Abstract
IL-35 is known as a regulatory cytokine produced by regulatory T cells. It has also been reported that IL-35 suppresses the proliferation of Th17 cells, which is involved in the pathogenesis of many autoimmune diseases. However, in rheumatoid arthritis patients, the role of IL-35 is controversial, and the role of IL-35 in bone metabolism has not been clarified. We investigated the effect of IL-35 on human osteoclast differentiation and activation. We first evaluated the effect of rhIL-35 on human osteoclastogenesis from monocytes cultured alone, induced by soluble-RANKL. We also examined the role of IL-35 on the bone-resorption function of mature osteoclasts. Furthermore, we analysed the molecular mechanism of IL-35 function in monocytes or pre-osteoclasts using RT-PCR. rhIL-35 significantly inhibited human osteoclastogenesis in a dose-dependent manner. In addition, rhIL-35 also significantly decreased the area of pit formation by mature osteoclasts. rhIL-35 significantly decreased mRNA expression of RANK in monocytes and RANK and FOS in pre-osteoclasts. Our current findings suggest that IL-35 inhibits osteoclastogenesis and osteoclast activation by inhibiting both RANK and FOS. IL-35 also has an inhibitory effect on osteoclastic-bone resorption, suggesting that IL-35 may have a therapeutic potential for RA.
Keywords: osteoclasts, monocytes, RANKL, Interleukin-35, osteoclastogenesis
Introduction
IL-35 is a recently identified cytokine belonging to the IL-12 family, which consists of an α chain (p35/IL-12α) and β chain (EBI3/IL-27β). The IL-35 receptor consists of IL-12Rβ2 (IL-12 component) and gp130 (IL-27 component), and activates the STAT1 and STAT4 signalling pathways [1]. The IL-35 α chain (p35) is ubiquitously expressed. In contrast, expression of the β chain (EBI3) is selective and highly inducible [2, 3]. IL-35 is a potent inhibitory cytokine produced by mouse and human regulatory T (Treg)-cell populations [4, 5]. In addition, many reports have demonstrated the regulatory effect of IL-35. IL-35 is involved in the suppressive function of a subgroup of Treg cells, known as iTr35 cells. IL-35 is also known to suppress Th17 cells and inhibit the proliferation of effector T cells [4, 6-11].
The role of IL-35 in human diseases has also been reported. Serum IL-35 levels were lower in multiple sclerosis (MS) patients, and treatment with interferon β, methylprednisolone (mPSL), or a combination of both showed beneficial effects via the upregulation of IL-35 production [12]. In addition, it was also found that IL-35 expression protects animals from autoimmune diabetes by decreasing T-cell infiltration and proliferation (via G1 arrest) [13].
However, in arthritis models, the role of IL-35 is controversial. Some studies have shown that IL-35 exacerbates arthritis [14, 15]. In contrast, Niedbala et al. demonstrated that intraperitoneal injection of rIL-35 significantly reduced the incidence and intensity of collagen-induced arthritis (CIA) [16]. Similar to mouse models, the effect of IL-35 in rheumatoid arthritis (RA) patients is also controversial. Filkova et al. reported that IL-35 dose-dependently induced release of IL-1β, IL-6, and MCP-1 from peripheral blood mononuclear cells, and that IL-35 was increased in the synovial tissue in RA patients [17]. Furthermore, the same group found that IL-35 levels were significantly elevated in the serum and synovial fluid of RA patients compared with osteoarthritis (OA) patients [18]. In contrast, serum IL-35 levels are significantly decreased in active RA patients, and IL-35 suppresses T-cell activation during the peripheral immune response of RA [19]. More recently, it has been reported that IL-35 suppresses RANKL expression and up-regulates OPG expression in fibroblast-like cells in mice [20]. However, the function of IL-35 in human osteoclastogenesis from monocytes cultured alone remains unclear. In the present study, we demonstrated that rhIL-35 inhibited human osteoclast differentiation and activation. Moreover, we also investigated the inhibitory effect of IL-35 on osteoclastogenesis.
Material and methods
Reagents
Recombinant human M-CSF (Leukoprol) was obtained from Yoshitomi Pharmaceutical (Osaka, Japan). Recombinant human soluble-receptor activator of NF-κB ligand (sRANKL) and recombinant human IL-35 (rhIL-35) were purchased from PeproTech EC Ltd. (London, United Kingdom). Microbeads for immunopurification were obtained from Miltenyi Biotec (Auburn, CA). Anti-human CD51/61 mAb was purchased from BD Bioscience Pharmingen (San Diego, CA).
Culture system for osteoclastogenesis in the absence of osteoblasts
Human peripheral blood was obtained from healthy volunteers. This study was approved by the Institutional Review Board. PBMC were isolated by Ficoll-Hypaque gradient centrifugation and resuspended in α-MEM (Gibco BRL, Gaithersburg, MD) supplemented with 10% foetal bovine serum (FBS) (JRH Biosciences, Lenexa, KS). CD14-positive cells, as monocytes, were separated from the PBMC by MACS and cultured for three days in 96-well plates (2.5 × 104 cells/0.2 ml/well; Corning, NY) with M-CSF (100 ng/ml). Next, the culture medium was completely replaced, and the adherent CD14-positive cells were further cultured in the presence of M-CSF and sRANKL (30 ng/ml) and with various concentrations of rhIL-35 for 10 days. The culture medium was replaced every 3 days, with fresh medium supplemented with the agents described above. As a negative control, human CD14-positive cells were cultured with only M-CSF for 14 days. As a positive control, human CD14-positive cells were cultured for the first three days in the presence of M-CSF, and the adherent CD14-positive cells were further cultured with M-CSF and sRANKL for 10 days.
Determination of osteoclast characteristics
Osteoclasts were immunohistochemically stained using antibodies against vitronectin receptor αvβ3 (CD51/61), as described previously [21]. We previously demonstrated that the multinuclear cells formed in the presence of M-CSF and sRANKL showed vitronectin receptor expression, tartrate-resistant acid phosphatase (TRAP) activity, and the ability to form resorption pits on Osteologic® plates (BD Biosciences, San Jose, CA) [22], therefore exhibiting the functions and properties of osteoclasts.
Mature osteoclast culture on Osteoassay™
CD14-positive cells, as monocytes, were separated from the PBMC by MACS and cultured for three days in six-well plates (7.0 × 105 cells/3ml/well; Corning, NY) with M-CSF (100 ng/ml). Next, the culture medium was completely replaced, and the adherent CD14-positive cells were further cultured in the presence of M-CSF and sRANKL (30 ng/ml) for 10 days. The culture medium was replaced every three days with fresh medium supplemented with the agents described above. As a negative control, human CD14-positive cells were cultured with only M-CSF for 14 days. Adherent mature osteoclasts were then washed with PBS. Cells were treated with trypsin (0.05%, Invitrogen) and EDTA (0.53 mM, Invitrogen) and separated from the culture plate after pipetting several times. Cell scrapers were not used. Mature osteoclasts were then cultured on Osteoassay™ discs (Corning, NY) with M-CSF (100 ng/ml) and sRANKL (30 ng/ml) in a CO2 incubator at 37°C. After three days, the cells were removed, and the areas of pit formation were evaluated.
RT-PCR
Monocytes
CD14-positive human monocytes were cultured in the presence of M-CSF (100 ng/ml) for three days and grown for an additional 24 hours with or without rhIL-35 (100 ng/ml). Total RNA was purified from these cells using the NucleoSpin® RNA kit (Takara Bio, Shiga, Japan). cDNA was obtained using the ReverTra Ace® qPCR RT Kit (Toyobo Life Science, Osaka, Japan). The amount of mRNA of NFATc1 (TaqManR Gene Expression Assay: Hs00542678_m1, Applied Biosystems), TNFRSF11A (RANK) (Hs00187189_m1), FOS (Hs99999140_m1), JUN (Hs99999141_s1), and GAPDH was measured using the Viia7 Real-Time PCR system (Applied Biosystems, Foster City, USA).
Pre-osteoclasts
CD14-positive human monocytes were cultured in the presence of M-CSF (100 ng/ml) for three days. Next, after culturing the cells in the presence of sRANKL (30 ng/ml) with or without rhIL-35 (100 ng/ml) for 24 hrs, total RNA was prepared from these pre-osteoclasts using the NucleoSpin® RNA kit (Takara Bio, Shiga, Japan). cDNA was obtained using the ReverTra Ace® qPCR RT Kit (Toyobo Life Science, Osaka, Japan). The amount of mRNA of NFATc1 (TaqManR Gene Expression Assay: Hs00542678_m1, Applied Biosystems), TNFRSF11A (RANK) (Hs00187189_m1), FOS (Hs99999140_m1), JUN (Hs99999141_s1), and GAPDH was measured using the Viia7 Real-Time PCR system (Applied Biosystems, Foster City, USA).
Statistical analysis
Data were analysed using the Mann-Whitney U test (Stat View®; Abacus Concepts, Berkeley, CA). P values less than 0.05 were considered significant. All values are represented as the mean ±SEM.
Results
rhIL-35 significantly inhibited human osteoclastogenesis
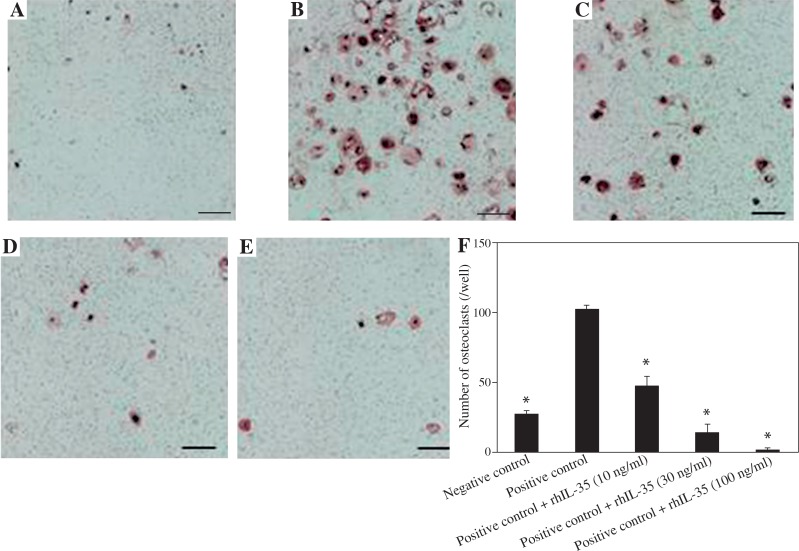
To investigate the effect of IL-35 on human osteoclastogenesis, we cultured cells in the presence of rhIL-35 and sRANKL. Compared with the negative (Fig. 1A) and positive controls (Fig. 1B), rhIL-35 dose-dependently inhibited the number of CD51/61-positive osteoclasts induced by sRANKL, as shown in Figures 1C-1E. Figure 1F shows that even at 10 ng/ml, rhIL-35 significantly decreased the number of osteoclasts.
Fig. 1.
rhIL-35 significantly inhibited human osteoclastogenesis. As negative and positive controls, human CD14-positive monocytes were cultured with M-CSF alone during the first three days, and the adherent cells were then cultured with either M-CSF alone (A – negative control) or M-CSF and sRANKL (30 ng/ml) (B – positive control) for the last 10 days. The osteoclasts were then stained for the vitronectin receptor. In the test conditions, rhIL-35 (10, 30, 100 ng/ml) (C, D, E) was added to the culture medium at the same time of sRANKL addition for the last 10 days. Original magnification, 100×. F) rhIL-35 dose-dependently inhibited the number of osteoclasts. Data are expressed as mean ±SEM for triplicate cultures (*p < 0.05 vs. positive control)
rhIL-35 significantly inhibited the activation of mature osteoclasts
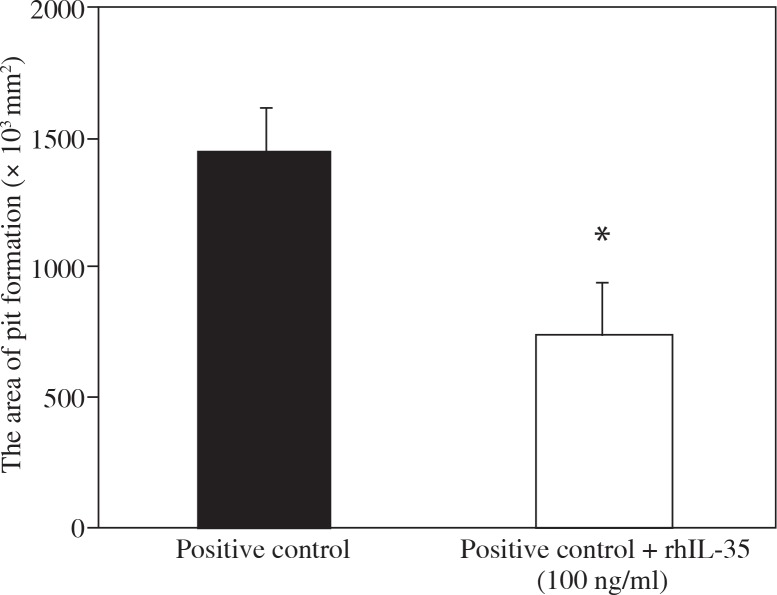
To elucidate whether rhIL-35 prevents osteoclast activation, we evaluated the area of pit formation after addition of rhIL-35 to mature osteoclasts by Osteoassay™. rhIL-35 significantly decreased the area of pit formation by mature osteoclasts, as shown in Figure 2, suggesting that IL-35 also has mature osteoclast activation inhibiting activity.
Fig. 2.
rhIL-35 decreased the area of pit formation by mature osteoclasts. Osteoclasts formed from human monocytes in the presence of M-CSF (100 ng/ml) and sRANKL (30 ng/ml) over 14 days. Mature osteoclasts were re-plated onto an OsteologicTM plate, and M-CSF, sRANKL, and rhIL-35 (100 ng/ml) were added. After four days, the area of pit formation was evaluated. Data are expressed as mean ±SEM for triplicate cultures (*p < 0.05 vs. positive control)
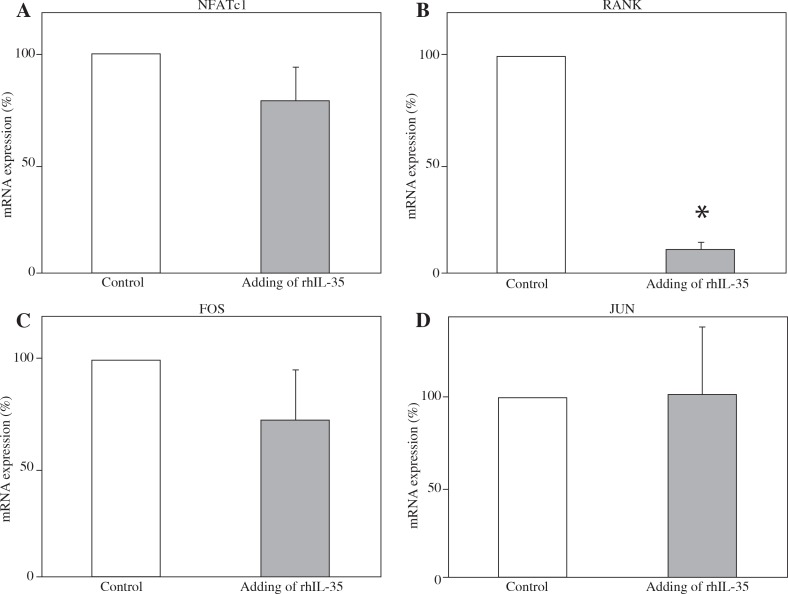
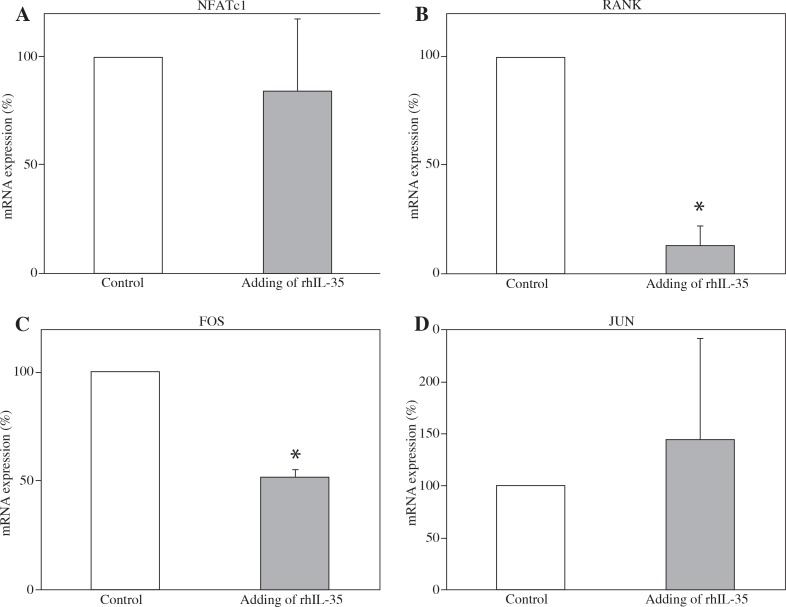
Addition of rhIL-35 reduced mRNA expression of RANK in monocytes and RANK and FOS in pre-osteoclasts
To clarify the mechanism of the inhibitory effect of IL-35 on osteoclastogenesis, we measured the mRNA expression of various proteins in monocytes and pre-osteoclasts after stimulation with rhIL-35. As shown in Figure 3, rhIL-35 significantly decreased mRNA expression of RANK in monocytes. In pre-osteoclasts, rhIL-35 significantly decreased the mRNA expression of RANK and FOS (Fig. 4). Interestingly, rhIL-35 did not decrease NFATc1 expression at the mRNA level in either monocytes or pre-osteoclasts.
Fig. 3.
rhIL-35 significantly decreased mRNA expression of RANK in monocytes. Effect of rhIL-35 on CD14-positive monocytes. Isolated human CD14-positive monocytes from four different donors were cultured with M-CSF (100 ng/ml) for three days. rhIL-35 (100 ng/ml) was then added for 24 hours. As a control, CD14-positive monocytes were cultured alone for 24 hours. The mRNA expression of various proteins was evaluated by RT-PCR. Percentages of mRNA expression are shown. In each panel, the right bar represents monocytes treated with rhIL-35, and the left bar represents monocytes alone (*p < 0.05). The SEM of the left bar (without rhIL-35) is zero because we normalised the values of the monocytes treated with rhIL-35 to monocytes alone
Fig. 4.
rhIL-35 significantly decreased mRNA expression of RANK and FOS in pre-osteoclasts. Effect of rhIL-35 on preosteoclasts. Isolated human CD14-positive monocytes from three different donors were cultured with M-CSF (100 ng/ml) for three days, followed by addition of sRANKL (30 ng/ml) for 24 hours. rhIL-35 (100 ng/ml) was added at the same time as sRANKL in the test conditions. As a control, CD14-positive monocytes were cultured with only sRANKL for 24 hours. The mRNA expression of various proteins was evaluated by RT-PCR. Percentages of mRNA expression are shown. In each panel, the right bar represents pre-osteoclasts treated with rhIL-35, and the left bar represents pre-osteoclasts alone (*p < 0.05). The SEM of the left bar (without rhIL-35) is zero because we normalised the values of the pre-osteoclasts treated with rhIL-35 to pre-osteoclasts alone
Discussion
This is the first report to demonstrate the inhibitory effect of IL-35 on human osteoclastogenesis. First, we demonstrated that rhIL-35 significantly decreased osteoclast differentiation induced by sRANKL in a dose-dependent manner. We have reported that pro-inflammatory cytokines, including TNFα and IL-17, induce human osteoclastogenesis [23, 24]. IL-35 is composed of an α chain (p35; IL-12 component) and β chain (EBI3; IL-27 component), and some studies have shown that IL-12 and IL-27 are involved in osteoclast differentiation. In light of our observation of the inhibitory effect of IL-35 on monocytes as osteoclast precursors, it is necessary to clarify whether the IL-12 component or IL-27 component is the main contributor to the abrogation of osteoclast differentiation. It has been reported that IL-27 directly inhibits human osteoclastogenesis from CD14-positive cells by suppressing NFATc1 or RANK expression [25]. On the other hand, IL-12 does not directly inhibit osteoclastogenesis in mouse monocytes cultured alone [26]. From these studies, it has been speculated that the inhibitory effect of rhIL-35 on human osteoclastogenesis may be due to the IL-27 subunit (EBI3) rather than the IL-12 subunit (p35).
In addition to IL-35 itself, the function of its receptors and their downstream signals must also be discussed. IL-35 receptor consists of IL-12Rβ2 (IL-12 component) and gp130 (IL-27 component), subsequently activating the STAT4 and STAT1 signalling pathways, respectively [1]. Furukawa et al. found that IL-27-induced inhibition of human osteoclast differentiation is mediated by STAT1 [27]. On the other hand, the direct role of STAT4 on monocytic osteoclastogenesis has not been reported. We are now investigating the contribution of STAT1 signalling to the inhibition of osteoclastogenesis in the presence of rhIL-35.
We found that rhIL-35 decreased the area of pit formation by mature osteoclasts. In the present study, it is notable that rhIL-35 inhibited osteoclast activation even after the monocytes differentiated into mature osteoclasts. We have also previously reported that IL-17 induces mature osteoclast activation by measuring actin ring formation [23]. We hypothesise that IL-35 also affects the cytoskeleton of mature osteoclasts.
Our analysis of the effect of rhIL-35 at the molecular level using RT-PCR demonstrated that the addition of rhIL-35 significantly decreased RANK expression in osteoclast precursors, including monocytes and pre-osteoclasts. Furthermore, in pre-osteoclasts, rhIL-35 significantly reduced FOS expression; however, the degree of reduction by rhIL-35 was greater in RANK than in FOS. Interestingly, rhIL-35 did not significantly reduce expression of NFATc1 in the current study. Kalliolias et al. reported that IL-27-induced inhibition of osteoclastogenesis is due to suppression of NFATc1 induction [25]. Thus, taken together, our findings suggest that IL-35 plays a direct role in the function of osteoclast precursors and activation of mature osteoclasts by preventing RANK-RANKL signalling.
Unfortunately, we could not substantiate our laboratory findings in vivo, because the clinical phenotype of IL-35 deficient or transgenic mice has not been reported so far. Further investigation will be needed to clarify the role of IL-35 on whole bone by using mouse models. In addition, future investigation of IL-35 using a co-culture system with osteoblasts and osteoclast precursors is also required. As an example, IL-6 has been reported to induce osteoclast differentiation with osteoblasts via inducing expression of RANKL in osteoblasts [28], whereas IL-6 inhibits osteoclastogenesis in monocytes cultured alone [29]. In addition, there is a possibility that IL-35 has different effects on osteoclastogenesis between humans and mice [30].
Whether IL-35 induces inflammatory bone loss in RA patients remains unclear. As mentioned in the Introduction, the role of IL-35 in arthritis is now being investigated. Some groups found that IL-35 plays an inflammatory role in arthritis [14, 15], while other groups suggested that IL-35 may potentially have an anti-inflammatory effect [16, 20, 31]. Recently, Li et al. showed that IL-35 increases the expression of RANKL and decreases the expression of OPG in both synoviocytes in CIA and cultured fibroblast like-cells [20]. Although they did not examine osteoclast differentiation, IL-35 may have inhibited osteoclastogenesis in their co-culture system of osteoblasts and monocytes. Considering the effect of IL-35 on monocytes as osteoclast precursors, our findings demonstrate, at least in part, that IL-35 plays a role as an anti-osteoclastogenic cytokine.
The role of IL-35 in RA patients is also controversial. Senolt’s group showed that IL-35 levels were increased in the synovial tissue, serum, and synovial fluid in RA patients compared with patients with other types of arthritis, including osteoarthritis [17, 18], and they speculated that IL-35 has a pro-inflammatory effect. In contrast, Nakano’s group demonstrated that serum IL-35 levels were decreased in RA patients compared with normal controls, and they concluded that IL-35 may be a negative regulator of immune responses in RA [19]. The reason for this discrepancy in the role of IL-35 level in RA remains unclear, but it may have been due to the patient population analysed or the methods used. Thus, further investigation is warranted to conclude the function of IL-35 in RA patients.
Conclusions
We found that rhIL-35 inhibited the sRANKL-induced differentiation and activation of human osteoclasts in monocytes cultured alone. Moreover, our findings also demonstrated that, at least in part, the inhibitory effect of IL-35 on osteoclast function was induced by decreasing RANK expression. These findings suggest that IL-35 is an anti-osteoclastogenic cytokine and that IL-35 may be a therapeutic target for prevention of joint destruction in RA patients.
Acknowledgement
We thank Ms. Hanae Kikuchi (Tokyo Women’s Medical University) for her valuable technical assistance.
Footnotes
The authors declare no conflict of interest.
References
- 1.Collison LW, Delgoffe GM, Guy CS, et al. The composition and signaling of the IL-35 receptor are unconventional. Nat Immunol. 2012;13:290–299. doi: 10.1038/ni.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 3.Bardel E, Larousserie F, Charlot-Rabiega P, et al. Human CD4+ CD25+ Foxp3+ regulatory T cells do not constitutively express IL-35. J Immunol. 2008;181:6898–6905. doi: 10.4049/jimmunol.181.10.6898. [DOI] [PubMed] [Google Scholar]
- 4.Collison LW, Vignali DA. Interleukin-35: odd one out or part of the family? Immunol Rev. 2008;226:248–262. doi: 10.1111/j.1600-065X.2008.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banchereau J, Pascual V, O’Garra A. From IL-2 to IL-37: the expanding spectrum of anti-inflammatory cytokines. Nat Immunol. 2012;13:925–931. doi: 10.1038/ni.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collison LW, Workman CJ, Kuo TT, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 8.Bettini M, Vignali DA. Regulatory T cells and inhibitory cytokines in autoimmunity. Curr Opin Immunol. 2009;21:612–618. doi: 10.1016/j.coi.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Astry B, Harberts E, Moudgil KD. A cytokine-centric view of the pathogenesis and treatment of autoimmune arthritis. J Interferon Cytokine Res. 2011;31:927–940. doi: 10.1089/jir.2011.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pillai MR, Collison LW, Wang X, et al. The plasticity of regulatory T cell function. J Immunol. 2011;187:4987–4997. doi: 10.4049/jimmunol.1102173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clavel G, Thiolat A, Boissier MC. Interleukin newcomers creating new numbers in rheumatology: IL-34 to IL-38. Joint Bone Spine. 2013;80:449–453. doi: 10.1016/j.jbspin.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Jafarzadeh A, Jamali M, Mahdavi R, et al. Circulating levels of interleukin-35 in patients with multiple sclerosis: evaluation of the influences of FOXP3 gene polymorphism and treatment program. J Mol Neurosci. 2015;55:891–897. doi: 10.1007/s12031-014-0443-z. [DOI] [PubMed] [Google Scholar]
- 13.Bettini M, Castellaw AH, Lennon GP, et al. Prevention of autoimmune diabetes by ectopic pancreatic β-cell expression of interleukin-35. Diabetes. 2012;61:1519–1526. doi: 10.2337/db11-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thiolat A, Denys A, Petit M, et al. Interleukin-35 gene therapy exacerbates experimental rheumatoid arthritis in mice. Cytokine. 2014;69:87–93. doi: 10.1016/j.cyto.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Kuo J, Nardelli DT, Warner TF, et al. Interleukin-35 enhances Lyme arthritis in Borrelia-vaccinated and -infected mice. Clin Vaccine Immunol. 2011;18:1125–1132. doi: 10.1128/CVI.00052-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niedbala W, Wei XQ, Cai B, et al. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol. 2007;37:3021–3029. doi: 10.1002/eji.200737810. [DOI] [PubMed] [Google Scholar]
- 17.Filková M, Vernerová Z, Hulejová H, et al. Pro-inflammatory effects of interleukin-35 in rheumatoid arthritis. Cytokine. 2015;73:36–43. doi: 10.1016/j.cyto.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Šenolt L, Šumová B, Jandová R, et al. Interleukin 35 Synovial Fluid Levels Are Associated with Disease Activity of Rheumatoid Arthritis. PLoS One. 2015;10:e0132674. doi: 10.1371/journal.pone.0132674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakano S, Morimoto S, Suzuki S, et al. Immunoregulatory role of IL-35 in T cells of patients with rheumatoid arthritis. Rheumatology (Oxford) 2015;54:1498–1506. doi: 10.1093/rheumatology/keu528. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Li D, Li Y, et al. Interleukin-35 upregulates OPG and inhibits RANKL in mice with collagen-induced arthritis and fibroblast-like synoviocytes. 2016. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Kotake S, Nanke Y, Mogi M, et al. IFN-gamma-producing human T cells directly induce osteoclastogenesis from human monocytes via the expression of RANKL. Eur J Immunol. 2005;35:3353–3363. doi: 10.1002/eji.200526141. [DOI] [PubMed] [Google Scholar]
- 22.Yago T, Nanke Y, Kawamoto M, et al. IL-23 induces human osteoclastogenesis via IL-17 in vitro, and anti-IL-23 antibody attenuates collagen-induced arthritis in rats. Arthritis Res Ther. 2007;9:R96.. doi: 10.1186/ar2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yago T, Nanke Y, Ichikawa N, et al. IL-17 induces osteoclastogenesis from human monocytes alone in the absence of osteoblasts, which is potently inhibited by anti-TNF-αlpha antibody: a novel mechanism of osteoclastogenesis by IL-17. J Cell Biochem. 2009;108:947–955. doi: 10.1002/jcb.22326. [DOI] [PubMed] [Google Scholar]
- 24.Kotake S, Udagawa N, Takahashi N, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalliolias GD, Zhao B, Triantafyllopoulou A, et al. Interleukin-27 inhibits human osteoclastogenesis by abrogating RANKL-mediated induction of nuclear factor of activated T cells c1 and suppressing proximal RANK signaling. Arthritis Rheum. 2010;62:402–413. doi: 10.1002/art.27200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagata N, Kitaura H, Yoshida N, et al. Inhibition of RANKL-induced osteoclast formation in mouse bone marrow cells by IL-12: involvement of IFN-gamma possibly induced from non-T cell population. Bone. 2003;33:721–732. doi: 10.1016/s8756-3282(03)00213-8. [DOI] [PubMed] [Google Scholar]
- 27.Furukawa M, Takaishi H, Takito J, et al. IL-27 abrogates receptor activator of NF-kappa B ligand-mediated osteoclastogenesis of human granulocyte-macrophage colony-forming unit cells through STAT1-dependent inhibition of c-Fos. J Immunol. 2009;183:2397–2406. doi: 10.4049/jimmunol.0802091. [DOI] [PubMed] [Google Scholar]
- 28.Palmqvist P, Persson E, Conaway HH, et al. IL-6, leukemia inhibitory factor, and oncostatin M stimulate bone resorption and regulate the expression of receptor activator of NF-kappa B ligand, osteoprotegerin, and receptor activator of NF-kappa B in mouse calvariae. J Immunol. 2002;169:3353–3362. doi: 10.4049/jimmunol.169.6.3353. [DOI] [PubMed] [Google Scholar]
- 29.Duplomb L, Baud’huin M, Charrier C, et al. Interleukin-6 inhibits receptor activator of nuclear factor kappaB ligand-induced osteoclastogenesis by diverting cells into the macrophage lineage: key role of Serine727 phosphorylation of signal transducer and activator of transcription 3. Endocrinology. 2008;149:3688–3697. doi: 10.1210/en.2007-1719. [DOI] [PubMed] [Google Scholar]
- 30.Kotake S, Nanke Y, Yago T, et al. Human osteoclastogenic T cells and human osteoclastology. Arthritis Rheum. 2009;60:3158–3163. doi: 10.1002/art.24886. [DOI] [PubMed] [Google Scholar]
- 31.Kochetkova I, Golden S, Holderness K, et al. IL-35 stimulation of CD39+ regulatory T cells confers protection against collagen II-induced arthritis via the production of IL-10. J Immunol. 2010;184:7144–7153. doi: 10.4049/jimmunol.0902739. [DOI] [PMC free article] [PubMed] [Google Scholar]