Abstract
Previous fundamental or clinical trials of dendritic cell (DC) vaccine against pancreatic ductal adenocarcinoma (PDAC) revealed the burgeoning neoadjuvant immunotherapy. Microarray studies indicated that multiple ingredients of the transfer growth factor beta (TGF-β) pathway were overexpressed in PDAC, which inhibited the intratumoral immune response. To explore whether the DC volume in tumor microenvironment contributes to the differentiation of T cell cohort and test the hypothesis that combining DC vaccine with TGF-β inhibitors will elevate the anti-tumor immune response, we managed to co-culture T cells in vitro with pancreatic cancer cells and DCs in different concentrations, and combine TGF-β blockage with DC vaccine therapy in a murine model of pancreatic cancer. In in vitro studies, we discovered that CD8+ T cytotoxic cell (Tc) presented a significant advantage and lower volume of CD4+ T helper cell (Th) existed with a certain elevated DC concentration (p < 0.05), associated with declined interleukin (IL)-10 and increased interferon (IFN)-γ, which suggested with the DC volume increasing, the enhancing immune effect may represent a great advantage in such a system (p < 0.05). When interfered with anti-TGF-β antibody or TGF-β cytokine, respectively, in the co-culture system, we found IFN-γ producing was extremely higher and T cell apoptosis relatively descent with TGF-β blockage (p < 0.05). The murine PDAC model demonstrated a survival advantage treated with anti-TGF-β antibody combined with DC vaccine when compared with monotherapy controls (p < 0.05). Therefore, these findings indicated that, through neutralizing TGF-β associated with DC vaccine, the anti-tumor immunity is highly elevated and this combinational therapy will provide an efficacious prospect.
Keywords: pancreatic ductal adenocarcinoma, DCs, TGF-beta, immunotherapy, prognosis
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is still one of the most aggressive cancers and is the fourth most common cause of cancer-related mortalities in the world, with an estimated incidence of 42,470 patients in the United States in 2009 and 35,240 deaths from this deadly disease [1-4]. Surgical resection is the only potentially curative treatment for pancreatic cancer, but a majority of cancers were diagnosed at a very advanced stage with broad invasion of adjacent structures or distant metastasis, which precludes the chance of curative tumor resection [1, 5]. For all stages combined, the overall 1-year survival rate is < 20% and the overall 5-year survival rate has remained dismally poor at about 8% [5, 6].
Tumor-infiltrating lymphocytes (TILs) play crucial roles in tumorigenesis, retarding tumor progression and metastasis and augmenting anti-tumor immune [7-9], which has been the focus of interest in the research of utilizing immunotherapies for treating various cancers. Studies confirmed that a sufficient number of spontaneous TILs presenting in tumor microenvironment is not only one of the essential factors for achieving an objective response to immune checkpoint blockade therapy, but also a pivotal prognostic factor for patients treated with anti- tumor immunotherapies. Dendritic cells (DCs) acting as the antigen-presenting cells are the important components among the immune cells in tumor microenvironment, which may play a crucial part in promoting the migration and infiltration of T cells in tumor microenvironment and regulating the differentiation of T cells in tumors. Our previous study found a significantly increased intratumoral infiltration of regulatory T cells (Treg) in human pancreatic cancers and a decreased infiltration of CD8+ T cells in intratumoral area of cancers [10], which may hint a limited positive cellular immune response presenting in tumor microenvironment and on the contrary, potential of Tregs-promoted tumor invasion [11, 12]. The transforming growth factor beta (TGF-β) signal pathway plays a vital role in cell differentiation and inflammation [13] and has been shown to be in favor of tumor progression. In Smad4-inactivated pancreatic cancer cells, TGF-βsignal pathway regulates a number of biological characteristics involved in extracellular matrix deposition, immunosuppression, etc. [14]. Therefore, this study was to explore the DC-induced differentiation of T cell cohort, and the effect of combination immunotherapy with DC vaccine and TGF-β blockage.
Material and methods
Pancreatic cancer cells
Mice pancreatic duct adenocarcinoma cells (Panc02, a kind gift from Johns Hopkins Hospital, Baltimore, USA) were maintained at 37°C and 5% CO2 in a complete medium containing RPMI-1640 medium (Gibco, Grand Island, New York, USA) supplemented with 10% fetal calf serum (Gibco), 100 U/ml penicillin and 100 U/ml streptomycin (Gibco).
Animals and in vivo experiments
Fifty female, 6-8-week-old C57BL/6 mice (Shanghai Slac Laboratory Animal Company, Shanghai, China) were maintained under specific pathogen-free conditions. All procedures were performed by following China Animal Welfare Guidelines. The protocol was approved by our Institutional Animal Care and Use Committee of the Fudan University, Shanghai, China.
Among the total mice, eight of which were administered for the isolation of DCs and T lymphocytes and the others (n = 42) were subcutaneously inoculated with 1 × 106 Panc02 cells or the same number of Panc02 cells mixed with 1 × 106 DCs in the back of the mice. 100 µg of anti-mouse TGF-β antibody (Clone 1D11) (Bio X Cell, West Lebanon, New Hampshire, USA) in 200 µl phosphate buffered solution was injected intraperitoneally twice a week at day 3 for 3 weeks.
Cell isolation and activation
Mice were anesthetized by intraperitoneal injection of 0.1 ml 2% sodium pentobarbital (Sigma, Saint Louis, Missouri, USA) solution and then euthanized with cervical dislocation. The collection of DCs was performed as previously described [15]. The cell suspensions in complete medium were filtrated with 70-µm cell strainer (BD Bioscience, Franklin Lakes, New Jersey, USA) and erythrocytes were lysed with lysing buffer (BD Bioscience). Cells were suspended in complete medium with IL-4 (10 ng/ml, R&D Systems, Tustin, California, USA) and granulocyte-monocyte colony-stimulating factor (GM-CSF, 10 ng/ml, R&D Systems). At day 7, lipopolysaccharide (1 µg/ml, Sigma) was added and at day 8 the suspending mature DCs were collected for further research. In addition, following the GentleMACS protocol of the digestion of the spleens, single-cell suspension was obtained with GentleMACS Dissociator (Miltenyi, Auburn, Washington, USA) and then filtrated with 70 µm cell strainer (BD Bioscience). Erythrocytes were lysed with lysing buffer (BD Bioscience). CD3+ T lymphocytes were isolated and purified with Mouse T Lymphocyte Enrichment Set (BD Bioscience).
Co-culturing and interfering
1 × 105 T lymphocytes were cultured in a 24-well plate with or without the presence of Panc02 cells or DCs. The T lymphocytes in group A were cultured without Panc02 cells, while the T lymphocytes in group B were co-cultured with 105 Panc02 cells. Cells in both groups were co-cultured in the subgroups of wells without DCs, with 104 DCs and 5 × 104 DC, respectively (Figs. 1A-B). Cells in each subgroup were cultured in quadruplicate wells with the complete medium supplemented with 2 ng/ml IL-2 (BD Bioscience), 10 ng/ml IL-4 (R&D Systems) and 10 ng/ml GM-CSF (R&D Systems) and then incubated for 48 hours at 37°C with 5% CO2. Subsequently, 10 µg/ml anti-TGF-β antibody (Clone 1D11) (R&D Systems) and 10 ng/ml TGF-β cytokines (R&D Systems) were respectively added to the complete medium with T lymphocytes and pancreatic cancer cell, and no treatment as a control, then incubated for 48 hours at 37°C with 5% CO2. Each experiment in quadruplicate wells was analyzed with its mean value, and all experiments were repeated four times.
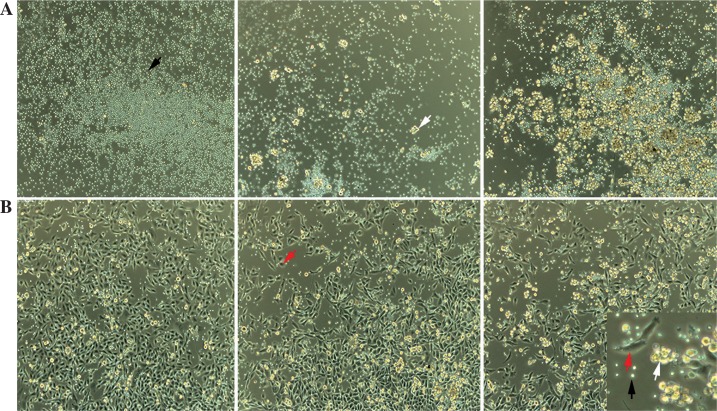
Fig. 1.
Co-culturing cells for 48 h. 1 × 105 T lymphocytes were incubated with dendritic cells (DCs) in the concentration of zero, 104 and 5 × 104 (A) and another group further added 1 × 105 Panc02 cells (B). The black arrow represented T lymphocytes, the white arrow represented DCs and the red arrow represented Panc02 cells (original ×200 magnification)
Flow cytometry
The suspension cells were harvested after co-culturing. The cell pellets were stained with FITC Annexin V Apoptosis Detection Kit (BD Bioscience), CD3-FITC (Biolegend, San Diego, California, USA), CD8a-PE-Cy7 (BD Bioscience), CD4-PerCP-Cy5.5 (BD Bioscience), and CD25-APC (BD Bioscience). The population of CD4+ and CD8+T cells or even regulatory T cells (Tregs) in lymphocytes was analyzed with FACS Aria II flow cytometer (BD Bioscience).
ELISA
The co-culture supernatants were collected for the analysis. The concentrations of TGF-β, IL-10 and IFN-γ (R&D Systems) in the medium were determined by ELISA analysis, according to the manufacturer’s instructions. TGF-β and IL-10 are the biomarker cytokines of Tregs and IFN-γ is the cytokine mainly excreted by CD8+ T cells and T helper type 1 cells. SoftMax 5.4 was used to plot a standard curve and the concentrations of the cytokines were calculated based on this curve.
Statistical analyses
The categoric value was presented as mean ±standard deviation). Statistical analyses were performed using the statistics software, SPSS 21.0 (IBM, Almon, New York, USA). One-way ANOVA or paired student t test was used to analyze the data with normal distribution and LSD or Tamhane method was administered for further analysis. Sixty-day free survival was carried out using the Kaplan-Meier method through GraphPad Prism 6.0 (GraphPad Software, San Diego, California, USA). P < 0.05 indicates statistical significance.
Results
Dendritic cells induce differentiation of T lymphocytes in the co-culturing environment
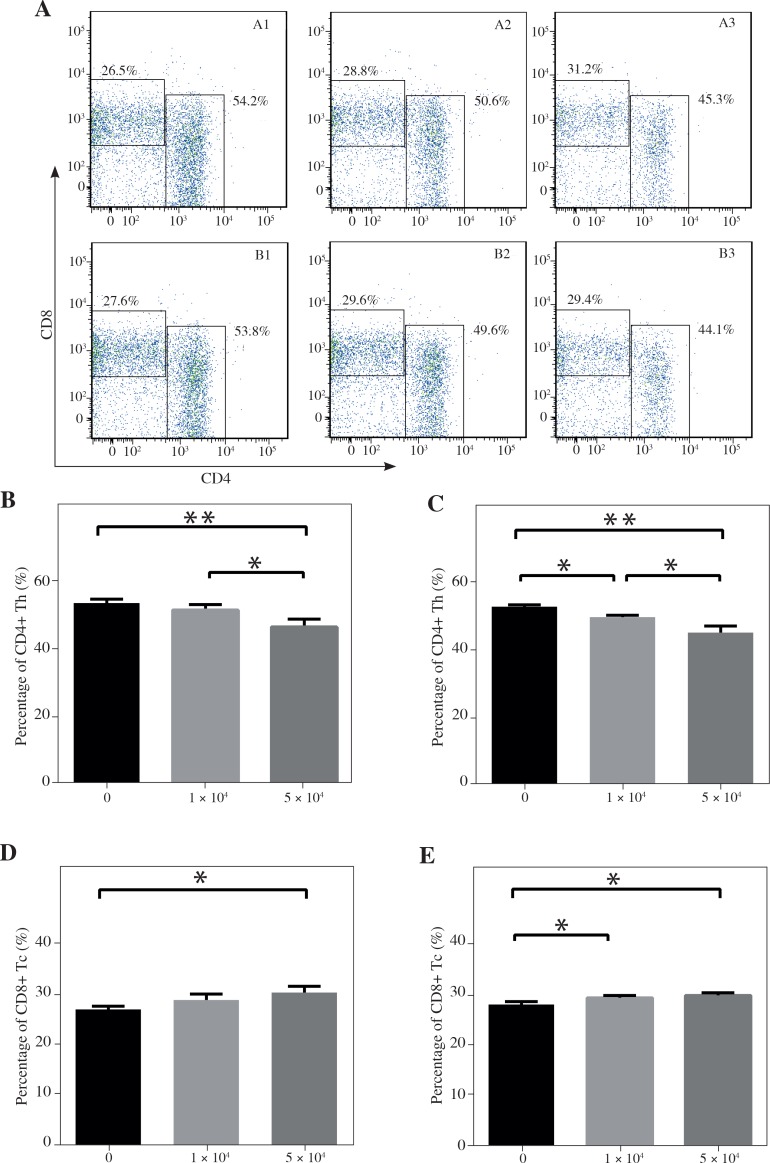
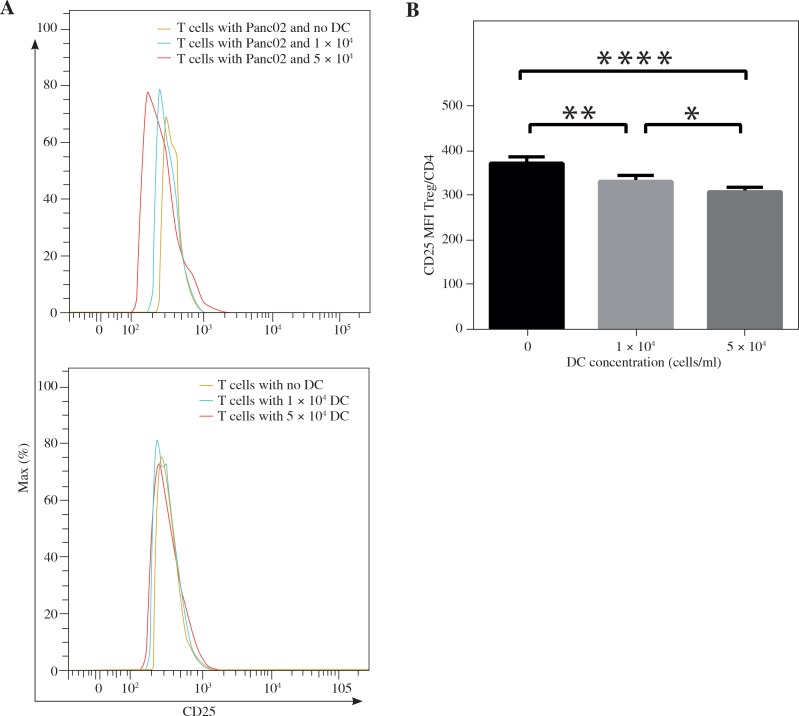
This study was to investigate whether DCs and pancreatic cancer cells have the regulatory effect on the differentiation of T lymphocytes in the co-culturing environment (Fig. 2A). We found that the percentage of CD4+ The cells among all CD3+ T lymphocytes was significantly lower in the group co-cultured with a high concentration of DCs compared with the group with a smaller number of DCs (46.30 ±2.27% vs. 51.27 ±1.61%, p < 0.01) and the group with no DCs (46.30 ±2.27% vs. 53.35 ±1.20%, p < 0.05) (Fig. 2B). In the groups with Panc02 cells, the percentage of CD4+ Th cells among CD3+ T lymphocytes was 53.17 ±1.20%, 50.00 ±0.57%, 45.45 ±1.91%, respectively, as the concentrations of DCs increased, which showed a significant difference between each group (p < 0.05) (Fig. 2C). On the contrary, the percentages of CD8+ Tc cells among CD3+ T lymphocytes were significantly higher in the group co-cultured with a high concentration of DCs alone compared with the group without DCs (30.33 ±1.17% vs. 26.95 ±0.64%, p < 0.05) (Fig. 2D). However, with the existence of Panc02 cells, the percentage of CD8+ Tc cells in the group without co-culturing with DCs is lower than the group with a high concentration of DCs (27.97 ±0.55% vs. 29.75 ±0.49%, p < 0.05) and the group with a low concentration of DCs (27.97 ±0.55% vs. 29.30 ±0.42%, p < 0.05) (Fig. 2E). Since the number of recorded CD4+ T cells was relatively small, the mean fluorescence intensity (MFI) of staining for CD25 marker on cells gated on CD4+ T cells was used to show the changing trend of Tregs. In the group with Panc02 cells, the MFI value was observed as 375.75 ±14.22, 334.75 ±13.94, 311.75 ±9.74 as DC volume enlarged, which revealed that the existence of DC in the tumor environment reduced the CD25 expressive intensity among CD4+ T cells (p < 0.001) and higher DC volume was associated with a lower expression of CD25 (p < 0.05) (Fig. 3A-B). However, there was no discovery in the group without Panc02 cells (Fig. 3A). We confirmed these changes by ELISA analysis that showed there is no significant change in TGF-β, decreased IL-10 and increased IFN-γ in the cell group with the increased number of DCs. As shown in Table 1, IL-10 and IFN-γ in the group without Panc02 cells all showed a great significance, so did TGF-β, IL-10 and IFN-γ in the group with Panc02 cells (p < 0.05 or p < 0.01). These data suggested that there was a high trend for DCs to induce the CD8+T cell differentiation, which was in favor of the anti-tumor immunity.
Fig. 2.
The differentiation of T lymphocytes during the co-culture with dendritic cells (DCs). The flow cytometry analysis of CD4+ and CD8+ T cells in CD3+ T cells were showed in (A). The percentage of CD4+ T cell and CD8+ T cells in CD3+ T cells co-culturing without Panc02 cells (B, D) or with Panc02 cells (C, E) was analyzed. *p < 0.05, **p < 0.01
Fig. 3.
The mean fluorescence intensity of CD25 expression in CD4+ T cells was showed in (A) and analyzed in (B) to reflect the changes of Tregs. *p < 0.05; **p < 0.01, ****p < 0.0001
Table 1.
The cytokine changes of TGF-β, IL-10 and IFN-γ in the co-culture system with dendritic cells concentration changing
| Group | TGF-β | IL-10 | IFN-γ | |||
|---|---|---|---|---|---|---|
| Concentration (pg/ml) | p value | Concentration (pg/ml) | p value | Concentration (pg/ml) | p value | |
| A1 | 750.78 ±14.71 | > 0.05 | 29.03 ±0.84 | < 0.05 | 4.38 ±1.19 | < 0.01 |
| A2 | 729.58 ±32.15 | 29.04 ±1.45 | 7.90 ±1.56 | |||
| A3 | 745.23 ±14.79 | 25.82 ±1.97 | 63.16 ±19.55 | |||
| B1 | 1217.16 ±17.09 | < 0.05 | 33.30 ±2.15 | < 0.01 | 8.17 ±2.61 | < 0.01 |
| B2 | 1214.91 ±32.72 | 27.79 ±1.72 | 25.40 ±12.88 | |||
| B3 | 1277.78 ±40.99 | 25.15 ±0.88 | 81.51 ±16.80 | |||
No Panc02 cell was added in group A, while 105 Panc02 cells were inoculated in group B. Both groups had 3 categories: A1/B1, A2/B2, and A3/B3, standing for no DC,104 DC and 5 × 104 DC
TGF-β blockade enhances antitumor antigen specific responses and inhibits T cells apoptosis
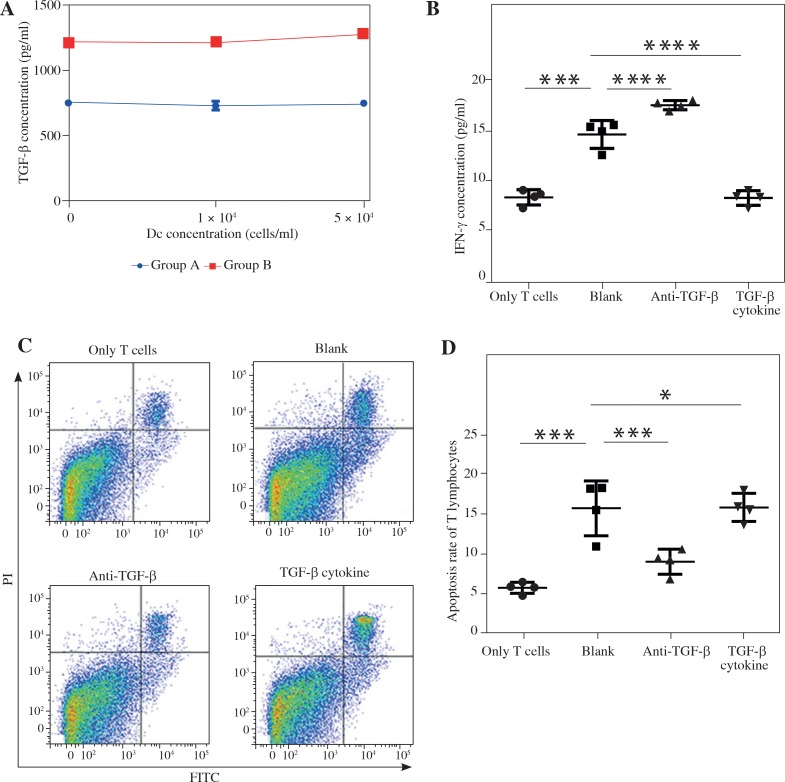
As shown in Figure 4A, an immense change of TGF-β between the tumor group and the non-tumor group (p < 0.01) confirmed that TGF-β was related to the tumorigenesis and progression and may be a potential therapeutic target in pancreatic cancer. Thus, to confirm that a high level of TGF-β secreted by pancreatic cancer cell attenuated the antitumor immunity, we managed to incubate T lymphocytes with Panc02 cells at the ratio of 5 : 1 and interfered with anti-TGF-β antibody or TGF-β to further stimulate the system. To analyze the anti-tumor immune response, IFN-γ production was recorded. The IFN-γ production in the group with TGF-β blockade was significantly higher compared to the group treated with TGF-β cytokines (17.46 ±0.46 vs. 8.25 ±0.73, p < 0.0001) and the control group (T lymphocytes and Panc02 cells with no treatment) (17.46 ±0.46 vs. 14.56 ±1.39, p < 0.001). Furthermore, we confirmed that, by contrast with the control group, T lymphocytes incubated alone secreted lower IFN-γ (8.31 ±0.76 vs. 14.56 ±1.39, p < 0.0001) (Fig. 4B). This indicated that T lymphocytes inhabiting in the tumor environment had a high possibility of obtaining a specific anti-tumor ability. When looking particularly at T cells apoptosis after incubation (Fig. 4C), we interestingly found that, after TGF-β blockade, the group had a significantly lower percentage of apoptosis of T lymphocytes than the control (8.99 ±1.57 vs. 15.63 ±3.42, p < 0.001) or TGF-β stimulation group (8.99 ±1.57 vs. 15.74 ±1.75, p < 0.001). In addition, we verified that in a tumor environment, T lymphocytes apoptosis was highly strengthened induced by the cancer cells (15.63 ±3.42 vs. 5.74 ±0.68, p < 0.0001) (Fig. 4D). Taken together, TGF-β signal pathway plays a vital role in anti-tumor immunity, and its blockade enhances antitumor antigen specific responses and improves the survival of T lymphocytes.
Fig. 4.
The functions and apoptosis of T lymphocytes with TGF-β blockade or stimulation co-culturing with Panc02 cells. ELISA analysis showed TGF-β was extremely higher in the group with Panc02 cells (A). The IFN-γ concentration reflected the ability of specific immune response with the invention of anti-TGF-β or TGF-β cytokines (B). Flow cytometry analysis displayed the changes of T lymphocytes apoptosis with the invention of anti-TGF-β or TGF-β cytokines (C, D). *p < 0.05, ***p < 0.001, ****p < 0.0001
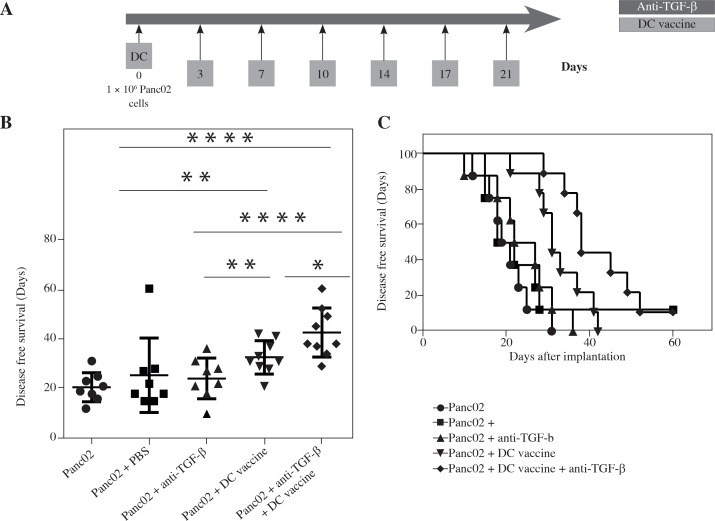
DC vaccine in combination with TGF-β blockade improves the disease-free survival (DFS) of PDAC in murine tumor models
We examined whether combining DC vaccine with a monoclonal anti-TGF-β neutralizing antibody, which blocks all 3 subtypes of the TGF-β (αTGF-β), TGF-β 1, 2 and 3, could promote the anti-tumor activity. For Panc02 mouse model, αTGF-β, PBS or no intervention was administered as control. αTGF-β or PBS control was administered twice a week for 3 weeks while DC was inoculated together with Panc02 cells on day 0 (Fig. 5A). During 60 days after inoculation, we recorded the day the tumor was detected as DFS operated by two surgeons. We found that the three control groups had no benefit on the DFS and their median DFS was 20.63 ±5.83, 25.38 ±14.95 and 24.13 ±8.13, respectively (p > 0.05). However, when Panc02 cells were inoculated with DCs, the median DFS was improved compared to Panc02 cells alone (32.56 ±6.64 vs. 20.63 ±5.83, p < 0.05). When further associated with TGF-β blockade, it significantly improved median DFS by leap and bounds compared to Panc02 cells alone (42.44 ±9.81 vs. 20.63 ±5.83, p < 0.0001) or DC vaccine therapy alone (42.44 ±9.81 vs. 32.56 ±6.64, p < 0.05) (Fig. 5B). We could conclude that the combinational therapy with DC vaccine and TGF-β blockade had an obvious effect on the DFS, which may even benefit for overall survival or cure rate (Fig. 5C).
Fig. 5.
The improvement of clinical outcomes in a PDAC mouse model with combination therapy of DC vaccine and TGF-β blockade. Schema of tumor implantation by the treatment with DC vaccine and TGF-β blockade (A). The days of mice that remained disease free following Panc02 tumor implantation with DC vaccine and/or αnti-TGF-β/PBS (B). Kaplan-Meier disease free survival curves of mice that were implanted with Panc02 tumor cells and treated with different combinations of DC vaccine, αnti-TGF-β and/ or PBS (C). *p < 0.05, **p < 0.01, ****p < 0.0001
Discussion
Tumor-infiltrating lymphocytes are the important immune cell components in the tumor microenvironment which act as the key role in the process of tumorigenesis, tumor progression and participating in every aspect of the response to anti-tumor immune therapy. Among the tumor-infiltrating lymphocytes, Tregs can adversely inhibit the tumor cell killing effect of CD8+T cells, which unexpectedly counteract the immune therapeutic effect [10, 16-18]. In the microenvironment of PDAC, DCs function as another essential component by recognizing the surface antigens of tumor cells and presenting to T cells including specific cellular immune response, chemotactic effect and so on. In human bladder transitional cell carcinoma, researchers found that the decrease in tumor-infiltrating DCs had a significant relation to tumor immune evasion or immune tolerance [19]. Our study shows that DCs inducing the differentiation of CD8+ Tc cells, while the percentage of CD4+ T cells relatively declined [20]. In the analysis of the CD4+ T cells, due to the limit of the recorded cell number, there was no apparent distinction of Treg. However, the MFI results showed that the expression of CD25 among CD4+ T cells was much lower after DC intervention with lymphocyte-tumor co-culture, which may suggest low differentiation or function to Tregs. Furthermore, in order to detect the function of T cells, the ELISA results showed that, in simulated tumor microenvironment, with existence of DCs, the change of TGF-β, IL-10 and IFN-γ further suggested that an increased DC concentration may enhance the differentiation of Tc cells and inhibit Tregs function to promote the specific cell killing and anti-tumor immune response [20, 21]. These results were consistent with the findings of Kindlund, which showed that Tregs secreting high levels of IL-10, low TGF-β and IFN-γ in local microenvironment of the gastric cancer mucosa inhibited T cell-mediated specific cell killing, thereby contributing to tumor growth and metastasis [22].
TGF-β has multiple functions in the progression of pancreatic cancer, including regulation of cell growth and differentiation, matrix deposition, epithelial-mesenchymal transition, immunosuppression and so on. TGF-β signaling pathway further shows significance in the development and progression of pancreatic cancer [23, 24]. TGF-β pathway was verified to suppress the growth of epithelial cells mediated by Smad signaling pathway, and the deletion or mutation of Smad/DPC4 gene may further promote its occurrence and metastasis [14]. According to our data, TGF-β was observed to be significantly increased in pancreatic cancer, which can play an efficient role in tumor progression. Through the intervention with anti-TGF-β antibodies or TGF-β cytokines in the lymphocyte-tumor co-culture system, we confirmed that IFN-γ was obviously increased with TGF-β blockade, which indicated the anti-tumor antigen specific responses enhancing. We further discovered the lower rate of T cell apoptosis after the TGF-β blockade. Above all, TGF-β blockade could decrease the tumor-infiltrating T cell apoptosis and improve the anti-tumor immune response. Some researchers have explored the relationship between TGF-β expression and clinical prognosis in pancreatic cancer patients. The results indicated that the patients with high levels of TGF-β in plasma and a high expression of TGF-βR2 and a low expression of Smad4 in tumor were related to the relatively poor prognosis [25]. Therefore, a number of relevant achievements had been obtained in attempting the therapy combined with TGF-β blockade for pancreatic cancer [26, 27].
Tumor-infiltrating DCs and TGF-β blockade all showed a great benefit against pancreatic cancer in vivo. In the mice model, mice with the therapy of DC vaccine combined with TGF-β antibodies obtained a large advantage of DFS or possible cure rate. In conclusion, combinational treatment with DC vaccine and TGF-β blockade may further effectively inhibit the differentiation of Tregs and promote Tc cells differentiation and functions to strengthen the immune response against pancreatic cancer, so that patients can survive for a long time with tumor, or even cure in cytology. Therefore, DC vaccine associated with TGF-β blockade may be useful as a promising therapeutic method for pancreatic cancer.
Acknowledgements
This work was supported by the following grants: the National Natural Science Foundation of China (no. 81272731), the Science and Technology Commission of Shanghai Municipality (no. 11JC1402502), and the Shanghai Pujiang Program (no. 16PJD013).
Footnotes
The authors declare no conflict of interest.
References
- 1.Martin RC, 2nd, McFarland K, Ellis S, et al. Irreversible electroporation therapy in the management of locally advanced pancreatic adenocarcinoma. J Am Coll Surg. 2012;215:361–369. doi: 10.1016/j.jamcollsurg.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 2.Thanos L, Poulou LS, Mailli L, et al. Image-guided radiofrequency ablation of a pancreatic tumor with a new triple spiral-shaped electrode. Cardiovasc Intervent Radiol. 2010;33:215–218. doi: 10.1007/s00270-009-9548-0. [DOI] [PubMed] [Google Scholar]
- 3.Carrafiello G, Ierardi AM, Fontana F, et al. Microwave ablation of pancreatic head cancer: safety and efficacy. J Vasc Interv Radiol. 2013;24:1513–1520. doi: 10.1016/j.jvir.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Casadei R, Ricci C, Pezzilli R, et al. A prospective study on radiofrequency ablation locally advanced pancreatic cancer. Hepatobiliary Pancreat Dis Int. 2010;9:306–311. [PubMed] [Google Scholar]
- 5.Carrafiello G, Ierardi AM, Piacentino F, et al. Microwave ablation with percutaneous approach for the treatment of pancreatic adenocarcinoma. Cardiovasc Intervent Radiol. 2012;35:439–442. doi: 10.1007/s00270-011-0189-8. [DOI] [PubMed] [Google Scholar]
- 6.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 7.Ino Y, Yamazaki-Itoh R, Shimada K, et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108:914–923. doi: 10.1038/bjc.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Protti MP, De Monte L. Immune infiltrates as predictive markers of survival in pancreatic cancer patients. Front Physiol. 2013;4:210. doi: 10.3389/fphys.2013.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotteas E, Saif MW, Syrigos K. Immunotherapy for pancreatic cancer. J Cancer Res Clin Oncol. 2016;142:1795–1805. doi: 10.1007/s00432-016-2119-2. [DOI] [PubMed] [Google Scholar]
- 10.Liu L, Zhao G, Wu W, et al. Low intratumoral regulatory T cells and high peritumoral CD8(+) T cells relate to long-term survival in patients with pancreatic ductal adenocarcinoma after pancreatectomy. Cancer Immunol Immunother. 2016;65:73–82. doi: 10.1007/s00262-015-1775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang Y, Xu X, Guo S, et al. An increased abundance of tumor-infiltrating regulatory T cells is correlated with the progression and prognosis of pancreatic ductal adenocarcinoma. PLoS One. 2014;9:e91551. doi: 10.1371/journal.pone.0091551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Du Y, Huang Z. CD4+CD25+ Treg derived from hepatocellular carcinoma mice inhibits tumor immunity. Immunol Lett. 2012;148:83–89. doi: 10.1016/j.imlet.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer. 2010;10:415–424. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- 14.Ijichi H. TGF-beta signaling pathway in pancreatic cancer cells. Nihon Rinsho. 2004;62:1241–1248. [PubMed] [Google Scholar]
- 15.Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony- stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosmaczewska A, Ciszak L, Potoczek S, et al. The significance of Treg cells in defective tumor immunity. Arch Immunol Ther Exp (Warsz) 2008;56:181–191. doi: 10.1007/s00005-008-0018-1. [DOI] [PubMed] [Google Scholar]
- 17.Khazaie K, von Boehmer H. The impact of CD4+CD25+ Treg on tumor specific CD8+ T cell cytotoxicity and cancer. Semin Cancer Biol. 2006;16:124–136. doi: 10.1016/j.semcancer.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto T, Yanagimoto H, Satoi S, et al. Circulating CD4+CD25+ regulatory T cells in patients with pancreatic cancer. Pancreas. 2012;41:409–415. doi: 10.1097/MPA.0b013e3182373a66. [DOI] [PubMed] [Google Scholar]
- 19.Xiang ST, Zhou SW, Guan W, et al. Expression of MUC1 and distribution of tumor-infiltrating dentritic cells in human bladder transitional cell carcinoma. Di Yi Jun Yi Da Xue Xue Bao. 2005;25:1114–1118. [PubMed] [Google Scholar]
- 20.Soruri A, Fayyazi A, Neumann C, et al. Ex vivo generation of human anti-melanoma autologous cytolytic T cells by dentritic cell/melanoma cell hybridomas. Cancer Immunol Immunother. 2001;50:307–314. doi: 10.1007/s002620100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhairavabhotla RK, Verm V, Tongaonkar H, et al. Role of IL-10 in immune suppression in cervical cancer. Indian J Biochem Biophys. 2007;44:350–356. [PubMed] [Google Scholar]
- 22.Kindlund B, Sjoling A, Yakkala C, et al. CD4+ regulatory T cells in gastric cancer mucosa are proliferating and express high levels of IL-10 but little TGF-beta. Gastric Cancer. 2017;20:116–125. doi: 10.1007/s10120-015-0591-z. [DOI] [PubMed] [Google Scholar]
- 23.Fullerton PT, Jr, Creighton CJ, Matzuk MM. Insights Into SMAD4 Loss in Pancreatic Cancer From Inducible Restoration of TGF-beta Signaling. Mol Endocrinol. 2015;29:1440–1453. doi: 10.1210/me.2015-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Truty MJ, Urrutia R. Basics of TGF-beta and pancreatic cancer. Pancreatology. 2007;7:423–435. doi: 10.1159/000108959. [DOI] [PubMed] [Google Scholar]
- 25.Javle M, Li Y, Tan D, et al. Biomarkers of TGF-beta signaling pathway and prognosis of pancreatic cancer. PLoS One. 2014;9:e85942. doi: 10.1371/journal.pone.0085942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soares KC, Rucki AA, Kim V, et al. TGF-beta blockade depletes T regulatory cells from metastatic pancreatic tumors in a vaccine dependent manner. Oncotarget. 2015;6:43005–43015. doi: 10.18632/oncotarget.5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greco SH, Tomkotter L, Vahle AK, et al. TGF-beta Blockade Reduces Mortality and Metabolic Changes in a Validated Murine Model of Pancreatic Cancer Cachexia. PLoS One. 2015;10:e0132786. doi: 10.1371/journal.pone.0132786. [DOI] [PMC free article] [PubMed] [Google Scholar]