Abstract
Alda-1, an aldehyde dehydrogenase 2 (ALDH2) agonist, has been demonstrated to reduce injury caused by acute myocardial infarction (MI) and ischemia/reperfusion. The present study aimed to investigate whether oral administration of Alda-1 improved long-term survival of rats with chronic heart failure (CHF) post-MI. MI model rats treated daily with Alda-1 exhibited an increase in 20-week survival rate compared with untreated MI rats. Alda-1 treatment decreased the heart weight/body weight ratio, collagen volume, left ventricular (LV) internal diameter at the end of diastole and LV internal diameter at the end of systole, while increasing LV ejection fraction with evident LV fractional shortening. Myocardial cell apoptosis index, the activity of caspase-3 and the expression of cleaved-caspase-3 were also reduced by Alda-1 treatment. The protective effects of Alda-1 were associated with reduced 4-hydroxynonenal accumulation. The results of the present study revealed that the long-term treatment with Alda-1 prevented the progression of ventricular remodeling and improved the long-term survival of rats with CHF post-MI.
Keywords: Alda-1, aldehyde dehydrogenase 2, myocardial infarction, long-term survival
Introduction
Coronary artery disease is a major problem worldwide account for the greatest proportion of cardiovascular diseases which cause 31.5% global deaths (1,2). With advances in the prevention, diagnosis and management of cardiovascular diseases, the mortality rate of acute myocardial infarction (AMI) has decreased. However, chronic heart failure (CHF) remains a major cause of morbidity and mortality in patients with post-infarcted hearts (3,4). In addition, treatments for CHF impose a heavy burden on society and monopolize numerous health care resources in industrialized countries (5). Therefore, the search for novel pharmacological approaches in the prevention and treatment of CHF is urgently needed.
Alcohol dehydrogenase 2 (ALDH2) is a member of the ALDH gene family and is a key enzyme in the metabolism of acetaldehyde and other toxic aldehydes (6). The cardioprotective role of ALDH2 in cardiac ischemic events was first reported by Chen et al (7). Subsequent studies have demonstrated that ALDH2 provides beneficial effects in alcoholic cardiomyopathy, ischemia-reperfusion (I/R) injury and heart failure (8–10). Knockout of ALDH2 was reported to exacerbate cardiac contractile dysfunction and promote apoptosis induced by endoplasmic reticulum stress induction, as manifested by the alterations in the ejection fraction and fractional shortening (11). Activation or overexpression of ALDH2 was demonstrated to protect against cardiac injury by diminishing AMI size, ameliorating cardiac dysfunction and preventing reperfusion arrhythmias (6,12,13).
Alda-1 is a selective agonist of ALDH2 (14), which increases productive substrate-enzyme interactions and protects ALDH2 from substrate-induced inactivation by binding near the exit of the substrate-binding tunnel (15). Previous studies have demonstrated that ALDH2 activation exhibits beneficial effects on I/R injury (9). In rats pre-treated with Alda-1 for 5 min in the left ventricle prior to ischemia, infarction damage was reduced by ~60% by clearing the toxic reactive aldehydes, such as 4-hydroxynonenal (4-HNE) the key mediator leading to oxidative stress (7). Additionally, in a rat model of MI, sustained treatment with Alda-1, either for 4 weeks starting at 24 h post-MI or for 6 weeks starting at 4 weeks following permanent MI, maintained mitochondrial bioenergetic status, prevented excessive oxidative stress and improved ventricular function and remodeling (10,13).
Improvement of long-term survival is the key goal of CHF drug therapy. Although certain therapeutic agents exhibited favorable effects on ventricular function and remodelling, they were associated with increased mortality rates. For example, the tumor necrosis factor antagonist etanercept is a cytokine inhibitor that is able to reverse ventricular remodeling over 3-months treatment; however, etanercept failed to demonstrate any long-term benefit in a 6-month long-term study (16,17). Peroxisome proliferator-activated receptor-γ (PPAR-γ) serves a prominent role in cardiac function, but the effects of PPAR-γ agonists in cardiac diseases remain controversial, as chronic PPAR-γ therapy may be deleterious (18). Systolic improvement therapy with digoxin, which reversed remodelling in dilated cardiomyopathy, was associated with a significant increase in mortality from all cardiac disease-associated causes among patients with atrial fibrillation as well as heart failure (19). The effects of long-term treatment with Alda-1 on CHF post-MI remain unclear. In the present study, Alda-1 treatment began 1 week following AMI and was sustained for 20 weeks. The effects were determined by investigating the mortality rate, cell apoptosis and collagen fiber formation, as well as toxic aldehyde clearance.
Materials and methods
Animals and surgical procedures
All experimental procedures involving animals were approved by the Animal Care and Use Committee at Southern Medical University (Guangzhou, China). A total of 66 specific-pathogen-free male Wistar rats (200–250 g, 7 weeks old) were obtained from the Experimental Animal Center of Southern Medical University after animal ethic approval (Guangzhou, China; Animal Quarantine Conformity Certificate no. 4402102052). The rats were maintained in a room with a 12-h light/dark cycle, constant temperature of 22–26°C, constant humidity of 40–60% and free access to tap water and food. To induce MI, left coronary artery ligation was performed as previously described (20). A total of ~20% of the rats (n=9) failed to survive the first week following ligation procedure owing to acute heart failure or malignant arrhythmia. All rats were examined by echocardiography 1-week post-MI surgery to eliminate unqualified rats that either did not develop sufficient MI (i.e. left ventricular ejection fraction >50% compared with the Sham group) or with severe complications. The rest of the successfully ligated rats were randomly assigned into two experimental groups: The MI group, in which MI was induced and rats were treated orally with 0.9% normal saline (1 ml/100 g/day), and the Alda-1-treated group, in which MI-induced rats were treated orally with Alda-1 (D43490, Merch Millipore, Darmstadt, Germany; 16 mg/kg/day) started from 1 week after MI surgery (14). Sham-operated rats served as the control group; they underwent surgery, but not left coronary artery ligation, and were treated orally with 0.9% normal saline (1 ml/100 g/day). Each group consisted of 18 rats and all animals underwent gavage administration for 20 weeks. All the procedures in this animal study were performed in accordance with the approval by the Institute of Animal Care and Use Committee of Southern Medical University. All the rats were weighed (body weight, BW), performed echocardiography and then euthanized with pentobarbitone sodium after 20 weeks treatment. Heart tissue was harvested, weighted (Heart weight, HW) and separated into two parts, which were snap froze in liquid nitrogen (stored at −80°C, for protein, enzyme and biomarker assay), and fixed in 10% neutral buffered formalin, embedded and cut into paraffin section afterwards (stored at room temperature for histopathology, immunohistochemistry and TUNEL apoptosis assay).
Transthoracic echocardiographic measurements
A non-invasive transthoracic echocardiography method was used to assess heart function at 1 week following surgery to exclude rats that failed in developing MI, and evaluate the morphology and function of the left ventricle at 20 week before rats' sacrifice as previously described (20).
Histological examination
The heart was fixed in 10% neutral-buffered formalin at room temperature for 48 h, embedded in paraffin and cut into 5 µm sections. The sections were subjected to hematoxylin & eosin (H&E) and Mallory's Trichrome staining, following standard procedures (21). All histopathological alterations were evaluated by two investigators that were blinded to the study. Five randomly selected fields from each section were examined at magnification, ×400 and analyzed using NIS-Elements F 3.2 software accompanied with microscope (Nikon ECLIPS Ti-S, Japan). In H&E staining paraffin sections, myocardial size was measured on the cross-section profile with idealized outline. In the Mallory's Trichrome staining paraffin sections, collagen volume fractions were semi-quantified with the quotient of the area of collagen divided by the total area occupied by heart tissue in each field.
ALDH2 enzymatic activity
ALDH2 Enzymatic Activity Assay kit (GenMed Scientifics Inc., Wilmington, DE, USA) was used to measure ALDH2 activity in tissue lysates, according to the manufacturer's protocol. Previously deep-frozen heart tissues (10 mg) were ground to a powder under liquid nitrogen and homogenized. The homogenates were sonicated on ice and then centrifuged at 10,000 × g for 30 min at 4°C. ALDH2 enzymatic activity was measured at 25°C in 1 ml reaction system containing 33 mM sodium pyrophosphate (pH 8.8), 0.8 mM NAD+, 15 µM propionaldehyde and 0.1 ml tissue homogenate. Reduced nicotinamide-adenine dinucleotide phosphate (NADH) production was determined spectrophotometrically by monitoring the alterations in absorbance intensity at 340 nm every 30 sec for 5 min. The ALDH2 reaction rates were expressed as µmol NADH/min/mg protein.
Immunohistochemistry
Immunohistochemical staining of 4-HNE and collagen types I and III was performed using the streptavidin peroxidase method. Polyclonal immunoglobulin G antibodies against 4-HNE (catalog no. ab46545, Abcam, Cambridge, UK), collagen type I (catalog no. ab34710, Abcam, Cambridge, UK) and collagen type III (catalog no. ab7778, Abcam, Cambridge, UK) were used at 1:100 dilution and incubated at 4°C overnight. Staining procedures were using StreptAvidin-Biotin Complex (SABC) Kit (SA1022, Boster Corporation, Wuhan, China) and DAB kit (AR1022, Boster Corporation, Wuhan, China) following the manufacturer's protocol. Nuclei were counterstained with H&E staining. Five randomly selected fields from each section were examined at magnification, ×400 and analyzed using NIS-Elements F 3.2 software accompanied with microscope (Nikon ECLIPS Ti-S, Japan). The positive contents were presented by the percentage of immunoreactive stained area, which were stained into brown. 4-HNE accumulation was calculated as the percentage of 4-HNE-positive stained cells/total cells.
Terminal deoxynucleotidyl-transferase-mediated dUTP nick end labeling (TUNEL) apoptosis assay
Apoptosis was detected using the TUNEL Detection kit (Roche Diagnostics GmbH, Mannheim, Germany), according to the manufacturer's protocol. The myocardium was stained with myosin-7 (MYH7; 1:200, cat. no. sc-168678, Santa Cruz Biotechnology, Inc., Dallas, Texas, USA) overnight at 4°C, and nuclei were stained with DAPI (0.5 µg/ml, cat. no. 10236276001, Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 5 min at room temperature. Fluorescence was detected by confocal microscopy. Whole cells with blue-colored nuclei were considered apoptotic. The results were semi-quantitatively scored by taking an average of the number of apoptotic cells per field at ×400 magnification; five fields were evaluated per tissue sample and cardiomyocyte apoptosis was represented as apoptosis index (AI), and calculated as follows: AI=number of TUNEL-positive cells/total number of cells.
Western blotting
Heart tissue (20 mg) protein samples were extracted and quantified, according to methods reported previously (22). The protein lysates (30 µg) were separated by 10% SDS-PAGE and electrotransferred onto polyvinylidene fluoride membranes and blocked with 5% nonfat milk at room temperature for 1 h. The membranes were incubated overnight with primary antibodies including anti-ALDH2 antibody (catalog no. 3221-1, Epitomics; Abcam), anti-4-HNE antibody (catalog no. ab46545; Abcam), anti-GAPDH catalog no. 5174, Cell Signaling Technology, Inc., Danvers, MA, USA) and anti-cleaved-caspase-3 (catalog no. 9661, Cell Signaling Technology, Inc., Danvers, MA, USA); all with dilution 1:2,000 at 4°C, followed by HRP-conjugated secondary antibodies (catalog no. 7074, Cell Signaling Technology, Inc., Danvers, MA, USA) with dilution 1:2,000 at room temperature for 1 h. Protein bands were visualized by Enhanced Chemiluminescence detection (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA). Images were captured and documented with the Image Station 2000MM CCD system (Kodak, Rochester, NY, USA).
Caspase-3 activity assay
A caspase-3 activity assay was performed using a colorimetric Caspase-3 Activity Assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), according to the manufacturer's protocol. Briefly, 200 µg tissue lysate was combined with 100 µl reaction buffer containing 5 µl caspase-3 substrate DEVD-pNA (4 mM), 1% NP-40, 20 mM Tris-HCl (pH 7.5), 137 mM n-acetyl-cysteine and 10% glycerol. The lysates were incubated at 37°C for 2 h in the dark and the absorbance was measured at a wavelength of 405 nm using a microplate reader, as previously described (23).
Serum B-type natriuretic peptide (BNP) concentration assay
The Rat BNP ELISA kit (cat. no. Ab108816, Abcam) was used to measure serum BNP, according to the manufacturer's protocol. Blood samples were collected from the abdominal aorta into plain blood collection tubes prior to the rats being sacrificed. Serum was separated from whole blood following clot formation and stored at −80°C. Briefly, serum samples and BNP standards were captured by a BNP specific antibody, which had been pre-coated onto the bottom of 96-well plates, and subsequently detected with a BNP specific biotinylated detection antibody, which was linked with a streptavidin-peroxidase conjugate. The chromogen substrate was catalysed by streptavidin-peroxidase to visualize colours into blue. The reactions were stopped and the absorbance was read on microplate reader at 450 nm wavelength to determine the final concentration based on the standard curve. Each sample was assayed with duplicates.
Statistical analysis
Data were presented as the mean ± standard deviation. Statistical analysis was performed using GraphPad Prism 7.0 (GraphPad Software, Inc., La Jolla, CA, USA). The statistical significance of each variable was estimated using one-way analysis of variance followed by a Bonferroni post hoc correction between all groups. Comparison of survival curves was performed by Kaplan-Meier and the Mantel-Cox log-rank test. P<0.05 was considered to indicate a statistically significant difference. Data of protein expression, enzyme activity, biomarker level and paraffin section staining are from a minimum of 3 independent experiments.
Results
Alda-1 treatment reduces CHF mortality in post-MI rats
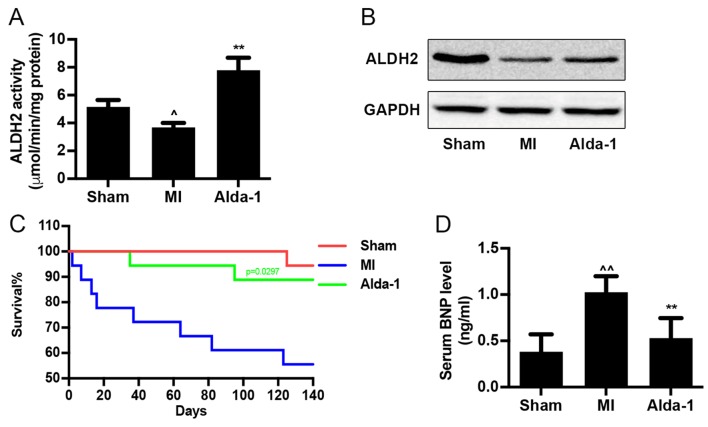
ALDH2 enzymatic activity was reduced in MI group (P<0.01; Fig. 1A), as well as ALDH2 expression (Fig. 1B). Alda-1 treatment significantly upregulated ALDH2 enzymatic activity (P<0.001; Fig. 1A), but did not affect ALDH2 protein expression (Fig. 1B) compared with the MI group. Survival curve analysis indicated that the mortality rates of the Sham group and MI group were 5.6 and 50%, respectively (Fig. 1C). Treatment with Alda-1 significantly improved the survival rate of chronic MI rats (16.6%; P=0.0297). In addition, results from the ELISA assay demonstrated that Alda-1 treatment significantly reduced BNP levels in the serum of MI rats (P<0.001; Fig. 1D).
Figure 1.
Long-term treatment with Alda-1 increases survival rate of CHF in post-MI rats. (A) ALDH2 activity in heart tissue from each group; 3 replicates of each sample, n=4/group. (B) ALDH2 protein expression levels were determined by western blot analysis using ALDH2 specific antibody; GAPDH was used as the loading control. (C) Kaplan-Meier survival curves and Mantel-Cox log rank test were used to examine the survival rates for rats in the Sham, MI and Alda-1 groups. Alda-1 treatment significantly improved 20-week survival rates; P=0.0297, Alda-1 vs. MI. Sham, n=17; MI, n=9; Alda-1, n=15. (D) BNP level in serum from each group; 3 replicates of each sample, n=4/group. ^P<0.01 vs. Sham, ^^P<0.001 vs. Sham, **P<0.001 vs. MI. ALDH2, alcohol dehydrogenase 2; BNP, B-type natriuretic protein; MI, myocardial infarction.
Alda-1 reduces heart size and improves cardiac function
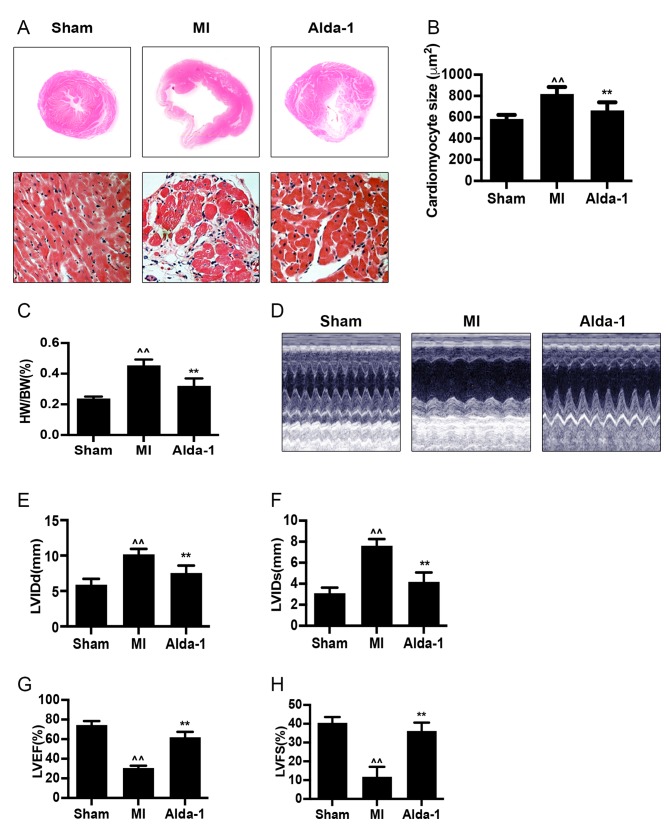
H&E staining of heart tissues demonstrated that the size of myocardial cells was increased (P<0.001; Fig. 2A and B) in the MI group. Compared with the Sham group, the heart weight/body weight (HW/BW) ratio of the MI group was significantly increased (P<0.001; Fig. 2C). The heart size of the chronic MI rats was detected by echocardiography in two-dimensional-guided M-mode of left ventricle. In MI group, visualization of wall movement weakening and dilated chamber; while in Alda-1 treatment group, changes induced by MI were reversed (Fig. 2D). The left ventricular dimension at the end of diastole (LVIDd) and left ventricular dimension at the end of systole (LVIDs) were significantly increased in MI group compared with the Sham group (P<0.001; Fig. 2E and F, respectively). In addition, the MI group exhibited significantly reduced left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS) compared with the Sham group (P<0.001; Fig. 2G and H, respectively). These results indicated that the ventricular remodeling model was successfully established, as previously reported (20). MI rats in the Alda-1 treatment group demonstrated a significantly lower HW/BW ratio and smaller cardiac myocytes compared with the MI group (P<0.001; Fig. 2C and D, respectively). Alda-1 treatment also significantly reduced LVIDd and LVIDs (P<0.001; Fig. 2E and F, respectively), whereas LVEF and LVFS were elevated (P<0.001; Fig. 2G and H, respectively) compared with the MI group.
Figure 2.
Long-term treatment with Alda-1 attenuates ventricular remodeling and improves cardiac function. (A) Representative H&E stained cross-section images of heart (top); representative H&E staining micrograph images of left ventricles in non-infarcted area in each group (bottom); magnification, ×400. (B) Myocardial size is summarized and demonstrated; n=4/group. (C) HW/BW ratios in each group. (D) Echocardiography was performed 20 weeks following treatment. Representative images of 2D-guided M-mode echocardiographic of the left ventricle in each group are exhibited. (E) Quantitative analysis of LVIDd. (F) Quantitative analysis of LVIDs. (G) Quantitative analysis of LVEF. (H) Quantitative analysis of LVFS. Sham, n=17; MI, n=9; Alda-1, n=15; ^^P<0.001 vs. Sham, **P<0.001 vs. MI. H&E, hematoxylin and eosin; HW/BW, heart weight/body weight; LVEF, left ventricular ejection fraction; LVFS, left ventricular fractional shortening; LVIDd, left ventricular dimension at end diastole; LVIDs, left ventricular dimension at end systole.
Alda-1 inhibits collagen formation in post-MI rats with CHF
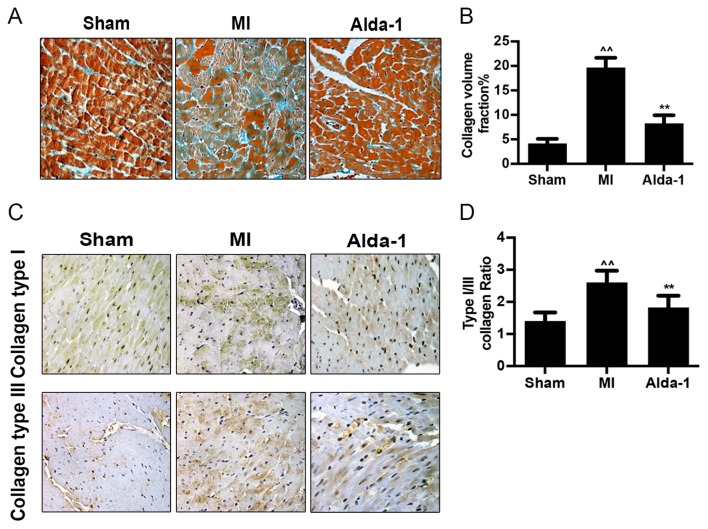
Mallory's trichrome staining demonstrated that collagen formation and collagen volume fraction were significantly increased in the MI group compared with the Sham group (P<0.001; Fig. 3A and B). Immunohistochemistry staining images illustrated that the expression of collagen types I and III in the MI group was elevated compared with expression in the Sham group (Fig. 3C). The increase of type I collagen exceeded that of type III, with an increased collagen type I/III ratio (P<0.001; Fig. 3D). Alda-1 treatment reduced collagen volume (P<0.001; Fig. 3B), collagen type I and type III expression (Fig. 3C) and collagen type I/III ratio (P<0.001; Fig. 3D), compared with untreated MI rats.
Figure 3.
Long-term treatment with Alda-1 inhibits collagen formation in chronic heart failure post-MI rats. (A) Representative Mallory's trichrome staining micrographs demonstrated collagen fibers in non-infracted area of left ventricles; magnification, ×400. (B) Quantitative analysis of fibrotic area; Mallory-positive stained area (light blue color) was normalized to total myocardial area; n=4/group. (C) Representative images of collagen type I and type III protein expression in each group. (D) The ratio of collagen type I/typeIII were summarized; n=4/group. ^^P<0.001 vs. Sham, **P<0.001 vs. MI. MI, myocardial infarction.
Alda-1 reduces myocyte apoptosis in post-MI rats with CHF
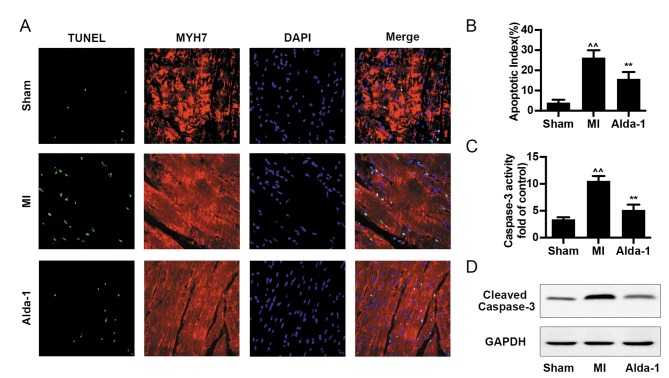
Cell apoptosis was analyzed by TUNEL assay, caspase-3 activity and cleaved-caspase-3 expression. The number of TUNEL-positive cells and the apoptotic index were increased in the MI group compared with the Sham group (P<0.001; Fig. 4A and B). Alda-1 treatment significantly reduced the levels cell apoptosis, including TUNEL-positive cell number and apoptotic index (P<0.001; Fig. 4A and B), caspase-3 activity (P<0.001; Fig. 4C) and cleaved-caspase-3 expression (Fig. 4D).
Figure 4.
Long-term treatment with Alda-1 reduces myocyte apoptosis in chronic heart failure post-MI rats. (A) Representative images of cardiomyocyte apoptosis by Terminal deoxynucleotidyl-transferase-mediated dUTP nick-end labeling assay in each group; myocytes were detected with anti-MYH7 antibody (red) and nuclei were counterstained with DAPI (blue). (B) Quantification of apoptosis from (A), expressed as percentage of apoptotic cells out of the total number of cardiac cells; n=4/group. (C) Caspase-3 activity in heart tissue from each group; 3 replicates of each sample, n=4/group. (D) Protein expression levels of cleaved-caspase-3 were determined by western blot analysis; GAPDH was used as the loading control. ^^P<0.001 vs. Sham, **P<0.001 vs. MI. MI, myocardial infarction; MYH7, myosin-7.
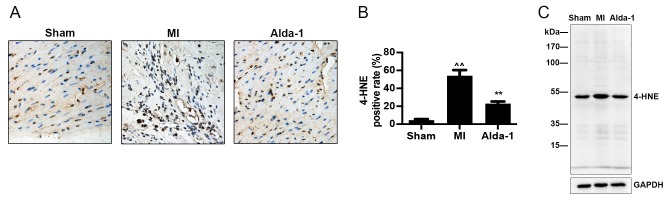
Alda-1 increases clearance of 4-HNE toxic aldehydes
Immunohistochemistry and western blotting demonstrated that 4-HNE modified protein expression was significantly increased in the MI group compared with the Sham group (P<0.001; Fig. 5A and B, respectively). The results also demonstrated that Alda-1 treatment reduced 4-HNE modified protein expression compared with the MI group (P<0.001; Fig. 5).
Figure 5.
Long-term treatment with Alda-1 increases toxic aldehydes 4-HNE clearance. (A) Accumulation of 4-HNE adducts in the Sham group, MI group, Alda-1 group were measured by immunochemistry and (B) quantification of 4-HNE accumulation was calculated as the percentage of 4-HNE-positive stained cells; n=4/group; ^^P<0.001 vs. Sham, **P<0.001 vs. MI. (C) Western blot representative image of 4-HNE modified proteins. 4-HNE, 4-hydroxynonenal; MI, myocardial infarction.
Discussion
In the present study, the effects of oral Alda-1 treatment on long-term survival and ventricular remodeling in post-MI model rats with CHF were investigated. The results demonstrated that Alda-1 treatment improved the long-term survival and ventricular remodeling in chronic MI rats. Ventricular remodelling following MI and the subsequent development into heart failure is a long pathological process. However, the association between ventricular remodeling and long-term survival is not consistent. Contemporary clinical drugs such as etanercept, digoxin and rosiglitazone exert preventive effects on ventricular remodelling without improvement of long-term survival (16–19). Therefore, the observations of long-term effect and its end point of mortality are also required in the pharmacological study of heart failure, other than general mechanism studies. Currently, the maximum length of Alda-1 treatment demonstrating improved ventricular function and remodelling is 6 weeks (10). The results of the present study showed the involvement of ALDH2 in a longer period (20 weeks), gave more powerful evidence of cardio protective effect of Alda-1.
Left ventricular hypertrophy, dilation and cavity distortion are the main features of ventricular remodeling (24). In the present study, cardiac structure was observed by echocardiography. MI model rats exhibited increased HW/BW ratio, LVIDs and LVIDd, and decreased LVEF and LVFS, which indicated that alteration of cardiac function in chronic MI promoted cardiac remodeling. MI rats treated with Alda-1 exhibited higher LVEF and LVFS and lower HW/BW ratio, LVIDd and LVIDs compared with untreated MI rats. These results indicated that the early activation of ALDH2, prior to pathological remodeling occurs, and extension of Alda-1 treatment course for 20 weeks or longer, may effectively prevent cardiac remodeling. In addition, the level of circulating BNP, a biomarker of cardiac hypertrophy and heart failure, was measured and the levels were consistent with the echocardiographic data, which suggested a favorable prognosis in MI rats receiving Alda-1 treatment.
Cardiac fibrosis, including fibroblast proliferation and the accumulation of extracellular matrix, serve a central and dynamic role in ventricular remodelling processes (25,26). Results from the present demonstrated increased collagen production in rats in the MI group was upregulated, along with an increased collagen type I to type III ratio; these effects were attenuated by Alda-1 treatment. These data demonstrated that long-term treatment with Alda-1 attenuated the processes mediating cardiac fibrosis in CHF post-MI model rats, which were in agreement with a previous study that reported Alda-1 treatment for 6 weeks in MI rats attenuated cardiac fibrosis (10).
Apoptosis serves a crucial role in I/R injury, cardiac remodeling and heart failure. Apoptosis progressively occurs from the first day following infarction (27,28). The loss of myocardial cells may lead to a decrease in cardiac function reserves in the surviving myocardium and results in heart failure (29). In the present study, it was demonstrated that myocardial apoptosis was at a high level at 20 weeks following MI. Alda-1 treatment decreased the apoptosis of myocardial cells, which indicated potential anti-apoptotic action is a key protective mechanism of Alda-1 against CHF.
In the present study, it was demonstrated that ALDH2 enzymatic activity was suppressed in the MI group and the mortality rate was increased with the elevated level of the reactive aldehydes 4-HNE, and these effects were dramatically attenuated by treatment with Alda-1. 4-HNE staining positive rates and TUNEL staining positive cells could be demonstrated in the heart following MI, which suggested that the overload of aldehydes may damage cardiac function and aggravate cardiac remodeling, as the toxic aldehydes may induce apoptosis in remote areas of the myocardium. ALDH2 enzymatic activity was suppressed when the heart was in ischemic conditions and this low ALDH2 activity accelerated 4-HNE accumulation and cardiac remodeling. The results coincided with previous study which indicated 4-HNE also directly inhibits ALDH2, forming a feedback loop (30). Alda-1 protects the heart by increasing enzymatic activity and indirectly clearing aldehydes. In addition, a previous study demonstrated that Alda-1 may prevent 4-HNE-induced inactivation of ALDH2 (7). However, in the present results, the activator of ALDH2 only affected ALDH2 enzymatic activity, but not ALDH2 expression.
In summary, to the best of our knowledge, the present study demonstrated for the first time that Alda-1 improves long-term survival and cardiac function, attenuates heart remodeling, apoptosis and fibrosis in rats with permanent MI. The results of the present suggest that Alda-1 may be a novel therapeutic agent for CHF post MI.
Acknowledgements
All the authors would like to acknowledge the contribution of Senior Laboratory Technician Yurao Chen as a valued mentor of animal study and histology in this research.
Funding
The present study was supported by The National Natural Science Foundation of China (grant nos. 81673805, 81373575, 81673949 and 81601779), The Guangdong Natural Science Foundation (grant nos. 2014A030313495 and 2014A030310210), The Science and Technology Planning Project of Guangdong Province (grant nos. 2014A020221013 and 2014A020221059), The Science Technology and Innovation Committee of Shenzhen (grant no. JCYJ20150630164505508), Planned Science Technology Project of Guangzhou (201804010064), Medical Science and Technology Research Project of Guangdong Province (grant no. A2016029) and The Traditional Chinese Medicine Bureau of Guangdong Province (grant no. 20161260 and 20162002).
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Authors' contributions
YZ and BL provided the concept, administration, supervision, resources and funding, and validated the data. YH, HC, XZ, ZT, HF and YT curated the data. YH, HC, XZ, ML, WJ, WY and YW analyzed the data. LX, WZ and BL analyzed the data with the software. YH, HC, XZ and BL prepared the figures. YH and HC wrote the manuscript. All authors reviewed and edited the final manuscript.
Ethics approval and consent to participate
All the procedures in this animal study were performed in accordance with the approval by the Institute of Animal Care and Use Committee of Southern Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, et al. Executive summary: Heart disease and stroke statistics-2014 update: A report from the American Heart Association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, et al. Heart disease and stroke statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Meara E, Thibodeau-Jarry N, Ducharme A, Rouleau JL. The epidemic of heart failure: A lucid approach to stemming the rising tide. Can J Cardiol. 2014;30(12 Suppl):S442–S454. doi: 10.1016/j.cjca.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al. Executive summary: Heart disease and stroke statistics-2013 update: A report from the American Heart Association. Circulation. 2013;127:143–152. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 5.Barquera S, Pedroza-Tobías A, Medina C, Hernández-Barrera L, Bibbins-Domingo K, Lozano R, Moran AE. Global overview of the epidemiology of atherosclerotic cardiovascular disease. Arch Med Res. 2015;46:328–338. doi: 10.1016/j.arcmed.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Ma H, Guo R, Yu L, Zhang Y, Ren J. Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischaemia/reperfusion injury: Role of autophagy paradox and toxic aldehyde. Eur Heart J. 2011;32:1025–1038. doi: 10.1093/eurheartj/ehq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–1495. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doser TA, Turdi S, Thomas DP, Epstein PN, Li SY, Ren J. Transgenic overexpression of aldehyde dehydrogenase-2 rescues chronic alcohol intake-induced myocardial hypertrophy and contractile dysfunction. Circulation. 2009;119:1941–1949. doi: 10.1161/CIRCULATIONAHA.108.823799. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Aldi S, Takano K, Tomita K, Koda K, Chan NY, Marino A, Salazar-Rodriguez M, Thurmond RL, Levi R. Histamine H4-receptors inhibit mast cell renin release in ischemia/reperfusion via protein kinase C ε-dependent aldehyde dehydrogenase type-2 activation. J Pharmacol Exp Ther. 2014;349:508–517. doi: 10.1124/jpet.114.214122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomes KM, Campos JC, Bechara LR, Queliconi B, Lima VM, Disatnik MH, Magno P, Chen CH, Brum PC, Kowaltowski AJ, et al. Aldehyde dehydrogenase 2 activation in heart failure restores mitochondrial function and improves ventricular function and remodelling. Cardiovasc Res. 2014;103:498–508. doi: 10.1093/cvr/cvu125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao J, Sun A, Xie Y, Isse T, Kawamoto T, Zou Y, Ge J. Aldehyde dehydrogenase-2 deficiency aggravates cardiac dysfunction elicited by endoplasmic reticulum stress induction. Mol Med. 2012;18:785–793. doi: 10.2119/molmed.2011.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koda K, Salazar-Rodriguez M, Corti F, Chan NY, Estephan R, Silver RB, Mochly-Rosen D, Levi R. Aldehyde dehydrogenase activation prevents reperfusion arrhythmias by inhibiting local renin release from cardiac mast cells. Circulation. 2010;122:771–781. doi: 10.1161/CIRCULATIONAHA.110.952481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes KM, Bechara LR, Lima VM, Ribeiro MA, Campos JC, Dourado PM, Kowaltowski AJ, Mochly-Rosen D, Ferreira JC. Aldehydic load and aldehyde dehydrogenase 2 profile during the progression of post-myocardial infarction cardiomyopathy: Benefits of Alda-1. Int J Cardiol. 2015;179:129–138. doi: 10.1016/j.ijcard.2014.10.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun L, Ferreira JC, Mochly-Rosen D. ALDH2 activator inhibits increased myocardial infarction injury by nitroglycerin tolerance. Sci Transl Med. 2011;3:107ra111. doi: 10.1126/scitranslmed.3002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perez-Miller S, Younus H, Vanam R, Chen CH, Mochly-Rosen D, Hurley TD. Alda-1 is an agonist and chemical chaperone for the common human aldehyde dehydrogenase 2 variant. Nat Struct Mol Biol. 2010;17:159–164. doi: 10.1038/nsmb.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bozkurt B, Torre-Amione G, Warren MS, Whitmore J, Soran OZ, Feldman AM, Mann DL. Results of targeted anti-tumor necrosis factor therapy with etanercept (ENBREL) in patients with advanced heart failure. Circulation. 2001;103:1044–1047. doi: 10.1161/01.CIR.103.8.1044. [DOI] [PubMed] [Google Scholar]
- 17.Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L, et al. Targeted anticytokine therapy in patients with chronic heart failure: Results of the Randomized Etanercept Worldwide Evaluation (RENEWAL) Circulation. 2004;109:1594–1602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 18.Blasi ER, Heyen J, Hemkens M, McHarg A, Ecelbarger CM, Tiwari S. Effects of chronic PPAR-agonist treatment on cardiac structure and function, blood pressure, and kidney in healthy sprague-dawley rats. PPAR Res. 2009;2009:237865. doi: 10.1155/2009/237865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitbeck MG, Charnigo RJ, Khairy P, Ziada K, Bailey AL, Zegarra MM, Shah J, Morales G, Macaulay T, Sorrell VL, et al. Increased mortality among patients taking digoxin-analysis from the AFFIRM study. Eur Heart J. 2013;34:1481–1488. doi: 10.1093/eurheartj/ehs348. [DOI] [PubMed] [Google Scholar]
- 20.Zhou YC, Liu B, Li YJ, Jing LL, Wen G, Tang J, Xu X, Lv ZP, Sun XG. Effects of buyang huanwu decoction on ventricular remodeling and differential protein profile in a rat model of myocardial infarction. Evid Based Complement Alternat Med. 2012;2012:385247. doi: 10.1155/2012/385247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pitarys CJ, II, Virmani R, Vildibill HD, Jr, Jackson EK, Forman MB. Reduction of myocardial reperfusion injury by intravenous adenosine administered during the early reperfusion period. Circulation. 1991;83:237–247. doi: 10.1161/01.CIR.83.1.237. [DOI] [PubMed] [Google Scholar]
- 22.Liu B, Zhang J, Liu W, Liu N, Fu X, Kwan H, Liu S, Liu B, Zhang S, Yu Z, Liu S. Calycosin inhibits oxidative stress-induced cardiomyocyte apoptosis via activating estrogen receptor-α/β. Bioorg Med Chem Lett. 2016;26:181–185. doi: 10.1016/j.bmcl.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Cai HB, Sun XG, Liu ZF, Liu YW, Tang J, Liu Q, Ji BM, Song YH, Zhou YC, Yang MH, Lv ZP. Effects of dahuangzhechong pills on cytokines and mitogen activated protein kinase activation in rats with hepatic fibrosis. J Ethnopharmacol. 2010;132:157–164. doi: 10.1016/j.jep.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 24.Tham YK, Bernardo BC, Ooi JY, Weeks KL, McMullen JR. Pathophysiology of cardiac hypertrophy and heart failure: Signaling pathways and novel therapeutic targets. Arch Toxicol. 2015;89:1401–1438. doi: 10.1007/s00204-015-1477-x. [DOI] [PubMed] [Google Scholar]
- 25.Jain M, Liao R, Ngoy S, Whittaker P, Apstein CS, Eberli FR. Angiotensin II receptor blockade attenuates the deleterious effects of exercise training on post-MI ventricular remodelling in rats. Cardiovasc Res. 2000;46:66–72. doi: 10.1016/S0008-6363(99)00429-0. [DOI] [PubMed] [Google Scholar]
- 26.Sun Y, Weber KT. Infarct scar: A dynamic tissue. Cardiovasc Res. 2000;46:250–256. doi: 10.1016/S0008-6363(00)00032-8. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Yang H, Song L, Li N, Han QY, Tian C, Gao E, Du J, Xia YL, Li HH. AGGF1 protects from myocardial ischemia/reperfusion injury by regulating myocardial apoptosis and angiogenesis. Apoptosis. 2014;19:1254–1268. doi: 10.1007/s10495-014-1001-4. [DOI] [PubMed] [Google Scholar]
- 28.Takemura G, Fujiwara H. Role of apoptosis in remodeling after myocardial infarction. Pharmacol Ther. 2004;104:1–16. doi: 10.1016/j.pharmthera.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Mill JG, Stefanon I, dos Santos L, Baldo MP. Remodeling in the ischemic heart: The stepwise progression for heart failure. Braz J Med Biol Res. 2011;44:890–898. doi: 10.1590/S0100-879X2011007500096. [DOI] [PubMed] [Google Scholar]
- 30.Doorn JA, Hurley TD, Petersen DR. Inhibition of human mitochondrial aldehyde dehydrogenase by 4-hydroxynon-2-enal and 4-oxonon-2-enal. Chem Res Toxicol. 2006;19:102–110. doi: 10.1021/tx0501839. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.