Abstract
Aim of the study
End stage renal disease (ESRD) patients on chronic haemodialysis (HD) are immuno-compromised and prone to infection. Toll-like receptors (TLRs) play a role as both primary sensors of pathogen invasion and activators of inflammatory reaction. To test if the immune impairment in HD patients is connected with the defective expression of the neutrophil TLRs, we aimed to examine their expression and chosen inflammatory indices.
Material and methods
We tested CD14, TLR4, and TLR9 expressions on neutrophils using flow cytometry. Soluble CD14, C-reactive protein (CRP), and mannose-binding lectin (MBL) concentrations were tested using the ELISA method in 31 ESRD patients on chronic haemodialysis programs and in 17 healthy control subjects.
Results
Neutrophil TLR4 and TLR9 expressions did not differ significantly compared to the controls. The ESRD patients had markedly increased CRP and sCD14 levels alongside decreased MBL concentrations and neutrophil CD14 expression. The TLR4 expression correlated well with both TLR9 and CD14 neutrophil expressions; however, the increased CRP in the blood did not correlate with the MBL concentration or TLR expression.
Conclusions
The chronic program of haemodialysis and biochemical disorders in ESRD patients result in a low-grade chronic inflammation with no significant impact on the expression of neutrophil TLR4 and TLR9.
Keywords: inflammation, toll-like receptors, innate immunity, neutrophils, haemodialysis
Introduction
Patients suffering from chronic kidney disease are subject to the severe impairment of both innate and adoptive immune responses, resulting a high prevalence of infection, this being the leading cause of death after cardiovascular events [1]. There are many theories within medical literature on the role of uremic toxins and the biochemical disorder in ESRD patients in the development of immune system dysfunction [2, 3]. The haemodialysis (HD) program removes uremic toxins and prolongs the life of ESRD patients, but they remain immunocompromised and are at high risk of death from cardiovascular diseases and infections given the pro-inflammatory, pro-oxidant effects of HD and accumulation of advance glycoxidation end-products and non-dialyzable uremic toxins [4-7]. Therefore, one of the main factors responsible for the immune system disorder appears to be the chronic inflammatory process induced by haemodialysis and by biochemical changes in ESRD patients [3].
Both soluble and cell membrane pattern recognition molecules (toll-like receptors) form an important first line of defence against invading pathogens acting as the main-pro-inflammatory alarmins [8]. Mannose binding lectin (MBL) – the circulating plasma pattern recognition modules – also constitutes a defence against infections and play a role in the innate immunity; its deficiencies in concentration and function are associated with a high incidence of infection [9]. Toll-like receptors on leucocytes initiate the inflammatory response to pathogens or to endogenous TLR ligands [10, 11]. Experiments on animal subjects and in vitro studies both imply that TLR4 is the principal leukocyte receptor for bacterial flagellin and lipopolysaccharides and plays a key role in the TLR4/NF-κB pathway inflammatory process [12].
TLR9 localised in the endosomal compartment in a number of cell types responds to the nonmethylated CpG-motif-containing DNA and also induces pro-inflammatory cytokine production in response to viral infection; however, according to some authors, it can also result in harmful effects on immunity during bacterial infections [13].
Some authors testing the innate immunity of patients undergoing haemodialysis have observed either significantly up-regulated TLR14 expression on monocytes 24 hours after haemodialysis or an increased percentage of TLR14-positive monocytes, proving their leading role as a triggering factor of low-grade inflammatory state noted in the patients [4, 14]. In other studies, the authors noted no significant changes or even down-regulation of the monocyte TLR14 expression after haemodialysis treatment in ESRD patients. This was explained as a result of chronic stimulation of the receptors by endotoxins from dialysate [15, 16].
Neutrophils are the first-line cells fighting pathogens, but information on their TLR functioning in ESRD patients is currently limited. The cells forming the largest white blood cell population have a significantly shorter life span than other white blood cells, and according to some researchers, the neutrophil TLR expression may be regulated differently when compared to monocytes [17]; therefore, we focused our attention on the expression of pattern recognition molecules on the neutrophils of the ESRD patients on a haemodialysis program.
In order to test if the immune impairment in haemodialysed patients is connected with the improper expression of neutrophil TLRs, we aimed to examine the receptor expression and chosen inflammatory indices in ESRD patients on a chronic haemodialysis program.
Material and methods
We tested 31 patients with stage V chronic kidney disease on a chronic haemodialysis program (10 females and 21 males, mean age 46.8 ±12.7 years.)
The renal failure was a result of:
chronic glomerulonephritis (10 cases),
hypertensive nephrosclerosis (8 cases),
diabetes nephropathy (6 cases),
reflux nephropathy (3 cases),
tubulo-interstitial nephritis (2 cases),
chronic vascular nephritis (1 case),
polycystic kidney disease (1 case).
Patients with lupus nephritis, unstable diabetes mellitus, acute inflammation, or an infection and taking recombinant erythropoietin within the month immediately before the test were excluded from the study group.
The chronic dialysis program with the use of poly sulfone membrane (Fresenius, Germany) consisted of five-hour dialysis sessions three times per week.
Haemodialysis adequacy tested according to the Clinical Practice Guidelines and Clinical Practice Recommendation were within the acceptable value if eKt/V > 1.2. Urea in the blood was measured with the Cobas 6000 apparatus and UREAL Cobas Systems kits (Roche, Germany)
The comparative group consisted of 17 healthy volunteers (seven females and 10 males, mean age 47.1 ±9.3 years.). Blood samples for biochemical and immunological analyses were taken by vein puncture from forearm cephalic vein into sterile heparinised tubes in the morning from the healthy control subjects and vascular access of the ESRD patients prior to initiation of haemodialysis.
Flow cytometry testing of CD14, TLR4, and TLR9 on PMNs
Membrane-anchored CD14, TLR4, and TLR9 expressions on peripheral blood neutrophils was tested by means of flow cytometry (FACscan Becton Dickinson). 20 ml blood samples were incubated for 20 minutes with a saturating concentration of respective antibodies after red blood cell lysis with FACS lysing solution (BD Bioscience) for 10 minutes in the dark. Following two washings in cold phosphate buffer saline, the cells were re-suspended in 0.5 ml PBS and subjected to analysis.
Based on the analysis of the physical parameters, forward-scatter (FSC), and side scatter (SSC) and using CD15-FITC monoclonal antibodies (mouse antihuman IgM, Dako Glostrup, Denmark), we identified the region of the fresh blood neutrophils. The neutrophil expression of CD14 was determined using a saturating concentration of phycoerythrin (PE) conjugated monoclonal mouse IgG2a antibody to human CD14 (Dako Glostrup, Denmark). The expression of TLR4 and TLR9 on neutrophils was determined using FITC-stained mouse antihuman TLR4 (ALX-804-419F-T100) and TLR9 (ALX-804-364F-C100) (Alexis Biochemicals, San Diego, USA). Cells before staining for TLR9 were permeabilised with Cytofix/Cytoperm (BD Pharmingen). Staining of the blood samples with monoclonal antibodies was performed for 20 minutes in the dark at room temperature. IgG2B (Dako Glostrup, Denmark) and IgG1-RPE monoclonal antibodies (Becton Dickinson, Mountain View, California, USA) were used as an isotope-matched negative control for CD14, TLR4, and TLR9, respectively.
The median fluorescence intensity (MFI) of the positively stained PMNs are presented as a measure of CD14, TLR4, and TLR expression reflecting the density of the molecules on the cells. MBL (Antibody Shop, Gentofte, Denmark) and soluble CD14 (Quantikine, R&D Systems) concentrations in the blood were measured using commercial enzyme-linked immunosorbent assay kits. All biochemical measurements were performed with a Technicon Ra100 automated analyser (Technicon Instruments Corp).
The study procedures followed were in accordance with the Helsinki Declaration of 1975 (revised in 2000), and the study protocol was approved by the Ethical Committee of the Medical University of Lodz (RNN/263/06/KB 13.06.2009), and all patients gave their written consent.
Statistical analysis was performed with the Statistica 9.0 package for Windows. The results are presented as mean and SD of biochemical and immunological data. The difference between the tested patients and the controls were measured by the Mann-Whitney U-test. Spearman’s rank correlation coefficient was used to measure the relationship between immune parameters and the tested basic inflammatory indices. P values > 0.05 were considered statistically significant.
Results
Our ESRD haemodialysed patients had serum creatinine and urea concentrations significantly higher than the healthy controls (Table 1). The significance of differences between GFR in the patients and the controls were not measured due to obvious reasons.
Table 1.
Biochemical parameters of 31 end-stage renal disease patients on a chronic chemodialysis program tested before subsequent haemodialysis and in 17 healthy volunteers
| Mean ±SD | Healthy controls (n = 17) | Patients (n = 31) | Significance |
|---|---|---|---|
| GFR (ml/min) | 103.3 ±13.1 | < 10 | – |
| Cr (mg/dl) | 0.8 ±0.1 | 6.9* ±2.2 | * = p < 0.001 vs. control |
| Urea (mg/dl) | 28.8 ±7.6 | 97.6* ±64.6 | * = p < 0.001 vs. control |
CRP and the soluble CD14 concentrations in the patients were significantly up-regulated when compared to the control group (Table 2). Although the biochemical parameters of blood proved the inflammatory state in our ESRD patients, the neutrophil TLR4 and TLR9 expression in the patients did not differ significantly compared to the controls (Table 2). CD14 expression on neutrophils and MBL concentration in the blood were markedly lower when compared to the control group (Table 2).
Table 2.
Inflammatory indices and pathogen recognition receptors in 31 end-stage renal disease patients on a chronic haemodialysis program tested before haemodialysis and in 17 healthy volunteers. Neutrophil expression of CD14, TLR4, and TLR9 are presented as medians of fluorescence intensity (MFI) reflecting the density of the molecules on neutrophils
| Parameter ±SD | Healthy controls | Patients | Significance |
|---|---|---|---|
| CRP (mg/ml in serum) | 1.2 ±1.3 | 9.1* ±16.7 | * = p < 0.001 vs. control |
| Neutrophil CD14 MFI | 17.7 ±8.1 | 14.5* ±5.3 | * = p < 0.05 vs. control |
| Neutrophil TLR4 MFI | 13.7 ±8.9 | 16.7 ±9.5 | NS |
| Neutrophil TLR 9 MFI | 36.5 ±34.1 | 57.3 ±79.6 | NS |
| sCD14 (mg/ml in serum) | 3.9 ±1.76 | 8.07* ±6.14 | * = p < 0.01 vs. control |
| MBL (mg/ml in serum) | 7.9 ±2.7 | 5.53* ±1.6 | * = p < 0.05 vs. control |
NS – not specified
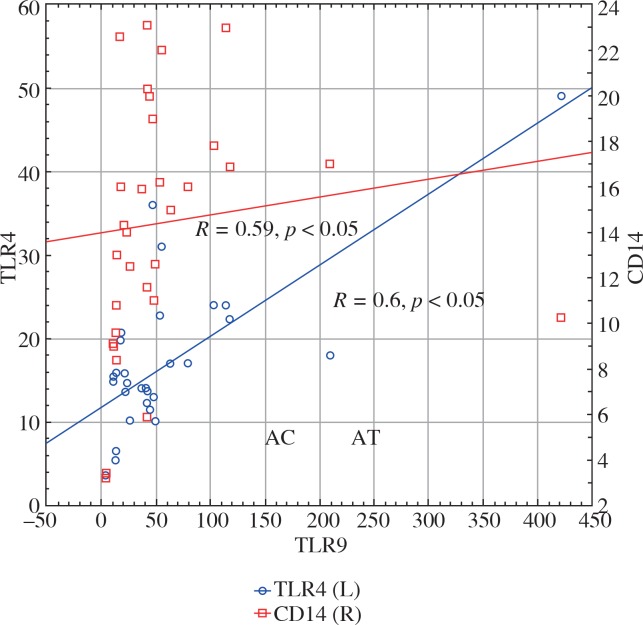
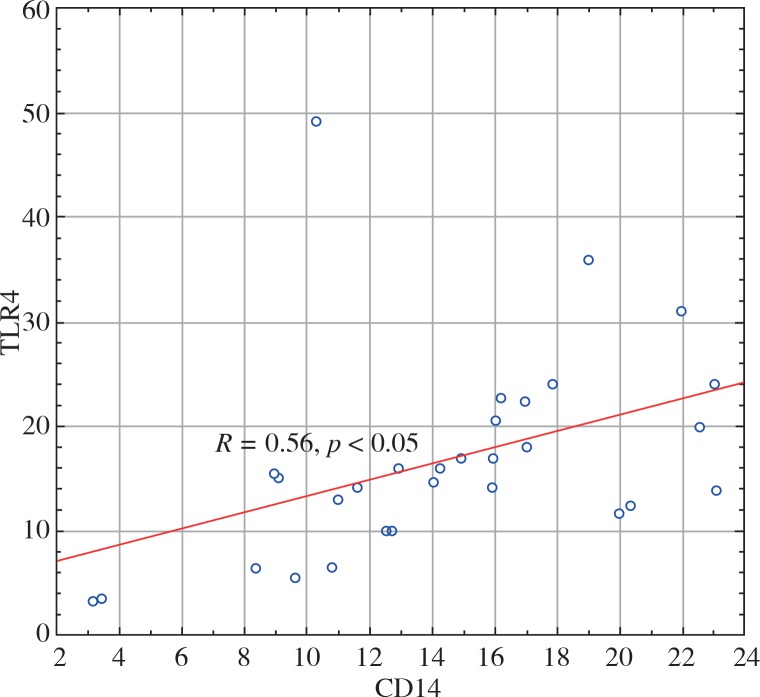
Neutrophil TLR9 expression in the patients correlated with both TLR4 and CD14 neutrophil expression (R = 0.6, p < 0.05 and R = 0.59, p < 0.05, respectively) (Fig. 1). TLR4 expression on neutrophils also positively correlated with CD14 expression on neutrophils in the HD patients (R = 0.56, p < 0.05) (Fig. 2).
Fig. 1.
Graph presenting the positive correlation between neutrophil TLR9 expression and neutrophil TLR4 (red squares, R = 0.59) and CD14 expression (blue circles, R = 0.6)
Fig. 2.
Graph presenting the positive correlation between TLR4 and neutrophil CD14 expression (circles, R = 0.56) in haemodialysed patients. The receptors expression are presented as median fluorescence intensity reflecting the density of the molecules on the cells
We did not observe any relation between sCD14 concentration and CD14 expression on neutrophils in either the patient or control group.
Discussion
Most papers on the effects of haemodialysis in ESRD patients on innate immunity report a significant drop of monocyte TLR4 expression as a result of haemodialysis-related chronic stimulation of the receptors, thus confirming the theory on its role in immunodeficiency and atherogenesis in patients [3, 15]. The authors observed that a chronic stimulation with dialysate toxins suppressed the TLR4 expression on monocytes [15]; however, others reported a stimulatory effect of the same clinical factors on both monocyte and neutrophil TLR4 expression in ESRD patients [4, 18]. We noted no significant difference in either TLR4 or TLR9 expression on neutrophils between the control group and our HD patients. Similar results suggesting the relative stability of these receptors’ expression on neutrophils under activation were observed by other authors, proving that even strong stimuli such as bacterial sepsis could not affect the receptors significantly [19]. The sole significant drop in the TLR4 neutrophil expression was seen in the survivor group seven days after the onset of sepsis. Moreover, the authors noted a marked decrease in CD14 neutrophil expression in all groups of septic patients [19]. The same downregulation of CD14 expression on neutrophils was noted in our HD patients tested two days after haemodialysis; this drop in the CD14 expression was accompanied by a significant rise in the soluble form of the receptor concentration in the serum. The marked rise of sCD14 molecules in the blood was also noted by other authors in acute bacterial infections and was suggested as a diagnostic marker for severe bacterial infections [20]. The co-occurrence of the significant rise in sCD14 molecules with marked increased in CRP concentration proves the inflammatory state in our HD patients.
The findings presented herein of a high positive correlation between CD14 and TLR4 neutrophil expression in our HD patients results from the fact that CD14 acts as a co-receptor and strongly interacts with TLR4 to transfer activation signals in response to stimuli [21].
We also noted in our patients a significantly decreased level of MBL concentration in the blood compared to the healthy controls; however, MBL levels for both fell within the normal range. Most researchers associate low concentrations of MBL in haemodialysed patients with a high risk of severe infection, therefore proving the importance of this parameter as a predictive all-cause mortality prognostic [22, 23]; nevertheless, this finding was contradicted by other researchers who did not find differences between MBL concentrations in healthy controls and HD patients. The latter finding raised doubts with regards to the relationship between lectin concentration and life-threatening infections [24].
Similarly to our results in HD patients, no changes in the neutrophil TLR9 expression were observed by other authors testing the wide spectrum of TLRs on monocytes and neutrophils in ESRD patients. Unlike our findings, however, they noted both significantly increased neutrophil TLR4 expression and reactivity resulting in a markedly higher production of pro-inflammatory cytokines [18].
Contradicting the previously cited papers [19], other authors imply that the TLR4 expression on neutrophils is more changeable when compared to that expressed on monocytes due either to their higher sensitivity to bacterial stimuli [17] or as a result of their higher resistance to dialyse membrane-induced shedding, as compared to TLR2 [14]. Nevertheless, the time-dependent changes of the neutrophil TLR4 receptor expression arising from the marked drop from upregulation to downregulation on the third day following the onset of disease may explain why we did not find significant changes of the toll-like receptor on neutrophils two days after haemodialysis [17]. Therefore, the lack of the receptor changes may result from both the testing of our patients 48 hours after the procedure and the very short lifespan of the cells (in haemodialysed patients the neutrophil lifespan is even shorter as a result of the proapoptic impact of uremic toxins on the cells [25]).
With that in mind, the short lifespan of neutrophils makes the cells the best target for measuring acute and short time stimuli such as the direct effect of a single haemodialysis. Despite the fact that the number of monocytes expressing TLR/CD14 positively correlates with a high sensitivity CRP concentration (proving the prevailing inflammatory state in the haemodialysed patients [26]), we observed no significant changes in TLR4 expression on neutrophils forming the biggest population of carriers of the pro-inflammatory alarmins [11].
One plausible theory is that the receptor expression depends on the time of the onset of stimulation and that the neutrophil TLR expression may be differently regulated by bacterial polysaccharides as compared to monocytes [17]; however, this makes our results difficult to explain.
The discrepancy in the papers cited in this article on the effect of uraemia and haemodialysis on leukocyte TLR expression was the primary reason for our research. Despite the limitations of our study (such as small size of the patient group and the relatively large distribution of the individual parameters of tested immune indices), we found data on low-grade inflammation in the patients and no marked changes in the tested neutrophil TLRs.
Conclusions
Low-grade chronic inflammation in HD patients expressed as the increased concentration of both CRP and sCD14 in blood proceeds without any marked changes in the expression of either TLR4 or TLR9.
Acknowledgements
The authors would like to thank Anna Augustyniak, who assisted in the laboratory tests.
Footnotes
The authors declare no conflict of interest.
References
- 1.Vaziri ND, Pahl MV, Crum A, Norris K. Effect of uremia on structure and function of immune system. J Ren Nutr. 2012;22:149–156. doi: 10.1053/j.jrn.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen G, Hörl WH. Immune dysfunction in uremia—An update. Toxins. 2012;4:962–990. doi: 10.3390/toxins4110962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betjes MG. Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol. 2013;9:255–265. doi: 10.1038/nrneph.2013.44. [DOI] [PubMed] [Google Scholar]
- 4.Kim HW, Woo YS, Yang HN, et al. Primed monocytes: putative culprits of chronic low-grade inflammation and impaired innate immune responses in patients on haemodialysis. Clin Exp Nephrol. 2011;15:258–263. doi: 10.1007/s10157-010-0379-8. [DOI] [PubMed] [Google Scholar]
- 5.Kato S, Chmielewski M, Honda H, et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008;3:1526–1533. doi: 10.2215/CJN.00950208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piroddi M, Bartolini D, Ciffolilli S, Galli F. Nondialyzable uremic toxins. Blood Purif. 2013;35(Suppl. 2):30–41. doi: 10.1159/000350846. [DOI] [PubMed] [Google Scholar]
- 7.Oleśkowska-Florek W, Połubinska A, Baum E, et al. Haemodialysis-induced changes in the blood composition affect function of the endothelium. Hemodial Int. 2014;18:650–656. doi: 10.1111/hdi.12148. [DOI] [PubMed] [Google Scholar]
- 8.Suresh R, Mosser DM. Pattern recognition receptors in innate immunity, host defense, and immunopathology. Adv Physiol Educ. 2013;37:284–291. doi: 10.1152/advan.00058.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisen DP, Dean MM, Boermeester MA, et al. Low serum mannose-binding lectin level increases the risk of death due to pneumococcal infection. Clin Infect Dis. 2008;47:510–516. doi: 10.1086/590006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prince LR, Whyte MK, Sabroe I, Parker LC. The role of TLRs in neutrophil activation. Curr Opin Pharmacol. 2011;11:397–403. doi: 10.1016/j.coph.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi F, Means TK, Luster A. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102:2660–2669. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 12.Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun. 2009;388:621–625. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 13.Plitas G, Burt BM, Nguyen HM, et al. Toll-like receptor 9 inhibition reduces mortality in polymicrobial sepsis. J Exp Med. 2008;205:1277–1283. doi: 10.1084/jem.20080162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koc M, Toprak A, Arikan H, et al. Toll-like receptor expression in monocytes in patients with chronic kidney disease and haemodialysis: relation with inflammation. Nephrol Dial Transplant. 2011;26:955–963. doi: 10.1093/ndt/gfq500. [DOI] [PubMed] [Google Scholar]
- 15.Kuroki Y, Tsuchida K, Go I, et al. A study of innate immunity in patients with end-stage renal disease: special reference to toll-like receptor-2 and -4 expression in peripheral blood monocytes of haemodialysis patients. Int J Mol Med. 2007;19:783–790. [PubMed] [Google Scholar]
- 16.Ando M, Shibuya A, Tsuchiya K, et al. Reduced expression of Toll-like receptor 4 contributes to impaired cytokine response of monocytes in uremic patients. Kidney Int. 2006;70:358–362. doi: 10.1038/sj.ki.5001548. [DOI] [PubMed] [Google Scholar]
- 17.Vallés PG, Melechuck S, González A, et al. Toll-like receptor 4 expression on circulating leucocytes in hemolytic uremic syndrome. Pediatr Nephrol. 2012;27:407–415. doi: 10.1007/s00467-011-2014-7. [DOI] [PubMed] [Google Scholar]
- 18.Gollapudi P, Yoon JW, Gollapudi S, et al. Leukocyte toll-like receptor expression in end-stage kidney disease. Am J Nephrol. 2010;31:247–254. doi: 10.1159/000276764. [DOI] [PubMed] [Google Scholar]
- 19.Silva SC, Baggio-Zappia GL, Brunialti MKC, et al. Evaluation of Toll-like, chemokine, and integrin receptors on monocytes and neutrophils from peripheral blood of septic patients and their correlation with clinical outcomes. Braz J Med Biol Res. 2014;47:384–393. doi: 10.1590/1414-431X20143190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marcos V, Latzin P, Hector A, et al. Expression, regulation and clinical significance of soluble and membrane CD14 receptors in pediatric inflammatory lung diseases. Respir Res. 2010;11:32. doi: 10.1186/1465-9921-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landmann R, Muller B, Zimmerli W. CD14, new aspects of ligand and signal diversity. Microbes Infect. 2000;2:295–304. doi: 10.1016/s1286-4579(00)00298-7. [DOI] [PubMed] [Google Scholar]
- 22.Akbari R, Mireskandari M, Alizadeh-Navaei R, Ghods A. Measurement of serum levels of mannose-binding lectin in haemodialysis patients: a comparison with healthy individuals. Iran J Kidney Dis. 2011;5:255–259. [PubMed] [Google Scholar]
- 23.Satomura A, Fujita T, Yanai M, et al. Functional mannose-binding lectin levels in patients with end-stage renal disease on maintenance haemodialysis. J Innate Immun. 2012;4:293–300. doi: 10.1159/000334601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishii M, Ohsawa I, Inoshita H, et al. Serum concentration of complement components of the lectin pathway in maintenance haemodialysis patients, and relatively higher levels of L-Ficolin and MASP-2 in Mannose-binding lectin deficiency. Ther Apher Dial. 2011;15:441–447. doi: 10.1111/j.1744-9987.2011.00936.x. [DOI] [PubMed] [Google Scholar]
- 25.Majewska E, Baj Z, Sulowska Z, et al. Effects of uraemia and haemodialysis on neutrophil apoptosis and expression of apoptosis-related proteins. Nephrol Dial Transplant. 2003;18:2582–2588. doi: 10.1093/ndt/gfg441. [DOI] [PubMed] [Google Scholar]
- 26.Lorenzen JM, David S, Richter A, et al. TLR-4+ peripheral blood monocytes and cardiovascular events in patients with chronic kidney disease – a prospective follow-up study. Nephrol Dial Transplant. 2011;26:1421–1424. doi: 10.1093/ndt/gfq758. [DOI] [PubMed] [Google Scholar]