morus cathayana. J. Nat. Prod. 2009;72:966–968. doi: 10.1021/np800789y. [DOI] [PubMed] [Google Scholar]
- 26.Dai S.J., Wu Y., Wang Y.H., He W.Y., Chen R.Y., Yu D.Q. New Diels-Alder type adducts from Morus macroura and their anti-oxidant activities. Chem. Pharm. Bull. 2004;52:1190–1193. doi: 10.1248/cpb.52.1190. [DOI] [PubMed] [Google Scholar]
- 27.Kim G.H., Kim J.E., Rhie S.J., Yoon S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015;24:325–340. doi: 10.5607/en.2015.24.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang D., Hao Z.Y., Zhang G.J., Zhang Q.J., Chen R.Y., Yu D.Q. Cytotoxic Triterpenoid Saponins from Lysimachia clethroides. J. Nat. Prod. 2011;74:2128–2136. doi: 10.1021/np2004038. [DOI] [PubMed] [Google Scholar]
- 29.Kang J., Tang Y., Liu Q., Guo N., Zhang J., Xiao Z., Chen R., Shen Z. Isolation, modification, and aldose reductase inhibitory activity of rosmarinic acid derivatives from the roots of Salvia grandifolia. Fitoterapia. 2016;112:197–204. doi: 10.1016/j.fitote.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 30.Zhang F., Yang Y.N., Song X.Y., Shao S.Y., Feng Z.M., Jiang J.S., Li L., Chen N.H., Zhang P.C. Forsythoneosides A-D, Neuroprotective Phenethanoid and Flavone Glycoside Heterodimers from the Fruits of Forsythia suspensa. J. Nat. Prod. 2015;78:2390–2397. doi: 10.1021/acs.jnatprod.5b00372. [DOI] [PubMed] [Google Scholar]
- 31.Ercisli S., Orhan E. Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. Food Chem. 2007;103:1380–1384. doi: 10.1016/j.foodchem.2006.10.054. [DOI] [Google Scholar]
- 32.Butta M.S., NazirbM A., Sultana M.T., Schroën K. Morus alba L. nature’s functional tonic. Trends Food Sci. Technol. 2008;19:505–512. doi: 10.1016/j.tifs.2008.06.002. [DOI] [Google Scholar]
- 33.Arabshahi-Delouee S., Urooj A. Antioxidant properties of various solvent extracts of mulberry (Morus indica L.) leaves. Food Chem. 2007;102:1233–1240. doi: 10.1016/j.foodchem.2006.07.013. [DOI] [Google Scholar]
- 34.Chang L.W., Juang L.J., Wang B.S., Wang M.Y., Tai H.M., Hung W.J., Chen Y.J., Huang M.H. Antioxidant and antityrosinase activity of mulberry (Morus alba L.) twigs and root bark. Food Chem. Toxicol. 2011;49:785–790. doi: 10.1016/j.fct.2010.11.045. [DOI] [PubMed] [Google Scholar]
- 35.Singab A.N., El-Beshbishy H.A., Yonekawa M., Nomura T., Fukai T. Hypoglycemic effect of Egyptian Morus alba root bark extract: Effect on diabetes and lipid peroxidation of streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2005;100:333–338. doi: 10.1016/j.jep.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 36.Doi K., Kojima T., Makino M., Kimura Y., Fujimoto Y. Studies on the Constituents of the Leaves of Morus alba L. Chem Pharm Bull. 2001;49:151–153. doi: 10.1248/cpb.49.151. [DOI] [PubMed] [Google Scholar]
- 37.Oku T., Yamada M., Nakamura M., Sadamori N., Nakamura S. Br Inhibitory effects of extractives from leaves of Morus alba on human and rat small intestinal disaccharidase activity. J. Nutr. 2006;95:933–938. doi: 10.1079/BJN20061746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.