Abstract
Cordyceps sinensis (CS) is a prominent medicinal herb in traditional Chinese medicine, and fermented CS is frequently used as a substitute for natural CS. Doxorubicin (DOX), an antitumor drug used in chemotherapy, is limited by its poor cardiotoxicity. The aim of the present study was to evaluate the protective effect of fermented CS against DOX-induced cardiotoxicity and the potential underlying mechanisms. Male Sprague-Dawley rats (180–200 g) were randomly assigned to seven different treatment groups: Normal control, DOX control, DOX+captopril (0.05 g/kg), 0.75, 1.5 and 3 g/kg DOX+CS, and the CS (1.5 g/kg) control. Histopathological changes, cardiac energy metabolism, cyclic adenosine monophosphate (cAMP) signaling and the associated mRNA expression of AMP-activated protein kinase (AMPK) were then evaluated. Fermented CS decreased the left ventricular weight index, heart weight index and mortality; however, it increased diastolic blood pressure and mean arterial pressure. In addition, it shortened the duration of the QRS complex and Sα-T segment, decreased serum creatine kinase (CK) and aspartate aminotransferase activity, inhibited histopathological changes and reduced brain natriuretic peptide content. Treatment with fermented CS also increased the activities of superoxide dismutase and glutathione peroxidase, reduced malondialdehyde content, increased the mitochondrial activities of Na+K+-adenosine 5′-triphosphate (ATP) ase, Ca2+Mg2+-ATPase and CK, and increased the creatine phosphate/ATP ratio and AMP/ATP ratio. Furthermore, it decreased the ATP/adenosine 5′-diphosphate (ADP) ratio, upregulated AMPKα2 expression, reduced the activity of serum phosphodiesterases (PDEs) and increased myocardial cAMP content. The results of the present study demonstrated that fermented CS attenuated DOX-induced cardiotoxicity by inhibiting myocardial hypertrophy and myocardial damage, ameliorating systolic function and the antioxidant enzyme system, improving cardiac energy metabolism, depressing the activities of PDEs, and by upregulating the cAMP and AMPK signaling pathways. Thus, fermented CS may be a candidate for the prevention of DOX-induced cardiotoxicity, cardiac energy impairment and against a number of cardiac diseases.
Keywords: doxorubicin, fermented Cordyceps sinensis, cardiotoxicity, cardiac energy metabolism, adenosine monophosphate-activated protein kinase α2, cyclic adenosine monophosphate
Introduction
Doxorubicin (DOX) is one of the most effective chemotherapy drugs for the treatment of hematological malignancies and several solid tumors including lymphomas, leukemia and breast cancer (1). However, DOX has limited clinical use as it induces severe cardiotoxicity, that leads to irreversible cardiac injuries such as heart failure and left ventricular dysfunction (2). DOX has a high affinity for binding to phospholipids in cardiolipin in the inner mitochondrial membrane (3). Therefore, the heart is susceptible to DOX-associated injury as it contains a greater number of mitochondria and fewer antioxidative enzymes than other organs. The mechanism underlying DOX-induced cardiotoxicity is multifactorial (4). It is primarily associated with the production of reactive oxygen species (ROS), apoptosis of cardiac cells, mitochondrial damage, impairment of cardiac energy metabolism and defects in the adenosine monophosphate-activated protein kinase (AMPK) signaling pathway (5,6).
Cordyceps sinensis (CS), a widely used traditional Chinese medicine (TCM), is the dried stroma and sporophore of Cordyceps sinensis (Berk.) Sacc. Which parasitizes on the larvae of Hepialidae. CS has been used extensively in TCM to treat coughs, shortness of breath and to improve quality of life. It is now commonly used as a health product in China (7), as it has broad pharmacological effects associated with anti-oxidative stress (OS), immunoregulatory, antisenescent, hypoglycemic, hypolipidemic and antitumor activities (8). As the primary sources of CS are restricted by the controlled natural conditions required, only an extremely limited amount of natural CS can be produced each year. Thus, it is very expensive and so, it is not widely available to general patients. Fermented CS, a substitute for natural CS, is produced by purifying and artificially fermenting the fungus isolated from fresh Qinghai CS. Fermented CS has been used extensively in supplementing medical treatments in traditional Chinese medicine, particularly in the adjuvant therapy of cardiac arrhythmia and chronic renal insufficiency (4). The chemical components and pharmacological effects of fermented CS are similar to those of natural CS, including anti-hyperglycemic actions (9), antitumor activity (10), anti-inflammation (11) and anti-OS (4).
Therefore, the aim of the study was to evaluate the protective effects of fermented CS against DOX-induced cardiotoxicity and the potential underlying mechanisms. The results suggested that fermented CS attenuated DOX-induced cardiotoxicity in rats by reducing mitochondrial damage and oxidative stress, depressing phosphodiesterase activities, upregulating the AMPK and cyclic (c)AMP signaling pathways, and ameliorating energy metabolism, myocardial damage, cardiac dysfunction and acute heart failure.
Materials and methods
Herbal materials and reagents
Fermented CS is produced by artificial fermentation, and CS mycelia, the raw material, is isolated from natural CS. Fermented CS is listed in the Pharmacopoeia of the People's Republic of China (2015 edition) (12), its chemical components are similar to those of natural CS (4). Fermented CS is produced by purifying and artificially fermenting the fungus isolated from fresh Qinghai CS. It was supplied by Hangzhou Zhongmei Huadong Pharmaceutical Co., Ltd. (batch no. 1410170; Zhejiang, China). Fermented CS contains 14.8% protein, 39.4% carbohydrate, 6.63% fat, 2.95% ash, 1.4% cordycepin, 6.4% H2O, 9.23% amino acid and 0.21% adenosine. The adenosine content in fermented CS was analyzed by high-performance liquid chromatography (HPLC; UltiMate 3000 HPLC system; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The mobile phase consisted of acetonitrile and water (containing 0.04 mol/l KH2PO4). A linear gradient elution program was applied as follows: 0–15 min 100% water, 15–45 min 0–15% acetonitrile, 100–85% water. The flow rate was 1.0 ml/min and the injection volume was 2 µl. The reference substance of adenosine and fermented CS were individually dissolved in 0.5% phosphoric acid to obtain a solution of 12 µg/ml. The detection wavelength was 223 nm and the chromatographic column used was the Ultimate AQ-C18 (4.6×150 mm, 3 µm, Welch Materials, Inc., Shanghai, China) with the injection volume of 10 µl with 0.2 µm precolumn inline filter maintained at 30°C. The adenosine content in 0.5 g fermented CS was quantitated as 1.07 mg, which was in accordance with the requirements of the Pharmacopoeia of People's Republic of China (2015 edition). The DOX Hydrochloride injection was obtained from Shenzhen Main Luck Pharmaceuticals Inc. (Guangdong, China). Captopril was supplied by Shanghai Hengshan Pharmaceuticals Co., Ltd. (Shanghai, China).
Animals
Male Sprague-Dawley rats (n=84; weight, 180–200 g; 8 weeks old) were supplied by Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China). Rats were kept in the Laboratory Animal Center of Shanghai Traditional Chinese Medicine (Shanghai, China; temperature 22–24°C, humidity 40±5% and 12 h light/dark cycle) with free access to food and water. All animals were used in accordance with the guidelines of the National Institutes of Health (NIH Publication no. 85-23, revised 1996) Guide for the Care and Use of Laboratory Animals (13), and were approved by the Animal Care and Use Committee of Shanghai University of Traditional Chinese Medicine (approval no. SZY2014029).
Experimental design
A total of 84 rats were randomly assigned to 7 groups which included the normal control group (n=10), the DOX control group (n=15), the DOX+captopril (0.05 g/kg) group (n=13), the 3 DOX+CS groups [0.75 (n=13), 1.5 (n=14) and 3 g/kg (n=14) of CS], and the CS (1.5 g/kg) control group (n=5).
Rats in the DOX control group, and the captopril and CS treatment groups were administered 6 doses of DOX (2.50 mg/kg/dose, via an intraperitoneal injection) at 48 h intervals in order to achieve a total dose of 15 mg/kg (487 mg/m2) which is above the threshold of DOX-induced cardiomyopathy (370 mg/m2) (14). Rats in the normal control and CS control groups received 0.9% NaCl via an intraperitoneal injection (solvent of DOX) in place of DOX. The volume of injection was 1.0 ml/kg each time.
Rats in the normal control and DOX control groups were intragastrically administered drinking water only, and the rats in the remaining 5 groups were administered with the corresponding drugs [captopril (0.05 g/kg) or CS (0.75, 1.5 or 3 g/kg)] in a volume of 10 ml/kg, starting 2 days prior to initial DOX administration and continuing for 23 days.
A total of 24 h following the last injection of DOX, blood samples (~500 µl each) were collected from the tail vein to measure the levels of cardiac enzymes in serum. Following the aforementioned intragastric drug administration for 23 days, the surviving animals (n=68) were fasted for 12 h. The number of surviving rats in each group was recorded to calculate the mortality. Subsequently, rats were anesthetized to measure blood pressure and electrocardiogram (ECG). Once blood pressure and ECG measurements were performed, the rats were sacrificed under anesthesia (urethane, i.p. injection, 1 g/kg). Blood samples were also collected following ECG and blood pressure measurement and centrifuged at 4°C and 2,325 × g for 10 min to recover the serum. The cardiac tissues were excised and the tissues of the left ventricle were separated. The upper part of left ventricle was fixed in 10% buffered neutral formalin solution (22–24°C, 1 week) for histopathological analysis. The serum and the remaining cardiac tissues were stored at −80°C for subsequent experiments.
Recording blood pressure and ECG
Blood pressure, including systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean arterial pressure (MAP), and ECG (position II) were recorded using a multi-channel biological signal analysis system (RM6240C; Chengdu Instrument Factory, Chengdu, China). Heart rate, and the duration of the QRS complex and Sα-T segment were measured. QRS complexes and the Sα-T segment have been reported to be reliable ECG parameters for the assessment of DOX-induced cardiotoxicity (15).
Assessment of general data and cardiac weight indexes
Once treatments and subsequent experiments were completed, total body weight (BW) and heart weight (HW) were measured following fasting for 12 h. Following sacrifice, the heart was removed and the left ventricle was separated from the adipose tissue and atria once blood samples were collected. The left ventricle weight (LVW) and HW were recorded, and then the LVW index (LVWI; the LVW/BW ratio) and the HW index (HWI; the HW/BW ratio) were calculated. The aforementioned fixed part of left ventricle was dehydrated in 70% ethanol, cleared in xylene and then embedded in paraffin. Sections (4-µm thick) were prepared and stained with hematoxylin-eosin (H&E) at room temperature for 1 day. A light microscope (UB202i; Chongqing COIC Industrial Co., Ltd., Chongqing, China) was used to capture the images of each sample.
Endogenous antioxidant enzymes and malondialdehyde (MDA)
Once serum was recovered from blood samples (centrifugation at 4°C and 2,325 × g for 10 min), total superoxide dismutase (T-SOD) activity, glutathione peroxidase (GSH-Px) activity and MDA content in serum were assayed using T-SOD assay kit (hydroxylamine method), MDA assay kit (TBA method) and assay kit (colorimetric method) kit, respectively (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). All assays were performed according to the manufacturer's instructions.
Measurement of serum cardiac enzymes
Blood samples were collected 24 h following the last DOX injection, serum was recovered and then the activities of creatine kinase (CK), lactate dehydrogenase (LDH) and aspartate aminotransferase (AST) in serum were analyzed using the Hitachi 7080 Biochemical Analyzer (Hitachi, Ltd., Tokyo, Japan). CK assay kit, LDH assay kit and AST assay kit were obtained from Shino-Test Corporation (Tokyo, Japan). All kits were performed according to the manufacturer's instructions.
Measurement of brain natriuretic peptide (BNP)
Serum BNP content was determined by ELISA using the aforementioned blood samples. The rat BNP ELISA kit (cat. no. JL15563) was purchased from Shanghai Jianglai Bioengineering Institute Co., Ltd. (Shanghai, China).
Measurement of adenosine 5′-triphosphate (ATP) ase and CK activities in myocardial mitochondria
Mitochondria were recovered from cardiac tissues by differential centrifugation (2,267 × g for 15 min at 4°C, followed by 10,000 × g for 20 min at 4°C). The activities of mitochondrial Na+K+-ATPase, Ca2+Mg2+-ATPase and CK were measured using Na+K+-ATPase assay kit and Ca2+Mg2+-ATPase assay kit, respectively. (Nanjing Jiancheng Bioengineering Institute Co., Ltd). All kits were used according to the manufacturer's instructions.
Measurement of adenine nucleotides and phosphocreatine (PCr) contents
ATP, adenosine 5′-diphosphate (ADP), AMP and PCr contents in cardiac tissues were assayed by HPLC (UltiMate 3000 HPLC system; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Chromatographic separation was achieved on an Ultimate AQ C18 column (4.6×150 mm, 3 µm; Welch Materials Co., Ltd., Shanghai, China) with 0.2 µm precolumn inline filter maintained at 15°C. The mobile phase consisted of (A) water (containing 10 mmol/l KH2PO4 and K2HPO4, pH 6.2) and (B) methanol. A linear gradient elution program was applied as follows: 0–2 min 100% A, 2–8.5 min 100–30% A and 0–30% B, 8.5–11 min 30% A and 70% B, 11–14 min 100% A. The flow rate was 1.0 ml/min and the injection volume was 2 µl. Detected wavelength: 210 nm. Total adenine nucleotides (ATP+ADP and +AMP), the PCr/ATP ratio, the ATP/ADP ratio and the AMP/ATP ratio were also calculated.
Myocardial mRNA expression of AMPKα2, peroxisome proliferator-activated receptor α (PPARα) and peroxisome proliferator-activated receptor γ coactivator-1α (PCG-1α)
The mRNA expression levels of AMPKα2, PPARα and PCG-1α were determined by reverse transcription-quantitative polymerase chain reaction. Total RNA was extracted from cardiac tissues by the Fresh Animal Tissue and Cell Sample Total RNA Extraction kit (cat. no. LN-0108A; Shanghai Novland Bio-pharmaceutical Tech. Co., Ltd., Shanghai, China) and centrifuged at 12,000 × g for 10 min at 4°C. Universal Reverse Transcription kit (Shanghai Novland Bio-pharmaceutical Tech. Co., Ltd., cat. no. LR-0103A) was used for reverse transcription and then real-time PCR kit (cat. no. LK-0101A; SYBR Green, Shanghai Novland Bio-pharmaceutical Tech. Co., Ltd., Shanghai, China) was used for qPCR. The temperature protocol for RT-PCR is 42°C for 30 min and 85°C for 10 min. The following thermocycling conditions were used for PCR: Initial denaturation at 95°C for 3 min; 40 cycles of 95°C for 12 sec, 60°C for 40 sec and 72°C extension for 30 sec. The method of quantification is according to the references (16). The following primers were used: AMPKα2 forward, 5-AAACACGGGAGGGTTGAAGAGG-3 and reverse, 5-ATGTGCCTGTGACAGTAGTCCACG-3; PPARα forward, 5-CATCTGTCCTCTCTCCCCACTTG-3 and reverse, 5-CCGAGGGACTGAGAAATCTCTTG-3; PCG-1α forward, 5-AAACCCTGCCATTGTTAAGACCG-3 and reverse, 5-CGTCTTTGTGGCTTTTGCTGTTG-3; and β-actin forward, 5-CGTAAAGACCTCTATGCCAACA-3 and reverse, 5-GGAGGAGCAATGATCTTGATCT-3. The mRNA expression levels were determined using the MX3000P qPCR System (Agilent Technologies GmbH, Waldbronn, Germany) and the results were normalized to those of β-actin.
Measurement of myocardial cyclic AMP (cAMP) and activity of serum phosphodiesterases (PDEs)
cAMP contents in cardiac tissues were assayed by HPLC, performed as previously described for adenine nucleotides. The activities of serum PDEs selected groups were assayed using PDEs assay kit (Shanghai Jianglai Bioengineering Institute Co., Ltd). In the 0.75 g/kg CS or captopril groups, the cAMP content were unaltered and therfore PDEs activity was not measured in the two groups.
Statistical analysis
All values were expressed as the mean ± standard error mean (n=9). The results were analyzed using SPSS v18.0 software (SPSS, Inc., Chicago, IL, USA). A one-way analysis of variance followed by Dunnett's post hoc test were performed for multiple comparisons. Mann-Whitney U rank-sum test was used as an alternate test for variance heterogeneity. P<0.05 was considered to indicate a statistically significant difference.
Results
General data and cardiac weight indexes
The DOX-treated control rats exhibited 40% mortality when comparing the original number of rats (n=15) to those that survived treatment (n=9), and when compared with the normal control group, a reduction in BW, and an increase in LVWI and HWI were observed (P<0.01). When compared with the DOX control group, rats treated with DOX and captopril or CS (3 g/kg) showed a significant decrease in LVWI and HWI (P<0.05 and P<0.01). In the DOX + 1.5 g/kg CS group, treatment did not result in mortality as all 14 rats survived. In addition, there were no marked differences in the cardiac weight indexes between the normal control group and the CS control group (Table I).
Table I.
Effect of fermented Cordyceps sinensis on mortality and cardiac weight indexes in doxorubicin-treated rats.
| Groups | No. of rats pre-treatment | No. of treatment induced mortalities | No. of surviving rats post-treatment | Mortality (%) | BW (g) | LVWI (mg/g) | HWI (mg/g) |
|---|---|---|---|---|---|---|---|
| Normal control | 10 | 0 | 10 | 0 | 286.2±6.93 | 2.22±0.05 | 2.79±0.05 |
| DOX control | 15 | 6 | 9 | 40.00a | 208.3±12.50b | 2.50±0.09b | 3.11±0.10b |
| DOX plus captopril | 13 | 3 | 10 | 23.08 | 224.8±8.25 | 2.18±0.05d | 2.73±0.06d |
| DOX plus 0.75 g/kg CS | 13 | 5 | 8 | 38.46 | 216.3±6.36 | 2.33±0.07 | 2.90±0.08 |
| DOX plus 1.50 g/kg CS | 14 | 0 | 14 | 0c | 211.0±7.64 | 2.40±0.05 | 3.00±0.08 |
| DOX plus 3.00 g/kg CS | 14 | 2 | 12 | 14.29 | 219.0±7.53 | 2.26±0.06d | 2.85±0.08c |
| CS control | 5 | 0 | 5 | 0 | 292.0±11.14 | 2.21±0.04 | 2.76±0.05 |
Rats were treated with DOX alone or in combination with 0.05 g/kg captopril, or with 0.75, 1.5 or 3 g/kg CS. The number of rats assigned to each group prior to treatment, and the number that survived and succumbed to treatment are presented as well as the percentage of mortality. BW, LVWI and HWI were also measured. Data are presented as the mean ± standard error mean.
P<0.05
P<0.01 vs. the normal control group
P<0.05
P<0.01 vs. the DOX control group. BW, body weight; LVWI, left ventricular weight index; HWI, heart weight index; CS, Cordyceps sinensis; DOX, doxorubicin.
Blood pressure
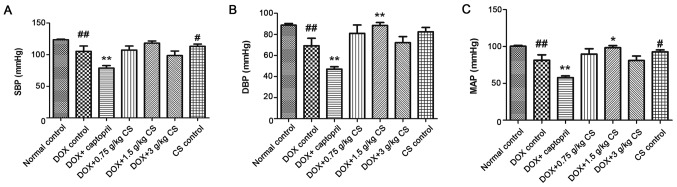
In the DOX control group, rat SBP, DBP and MAP were significantly reduced when compared with the normal control group (P<0.01). In addition, combined treatment with captopril significantly decreased SBP, DBP and MAP when compared with the DOX control group (P<0.01). The DBP and MAP of the DOX + 1.5 g/kg CS group were significantly increased when compared with the DOX control group (P<0.05 and P<0.01). Furthermore, when compared with the normal control group, rat SBP and MAP were significantly lower in CS control group (P<0.05; Fig. 1).
Figure 1.
Effect of fermented CS on BP in DOX-treated rats. (A) SBP, (B) DBP and (C) MAP were recorded in rats following treatment with DOX alone or in combination with 0.05 g/kg captopril, or with 0.75, 1.5 or 3 g/kg CS. Data are presented as the mean ± standard deviation. #P<0.05 and ##P<0.01 vs. the normal control group; *P<0.05 and **P<0.01 vs. the DOX control group. CS, Cordyceps sinensis; DOX, doxorubicin; BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure.
Evaluation of ECG
The DOX control group exhibited a significantly reduced heart rate, and a prolonged QRS complex and Sα-T segment, when compared with the normal control group (P<0.01). When compared with the DOX control group, rats co-treated with captopril had significantly increased heart rates (P<0.05), and decreased QRS complex and Sα-T segment durations (P<0.05 and P<0.01). In addition, rats co-treated with 1.5 g/kg CS had decreased Sα-T segment durations (P<0.05), and rats co-treated with 3 g/kg CS showed markedly shorter QRS complex and Sα-T segment durations (P<0.05 and P<0.01). However, no differences were observed for all ECG measurements the between normal control and CS control groups (Fig. 2).
Figure 2.
Effect of fermented CS (0.75, 1.5 or 3 g/kg) on ECG parameters in DOX-treated rats. (A) Heart rate, (B) the duration of the QRS complexes and the (C) Sα-T segment were measured via ECG procedures in order to assess DOX-induced cardiotoxicity. Data are presented as the mean ± standard deviation. ##P<0.01 vs. the normal control group; *P<0.05 and **P<0.01 vs. the DOX control group. CS, Cordyceps sinensis; ECG, electrocardiography; DOX, doxorubicin.
Serum activities of CK, LDH, and AST
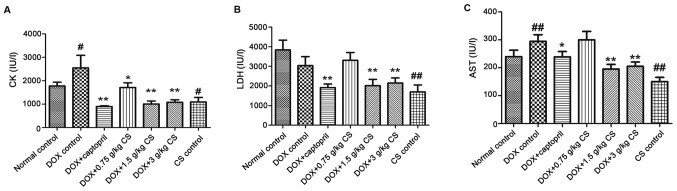
The activities of CK and AST in the DOX control group were significantly increased when compared with the normal control group (P<0.05 and P<0.01). The activities of CK, LDH and AST in the DOX + captopril or CS (1.5 and 3 g/kg) groups were markedly lower than those of the DOX control group (P<0.05 and P<0.01). In addition, CK activity was significantly decreased when comparing the DOX + captopril or CS (0.75 g/kg) groups with the DOX control group (P<0.05). When compared with normal control group, rats in CS control group exhibited a significant decrease in CK, LDH and AST activities (P<0.05 and P<0.01; Fig. 3).
Figure 3.
Effect of fermented CS on the activities of CK, LDH and AST in the serum of DOX-treated rats. Blood samples were collected from rats treated with DOX alone, or in combination with 0.05 g/kg captopril, or with 0.75, 1.5 or 3 g/kg CS in order to measure the levels of (A) CK, (B) LDH, and (C) AST in rat serum. Data are presented as the mean ± standard deviation. #P<0.05 and ##P<0.01 vs. the normal control group; *P<0.05 and **P<0.01 vs. the DOX control group. CS, Cordyceps sinensis; DOX, doxorubicin; CK, creatine kinase; LDH, lactate dehydrogenase; AST, aspartate aminotransferase.
Histopathological analysis
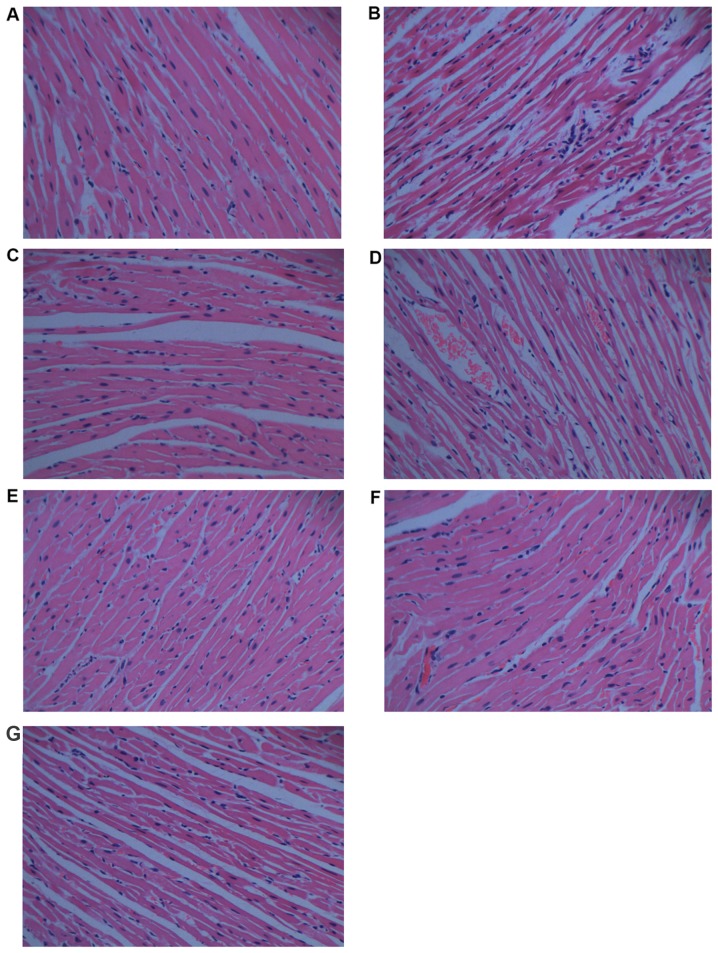
Histopathological analysis is shown in Fig. 4. When compared with the normal control group (Fig. 4A), the histological section of the cardiac tissue in rats treated with DOX (Fig. 4B) shows widespread vacuolization, marked myocardial degeneration, and some necrotic cardiomyocytes. DOX treatment with 0.75 g/kg CS (Fig. 4D) and with 1.5 g/kg CS (Fig. 4E) also induced vacuolization and moderate myocardial degeneration. The DOX + captopril group (Fig. 4C) and DOX + 3 g/kg CS group (Fig. 4F) presented only moderate vacuolization. In addition, no marked differences were observed in the CS control group (Fig. 4G).
Figure 4.
Effect of fermented CS on histopathology in DOX-treated rats. Cardiac tissues were collected and stained with hematoxylin and eosin for histopathologic analysis (magnification, ×400). (A) Normal control group, (B) doxorubicin control group, (C) DOX plus 0.05 g/kg captopril group, (D) DOX plus 0.75 g/kg CS group, (E) DOX plus 1.5 g/kg CS group, (F) DOX plus 3 g/kg CS group and (G) CS control group. CS, Cordyceps sinensis; DOX, doxorubicin.
Serum BNP content
The BNP content of the DOX control group was significantly higher than that of the normal control group (P<0.01). Co-treatment with DOX and captopril or CS (0.75, 1.5 and 3 g/kg) significantly reduced the BNP content when compared with DOX treatment alone (P<0.01). No marked difference in BNP content was observed between the normal control and CS control groups (Fig. 5).
Figure 5.
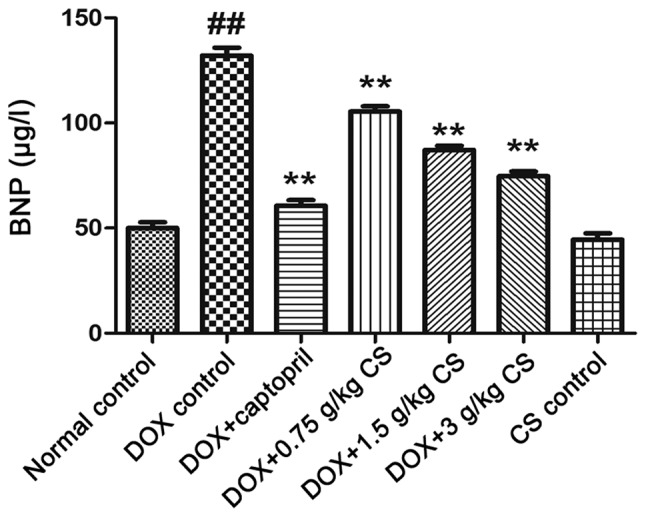
Effect of fermented Cordyceps sinensis on serum BNP content in DOX-treated rats. Blood samples were collected from rom rats treated with DOX alone, or in combination with 0.05 g/kg captopril, or with 0.75, 1.5 or 3 g/kg CS in order to measure the levels of BNP in rat serum via ELISA. Data are presented as the mean ± standard deviation. ##P<0.01 vs. the normal control group; **P<0.01 vs. the DOX control group. CS, Cordyceps sinensis; DOX, doxorubicin; BNP, brain natriuretic peptide.
Serum activities of T-SOD and GSH-Px, and MDA content
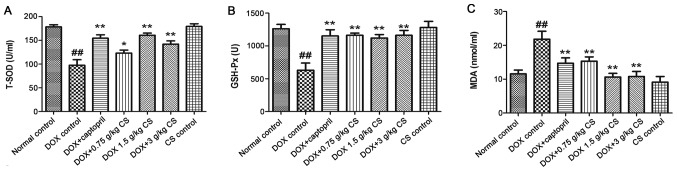
The DOX control treated rats exhibited significantly decreased T-SOD and GSH-Px activities, and increased MDA content when compared with the normal control (P<0.01). In rats co-treated with captopril or CS (0.75, 1.5 and 3 g/kg), the T-SOD and GSH-Px activities were increased, and MDA content was significantly decreased, when compared with those observed in the DOX control group (P<0.05 and P<0.01). There were no marked differences in the serum activities of T-SOD and GSH-Px, and MDA content between the normal control and CS control groups (Fig. 6).
Figure 6.
Effect of fermented CS on T-SOD and GSH-Px activities, and MDA content in DOX-treated rats. Serum was recovered from blood samples in order to measure (A) T-SOD and (B) GSH-Px activities, and (C) MDA content in rats treated with DOX alone, or in combination with 0.05 g/kg captopril, or with 0.75, 1.5 or 3 g/kg CS. Data are presented as the mean ± standard deviation. ##P<0.01 vs. the normal control group; *P<0.05 and **P<0.01 vs. the DOX control group. CS, Cordyceps sinensis; DOX, doxorubicin; T-SOD, total superoxide dismutase; GSH-Px, glutathione peroxidase; MDA, malondialdehyde.
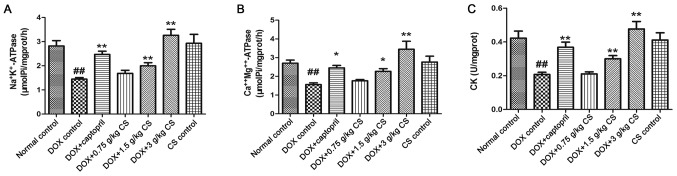
Activities of Na+K+-ATPase, Ca2+Mg2+-ATPase and CK in myocardial mitochondria
In the DOX control group, the rats exhibited significantly reduced mitochondrial activities of Na+K+-ATPase, Ca2+Mg2+-ATPase and CK (P<0.01). When compared with the DOX control group, the mitochondrial activities of Na+K+-ATPase, Ca2+Mg2+-ATPase and CK in the DOX + captopril or CS (1.5 and 3 g/kg) groups were significantly increased (P<0.05 and P<0.01). However, no marked differences in CK or ATPase activities were observed between the normal control and CS control groups (Fig. 7).
Figure 7.
Effect of fermented CS on the mitochondrial activities of ATPases and CK in DOX-treated rats. Mitochondria were recovered from cardiac tissues by differential centrifugation in order to measure the activities of mitochondrial (A) Na+K+-ATPase, (B) Ca2+Mg2+-ATPase and (C) CK. Data are presented as the mean ± standard deviation. ##P<0.01 vs. the normal control group; *P<0.05 and **P<0.01 vs. the DOX control group. CS, Cordyceps sinensis; DOX, doxorubicin; ATP, adenosine-triphosphate; CK, creatine kinase.
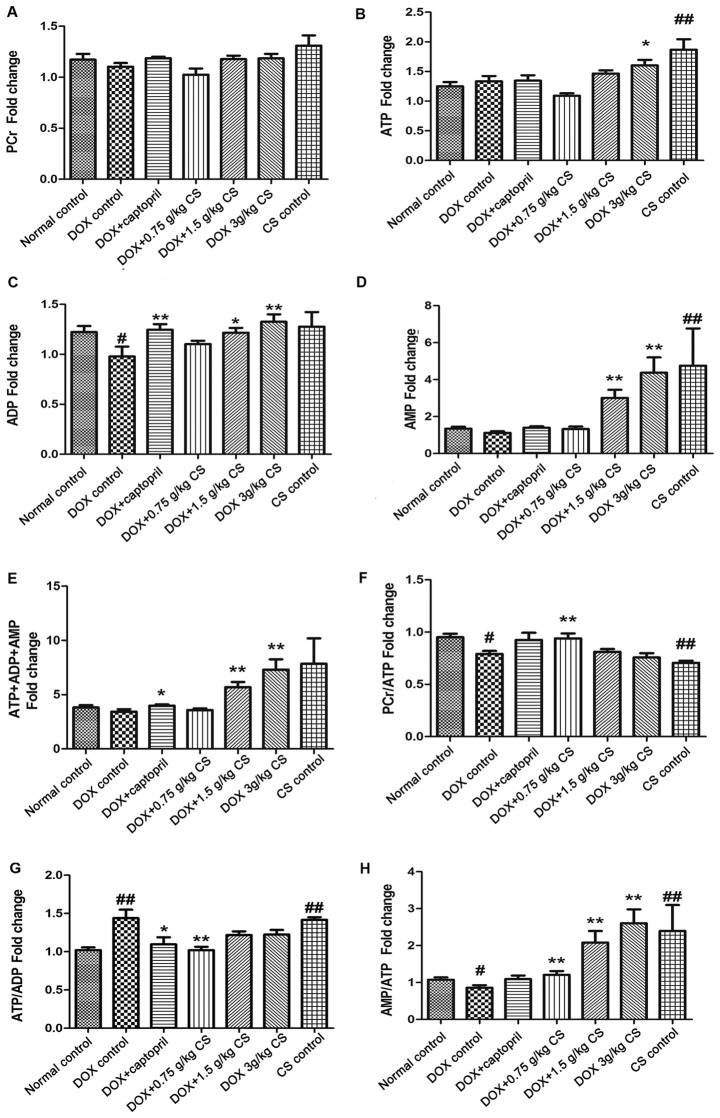
Adenine nucleotides (ATP, ADP and AMP) and PCr contents
In the DOX control group, the ADP content and the PCr/ATP and AMP/ATP ratios were significantly reduced (P<0.05), and the ATP/ADP ratio was significantly increased, when compared with the normal control group (P<0.01). When compared with the DOX control group, rats co-treated with captopril presented significantly increased ADP and total adenine nucleotides (ATP+ADP+AMP fold-change) contents (P<0.05 and P<0.01), and a decreased ATP/ADP ratio (P<0.05). Rats co-treated with 0.75 g/kg CS exhibited increased PCr/ATP and AMP/ATP ratios (P<0.05) and a reduced ATP/ADP ratio (P<0.01). Co-treatment with 1.5 g/kg CS increased ADP, AMP and total adenine nucleotides content, and the AMP/ATP ratio (P<0.05 and P<0.01). In addition, DOX + 3 g/kg CS increased the ATP, ADP, AMP and total adenine nucleotides contents, and the AMP/ATP ratio (P<0.05 and P<0.01). When compared with the normal control group, rats in the CS control group had significantly increased ATP and AMP contents, and ATP/ADP and AMP/ATP ratios; however, the PCr/ATP ratio was significantly decreased (P<0.01; Fig. 8). No differences were observed across the groups in PCr content.
Figure 8.
Effect of fermented CS on adenine nucleotides and PCr contents in DOX-treated rats. Cardiac tissues were collected from DOX treated rats to measure the (A) PCr, (B) ATP, (C) ADP, (D) AMP and (E) ATP+ADP+AMP (total adenine nucleotides) contents, and the (F) PCr/ATP ratio, (G) ATP/ADP ratio and (H) AMP/ATP ratio via high-performance liquid chromatography. The contents of adenine nucleotides and PCr in each group were expressed as the fold change of the normal control group. Data are presented as the mean ± standard deviation. #P<0.05 and ##P<0.01 vs. the normal control group; *P<0.05 and **P<0.01 vs. the DOX control group. CS, Cordyceps sinensis; DOX, doxorubicin; PCr, phosphocreatine; ATP adenosine triphosphate; ADP, adenosine diphosphate; AMP, adenosine monophosphate.
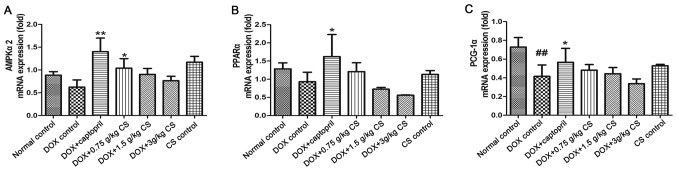
mRNA expression of AMPKα2, PPARα and PCG-1α
When compared to the normal control group, PCG-1α mRNA expression was significantly reduced in the DOX control treated rats (P<0.01). Rats co-treated with captopril had significantly increased AMPKα2, PPARα and PCG-1α mRNA expression when compared with the levels presented by the DOX control group (P<0.05 and P<0.01). In the DOX + 0.75 g/kg CS treatment group, the AMPKα2 mRNA expression was significantly greater than that of the DOX control group (P<0.05). However, no marked difference in mRNA expression was observed between the normal control and CS control groups (Fig. 9).
Figure 9.
Effect of fermented CS on the mRNA expressions of AMPKα2, PPARα and PCG-1α in the cardiac tissue of DOX-treated rats. The mRNA expression of (A) AMPKα2, (B) PPARα and (C) PCG-1α in cardiac tissues were determined by reverse transcription-quantitative polymerase chain reaction. The relative mRNA expression in each group was expressed as the fold change of the normal control group. Data are presented as the mean ± standard deviation. ##P<0.01 vs. the normal control group; *P<0.05 and **P<0.01 vs. the DOX control group. CS, Cordyceps sinensis; DOX, doxorubicin; AMPKα2, AMP-activated protein kinase catalytic subunit α2; PPARα, peroxisome proliferator-activated receptor α; PCG-1α, peroxisome proliferator-activated receptor γ coactivator 1α.
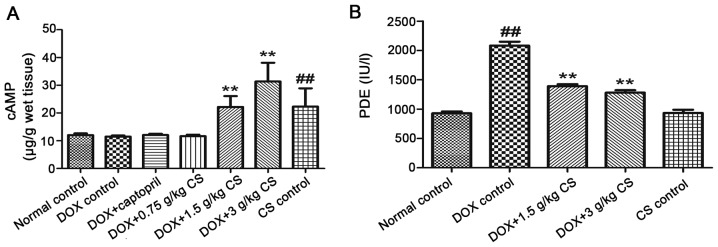
Myocardial cAMP contents and serum PDE activity
When compared with the normal control group, the DOX control treated rats exhibited an increase in the activity of serum PDEs (P<0.01). In CS (1.5 and 3 g/kg) treated rats, the content of myocardial cAMP and serum PDE activity were significantly lower than that observed in the DOX control group (P<0.01). In addition, when compared with the normal control group, rats in the CS control group had significantly increased myocardial cAMP content (P<0.01; Fig. 10).
Figure 10.
Effect of fermented CS on the myocardial cAMP contents and the activities of serum PDEs in DOX-treated rats. (A) Myocardial cAMP contents and (B) the activities of PDEs in the cardiac tissue of rats treated with DOX alone, or in combination with 0.05 g/kg captopril, or with 0.75, 1.5 or 3 g/kg CS. Data are presented as the mean ± standard deviation. ##P<0.01 vs. the normal control group; **P<0.01 vs. the DOX control group. CS, Cordyceps sinensis; DOX, doxorubicin; cAMP, cyclic adenosine monophosphate; PDEs, phosphodiesterases.
Discussion
DOX-induced cumulative cardiotoxicity is the greatest problem to overcome in order to improve clinical application. DOX can induce cardiac and systemic toxicity (17), while the cardiomyopathy induced by DOX serves a key role in the mortality of experimental animals following treatment with DOX (18,19). The experimental model used in the present study has been reported previously (19). CS, a TCM, has a variety of pharmacological effects. A previous study demonstrated that the extracts from the mycelia of CS have cardiovascular protective and antioxidant activities (20). It has also been demonstrated that oral administration of CS significantly attenuated liver and cardiac-associated injuries in rats with anti-chronic kidney disease (21). In the present study, rats were treated with a cumulative dose of 15 mg/kg of DOX to mimic the DOX-induced cumulative cardiotoxicity observed in clinical treatments. This dose is above the threshold at which doxorubicin cardiomyopathy is expected to clinically occur (14). It was observed that 40% of the rats in the DOX control group, succumbed to treatment prior to the termination of the experiment, which is similar to the results of Kelishomi et al (17). There was quite a low mortality in DOX + 3 g/kg CS treated animals, and none of rats succumbed in the DOX + 1.5 g/kg CS treated. This result indicated that treatment with fermented CS may enhance the survival of DOX-treated rats. In addition, a significant decrease in BW was observed in the DOX control group. This reduction may be attributable to an inhibition of protein synthesis, mucositis and a reduction in food intake due to DOX treatment (18).
DOX-induced cardiomyopathy is a transition between myocardial hypertrophy and heart failure (22). It is a severe adverse reaction of DOX, and is usually accompanied by myocardial hypertrophy. In clinical treatments, myocardial hypertrophy is associated with mortality in patients (23). Myocardial hypertrophy is primarily evaluated by measuring HWI and LVWI in animals (24). In the present study, an increase in HWI and LVWI was observed following DOX treatment which demonstrated that myocardial hypertrophy is induced by DOX; this result is in accordance with a previous study involving DOX-treated rats (22). In the DOX + 3 g/kg CS group, the increase in the HWI and LVWI was attenuated. This result indicated that a high dose of fermented CS may contribute to the inhibition of myocardial hypertrophy, potentially leading to the attenuation of DOX-induced cardiomyopathy.
Systolic and diastolic functioning of heart are closely associated with cardiac dysfunction. Previous studies have reported that DOX treatment induced diastolic dysfunction, leading to alterations in systolic function (25,26). These alterations included a reduction in BP. In the present study, a significant decrease in SBP, DBP and MAP was observed in DOX-treated rats. This decrease is similar to results observed previously (27), and is potentially caused by DOX-induced myofibril disruption (28). Rats treated with DOX + 1.5 g/kg CS had significantly elevated DBP and MAP, suggesting that fermented CS may improve systolic and diastolic function, and may have a potential role in therapies for DOX-induced cardiac dysfunction.
ECGs can reflect the electrical activity of the heart and identify damage to the myocardium in specific areas. It is also one of the most classic methods applied to diagnose heart injuries (29). Larsen et al (30) reported that ECG analysis can be used to evaluate DOX-induced cardiotoxicity in clinical practice. According to previous studies, the duration of the QRS complexes and Sα-T segment are the most reliable ECG parameters in the assessment of DOX-induced cardiotoxicity in rats (14,31). The duration of the transmembrane action potential is easily affected by DOX treatment, thus, all ECG alterations are accompanied by a prolongation of action potential duration (31). In the present study, the prolongation of the duration of the QRS complexes and Sα-T segment, and a reduction in heart rate, were observed following DOX treatment. This result is in agreement with previous research (14). In rats treated with 3 g/kg CS, there was a decrease in the prolongation of QRS complexes and Sα-T segment, and rats treated with 1.5 g/kg CS also exhibited a reduction in the prolongation of the Sα-T segment. These results demonstrated that fermented CS may be able to attenuate DOX-induced cardiotoxicity.
The cardiac enzymes, CK, LDH and AST, are abundant in cardiac muscle, particularly CK and LDH. In clinical settings, an elevation of cardiac enzymes in serum is considered a vital biomarker of myocardial damage, indicating a number of cardiac diseases, such as myocarditis and myocardial infarction (32). Detecting the activities of these enzymes in serum is essential for assessing myocardial damage. DOX treatment can increase the activities of cardiac enzymes in serum during clinical therapy (33). The increase in serum cardiac enzymes in DOX-treated rats, due to DOX-induced cardiac injury, has been reported previously (34). Histological changes also serve an important role in evaluating DOX cardiotoxicity. In the present study, the increase in CK and AST in the serum and the microscopic findings indicated that DOX-induced myocardial damage. In the majority of the CS treated animals, the secretion of LDH, AST and CK, and the histological changes observed (including vacuolization, myocardial degeneration and necrotic cardiomyocytes) were significantly attenuated, indicating that fermented CS may be able to inhibit DOX-induced myocardial necrosis, leading to cardiac protection from DOX-induced myocardial damage.
As a sensitive biomarker of heart failure, BNP is secreted by cardiomyocytes and is frequently used in emergency settings (35). During heart failure, a large quantity of BNP is released into the blood; due to this, it can be used for diagnosing heart failure induced by DOX (36). In the present study, DOX treatment significantly increased serum BNP, thereby demonstrating that acute heart failure may be caused by DOX. Rats treated with CS exhibited a decrease in serum BNP, indicating that fermented CS may be able to protect the heart from DOX-induced cardiotoxicity and acute heart failure.
OS, the basis of a number of diseases, is considered to be a direct factor of many types of cardiac damage (37), such as cardiomyopathy and cardiac dysfunction. It is generally believed that OS serves a vital role in the mechanism underlying DOX-induced cardiotoxicity (6,27). In some previous studies, DOX promoted the production of ROS and MDA (6), and inhibited the activities of SOD and GSH-Px (6), resulting in damage to myocardial mitochondria (38). As a final product of lipoperoxidation, MDA is a reliable biomarker for evaluating the degree of oxidative damage (39). SOD and GSH-Px are involved in a critical defense mechanism that protects the body from the harmful effects of ROS (40). Thus, the degree of OS is assessed by measuring the activities of T-SOD and GSH-Px, and MDA content. In the present study, the decrease in the activities of T-SOD and GSH-Px, and the increase in MDA content demonstrated that DOX treatment induced OS. An elevation in the activities of T-SOD and GSH-Px, and a reduction in MDA content were observed in the DOX + CS (0.75, 1.5 and 3 g/kg) groups. These results indicated that fermented CS may significantly inhibit the oxidative damage caused by treatment with DOX, particularly by improving the activities of antioxidant enzymes. It can be speculated that the anti-OS effect of fermented CS may serve an important role in protecting the heart against DOX-induced cardiotoxicity.
ATPases, containing Na+K+-ATPase and Ca2+Mg2+-ATPase, are primarily found in mitochondria. These enzymes can maintain the cell membrane potential, catalyze the decomposition of ATP into ADP and release energy (41). They are also important for muscle tissues, such as cardiac muscle, as a decline in mitochondrial ATPases can lead to energy impairment. Mitochondrial CK is a modulator of the energy reservoir, that converts creatine to phosphocreatine (41). A reduction in mitochondrial CK activity can affect energy metabolism and the electron transport chain (42). Cardiac energy impairment is one of the main characteristics of DOX-induced mitochondrial ultrastructural damage (1). In the present study, the reduction in the activities of mitochondrial ATPases and CK demonstrated that DOX treatment induced mitochondrial ultrastructural damage in the heart, which is in agreement with a previous study (22). Rats treated with CS exhibited an increase in the mitochondrial activities of ATPases and CK. This result indicated that fermented CS may significantly inhibit DOX-induced mitochondrial ultrastructural damage, and may have a positive effect in therapies for cardiac energy impairment induced by DOX as well as other causes.
Cardiac energy metabolism is associated with a number of cardiac diseases, such as heart failure and heart remodeling. Due to the high energy requirements, a large amount of ATP is transported to cardiac muscles every day (43). ATP is predominantly produced by carbohydrate oxidation and fatty acid (FA) oxidation from ADP or AMP, as well as other donors. Thus, total adenine nucleotides (ATP, ADP and AMP) are important in energy metabolism (44). In the process of cardiac energy metabolism, FAs are the primary substrates that provide ATP under normal conditions; however, glucose is frequently used during heart failure (43). The ATP/ADP ratio serves an important role in indicating the balance of FA and glucose utilization. During heart failure, mitochondrial damage frequently leads to a low production of mitochondrial ATP and a decrease in the ATP/ADP ratio that favors enhanced glycolysis (45). However, in a previous study investigating heart failure, the ATP/ADP ratio was observed to be increased (46). The decrease in glucose utilization is potentially due to an impairment of the expression of associated genes (47). PCr is able to donate phosphate groups to ADP to form ATP under anaerobic conditions. By contrast, excess ATP can be used to produce PCr from creatine; a reduction in PCr will accelerate the depletion of ATP (48), particularly during early heart failure. Thus, the PCr/ATP ratio is a key feature used to determine the extent of energy impairment. AMPK is mainly regulated by AMP, and an increase in the AMP/ATP ratio can activate the expression of AMPK (49). In some previous studies, DOX induced a number of alterations in cardiac energy metabolism (1,22,42). These alterations mainly included a reduction in the PCr/ATP ratio and a depletion of total adenine nucleotides. In the present study, an increase in the ATP/ADP ratio of DOX-treated rats was observed, similar to the results reported by Amorim et al (46). A reduction in ADP, the PCr/ATP ratio and the AMP/ATP ratio were also identified, demonstrating that DOX treatment induced cardiac energy impairment. In addition, CS control rats exhibited an increase in the AMP/ATP and ATP/ADP ratios, which indicated that fermented CS may potentially increase AMPK expression and FA utilization in the healthy heart. In CS treated animals, the levels of total adenine nucleotides, PCr/ATP ratio and AMP/ATP ratio were significantly improved, indicating that fermented CS may be able to attenuate the cardiac energy impairment induced by DOX; this improvement may also be associated with the AMPK signaling pathway.
AMPK, an important energy regulator and sensor, is a heterotrimer consisting of α, β and γ subunits; AMPKα2 is the main subunit in cardiac muscle. AMPK can increase energy production, maintain myocardial energy homeostasis and inhibit apoptosis, thus, a previous study suggested that it may be considered as a potential therapeutic target in a number of cardiac diseases including heart failure, myocardial infarction and cardiac ischemia (50). PPARα, a major regulator of lipid metabolism, is predominantly found in the liver, followed by the heart and kidneys (51). PPARα can promote the utilization, catabolism and uptake of FAs; a reduction in cardiac PPARα serves an important role in decreasing FA utilization (52). The expression of PPARα is dependent on AMPK (53). Agonists of PPARα are able to activate AMPK in order to increase energy production, such as via fatty acid oxidation (53). PCG-1α, a regulator of energy metabolism and mitochondrial function (54), can activate oxidative phosphorylation. The gene silencing of PCG-1α will inhibit oxidative phosphorylation, downregulate the mRNA expression of mitochondria and accelerate heart failure (55). Previous studies have reported that DOX induces a reduction in the genetic expression of AMPKα2, PPARα and PCG-1α, leading to an impairment of energy metabolism (4,56). In the present study, a significant decrease in PCG-1α mRNA expression indicated that DOX treatment induced an impairment in energy metabolism; DOX treatment also decreased the mRNA expression of AMPKα2 and PPARα to certain extent. In DOX + 0.75 g/kg CS group, an increase in AMPKα2 mRNA expression was observed, while the mRNA expression of PPARα and PCG-1α was marginally improved. These results revealed that fermented CS may potentially contribute towards the regulation of the AMPK signaling pathway. In addition, fermented CS may improve cardiac energy metabolism, as indicated by a significant increase in the mitochondrial capacities of ATPases combined with improved total adenine nucleotides contents and PCr/ATP ratio. It may also be involved in the regulation of the AMPK signaling pathway.
cAMP, a second messenger derived from ATP, can activate protein kinases, and regulate glucagon and adrenaline, thereby improving energy metabolism, and preventing myocardial ischemia and myocardial damage (57). The cAMP signaling pathway serves an important role in a number of biological processes, and is converted into AMP by PDE enzymes (58). PDEs include a group of enzymes that dephosphorylate cAMP and cyclic guanosine monophosphate (cGMP) (59). PDEs are important regulators of these second messenger molecules; inhibition of PDEs can enhance the effects of biological processes mediated by cGMP or cAMP (60). Therefore, PDEs and inhibitors of PDEs are important in clinical therapies (61). PDE activities are known to be increased under specific conditions, such as during ischemia and spinal cord injury (61). In the present study, an increase in serum PDE activities was associated with DOX treatment in rats. Rats co-treated with CS exhibited a significant increase in myocardial cAMP content and a marked decrease in serum PDEs. CS control rats also presented a significant increase in myocardial cAMP content. These results revealed that fermented CS may be able to increase myocardial cAMP, potentially induced via the inhibition of PDE activities. This may be one of the effective targets of fermented CS, which is contributed to an improvement in energy metabolism and ameliorated heart failure. Additional studies are required to evaluate whether or not fermented CS is a PDE inhibitor.
In conclusion, fermented CS ameliorated the cardiotoxicity induced by DOX in rats. The results demonstrated that CS treatment was able to inhibit myocardial hypertrophy and myocardial damage, improve the antioxidant enzyme system and energy metabolism, and inhibit PDE activities. Its potential effects on the upregulation of the AMPK and cAMP signaling pathways were also revealed. Therefore, fermented CS may be a promising adjuvant of DOX for clinical application. It may also be a candidate for the prevention of a number of cardiac diseases. However, further studies are required to confirm the accurate targets of fermented CS in the AMPK and/or cAMP signaling pathways.
Acknowledgements
The present study was supported by the Shanghai University of Traditional Chinese Medicine Budget Project (grant no. 2014YSN07). The authors would also like to thank Professor Chang-Xun Chen (Department of Pharmacology, School of Pharmacy, Shanghai University of Traditional Chinese Medicine, Shanghai, China) for his helpful suggestions and guidance.
References
- 1.Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL. Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. 2012;52:1213–1225. doi: 10.1016/j.yjmcc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Carvalho C, Santos RX, Cardoso S, Correia S, Oliveira PJ, Santos MS, Moreira PI. Doxorubicin: The good, the bad and the ugly effect. Curr Med Chem. 2009;16:3267–3285. doi: 10.2174/092986709788803312. [DOI] [PubMed] [Google Scholar]
- 3.Parker MA, King V, Howard KP. Nuclear magnetic resonance study of doxorubicin binding to cardiolipin containing magnetically oriented phospholipid bilayers. Biochim Biophys Acta. 2001;1514:206–216. doi: 10.1016/S0005-2736(01)00371-6. [DOI] [PubMed] [Google Scholar]
- 4.Wu R, Gao JP, Wang HL, Gao Y, Wu Q, Cui XH. Effects of fermented Cordyceps sinensis on oxidative stress in doxorubicin treated rats. Pharmacogn Mag. 2015;11:724–731. doi: 10.4103/0973-1296.165562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tokarska-Schlattner M, Wallimann T, Schlattner U. Alterations in myocardial energy metabolism induced by the anti-cancer drug doxorubicin. C R Biol. 2006;329:657–668. doi: 10.1016/j.crvi.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Lebrecht D, Setzer B, Ketelsen UP, Haberstroh J, Walker UA. Time-dependent and tissue-specific accumulation of mtDNA and respiratory chain defects in chronic doxorubicin cardiomyopathy. Circulation. 2003;108:2423–2429. doi: 10.1161/01.CIR.0000093196.59829.DF. [DOI] [PubMed] [Google Scholar]
- 7.Li SP, Li P, Lai CM, Gong YX, Kan KK, Dong TT, Tsim KW, Wang YT. Simultaneous determination of ergosterol, nucleosides and their bases from natural and cultured Cordyceps by pressurised liquid extraction and high-performance liquid chromatography. J Chromatogr A. 2004;1036:239–243. doi: 10.1016/j.chroma.2004.02.080. [DOI] [PubMed] [Google Scholar]
- 8.Zhu JS, Halpern GM, Jones K. The scientific rediscovery of an ancient Chinese herbal medicine: Cordyceps sinensis: Part I. J Altern Complement Med. 1998;4:289–303. doi: 10.1089/acm.1998.4.429. [DOI] [PubMed] [Google Scholar]
- 9.Lo HC, Hsu TH, Tu ST, Lin KC. Anti-hyperglycemic activity of natural and fermented Cordyceps sinensis in rats with diabetes induced by nicotinamide and streptozotocin. Am J Chin Med. 2006;34:819–832. doi: 10.1142/S0192415X06004314. [DOI] [PubMed] [Google Scholar]
- 10.Ji J, Liu J, Liu H, Wang Y. Effects of fermented mushroom of cordyceps sinensis, rich in selenium, on uterine cervix cancer. Evid Based Complement Alternat Med. 2014;2014:173180. doi: 10.1155/2014/173180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin XX, Xie QM, Shen WH, Chen Y. Effects of fermented Cordyceps powder on pulmonary function in sensitized guinea pigs and airway inflammation in sensitized rats. Zhongguo Zhong Yao Za Zhi. 2001;26:622–625. (In Chinese) [PubMed] [Google Scholar]
- 12.National Pharmacopoeia Committee: Pharmacopoeia of the people's Republic of China. China Medical Science Press; Beijing: 2015. pp. 832–833. [Google Scholar]
- 13.Council NR. Guide for the care and use of laboratory animals. The National Academies Press; Washington, DC: 1996. [PubMed] [Google Scholar]
- 14.Xiao J, Sun GB, Sun B, Wu Y, He L, Wang X, Chen RC, Cao L, Ren XY, Sun XB. Kaempferol protects against doxorubicin-induced cardiotoxicity in vivo and in vitro. Toxicology. 2012;292:53–62. doi: 10.1016/j.tox.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 15.Danesi R, Del Tacca M, Soldani G. Measurement of the S alpha T segment as the most reliable electrocardiogram parameter for the assessment of adriamycin-induced cardiotoxicity in the rat. J Pharmacol Methods. 1986;16:251–259. doi: 10.1016/0160-5402(86)90046-X. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Kelishomi RB, Ejtemaeemehr S, Tavangar SM, Rahimian R, Mobarakeh JI, Dehpour AR. Morphine is protective against doxorubicin-induced cardiotoxicity in rat. Toxicology. 2008;243:96–104. doi: 10.1016/j.tox.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 18.Herman EH, Zhang J, Chadwick DP, Ferrans VJ. Comparison of the protective effects of amifostine and dexrazoxane against the toxicity of doxorubicin in spontaneously hypertensive rats. Cancer Chemother Pharmacol. 2000;45:329–334. doi: 10.1007/s002800050048. [DOI] [PubMed] [Google Scholar]
- 19.Rossi F, Filippelli W, Russo S, Filippelli A, Berrino L. Cardiotoxicity of doxorubicin: Effects of drugs inhibiting the release of vasoactive substances. Pharmacol Toxicol. 1994;75:99–107. doi: 10.1111/j.1600-0773.1994.tb00330.x. [DOI] [PubMed] [Google Scholar]
- 20.Yan XF, Zhang ZM, Yao HY, Guan Y, Zhu JP, Zhang LH, Jia YL, Wang RW. Cardiovascular protection and antioxidant activity of the extracts from the mycelia of Cordyceps sinensis act partially via adenosine receptors. Phytother Res. 2013;27:1597–1604. doi: 10.1002/ptr.4899. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Zhong F, Tang XL, Lian FL, Zhou Q, Guo SM, Liu JF, Sun P, Hao X, Lu Y, et al. Cordyceps sinensis protects against liver and heart injuries in a rat model of chronic kidney disease: A metabolomic analysis. Acta Pharmacol Sin. 2014;35:697–706. doi: 10.1038/aps.2013.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed LA, El-Maraghy SA. Nicorandil ameliorates mitochondrial dysfunction in doxorubicin-induced heart failure in rats: Possible mechanism of cardioprotection. Biochem Pharmacol. 2013;86:1301–1310. doi: 10.1016/j.bcp.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Schillaci G, Verdecchia P, Porcellati C, Cuccurullo O, Cosco C, Perticone F. Continuous relation between left ventricular mass and cardiovascular risk in essential hypertension. Hypertension. 2000;35:580–586. doi: 10.1161/01.HYP.35.2.580. [DOI] [PubMed] [Google Scholar]
- 24.Kuzman JA, O'Connell TD, Gerdes AM. Rapamycin prevents thyroid hormone-induced cardiac hypertrophy. Endocrinology. 2007;148:3477–3484. doi: 10.1210/en.2007-0099. [DOI] [PubMed] [Google Scholar]
- 25.Almeida AL, dos Santos Júnior EG. Subclinical ventricular dysfunction detected by speckle-tracking two years after use of anthracycline-reply. Arq Bras Cardiol. 2015;105:207. [PubMed] [Google Scholar]
- 26.Tassan-Mangina S, Codorean D, Metivier M, Costa B, Himberlin C, Jouannaud C, Blaise AM, Elaerts J, Nazeyrollas P. Tissue Doppler imaging and conventional echocardiography after anthracycline treatment in adults: Early and late alterations of left ventricular function during a prospective study. Eur J Echocardiogr. 2006;7:141–146. doi: 10.1016/j.euje.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Ozdogan K, Taskin E, Dursun N. Protective effect of carnosine on adriamycin-induced oxidative heart damage in rats. Anadolu Kardiyol Derg. 2011;11:3–10. doi: 10.5152/akd.2011.003. [DOI] [PubMed] [Google Scholar]
- 28.Weinberg LE, Singal PK. Refractory heart failure and age-related differences in adriamycin-induced myocardial changes in rats. Can J Physiol Pharmacol. 1987;65:1957–1965. doi: 10.1139/y87-305. [DOI] [PubMed] [Google Scholar]
- 29.Guo X, Xue M, Li CJ, Yang W, Wang SS, Ma ZJ, Zhang XN, Wang XY, Zhao R, Chang BC, Chen LM. Protective effects of triptolide on TLR4 mediated autoimmune and inflammatory response induced myocardial fibrosis in diabetic cardiomyopathy. J Ethnopharmacol. 2016;193:333–344. doi: 10.1016/j.jep.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 30.Larsen RL, Jakacki RI, Vetter VL, Meadows AT, Silber JH, Barber G. Electrocardiographic changes and arrhythmias after cancer therapy in children and young adults. Am J Cardiol. 1992;70:73–77. doi: 10.1016/0002-9149(92)91393-I. [DOI] [PubMed] [Google Scholar]
- 31.Villani F, Galimberti M, Monti E, Cova D, Lanza E, Rozza-Dionigi A, Favalli L, Poggi P. Effect of ICRF-187 pretreatment against doxorubicin-induced delayed cardiotoxicity in the rat. Toxicol Appl Pharmacol. 1990;102:292–299. doi: 10.1016/0041-008X(90)90028-S. [DOI] [PubMed] [Google Scholar]
- 32.Bagai A, Schulte PJ, Granger CB, Mahaffey KW, Christenson RH, Bell G, Lopes RD, Green CL, Lincoff AM, Armstrong PW, Roe MT. Prognostic implications of creatine kinase-MB measurements in ST-segment elevation myocardial infarction patients treated with primary percutaneous coronary intervention. Am Hear J. 2014;168(503–511):e2. doi: 10.1016/j.ahj.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Pongprot Y, Sittiwangkul R, Charoenkwan P, Silvilairat S. Use of cardiac markers for monitoring of doxorubixin-induced cardiotoxicity in children with cancer. J Pediatr Hematol Oncol. 2012;34:589–595. doi: 10.1097/MPH.0b013e31826faf44. [DOI] [PubMed] [Google Scholar]
- 34.Abdel-Raheem IT, Taye A, Abouzied MM. Cardioprotective effects of nicorandil, a mitochondrial potassium channel opener against doxorubicin-induced cardiotoxicity in rats. Basic Clin Pharmacol Toxicol. 2013;113:158–166. doi: 10.1111/bcpt.12078. [DOI] [PubMed] [Google Scholar]
- 35.Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, Clausell N, Diaz R, Fleg JL, Gordeev I, et al. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: A randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Hear J. 2011;162(966–972):e10. doi: 10.1016/j.ahj.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Chen Y, Zhang M, Tang Y, Xie Y, Huang X, Li Y. Doxorubicin induces sarcoplasmic reticulum calcium regulation dysfunction via the decrease of SERCA2 and phospholamban expressions in rats. Cell Biochem Biophys. 2014;70:1791–1798. doi: 10.1007/s12013-014-0130-2. [DOI] [PubMed] [Google Scholar]
- 37.Forgione MA, Cap A, Liao R, Moldovan NI, Eberhardt RT, Lim CC, Jones J, Goldschmidt-Clermont PJ, Loscalzo J. Heterozygous cellular glutathione peroxidase deficiency in the mouse: Abnormalities in vascular and cardiac function and structure. Circulation. 2002;106:1154–1158. doi: 10.1161/01.CIR.0000026820.87824.6A. [DOI] [PubMed] [Google Scholar]
- 38.Sharma M, Kishore K, Gupta SK, Joshi S, Arya DS. Cardioprotective potential of ocimum sanctum in isoproterenol induced myocardial infarction in rats. Mol Cell Biochem. 2001;225:75–83. doi: 10.1023/A:1012220908636. [DOI] [PubMed] [Google Scholar]
- 39.Traverso N, Menini S, Maineri EP, Patriarca S, Odetti P, Cottalasso D, Marinari UM, Pronzato MA. Malondialdehyde, a lipoperoxidation-derived aldehyde, can bring about secondary oxidative damage to proteins. J Gerontol A Biol Sci Med Sci. 2004;59:B890–B895. doi: 10.1093/gerona/59.9.B890. [DOI] [PubMed] [Google Scholar]
- 40.Anwar S, Khan MA, Sadaf A, Younus H. A structural study on the protection of glycation of superoxide dismutase by thymoquinone. Int J Biol Macromol. 2014;69:476–481. doi: 10.1016/j.ijbiomac.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Martin SS, Senior AE. Membrane adenosine triphosphatase activities in rat pancreas. Biochim Biophys Acta. 1980;602:401–418. doi: 10.1016/0005-2736(80)90320-X. [DOI] [PubMed] [Google Scholar]
- 42.Tokarska-Schlattner M, Zaugg M, Zuppinger C, Wallimann T, Schlattner U. New insights into doxorubicin-induced cardiotoxicity: The critical role of cellular energetics. J Mol Cell Cardiol. 2006;41:389–405. doi: 10.1016/j.yjmcc.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Azevedo PS, Minicucci MF, Santos PP, Paiva SA, Zornoff LA. Energy metabolism in cardiac remodeling and heart failure. Cardiol Rev. 2013;21:135–140. doi: 10.1097/CRD.0b013e318274956d. [DOI] [PubMed] [Google Scholar]
- 44.Brautbar N, Baczynski R, Carpenter C, Moser S, Geiger P, Finander P, Massry SG. Impaired energy metabolism in rat myocardium during phosphate depletion. Am J Physiol. 1982;242:F699–F704. doi: 10.1152/ajprenal.1982.242.6.F699. [DOI] [PubMed] [Google Scholar]
- 45.Maldonado EN, Lemasters JJ. ATP/ADP ratio, the missed connection between mitochondria and the Warburg effect. Mitochondrion. 2014;19:78–84. doi: 10.1016/j.mito.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amorim PA, Nguyen TD, Schwarzer M, Mohr FW, Schrepper A, Doenst T. Myocardial infarction in rats causes partial impairment in insulin response associated with reduced fatty acid oxidation and mitochondrial gene expression. J Thorac Cardiovasc Surg. 2010;140:1160–1167. doi: 10.1016/j.jtcvs.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 47.Kato T, Niizuma S, Inuzuka Y, Kawashima T, Okuda J, Tamaki Y, Iwanaga Y, Narazaki M, Matsuda T, Soga T, et al. Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure. Circ Hear Fail. 2010;3:420–430. doi: 10.1161/CIRCHEARTFAILURE.109.888479. [DOI] [PubMed] [Google Scholar]
- 48.Brennan JP, Southworth R, Medina RA, Davidson SM, Duchen MR, Shattock MJ. Mitochondrial uncoupling, with low concentration FCCP, induces ROS-dependent cardioprotection independent of KATP channel activation. Cardiovasc Res. 2006;72:313–321. doi: 10.1016/j.cardiores.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 49.Gowans GJ, Hardie DG. AMPK: A cellular energy sensor primarily regulated by AMP. Biochem Soc Trans. 2014;42:71–75. doi: 10.1042/BST20130244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao S, Li H, Feng XJ, Li M, Liu ZP, Cai Y, Lu J, Huang XY, Wang JJ, Li Q, et al. α-Enolase plays a catalytically independent role in doxorubicin-induced cardiomyocyte apoptosis and mitochondrial dysfunction. J Mol Cell Cardiol. 2015;79:92–103. doi: 10.1016/j.yjmcc.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 51.Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): Tissue distribution of PPAR-alpha, -beta and -gamma in the adult rat. Endocrinology. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 52.Karbowska J, Kochan Z, Smolenski RT. Peroxisome proliferator-activated receptor alpha is downregulated in the failing human heart. Cell Mol Biol Lett. 2003;8:49–53. [PubMed] [Google Scholar]
- 53.Chen WL, Chen YL, Chiang YM, Wang SG, Lee HM. Fenofibrate lowers lipid accumulation in myotubes by modulating the PPARα/AMPK/FoxO1/ATGL pathway. Biochem Pharmacol. 2012;84:522–531. doi: 10.1016/j.bcp.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 54.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 55.Finck BN, Kelly DP. PGC-1 coactivators: Inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang DX, Zhao HP, Pan CS, Liu YY, Wei XH, Yang XY, Chen YY, Fan JY, Wang CS, Han JY, Li PP. QiShenYiQi Pills, a compound chinese medicine, ameliorates doxorubicin-induced myocardial structure damage and cardiac dysfunction in rats. Evid Based Complement Altern Med. 2013;2013:480597. doi: 10.1155/2013/480597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perera RK, Nikolaev VO. Compartmentation of cAMP signalling in cardiomyocytes in health and disease. Acta Physiol (Oxf) 2013;207:650–662. doi: 10.1111/apha.12077. [DOI] [PubMed] [Google Scholar]
- 58.Meier S, Andressen KW, Aronsen JM, Sjaastad I, Hougen K, Skomedal T, Osnes JB, Qvigstad E, Levy FO, Moltzau LR. PDE3 inhibition by C-type natriuretic peptide-induced cGMP enhances cAMP-mediated signaling in both non-failing and failing hearts. Eur J Pharmacol. 2017;812:174–183. doi: 10.1016/j.ejphar.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 59.Levy FO. Cardiac PDEs and crosstalk between cAMP and cGMP signalling pathways in the regulation of contractility. Naunyn Schmiedebergs Arch Pharmacol. 2013;386:665–670. doi: 10.1007/s00210-013-0874-z. [DOI] [PubMed] [Google Scholar]
- 60.Guo L, Luo L, Ju R, Chen C, Zhu L, Li J, Yu X, Ye C, Zhang D. Carboxyamidotriazole: A novel inhibitor of both cAMP-phosphodiesterases and cGMP-phosphodiesterases. Eur J Pharmacol. 2015;746:14–21. doi: 10.1016/j.ejphar.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 61.Li LX, Cheng YF, Lin HB, Wang C, Xu JP, Zhang HT. Prevention of cerebral ischemia-induced memory deficits by inhibition of phosphodiesterase-4 in rats. Metab Brain Dis. 2011;26:37–47. doi: 10.1007/s11011-011-9235-0. [DOI] [PubMed] [Google Scholar]