Abstract
Fibromyalgia (FM) is a complex syndrome characterized by chronic widespread pain, hyperalgesia, and other disabling symptoms. Although the brain response to experimental pain in FM patients has been the object of intense investigation, the biological underpinnings of painful after-sensations (PAS), and their relation to negative affect have received little attention. In this cross-sectional cohort study, subjects with FM (n = 53) and healthy controls (n = 17) were assessed for PAS using exposure to a sustained, moderately painful cuff stimulus to the leg, individually calibrated to a target pain intensity of 40 of 100. Despite requiring lower cuff pressures to achieve the target pain level, FM patients reported more pronounced PAS 15 seconds after the end of cuff stimulation, which correlated positively with clinical pain scores. Functional magnetic resonance imaging revealed reduced deactivation of the medial temporal lobe (MTL; amygdala, hippocampus, parahippocampal gyrus) in FM patients, during pain stimulation, as well as in the ensuing poststimulation period, when PAS are experienced. Moreover, the functional magnetic resonance imaging signal measured during the poststimulation period in the MTL, as well as in the insular and anterior middle cingulate and medial prefrontal cortices, correlated with the severity of reported PAS by FM patients. These results suggest that the MTL plays a role in PAS in FM patients.
Perspective
PAS are more common and severe in FM, and are associated with clinical pain and catastrophizing. PAS severity is also associated with less MTL deactivation, suggesting that the MTL, a core node of the default mode network, may be important in the prolongation of pain sensation in FM.
Keywords: Human, psychophysics, neuroimaging, psychosocial, temporal summation, sensitization, default mode network
Historically, many in the medical community have viewed fibromyalgia (FM) with skepticism. Clear peripheral signs in FM are difficult to discern, and an unfortunate consequence that many patients anecdotally report is a lingering tendency of some medical practitioners to underestimate their pain. Over the past 2 decades, an improvement in the specificity of diagnostic criteria for FM,81 along with the accumulation of evidence of objective anatomical, functional, and neurochemical alterations in the central nervous system (eg, changes in brain morphometry, functional connectivity, and concentration of various neurotransmitters and metabolites),19,20,31,36,37,41,45,49,50,57,79 as well as more recent recognition of peripheral changes in at least a subgroup of FM patients,59,77 has helped to increase the acceptance of FM as a clinically recognized pain disorder with a neurobiological basis. Formal Quantitative Sensory Testing (QST) studies have also shown that individuals with FM have greater sensitivity (compared with pain-free control subjects) to a broad variety of standardized noxious stimuli, and importantly, have implicated alterations in the central nervous system as a possible substrate for differences in pain sensitivity between FM and non-FM. Specifically, FM patients exhibit a tendency toward greater central sensitization-like processes, such as temporal summation of pain (TSP).62,69,71 Amplified TSP in FM patients is a phenomenon that has been well studied using a variety of stimulus modalities, including repetitive or prolonged75 heat, pressure, or pin prick mechanical stimuli.5,71,72
Painful after-sensations (PAS), defined as painful sensations persisting beyond the offset of a noxious stimulus, represent a clinically relevant, but less well studied, example of the sensitization-related processes that appear to be enhanced in FM. These PAS, which some authors have also called ‘windup after-sensations’ or ‘prolonged pain after-sensations,’2,30,64,65,67,70,72,73 are typically experimentally measured 15 or 30 seconds after the removal of a nociceptive stimulus (or train of stimuli) of relatively long duration. PAS are enhanced in individuals with neuropathic pain compared with control subjects,30 and are more frequent and pronounced in postmastectomy patients with persistent postsurgical pain than those without it.67 Importantly, a handful of studies have shown that PAS are more pronounced in FM patients than in control subjects.12,62,70,72 Additionally, PAS appear to be more closely correlated to pain severity than other QST measures, including indices of temporal summation,2,65,73 underlining their potential clinical relevance. Whereas PAS have been studied by several groups psychophysically, their associated brain activity has not been investigated. Furthermore, although negative cognitive and affective factors such as catastrophizing, anxiety, and fear are known to contribute to pain-facilitatory processes,24,25,63,66,80 it is unclear from previous work how negative affect relates to the experience of PAS.
In the current study, we evaluated the incidence and severity of PAS in FM, hypothesizing that PAS would be associated with clinical pain and catastrophizing, as well as differential brain activation, assessed using functional magnetic resonance imaging (fMRI), compared with control subjects.
Methods
This cross-sectional cohort study included patients suffering from FM, as well as healthy control volunteers. Study design and procedures were reviewed and approved by the institutional review board. Subjects were recruited through online advertising and flyers posted in the Boston community. Patients were eligible if they held a diagnosis of FM meeting criteria outlined by Wolfe.81 Exclusion criteria included: 1) history of significant neurologic disorder, 2) history of anxiety disorders or significant anxiety symptoms interfering with magnetic resonance imaging (MRI) procedures, 3) history of significant cardiac events, 4) history of significant head injury, 5) current treatment with opioids, 6) current/recent use of recreational drugs, and 7) implanted metallic objects and other typical contraindications for MRI, and 8) pregnancy. Patients’ medication regimens included gabapentin, a variety of antidepressants, nonsteroidal anti-inflammatory drugs, and acetaminophen, and were not altered during the course of this study. Healthy control subjects were recruited so as to achieve a balance of age and sex between FM and control groups. Subjects attended 2 study visits. The first visit consisted of a behavioral assessment, performed in a single, large, urban, university-based pain management center (Brigham and Women’s Hospital). The second was a neuroimaging visit, performed at the A.A. Martinos Center for Biomedical Imaging at Massachusetts General Hospital, an average of 2 weeks later (mean duration between visits ± SD: 15.1 ± 16.2 days).
Behavioral Visit
At the beginning of the visit, all participants provided informed consent. FM patients (n = 53) and healthy control subjects (n = 17) then rated the severity and extent of their clinical pain by using a numeric rating scale (NRS; 0–100, with anchors 0 = no pain, 100 = most intense pain imaginable; numbers indicated verbally), the Brief Pain Inventory,76 the neuropathic pain questionnaire,13 as well as the Widespread Pain Inventory and the Symptom Severity index, which are used in the diagnostic criteria for FM.81 Negative affect and cognition were assessed using the Beck Depression Inventory,11 Pain Catastrophizing Scale (PCS),74 and an anxiety NRS. Subjects also completed a questionnaire at the end of the QST session, the Situational PCS (SPCS),16 which assessed catastrophizing during the painful events of the testing session itself, and is distinct from the PCS.17 Clinical pain NRS rating was also assessed at several points between QST. Pain scores in response to QST were also rated using a NRS (0–100). QST was performed by a single practitioner during the study visit for consistency, and the order of tests was kept constant across participants, as follows:
Mechanical pain thresholds were assessed using a digital pressure algometer (Somedic AB, Sösdala, Sweden) bilaterally at the trapezius muscle and the metacarpophalangeal joint of the thumb.
Cuff pain, temporal summation, and PAS were assessed using a 13.5-cm wide velcro-adjusted blood pressure cuff placed around the left leg (gastrocnemius muscle belly) and connected to a rapid cuff inflator (Hokanson Inc, Bellevue, WA). The cuff was inflated to a pressure corresponding to approximately 40 of 100 pain intensity for each patient. After determination of this 40 of 100 pressure, the cuff was deflated for a rest period and then again inflated to this value and held for a longer duration (2 minutes), after which it was rapidly deflated. Patients were asked to rate the pain experienced from this stimulus upon initial inflation, at 60 seconds, and at 120 seconds. Temporal summation was measured as the difference between pain rating at 120 seconds and initial pain rating. Additionally, the patients were asked to rate any ongoing pain at the cuff site 15 seconds after cuff deflation (PAS).
MRI Visit
FM patients (n = 43) and healthy control subjects (n = 15) also participated in the imaging visit. Of these, 5 subjects with FM were excluded from the analyses: 3 because of differences in the fMRI scanning parameters used, 1 because of technical difficulties with the scanner, and 1 because of falling asleep during the scan. These exclusions did not substantially alter the clinical and demographic profile of participants. In addition, 3 of the participants completed only 3 of 4 runs, because either they fell asleep during the scanning procedures (n = 1), or their clinical pain was excessive and prevented them from proceeding further with the scan (n = 2).
fMRI data were acquired using a 3T Tim Trio MRI System (Siemens Healthcare GmbH, Erlangen, Germany), equipped for echoplanar imaging with a 32-channel head coil. During 4 blood-oxygen level-dependent (BOLD) fMRI runs (time repetition/time echo [TR/TE] = 2 seconds/30 ms, 37 slices, voxel size = 3.1×3.1×3.6 mm), patients received a total of eight 75- to 105-second (average ± SD: 90 ± 10 seconds) cuff pain stimuli (2 per run) individually calibrated to elicit target intensity ratings of approximately 40 of 100, on the left calf. To evaluate the effect of attentional focus on pain processing (an ancillary aim of the original study), we varied subjects’ focus of attention across the 4 runs; in 2 runs subjects were instructed to keep their eyes open and to look at a fixation cross and in 2 runs subjects were instructed to keep their eyes closed and to focus on a pleasant visual image in a pseudorandomized order. In an effort to avoid disruption of the imagery task during the imaging runs, subjects were asked to keep their eyes closed, and only expressed ‘average’ pain intensity (0 = no pain; 100 = the most intense pain imaginable) and unpleasantness ratings (0 = not unpleasant at all; 100 = extremely unpleasant) at the end of each run. Because mixed factorial analysis of variance (ANOVA; Group × Focus condition × Time) revealed that neither the effect of time on these pain ratings was significant (F1,54 = .357, P = .553), nor was there a significant Group × Time interaction (F1,52 = .392, P = .534), and focus condition did not have any significant differences across groups (Group × Focus condition interaction: F1,52 = .395, P = .533), all 4 imaging runs were collapsed into 1 average for further analyses, and the effect of attentional focus will not be discussed further. We also collected anatomical MRI data, using a multiecho magnetization-prepared rapid gradient-echo imaging pulse sequence (TR/TE1/TE2/TE3/TE4 = 2,530/1.64/3.5/5.36/7.22 ms, flip angle = 7°, voxel size = 1 mm isotropic).
Statistics
Analysis of behavioral data was performed using SPSS (version 22; IBM Corp, Armonk, NY). Data for continuous variables are presented as means and SDs, and data for categorical variables are presented as percentages. A temporal summation score was computed by subtracting a patient’s end pressure pain rating from their initial pressure pain rating during the prolonged painful cuff stimulus. To determine significant group differences in reported clinical symptoms, psychosocial and psycho-physical variables, independent samples t-test or Fisher/χ2 tests were performed. To examine whether pressure pain ratings varied as a function of cuff time, or subject group, mixed-model ANOVA was conducted. Additionally, a paired samples t-test between initial and final pain ratings was conducted to assess temporal summation. To compare PAS ratings between groups, an independent samples t-test was used. To investigate the inter-relationships between PAS and other variables among the subjects with FM, Pearson correlation coefficients were calculated. PAS from the behavioral visit were also used in the imaging regression analysis. Significance for all tests was set at α = .05.
fMRI data processing was carried out using FMRI Expert Analysis Tool version 6.00, part of FMRIB Software Library (FSL; www.fmrib.ox.ac.uk/fsl). Data were first corrected for slice timing (slicetimer) and motion (MCFLIRT), and were then skull stripped (BET), realigned to a single fMRI volume within-session (FLIRT), grand-mean intensity normalized by a single multiplicative factor, high-pass temporal filtered (Gaussian-weighted least-squares straight line fitting, with σ = 136 to 164 seconds depending on the run, and estimated using FSL’s cutoffcalc tool) and spatially smoothed (full width at half maximum = 5 mm). Time-series statistical analysis was carried out using FILM with local autocorrelation correction. Cortical surface reconstruction was performed using FreeSurfer (http://surfer.nmr.mgh.harvard.edu) for improved structural-functional coregistration, which was carried out using FreeSurfer’s bbregister tool.33 To ensure that any effect observed in the fMRI data would not be confounded by differences in head motion, we computed for each subject the maximum rotation (in radians [rad]) and the maximum translation (in millimeters) along the x, y, and z axes, and compared them across groups. Maximum rotation values (mean ± SD) were .023 ± .012, .011 ± .009, and .014 ± .012 rad for controls and .028 ± .027, .010 ± .009, and .013 ± .011 rad for FM patients. Maximum translation values were .649 ± .489, .353 ± .160, and 1.332 ± .581 for the controls and .704 ± .634, .682 ± .715, and 1.504 ± 1.827 for FM patients. None of the group differences for each of the 6 motion parameters were statistically significant (Ps = .45, .93, and .53 for the rotations; .76, .09, and .72 for the translations).
A first-level within-subject general linear model (GLM) analysis was performed by modeling the sustained tonic response as a boxcar function (beginning from cuff inflation to cuff deflation), and the 15-second period after stimulus offset. The latter regressor was used in our design matrix because our behavioral data (see Results) in this as well as other studies67 indicated the presence of PAS during this period. In addition, the stimulus onset and offset transients were modeled using stick functions (1 TR each), similar to previous investigations.10,44,78 These regressors were included to improve the overall modeling of brain activity, because previous work has shown that task/stimulus transitions give rise to transient BOLD responses which are, at least in part, spatially separable from the tonic/sustained brain responses observed throughout the presentation of the task or stimulus.23,29,44,60,78 Such modeling is arguably more important in the presence of relatively long stimulus blocks, as was the case in our study. The inclusion of the offset regressor, in particular, improved the separation between brain responses to the discrete act of cuff deflation versus the more prolonged 15-second poststimulus period with the noted PAS. A canonical double-g hemodynamic response function was convolved with all of the mentioned regressors. Finally, 6 head motion parameters (3 translations and 3 rotations), as well as a regressor of no interest for each volume deemed to be an outlier in terms of motion (as computed using fsl_motions_outliers) were also included in the design matrix to minimize the effect of motion in the estimation of our brain responses to our variables of interest. The first-level parameter estimate and corresponding variance maps were registered to the Montreal Neurological Institute 152 standard space using the FMRIB Nonlinear Image Registration Tool for group analyses. Group differences in the 15-second poststimulus period, as well as the association between this activity and the behavioral ratings of PAS (for subjects with FM), were then assessed in whole-brain voxelwise GLMs, using FMRIB Local Analysis of Mixed Effects 112, with automatic outlier detection enabled. The resulting statistical map was cluster-corrected for multiple comparisons using the FSL default cluster-forming voxelwise threshold of Z > 2.3, and a (corrected) cluster significance threshold of P < .05. For illustrative purposes, the average percent signal change was extracted from the statistically significant clusters, masked by anatomical labels obtained from the Harvard Oxford Atlas (thresholded at the arbitrary value of 30). The medial temporal lobe (MTL) label was obtained by fusing the amygdala, hippocampus, and parahippocampal gyrus labels from the atlas.
Results
Demographic, Psychosocial, Psychophysical, and Clinical Pain Characteristics of Subjects
Subjects with FM and healthy control subjects were comparable in age, sex, ethnicity, and education (Table 1). However, as expected, subjects with FM differed from healthy control subjects in their degree of pain and physical symptoms, including pain severity, pain interference, fatigue, widespread pain, and symptom severity, according to commonly used clinical assessment tools for FM. Additionally, subjects with FM reported a greater degree of depressive symptoms, and higher ‘trait’ pain catastrophizing (although not different ‘situational’ catastrophizing during QST) than control subjects (Table 1). Upon psychophysical testing, subjects with FM reported greater pain sensitivity overall, with lower pressure pain thresholds at multiple body locations, and higher PAS ratings (Table 2).
Table 1.
Demographic, Clinical, and Psychosocial Characteristics
| Factor | Control | FM | P |
|---|---|---|---|
| Demographic | |||
| Age | 44.1 ± 14.8 | 46.3 ± 11.4 | .52 |
| emale sex | 71 | 87 | .15 |
| Ethnicity (% non-Caucasian) | 11.7 | 20.7 | .50 |
| Education (7-point scale, range = some high school to doctoral degree) | 5.0 ± .5 | 4.4 ± 1.3 | .10 |
| Clinical pain characteristics | |||
| Pain severity (BPI; NRS 0–10) | .29 ± .51 | 5.4 ± 2.1 | <.001 |
| Pain interference (BPI; NRS 0–10) | .01 ± .05 | 5.8 ± 2.1 | <.001 |
| Fatigue (NRS; 0–100) | 15.8 ± 18.4 | 66.6 ± 21.4 | <.001 |
| Widespread pain index (FM diagnosis ≥7) | .4 ± .8 | 10.9 ± 2.6 | <.001 |
| Symptom severity sum (FM diagnosis ≥5) | 1.5 ± 1.7 | 9.3 ± 1.9 | <.001 |
| Neuropathic Pain Questionnaire (0–10) | .09 ± .18 | 4.2 ± 1.8 | <.001 |
| Psychosocial characteristics | |||
| Depression (BDI; 0–63) | 3.1 ± 3.8 | 15.2 ± 8.2 | <.001 |
| Catastrophizing (PCS; 0–56) | 5.6 ± 5.8 | 23.3 ± 13.0 | <.001 |
| Situational catastrophizing score (0–10) | 3.3 ± 4.1 | 4.7 ± 4.9 | .28 |
Abbreviations: BPI, Brief Pain Inventory; BDI, Beck Depression Inventory.
NOTE. Data are presented as mean ± SD or %, except where otherwise stated. Bold indicates statistical significance. FM patients had more widespread pain, fatigue, and higher trait, but not situational, catastrophizing or depression scores than control subjects; Fisher exact test, Pearson χ2, or independent samples t-test were used for group comparisons, as appropriate.
Table 2.
Psychophysical Characteristics
| Quantitative Sensory Test | Control | FM | P |
|---|---|---|---|
| Cuff pressure (mm Hg) producing 40/100 pain intensity | 160 ± 74 | 100 ± 43 | .005 |
| Pressure pain threshold thumb (mm Hg) | 337 ± 92 | 258 ± 87 | .004 |
| Pressure pain threshold trapezius (mm Hg) | 361 ± 131 | 250 ± 131 | .005 |
| PAS rating 15 seconds after cuff deflation (NRS 0–100) | 2.1 ± 7.3 | 13.6 ± 20.4 | .001 |
NOTE. Data are presented as mean ± SD except where otherwise noted. FM patients had lower pain thresholds and higher pain ratings than their age- and sex-matched control subjects; independent samples t-test was used for group comparisons.
Temporal Summation of Prolonged Cuff Pain in FM and Control Subjects
To assess the degree of TSP in subjects, a prolonged, moderately painful pressure of inflatable cuff was applied to the left leg, using an individually-determined pressure to produce a 40 of 100 NRS rating for each subject at cuff onset. Consistent with other QST studies, significantly less pressure was needed to produce a 40 of 100 level of pain in FM subjects than control subjects (Table 2). This pressure was then used in a 2-minute long sustained cuff inflation, with patients rating pain at 0, 60, and 120 seconds after stimulation onset. Mixed model ANOVA revealed that pain scores significantly increased over time (F66,2 = 9.135, P < .001), and that there was a significant effect of group on pain scores (F66,1 = 5.303, P = .024), indicating that those with FM had significantly higher pain scores. A paired sample t-test showed a significant increase in pain scores from baseline to 120 seconds in the FM group (t51 = 4.9, P < .001), but not in the control group (t16 = 1.1, P = .30; Fig 1A). Notably, there was some variability among individuals within both groups, in terms of whether TSP occurred (ie, last score higher than initial score), pain remained the same throughout the duration of the cuff stimulus, or habituation occurred (ie, last score lower than initial score). Among the FM patients, 69% had temporal summation, 13% had no change, and 17% habituated. Among control subjects, 53% had temporal summation, 12% reported no change, and 35% habituated. However, these proportions were not statistically different between groups (χ2 = 2.46, P = .293; Fig 1B).
Figure 1.
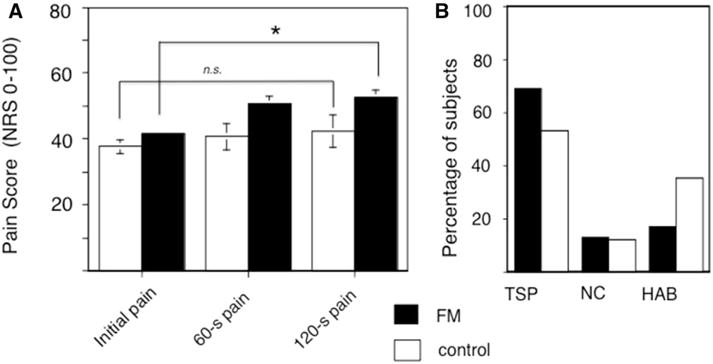
Temporal summation to prolonged moderately painful leg cuff in subjects with FM and control subjects. A cuff was applied to the left leg and inflated to a pressure corresponding to 40/100 pain for each subject. This pressure was then held for a 2-minute period and subjects asked to rate the pain on an NRS (0–100). (A) Pain ratings at 0, 60, and 120 seconds, showing significant increase in cuff scores in FM patients, but not control subjects (mean and standard error shown). (B) Variability of response to sustained painful cuff, showing the proportion of subjects with increase in score between initiation and end of cuff stimulus (TSP), no change (NC), and with decrease in score between initiation and end of cuff stimulus (habituation; HAB), although there was no difference in these groupings between FM and control participants (χ2 = 2.46, P = .293). *P < .05.
PAS in FM and Control Subjects After Prolonged Cuff Stimulus
Subjects were also asked to rate the degree of pain they experienced 15 seconds after the deflation of the painful cuff stimulus (PAS). Interestingly, despite experiencing a significantly lower cuff pressure over these 2 minutes (mean cuff inflation = approximately 100 mm Hg for FM subjects, mean cuff inflation = approximately 160 mm Hg for control subjects), subjects with FM more frequently reported PAS at 15 seconds (50% of FM vs 12% of control subjects, χ2 = 7.77, P = .005; Fig 2B). The average severity of PAS was also significantly higher in subjects with FM (t67 = 3.5, P = .001; Fig 2A). We then examined the relation of PAS severity with other relevant QST parameters including TSP, general and more specific measures of clinical pain, and measures of pain catastrophizing, in FM patients. PAS severity was correlated with overall cuff pain ratings but not to the degree of TSP. PAS severity was also significantly correlated with clinical pain as rated on the Brief Pain Inventory, but not to measures of pain catastrophizing (Table 3).
Figure 2.
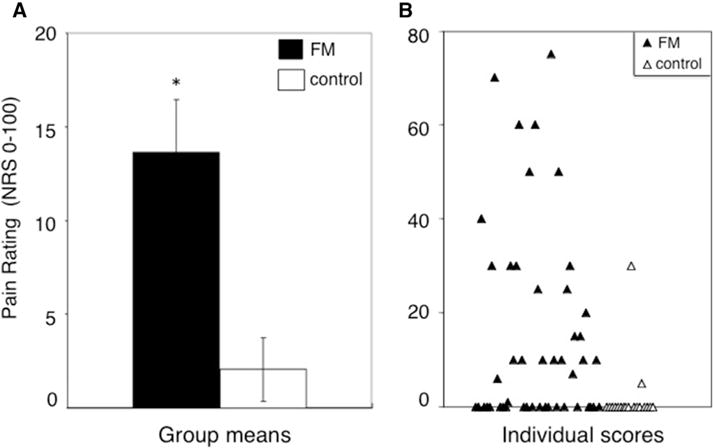
PAS in subjects with FM and control participants. Fifteen seconds after deflation of the painful leg cuff, subjects were asked to rate any ongoing pain in their leg. (A) Subjects with FM had a significantly higher rating of PAS than control participants (mean and standard error shown). (B) Variability of PAS ratings in subjects with FM (closed triangles) and control subjects (open triangles). *P < .05.
Table 3.
Correlation of PAS With Psychophysical, Clinical and Psychosocial Characteristics
| Variable | Correlation with Pas Pearson Correlation Coefficient | P |
|---|---|---|
| Psychophysical characteristics | ||
| Cuff pressure (mm Hg) producing 40/100 pain intensity | −.365 | .008 |
| Average cuff pain over 2-minute inflation | .330 | .017 |
| Temporal summation of pain | .187 | .185 |
| Clinical pain characteristics | ||
| BPI severity | .380 | .005 |
| BPI interference | .303 | .029 |
| Widespread pain index | .199 | .171 |
| Symptom severity sum | −.064 | .664 |
| Psychosocial characteristics | ||
| Pain catastrophizing (PCS) | .202 | .151 |
| Situational pain catastrophizing (SPCS) | .204 | .146 |
Abbreviations: BPI, Brief Pain Inventory; PCS, Pain Catastrophizing Scale. NOTE. Bold indicates statistical significance. Painful aftersensations were correlated with other psychosocial measures of pain sensitivity, clinical pain and FM symptom measures, as well as a measure of trait and state catastrophizing in FM subjects.
fMRI Response to Pain and PAS
The group difference maps for brain responses to the prolonged painful cuff stimulus, as well as to the 15-second poststimulus offset, are shown in Fig 3 (see Table 4 for cluster information). Group maps for the various regressors modeled in the GLM are presented in Supplementary Fig 1.
Figure 3.
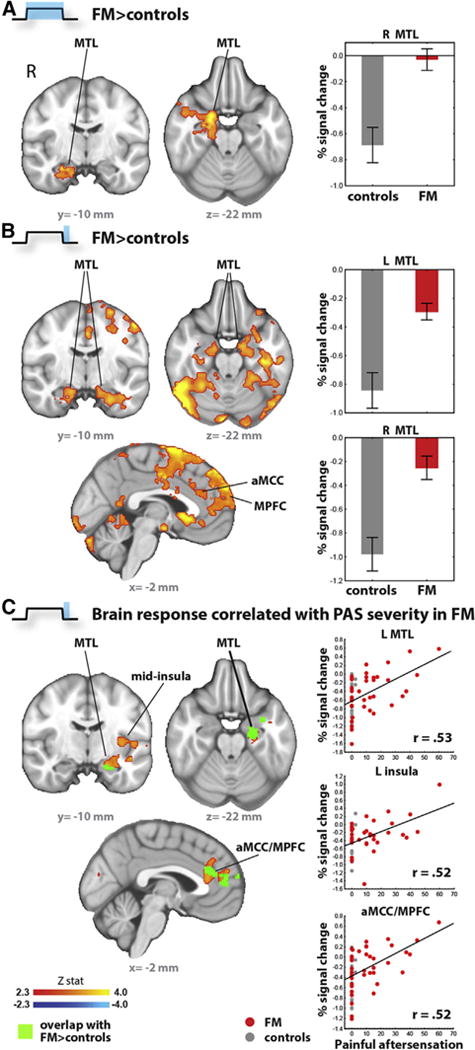
Group differences in brain responses during and after painful cuff stimulus. (A) Patients showed markedly dampened deactivations during pain stimulation, and (B) in the 15-second period after stimulus offset. (C) Brain activity during the postoffset period was statistically associated with the reports of PAS measured in the behavioral visit, including in regions showing group differences (green). Scatter plots are presented for illustrative purposes. Abbreviations: aMCC, anterior middle cingulate cortex; L, left; R, right.
Table 4.
Group Differences in Brain Responses to Cuff Stimulation Blocks
| Cluster Size, Number of Voxels | cluster P |
Local Maxima
|
||||
|---|---|---|---|---|---|---|
| Z | MNI X, MM | MNI Y, MM | MNI Z, MM | Label | ||
| FM > CTRL | ||||||
| 1,329 | .000757 | 4.33 | 20 | 2 | −24 | R Entorhinal cortex |
| 4.2 | 20 | −2 | −22 | R amygdala | ||
| 3.98 | 38 | 18 | −42 | R temporal pole | ||
| 3.84 | 20 | −2 | −34 | R parahippocampal gyrus | ||
| 3.56 | 26 | −10 | −22 | R hippocampus | ||
| 3.05 | 40 | 0 | −42 | R inferior temporal gyrus | ||
| CTRL > FM | ||||||
| n.s. | ||||||
Abbreviations: MNI, Montreal Neurological Institute; CTRL, control; R, right; n.s., not significant.
Whole-brain voxelwise group comparisons revealed that FM patients demonstrated significantly dampened deactivation (less negative BOLD signal) of the MTL (amygdala, hippocampus, parahippocampal gyrus, entorhinal cortex) during cuff pain stimulation (Fig 3A), as well as during the 15-second period after cuff deflation (postoffset period) which corresponds to timing of PAS (Fig 3B). This group difference reached statistical significance for the right MTL during the pain stimulation, and bilaterally during the post-offset period. Additional regions showing higher BOLD signal in FM patients during the postoffset period included the anterior middle cingulate cortex, the medial prefrontal cortex (mPFC), the supplementary and presupplementary motor cortices, the ventral striatum/nucleus accumbens, the frontal pole, the posterior parietal cortex, and the cerebellum (Fig 3B, Table 5).
Table 5.
Group Differences in Brain Responses to Postoffset Period
| Cluster Size, Number of Voxels | Cluster P |
Local Maxima
|
||||
|---|---|---|---|---|---|---|
| Z | x | y | z | Label | ||
| FM > CTRL | ||||||
| 17,473 | 7.99E-26 | 6.18 | 54 | −56 | −20 | R inferior temporal gyrus |
| 5.33 | −16 | 62 | 20 | L frontal pole | ||
| 4.77 | 48 | −74 | −32 | R cerebellum (hemisphere) | ||
| 4.7 | 8 | 36 | −8 | R subgenual cingulate cortex | ||
| 4.64 | −2 | 2 | 72 | L supplementary motor area | ||
| 4.55 | −22 | 40 | 50 | L superior frontal gyrus | ||
| 4.38 | 20 | 64 | 24 | R frontal pole | ||
| 4.23 | −4 | −6 | 42 | L posterior middle cingulate cortex | ||
| 4.2 | −28 | −8 | 66 | L premotor cortex | ||
| 4.18 | −52 | −6 | 50 | L precentral gyrus | ||
| 4.09 | −36 | −22 | −22 | L fusiform cortex | ||
| 4.07 | −46 | −72 | −24 | L cerebellum (hemisphere) | ||
| 4.06 | −28 | 12 | −16 | L frontoinsular cortex | ||
| 4.02 | −6 | 44 | 8 | L pregenual anterior cingulate cortex | ||
| 3.97 | 16 | 44 | 50 | R superior frontal gyrus | ||
| 3.89 | −32 | −20 | 42 | L postcentral gyrus | ||
| 3.88 | 20 | −14 | −26 | R entorhinal cortex | ||
| 3.68 | −10 | 28 | 28 | L anterior middle cingulate cortex | ||
| 3.64 | 22 | −20 | −16 | R hippocampus | ||
| 3.59 | −14 | −8 | −20 | L amygdala | ||
| 3.43 | 30 | −24 | −24 | R parahippocampal gyrus | ||
| 3.32 | −16 | −12 | −18 | L hippocampus | ||
| 3.32 | 8 | 8 | −10 | R nucleus accumbens | ||
| 3.27 | −6 | 14 | −4 | L nucleus accumbens | ||
| 2.95 | −18 | −6 | −32 | L entorhinal cortex | ||
| 2.82 | 16 | −6 | −20 | R amygdala | ||
| 702 | .0305 | 4 | 0 | −60 | 62 | Precuneus |
| 3.56 | 8 | −66 | 66 | R superior parietal lobule | ||
| CTRL > FM | ||||||
| n.s. | ||||||
Abbreviations: CTRL, control; R, right; L, left; n.s., not significant.
In FM patients, the magnitude of PAS measured at the behavioral visit was correlated with brain activity during the corresponding 15-second period after cuff deflation in several regions, including the left MTL, the left middle insular and frontoinsular cortex, dorsal anterior cingulate cortex (dACC)/mPFC, frontal pole, the putamen, and the occipital cortex (Fig 3C, Table 6). Among these regions, the MTL and dACC/mPFC clusters overlapped (shown in green) with those observed when comparing groups during the postoffset period.
Table 6.
Correlations Between PAS and Brain Responses to Postoffset Period
| Cluster Size, Number of Voxels | Cluster P |
Local Maxima
|
||||
|---|---|---|---|---|---|---|
| Z | x | y | z | Label | ||
| Positive correlations | ||||||
| 1,485 | .000269 | 3.69 | −24 | 16 | −8 | L putamen |
| 3.57 | −56 | 36 | 0 | L ventrolateral prefrontal cortex | ||
| 3.48 | −20 | −4 | −16 | L amygdala | ||
| 3.35 | −12 | 20 | 2 | L head of the caudate | ||
| 3.19 | −42 | −6 | −12 | L planum polare | ||
| 3.15 | −22 | −16 | −20 | L hippocampus | ||
| 3.03 | −36 | −10 | 6 | L posterior insula | ||
| 1,127 | .00201 | 3.74 | 14 | −98 | 14 | R occipital pole |
| 3.54 | −8 | −94 | 4 | L occipital pole | ||
| 855 | .0107 | 3.79 | −4 | 52 | 24 | L medial frontal gyrus |
| 3.74 | 0 | 48 | 14 | Anterior middle cingulate/paracingulate gyrus | ||
| Negative correlations | ||||||
| n.s. | ||||||
Abbreviations: L, left; R, right; n.s., not significant.
Discussion
We found that PAS after a perceptually matched, moderately painful mechanical stimulus were more intense and of higher incidence in subjects with FM than in healthy controls. Similar to previous investigators,2,25,65 we also found that PAS were significantly correlated with clinical pain severity in FM (ie, participants with higher ratings of the intensity of PAS also reported more severe daily pain). Although PAS and catastrophizing scores were higher in subjects with FM, among FM subjects PAS severity was not correlated with trait (PCS) and state (SPCS) catastrophizing scores. Interestingly, brain activity during the time period when PAS were measured (ie, 15 seconds after prolonged moderately painful cuff stimuli) differed between FM and healthy control subjects. Specifically, healthy control subjects, who reported lower incidence and severity of PAS and lower catastrophizing, showed greater deactivation in the MTL, including amygdala, hippocampus, and parahippocampal gyrus. Moreover, the fMRI signal in the MTL and dACC/MPFC correlated with PAS severity. These findings may suggest that a relative lack of deactivation in these areas may be important to the experience of PAS, and possibly to pain sensitization in the temporal dimension (pain prolongation) in FM.
Although most studies of temporal summation in FM have used a cutaneous heat stimulus, repeated or sustained pressure stimuli also produce TSP in FM.43,69,72 Furthermore, phasic as well as tonic application of a painful stimulus can produce temporal summation,3 and both modes of application show a close correlation with psychosocial factors such as catastrophizing.32 As in the current study, lower pressures are typically required to produce pain in FM patients compared with control subjects.40,52,69 Thus, with similar basal mean arterial pressures between FM and control subjects, one would expect that the degree of ischemia from cuff inflation would be more pronounced in control subjects (average systolic blood pressure 120.31 vs 120.24, t66 = −.019, P = .985). However, we cannot exclude differences in peripheral pressure sensitivity and perfusion dynamics as accounting for group differences in this paradigm. Our moderately painful (40 of 100) prolonged cuff stimulus produced temporal summation in most patients with FM, consistent with previous studies that observed a modest increase in temporal summation in FM as a group over control subjects with stimulus matching.43,69,72 The duration of the painful cuff stimulus we used was relatively long compared with some other measures of temporal summation,5 for the purpose of increasing the likelihood of detecting temporal summation as well as PAS. Although there was some variability in the response to a prolonged cuff stimulus in both groups, FM patients reported increased incidence and severity of PAS compared with control subjects, similar to previous reports.69 TSP and PAS, although collected in tandem in a combined psychophysical test, may represent separable psychophysical phenomena,61 and, importantly, this study noted no correlation between measurements of an individual’s TSP and PAS (Table 3). Whereas TSP measures augmentation of pain in terms of increased amplitude of pain with a prolonged or repeated stimulus, PAS measures increased duration of pain beyond the end of the pain stimulus. To the extent that both tests attempt to assess pain plasticity at a central level, it is not surprising that they are both amplified in FM patients, because FM is increasingly recognized as a pain disorder with a prominent component of central sensitization. Importantly, variability in the degree of TSP and PAS measured using QSTcan be observed generally among individuals, as well as between groups such as men/women28 or older/younger adults.35 This individual variability has been shown to distinguish those who develop postsurgical pain from those who do not,67 and to predict risk for opiate misuse in individuals with chronic pain.26
The degree to which psychosocial processes such as catastrophizing influence an individual’s experience of pain also varies among individuals. These processes likely exert their influence on pain-related outcomes at a variety of central nervous system sites.21 Interestingly, previous studies have also reported a relationship between measures of pain augmentation (such as TSP and PAS), and indices of negative affect, anxiety, and fear of pain.25,63,66,80 In the present study, we similarly found a significantly higher report of pain-related catastrophizing as well as PAS among subjects with FM, whether measured as a stable trait with the PCS, or measured “situationally” in reference to experimental pain stimulation (SPCS). However, within the FM subject cohort, there was not a significant correlation between PAS and measures of catastrophizing (Table 3). Because of the cross-sectional nature of this study, we are unable to determine the temporal association between amplified PAS, and FM-relevant clinical outcomes such as the spatial extent of clinical pain and FM symptom severity. However, the incidence of higher PAS as well as catastrophizing in subjects with FM, together with a concurrent decreased activation in MTL during the period immediately after the offset of a noxious stimulus, suggests at least the possibility that altered brain processing in these regions may represent an underlying pathophysiological process that contributes to a variety of clinical manifestations of chronic pain (eg, amplified pain sensitivity, perceptions of pain that outlast the stimulus, spreading pain, elevated pain-related distress, etc).
Whereas PAS have been reported in several chronic pain disorders,27,30,58,69 the neural correlates of PAS have not previously been elucidated in the brain, although a recent study showed higher dorsal horn spinal activation in FM, who also had higher PAS.12 The present study found that PAS, which were prominent in subjects with FM, and more rare in control subjects, were associated with differences in MTL response (specifically, deactivation). Of note, MTL alterations have been previously reported in FM. For instance, the parahippocampus was shown to have significantly reduced gray matter density,46,82 reduced cerebral blood flow,34 as well as reduced binding potential to dopamine tracers,83 which correlated with self-reported pain1 in FM patients. Anatomofunctional changes have also been reported in patients with chronic pain in the amygdala,22,42,51 including decreases in gray matter volume,15,53 increases in fractional anisotropy,53 and alterations of power spectral density of low frequency.42 Finally, studies have also reported FM-related changes in the hippocampus, including hippocampal atrophy,55 reduced activation during a cognitive performance task,54 reduced levels of N-acetyl aspartate, a measure of neuronal integrity,4 as well as reduced binding potential for m-opioid receptor ligands.36 Our study further uncovers a link between altered functional response in the MTL in FM patients and their experience of PAS. In addition, post-stimulus brain activity in the insula, anterior middle cingulate cortex, and MPFC correlated with severity of PAS. Although these brain regions have been associated with hyperalgesia in studies evaluating brain response to experimental pain administration, to our knowledge their role in PAS has not previously been evaluated.
Interestingly, MTL regions and MPFC are ‘core’ structures of the default-mode network (DMN),14 whereas anterior insula and anterior middle cingulate cortex are key nodes of the salience network (SN).68 The DMN and SN networks are key subsystems of what has been recently labeled the ‘pain connectome,’47 and are thought to dynamically contribute to the experience of experimental pain by modulating one’s propensity to attend to a noxious stimulus. For instance, when attention is maintained on a nociceptive stimulus, SN regions show increased activity and DMN regions show decreased activity. Conversely, when attention fluctuates away from a nociceptive stimulus, SN regions show relatively reduced activation, and DMN regions show reduced deactivation.46,48 Although this often antagonistic interplay between SN and DMN is commonly observed in healthy volunteers, it becomes disrupted in the context of chronic pain. For instance, Napadow and colleagues57 have shown that the insula, a core SN region, becomes abnormally connected to the DMN in FM patients, and that the stronger the functional connectivity between insula and DMN, the greater the severity of clinical pain reported. Aberrantly increased connectivity between DMN regions and the insula has been replicated in patients with other disorders, including chronic low back pain, complex regional pain syndromes, and osteoarthritis.9,52 In the present study, we showed that alterations in the response of DMN and SN regions are also associated with PAS. Whereas additional studies are needed to further characterize this association, these findings add to the body of evidence implicating these networks in chronic pain disorders, including FM18,56,57 and others.6–8,38,52,75
Limitations
Whereas the presence of PAS and related brain activity may be interpreted as a manifestation of central sensitization, it is possible that other factors may contribute the group differences in psychophysical and imaging data reported. For instance, it is possible that local changes potentially associated with cuff algometry (eg, group differences in the latency and magnitude of muscle ischemia) may have a role. However, although we were not able to directly measure tissue ischemia, it should be noted that systolic blood pressure was similar between groups. Furthermore, because of our percept-matching design, patients with FM received significantly lower cuff pressures than control subjects. Nonetheless, future studies should assess the role of peripheral mechanisms mediating PAS in FM. Another potential limitation of the present study is that the neurobiological substrates of TSP obtained in our study are more uncertain than if we had adopted a more conventional wind-up like behavioral paradigm (which is more clearly known to be mediated by c-fiber-induced wind-up of dorsal horn neurons). Nonetheless, whereas the neurobiological correlates of TSP induced by tonic stimuli may not have been as well studied as in the context of wind-up paradigms, previous neurophysiological investigations have indeed shown a contribution of c-fibers to sensitization in the context of sustained mechanical stimulation.3 It is also important to note that control subjects, despite being exposed to a higher pressure cuff stimulus to produce moderate pain (40 of 100), did not develop the same degree of TSP over the course of the stimulus as did FM subjects as a group, thus potentially confounding the interpretation of differences in PAS. Viewing this difference in the propensity to develop TSP as a confound, however, rests on the supposition that TSP is necessary to produce the experience of PAS, which a recent study suggests.39 Interestingly, however, we did not observe a significant correlation between the degree of TSP and PAS within individual subjects, thus suggesting that these are at least partially separable phenomena. It should also be recognized that, although TSP and PAS have been observed with a variety of stimulus modalities (ie, thermal or mechanical), it is as yet unclear whether the same mechanisms underlie TSP and PAS in these different behavioral paradigms. Finally, another limitation of our study is that the PAS and brain imaging data were collected in separate visits. Although collecting ratings in real-time would have allowed a more direct assessment of the association between these variables, we decided against this option to reduce interference of real-time pain rating with fMRI data collection. Last, although we observed differences between FM and control subjects in this study, we cannot claim that the effects observed are specific to FM. Future studies including an additional group of patients with a different etiology of chronic pain could potentially address the question of whether the observed differences are unique to FM.
Conclusions
We observed that PAS after a prolonged pressure pain stimulus were more prevalent as well as more severe in subjects with FM. Importantly, we found that these PAS were correlated with the degree of clinical symptoms reported in these subjects, consistent with previous findings that PAS were the most robust psychophysical predictor of clinical pain in FM.26 As with TSP, the severity of PAS varied between subjects, and higher PAS severity was correlated with higher pain scores. This first study of the neural correlates of PAS found that PAS severity corresponded to less deactivation in the MTL, suggesting that altered brain processing in these regions may represent an underlying pathophysiological process that contributes, at least in part, to pain amplification and prolongation in FM.
Supplementary Material
Acknowledgments
The authors thank the subjects for their willingness and time to participate in the study.
This work was supported by the National Institutes of Health, National Center for Complementary and Integrative Health (R01-AT007550, P01-AT006663), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01-AR064367), and the National Center for Research Resources (P41RR14075, S10RR021110, S10RR023043).
Footnotes
The authors have no conflicts of interest to declare.
Supplementary data accompanying this article are available online at www.jpain.org and www.sciencedirect.com.
Supplementary Data
Supplementary data related to this article can be found online at http://dx.doi.org/10.1016/j.jpain.2017.02.437.
References
- 1.Albrecht DS, MacKie PJ, Kareken DA, Hutchins GD, Chumin EJ, Christian BT, Yoder KK. Differential dopamine function in fibromyalgia. Brain Imaging Behav. 2016;10:829–839. doi: 10.1007/s11682-015-9459-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson RJ, McCrae CS, Staud R, Berry RB, Robinson ME. Predictors of clinical pain in fibromyalgia: Examining the role of sleep. J Pain. 2012;13:350–358. doi: 10.1016/j.jpain.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrew D, Greenspan JD. Peripheral coding of tonic mechanical cutaneous pain: Comparison of nociceptor activity in rat and human psychophysics. J Neurophysiol. 1999;82:2641–2648. doi: 10.1152/jn.1999.82.5.2641. [DOI] [PubMed] [Google Scholar]
- 4.Aoki Y, Inokuchi R, Suwa H. Reduced N-acetylaspartate in the hippocampus in patients with fibromyalgia: A meta-analysis. Psychiatry Res. 2013;213:242–248. doi: 10.1016/j.pscychresns.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Arendt-Nielsen L. Central sensitization in humans: Assessment and pharmacology. Handb Exp Pharmacol. 2015;227:79–102. doi: 10.1007/978-3-662-46450-2_5. [DOI] [PubMed] [Google Scholar]
- 6.Baliki MN, Baria AT, Apkarian AV. The cortical rhythms of chronic back pain. J Neurosci. 2011;31:13981–13990. doi: 10.1523/JNEUROSCI.1984-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, Apkarian AV. Chronic pain and the emotional brain: Specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26:12165–12173. doi: 10.1523/JNEUROSCI.3576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: Chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008;28:1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baliki MN, Mansour AR, Baria AT, Apkarian AV. Functional reorganization of the default mode network across chronic pain conditions. PLoS One. 2014;9:e106133. doi: 10.1371/journal.pone.0106133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becerra L, Navratilova E, Porreca F, Borsook D. Analogous responses in the nucleus accumbens and cingulate cortex to pain onset (aversion) and offset (relief) in rats and humans. J Neurophysiol. 2013;110:1221–1226. doi: 10.1152/jn.00284.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 12.Bosma RL, Mojarad EA, Leung L, Pukall C, Staud R, Stroman PW. FMRI of spinal and supra-spinal correlates of temporal pain summation in fibromyalgia patients. Hum Brain Mapp. 2016;37:1349–1360. doi: 10.1002/hbm.23106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, Cunin G, Fermanian J, Ginies P, Grun-Overdyking A, Jafari-Schluep H, Lantéri-Minet M, Laurent B, Mick G, Serrie A, Valade D, Vicaut E. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4) Pain. 2005;114:29–36. doi: 10.1016/j.pain.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 15.Burgmer M, Gaubitz M, Konrad C, Wrenger M, Hilgart S, Heuft G, Pfleiderer B. Decreased gray matter volumes in the cingulo-frontal cortex and the amygdala in patients with fibromyalgia. Psychosom Med. 2009;71:566–573. doi: 10.1097/PSY.0b013e3181a32da0. [DOI] [PubMed] [Google Scholar]
- 16.Campbell CM, Kronfli T, Buenaver LF, Smith MT, Berna C, Haythornthwaite JA, Edwards RR. Situational versus dispositional measurement of catastrophizing: Associations with pain responses in multiple samples. J Pain. 2010;11:443–453. doi: 10.1016/j.jpain.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell CM, McCauley L, Bounds SC, Mathur VA, Conn L, Simango M, Edwards RR, Fontaine KR. Changes in pain catastrophizing predict later changes in fibromyalgia clinical and experimental pain report: Cross-lagged panel analyses of dispositional and situational catastrophizing. Arthritis Res Ther. 2012;14:R231. doi: 10.1186/ar4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cifre I, Sitges C, Fraiman D, Munoz MA, Balenzuela P, Gonzalez-Roldan A, Martinez-Jauand M, Birbaumer N, Chialvo DR, Montoya P. Disrupted functional connectivity of the pain network in fibromyalgia. Psychosom Med. 2012;74:55–62. doi: 10.1097/PSY.0b013e3182408f04. [DOI] [PubMed] [Google Scholar]
- 19.Clauw DJ. Fibromyalgia: A clinical review. JAMA. 2014;311:1547–1555. doi: 10.1001/jama.2014.3266. [DOI] [PubMed] [Google Scholar]
- 20.Cook DB, Stegner AJ, McLoughlin MJ. Imaging pain of fibromyalgia. Curr Pain Headache Rep. 2007;11:190–200. doi: 10.1007/s11916-007-0190-8. [DOI] [PubMed] [Google Scholar]
- 21.Darnall BD. Pain psychology and pain catastrophizing in the perioperative setting: A review of impacts, interventions, and unmet needs. Hand Clin. 2016;32:33–39. doi: 10.1016/j.hcl.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dehghan M, Schmidt-Wilcke T, Pfleiderer B, Eickhoff SB, Petzke F, Harris RE, Montoya P, Burgmer M. Coordinate-based (ALE) meta-analysis of brain activation in patients with fibromyalgia. Hum Brain Mapp. 2016;37:1749–1758. doi: 10.1002/hbm.23132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Downar J, Mikulis DJ, Davis KD. Neural correlates of the prolonged salience of painful stimulation. Neuroimage. 2003;20:1540–1551. doi: 10.1016/s1053-8119(03)00407-5. [DOI] [PubMed] [Google Scholar]
- 24.Edwards RR, Mensing G, Cahalan C, Greenbaum S, Narang S, Belfer I, Schreiber KL, Campbell C, Wasan AD, Jamison R. Alteration in pain modulation in women with persistent pain after lumpectomy: Influence of catastrophizing. J Pain Symptom Manage. 2013;46:30–42. doi: 10.1016/j.jpainsymman.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edwards RR, Smith MT, Stonerock G, Haythornthwaite JA. Pain-related catastrophizing in healthy women is associated with greater temporal summation of and reduced habituation to thermal pain. Clin J Pain. 2006;22:730–737. doi: 10.1097/01.ajp.0000210914.72794.bc. [DOI] [PubMed] [Google Scholar]
- 26.Edwards RR, Wasan AD, Michna E, Greenbaum S, Ross E, Jamison RN. Elevated pain sensitivity in chronic pain patients at risk for opioid misuse. J Pain. 2011;12:953–963. doi: 10.1016/j.jpain.2011.02.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eide PK, Rabben T. Trigeminal neuropathic pain: Pathophysiological mechanisms examined by quantitative assessment of abnormal pain and sensory perception. Neurosurgery. 1998;43:1103–1110. doi: 10.1097/00006123-199811000-00055. [DOI] [PubMed] [Google Scholar]
- 28.Fillingim RB, Maixner W, Kincaid S, Silva S. Sex differences in temporal summation but not sensory-discriminative processing of thermal pain. Pain. 1998;75:121–127. doi: 10.1016/S0304-3959(97)00214-5. [DOI] [PubMed] [Google Scholar]
- 29.Fox MD, Snyder AZ, Barch DM, Gusnard DA, Raichle ME. Transient BOLD responses at block transitions. Neuroimage. 2005;28:956–966. doi: 10.1016/j.neuroimage.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 30.Gottrup H, Kristensen AD, Bach FW, Jensen TS. Aftersensations in experimental and clinical hypersensitivity. Pain. 2003;103:57–64. doi: 10.1016/s0304-3959(02)00415-3. [DOI] [PubMed] [Google Scholar]
- 31.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333–1343. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 32.Granot M, Granovsky Y, Sprecher E, Nir RR, Yarnitsky D. Contact heat-evoked temporal summation: Tonic versus repetitive-phasic stimulation. Pain. 2006;122:295–305. doi: 10.1016/j.pain.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48:63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guedj E, Taieb D, Cammilleri S, Lussato D, de Laforte C, Niboyet J, Mundler O. 99mTc-ECD brain perfusion SPECT in hyperalgesic fibromyalgia. Eur J Nucl Med Mol Imaging. 2007;34:130–134. doi: 10.1007/s00259-006-0174-7. [DOI] [PubMed] [Google Scholar]
- 35.Harkins SW, Davis MD, Bush FM, Kasberger J. Suppression of first pain and slow temporal summation of second pain in relation to age. J Gerontol. 1996;51:260–265. doi: 10.1093/gerona/51a.5.m260. [DOI] [PubMed] [Google Scholar]
- 36.Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK. Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci. 2007;27:10000–10006. doi: 10.1523/JNEUROSCI.2849-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris RE, Sundgren PC, Craig AD, Kirshenbaum E, Sen A, Napadow V, Clauw DJ. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum. 2009;60:3146–3152. doi: 10.1002/art.24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hemington KS, Wu Q, Kucyi A, Inman RD, Davis KD. Abnormal cross-network functional connectivity in chronic pain and its association with clinical symptoms. Brain Struct Funct. 2016;221:4203–4219. doi: 10.1007/s00429-015-1161-1. [DOI] [PubMed] [Google Scholar]
- 39.Janal MN, Raphael KG, Cook DB, Sirois DA, Nemelivsky L, Staud R. Thermal temporal summation and decay of after-sensations in temporomandibular myofascial pain patients with and without comorbid fibromyalgia. J Pain Res. 2016;9:641–652. doi: 10.2147/JPR.S109038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen KB, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Giesecke T, Mainguy Y, Gracely R, Ingvar M. Evidence of dysfunctional pain inhibition in fibromyalgia reflected in rACC during provoked pain. Pain. 2009;144:95–100. doi: 10.1016/j.pain.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 41.Jensen KB, Srinivasan P, Spaeth R, Tan Y, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Vitton O, Gracely R, Ingvar M, Kong J. Overlapping structural and functional brain changes in patients with long-term exposure to fibromyalgia pain. Arthritis Rheum. 2013;65:3293–3303. doi: 10.1002/art.38170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim JY, Kim SH, Seo J, Kim SH, Han SW, Nam EJ, Kim SK, Lee HJ, Lee SJ, Kim YT, Chang Y. Increased power spectral density in resting-state pain-related brain networks in fibromyalgia. Pain. 2013;154:1792–1797. doi: 10.1016/j.pain.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 43.Kim J, Loggia ML, Cahalan CM, Harris RE, Beissner F, Garcia RG, Kim H, Barbieri R, Wasan AD, Edwards RR, Napadow V. The somatosensory link in fibromyalgia: Functional connectivity of the primary somatosensory cortex is altered by sustained pain and is associated with clinical/autonomic dysfunction. Arthritis Rheumatol. 2015;67:1395–1405. doi: 10.1002/art.39043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Konishi S, Donaldson DI, Buckner RL. Transient activation during block transition. Neuroimage. 2001;13:364–374. doi: 10.1006/nimg.2000.0691. [DOI] [PubMed] [Google Scholar]
- 45.Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: Premature aging of the brain? J Neurosci. 2007;11:4004–4007. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kucyi A, Davis KD. Dynamic functional connectivity of the default mode network tracks daydreaming. Neuroimage. 2004;100:471–480. doi: 10.1016/j.neuroimage.2014.06.044. [DOI] [PubMed] [Google Scholar]
- 47.Kucyi A, Davis KD. The dynamic pain connectome. Trends Neurosci. 2015;38:86–95. doi: 10.1016/j.tins.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 48.Kucyi A, Salomons TV, Davis KD. Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proc Natl Acad Sci U S A. 2013;110:18692–18697. doi: 10.1073/pnas.1312902110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Larson AA, Giovengo SL, Russell IJ, Michalek JE. Changes in the concentrations of amino acids in the cerebrospinal fluid that correlate with pain in patients with fibromyalgia: Implications for nitric oxide pathways. Pain. 2000;87:201–211. doi: 10.1016/S0304-3959(00)00284-0. [DOI] [PubMed] [Google Scholar]
- 50.Legangneux E, Mora JJ, Spreux-Varoquaux O, Thorin I, Herrou M, Alvado G, Gomeni C. Cerebrospinal fluid biogenic amine metabolites, plasma-rich platelet serotonin and [3H] imipramine reuptake in the primary fibromyalgia syndrome. Rheumatology. 2001;40:290–296. doi: 10.1093/rheumatology/40.3.290. [DOI] [PubMed] [Google Scholar]
- 51.Loggia ML, Berna CJ, Cahalan CM, Gollub RL, Wasan AD, Harris RE, Edwards RR, Napadow V. Disrupted brain circuitry for pain-related reward/punishment in fibromyalgia. Arthritis Rheumatol. 2014;66:203–212. doi: 10.1002/art.38191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loggia ML, Kim J, Gollub RL, Vangel MG, Kirsch I, Kong J, Wasan AD, Napadow V. Default mode network connectivity encodes clinical pain: An arterial spin labeling study. Pain. 2013;154:24–33. doi: 10.1016/j.pain.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lutz J, Jäger L, de Quervain D, Krauseneck T, Padberg F, Wichnalek M, Beyer A, Stahl R, Zirngibl B, Morhard D, Reiser M, Schelling G. White and gray matter abnormalities in the brain of patients with fibromyalgia: A diffusion-tensor and volumetric imaging study. Arthritis Rheum. 2008;58:3960–3969. doi: 10.1002/art.24070. [DOI] [PubMed] [Google Scholar]
- 54.Martinsen S, Flodin P, Berrebi J, Löfgren M, Bileviciute-Ljungar I, Ingvar M, Fransson P, Kosek E. Fibromyalgia patients had normal distraction related pain inhibition but cognitive impairment reflected in caudate nucleus and hippocampus during the Stroop Color Word Test. PLoS One. 2014;9:e108637. doi: 10.1371/journal.pone.0108637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCrae CS, O’Shea AM, Boissoneault J, Vatthauer KE, Robinson ME, Staud R, Perlstein WM, Craggs JG. Fibromyalgia patients have reduced hippocampal volume compared with healthy controls. J Pain Res. 2015;8:47–52. doi: 10.2147/JPR.S71959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Napadow V, Kim J, Clauw DJ, Harris RE. Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis Rheum. 2012;64:2398–2403. doi: 10.1002/art.34412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62:2545–2555. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Noordenbos W. Pain. Amsterdam: Elsevier; 1959. [Google Scholar]
- 59.Oaklander AL, Herzog ZD, Downs HM, Klein MM. Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain. 2013;154:2310–2316. doi: 10.1016/j.pain.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paret C, Kluetsch R, Ruf M, Demirakca T, Kalisch R, Schmahl C, Ende G. Transient and sustained BOLD signal time courses affect the detection of emotion-related brain activation in fMRI. Neuroimage. 2014;103:522–532. doi: 10.1016/j.neuroimage.2014.08.054. [DOI] [PubMed] [Google Scholar]
- 61.Price DD, Hayes RL, Ruda M, Dubner R. Neural representation of cutaneous aftersensations by spinothalamic tract neurons. Fed Proc. 1978;37:2237–2239. [PubMed] [Google Scholar]
- 62.Price DD, Staud R, Robinson ME, Mauderli AP, Cannon R, Vierck CJ. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain. 2002;99:49–59. doi: 10.1016/s0304-3959(02)00053-2. [DOI] [PubMed] [Google Scholar]
- 63.Robinson ME, Bialosky JE, Bishop MD, Price DD, George SZ. Supra-threshold scaling, temporal summation, and after-sensation: Relationships to each other and anxiety/fear. J Pain Res. 2010;3:25–32. doi: 10.2147/jpr.s9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sarlani E, Grace EG, Reynolds MA, Greenspan JD. Sex differences in temporal summation of pain and aftersensations following repetitive noxious mechanical stimulation. Pain. 2004;109:115–123. doi: 10.1016/j.pain.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 65.Sato H, Saisu H, Muraoka W, Nakagawa T, Svensson P, Wajima K. Lack of temporal summation but distinct after-sensations to thermal stimulation in patients with combined tension-type headache and myofascial temporomandibular disorder. J Orofac Pain. 2012;26:288–295. [PubMed] [Google Scholar]
- 66.Schreiber KL, Campbell C, Martel MO, Greenbaum S, Wasan AD, Borsook D, Jamison RN, Edwards RR. Distraction analgesia in chronic pain patients: The impact of catastrophizing. Anesthesiology. 2014;121:1292–1301. doi: 10.1097/ALN.0000000000000465. [DOI] [PubMed] [Google Scholar]
- 67.Schreiber KL, Martel MO, Shnol H, Shaffer JR, Greco C, Viray N, Taylor LN, McLaughlin M, Brufsky A, Ahrendt G, Bovbjerg D, Edwards RR, Belfer I. Persistent pain in postmastectomy patients: Comparison of psychophysical, medical, surgical, and psychosocial characteristics between patients with and without pain. Pain. 2013;154:660–668. doi: 10.1016/j.pain.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Staud RC, Cannon RL, Mauderli AP, Robinson ME, Price DD, Vierck CJ., Jr Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain. 2003;102:87–95. doi: 10.1016/s0304-3959(02)00344-5. [DOI] [PubMed] [Google Scholar]
- 70.Staud R, Price DD, Robinson ME, Vierck CJ., Jr Body pain area and pain-related negative affect predict clinical pain intensity in patients with fibromyalgia. J Pain. 2004;5:338–343. doi: 10.1016/j.jpain.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 71.Staud R, Robinson ME, Price DD. Temporal summation of second pain and its maintenance are useful for characterizing widespread central sensitization of fibromyalgia patients. J Pain. 2007;8:893–901. doi: 10.1016/j.jpain.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91:165–175. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 73.Staud R, Weyl EE, Riley JL, 3rd, Fillingim RB. Slow temporal summation of pain for assessment of central pain sensitivity and clinical pain of fibromyalgia patients. PLoS One. 2014;18:e89086. doi: 10.1371/journal.pone.0089086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- 75.Tagliazucchi E, Balenzuela P, Fraiman D, Chialvo DR. Brain resting state is disrupted in chronic back pain patients. Neurosci Lett. 2010;485:26–31. doi: 10.1016/j.neulet.2010.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5:133–137. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 77.Öçeyler N, Zeller D, Kahn AK, Kewenig S, Kittel-Schneider S, Schmid A, Casanova-Molla J, Reiners K, Sommer C. Small fibre pathology in patients with fibromyalgia syndrome. Brain. 2013;136:1857–1867. doi: 10.1093/brain/awt053. [DOI] [PubMed] [Google Scholar]
- 78.Uludağ K. Transient and sustained BOLD responses to sustained visual stimulation. Magn Reson Imaging. 2008;26:863–869. doi: 10.1016/j.mri.2008.01.049. [DOI] [PubMed] [Google Scholar]
- 79.Vaerøy H, Helle R, Førre O, Kass E, Terenius L. Elevated CSF levels of substance P and high incidence of Raynaud phenomenon in patients with fibromyalgia: New features for diagnosis. Pain. 1988;32:21–26. doi: 10.1016/0304-3959(88)90019-X. [DOI] [PubMed] [Google Scholar]
- 80.Wideman TH, Finan PH, Edwards RR, Quartana PJ, Buenaver LF, Haythornthwaite JA, Smith MT. Increased sensitivity to physical activity among individuals with knee osteoarthritis: Relation to pain outcomes, psychological factors, and responses to quantitative sensory testing. Pain. 2014;155:703–711. doi: 10.1016/j.pain.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 81.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62:600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 82.Wood PB, Glabus MF, Simpson R, Patterson JC., 2nd Changes in gray matter density in fibromyalgia: Correlation with dopamine metabolism. J Pain. 2009;10:609–618. doi: 10.1016/j.jpain.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 83.Wood PB, Patterson JC, 2nd, Sunderland JJ, Tainter KH, Glabus MF, Lilien DL. Reduced presynaptic dopamine activity in fibromyalgia syndrome demonstrated with positron emission tomography: A pilot study. J Pain. 2007;8:51–58. doi: 10.1016/j.jpain.2006.05.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


