Abstract
Alpha circuits (8–12 Hz), necessary for basic and complex brain processes, are abnormal in autism spectrum disorder (ASD). The present study obtained estimates of resting-state (RS) alpha activity in children with ASD and examined associations between alpha activity, age, and clinical symptoms. Given that the thalamus modulates cortical RS alpha rhythms, associations between thalamic structure and alpha activity were examined. RS magnetoencephalography was obtained from 47 typically-developing children (TDC) and 41 children with ASD. RS alpha activity was measured using distributed source localization. Left and right thalamic volume measurements were also obtained. In both groups, the strongest alpha activity was observed in Calcarine Sulcus regions. In Calcarine regions, only TDC showed the expected association between age and alpha peak frequency. ASD had more alpha activity than TDC in regions bordering the Central Sulcus as well as parietal association cortices. In ASD, whereas greater left Central Sulcus relative alpha activity was associated with higher Social Responsiveness Scale (SRS) scores, greater Calcarine region relative alpha activity was associated with lower SRS scores. Although thalamic volume group differences were not observed, relationships between thalamic volume and Calcarine alpha power were unique to TDC. The present study also identified a failure to shift peak alpha frequency as a function of age in primary alpha-generating areas in children with ASD. Findings suggested that increased RS alpha activity in primary motor and somatosensory as well as parietal multimodal areas—with increased alpha thought to reflect greater inhibition—might impair the ability to identify or interpret social cues. Finally, to our knowledge, this is the first study to report associations between thalamic volume and alpha power, an association observed only in TDC. The lack of thalamic and alpha associations in ASD suggests thalamic contributions to RS alpha abnormalities in ASD.
Keywords: Autism spectrum disorder, Alpha, Resting-state, Magnetoencephalography, Thalamus
Introduction
Background
In the resting-state (RS), parietal-occipital 8–12 Hz oscillations are the dominant rhythm. This rhythm is often referred to as the alpha or Berger rhythm (1929). RS alpha oscillations are strongest when at rest but modulated when performing tasks. For example, although alpha-band oscillations are typically thought to have an inhibitory function (e.g., Pfurtscheller 2006; Pfurtscheller et al. 1996), increasing evidence shows that via modulating attention processes (suppression and selection) alpha-band activity is associated with fundamental cognitive processes such as the ability to be consciously oriented in time, space, and context (for a review, see Klimesch 2012). Recent studies also indicate that via neural oscillations the brain generates it’s own temporal structure, with alpha rhythms—in particular the peaks-and-troughs of alpha oscillations—providing the timing for communication within and between brain regions (e.g., Osipova et al. 2008; VanRullen and Koch 2003). Given that alpha rhythms and the circuits associated with alpha oscillations provide a scaffold for basic and complex brain processes, an examination of alpha rhythms in neurodevelopmental disorders is of interest.
Neural oscillatory processes are abnormal in autism spectrum disorder (ASD), with characterizing neural abnormalities in ASD considered a promising route to understanding ASD-related brain dysfunction (and with normalizing neural activity a primary treatment target). In a previous study, our laboratory observed increased RS parietal-occipital alpha power in non-medicated children with ASD, with increased alpha associated with higher scores on the Social Responsiveness Scale (SRS) (Cornew et al. 2012). The present study sought to replicate and extend these findings, examining a new and larger sample of ASD and typically developing children (TDC). Rather than obtaining lobar measures of alpha activity, RS alpha activity was analyzed using a lead-field-based source localization method, Vector-based Spatio-temporal Analysis using L1-minimum norm (VESTAL; Huang et al. 2012) to obtain measures of alpha activity throughout the brain. Finally, as cortical RS alpha oscillations are highly dependent on the thalamus and given thalamic abnormalities in ASD (see “Discussion”), the present study also examined the hypothesis that cortical alpha oscillatory activity is associated with thalamic structure.
Methods and Materials
Subjects
Sixty-three male TDC and 58 ASD subjects were recruited for a study using multiple tasks to examine brain activity in ASD. Sixteen TDC and 17 ASD subjects elected not to do the eyes-closed RS task or did not keep their eyes closed during the task. Thus, in the present study, RS data was available from forty-seven male TDC (3 left-handed) and forty-one male ASD (3 left-handed, 2 ambidextrous) participants aged 6–14 years. Participants were selected according to the following criteria: (1) no history of traumatic brain injury or other significant medical or neurological abnormality, (2) no active psychosis, (3) no MRI contraindications and (4) no known drug or alcohol use within 24 hours of any study procedure. Members of the TDC group were evaluated by licensed clinical psychologists who ruled out the presence of DSM-IV-TR Axis I disorders based on clinical judgment, review of the child’s medical history form and parent interview. Current diagnosis of ASD was confirmed by expert clinical judgment based on NIH CPEA guidelines, including the Autism Diagnostic Observation Schedule—Generic (Lord et al. 2000) and Autism Diagnostic Interview—Revised (Lord et al. 1994) and with consensus diagnostic agreement between at least two experienced clinicians.
As shown in Table 1, groups did not differ in age or IQ. As expected, the participants with ASD had higher SRS scores than the TDC. Of the ten participants with ASD on medications, the following medications were taken: 1 on Zoloft, 3 on Prozac, 1 on Strattera, 1 on Vyvanse, 1 on Daytrana, 1 on Adderall, 2 on Focalin. No children in the TDC group reported medication use.
Table 1.
Demographic information
| TDC (N = 47) |
ASD (N = 41) |
|||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age | 10.48 | 1.71 | 10.31 | 1.84 |
| SRS | 41.02 | 6.21 | 78.01 | 9.96 |
| DASII GCA | 111.83 | 15.04 | 106.03 | 20.21 |
Group differences in age, t(86) = 0.44, and IQ, t(82) = 1.51, were not significant (p > 0.05)
ASD had higher SRS scores, t(84) = 21.01, p < 0.001)
MEG and MRI Data Acquisition, Co-registration, and MEG Forward Modeling with BEM
MEG data was recorded continuously using a 306-channel Vector-View system (Elekta-Neuromag, Helsinki, Finland) with a sampling rate of 1,000 Hz and a bandpass of 0.1–330 Hz. Electro-oculogram (EOG) (vertical EOG on the upper and lower left sides) and electrocardiogram (ECG) were also obtained. The subjects’ head position was monitored using head position indicator (HPI) coils attached to the scalp. During the 5-min RS eyes-closed exam, participants were asked to rest with their “eyes gently closed, like when you are sleeping”. To help remind participants to keep their eyes closed during the eyes-closed exam subjects wore a sleeping mask. During the recording the EOG channel was monitored and if subjects opened their eyes during the exam they were reminded to close their eyes.
After the MEG session, structural magnetic resonance imaging (sMRI) provided T1-weighted, 3-D anatomical images using a 3T Siemens Verio scanner (voxel size 0.8 × 0.8 × 0.9 mm3). To coregister MEG and sMRI data, three anatomical landmarks (nasion and right and left preauriculars) as well as an additional 200+ points on the scalp and face were digitized for each subject using the Probe Position Identification (PPI) System (Polhemus, Colchester, VT). To co-register the MEG and sMRI data, a transformation matrix that involved rotation and translation between the MEG and sMRI coordinate systems was obtained via a least-squares match of the PPI points to the surface of the scalp and face. A realistic boundary element method (BEM) head model was used for the MEG forward calculation (Huang et al. 2007; Mosher et al. 1999), with the BEM mesh constructed by tessellating the inner skull surface from the T1-weighted sMRI into ~6,000 triangular elements with ~5 mm size.
Frequency-Domain VESTAL Source Imaging for Oscillatory MEG Signals
MEG data were corrected for head motion using Max- Move, and Maxfilter was used for noise reduction using a signal space separation method with a temporal extension (tSSS; Taulu and Simola 2006). After motion correction and tSSS, MEG data were visually inspected for muscle and eye-blink artifact, and time segments with artifact removed. Groups did not differ on the total amount of artifact-free data available (t = 1.56, p > 0.05).
The RS eyes-closed data were filtered to examine alpha activity (8–12 Hz). MEG data were divided into 2.5-s epochs with 50 % overlap. For each epoch a Fast Fourier Transform (FFT) provided real and imaginary sensor-space coefficients for 10 equally-spaced bins (0.44 Hz resolution) with a starting frequency of 7.82 Hz and an end frequency of 12.21 Hz. The frequency-domain VESTAL source grid (5 mm isotropic resolution) was obtained by sampling gray-matter areas from the T1-weighted MRI of each subject. The sensor-space frequency-domain data were used by frequency-domain VESTAL to obtain the alpha amplitude (root mean squared) at each source location, which were then used to generated MEG alpha source images. Additional details of frequency-domain VESTAL source imaging are provided in Huang et al. (2012).
Group Statistics of VESTAL Solution
For group analyses, each subject’s alpha image was registered into a common (template) coordinate frame to facilitate voxelwise comparisons. This was accomplished by determining a non-linear transformation between the subject’s sMRI and that of a template subject (TD male, aged 9.74 years), using FSL’s FNIRT (http://www.fmrib.ox.ac.uk/fsl/fnirt). Using this transformation, the alpha image was transformed into the template space, resampled to a 1 mm spatial resolution and Gaussian spatial smoothing (sigma 3 mm) was applied.
Between-group t tests compared alpha VESTAL volumes. In the children with ASD, correlations examined associations between alpha activity and SRS scores. For all whole-brain analyses, the cluster size needed to obtain a family-wise correction was determined using a standard fMRI package (AFNI AlphaSim, B. Douglas Ward, 2000). As ASD RS alpha anomalies have been reported in absolute and relative power (e.g., Coben et al. 2008; Cornew et al. 2012), alpha group differences were computed for absolute and relative alpha power. Absolute power and relative power are related but distinct, with group differences in absolute power indicating differences in the raw power spectra, and group differences in relative power indicating group differences in the distributions of power. Thus, it is possible that group differences in absolute and relative power may differ. To compute relative alpha power, at each source voxel relative power was calculated by dividing alpha power (8–12 Hz) by total power (1–50 Hz).
In the present study, as relative and absolute alpha power findings were generally the same, relative alpha power findings are reported in the main manuscript and absolute power findings reported in the Online Supplement. In the Results section, the few instances where relative and absolute alpha power findings differed are noted.
Estimates of Thalamic Volume
Subcortical parcellations were computed within each hemisphere using the FIRST subcortical segmentation tool in FSL (Patenaude et al. 2011). Right and left thalamic volumes were calculated from the resulting binary masks. Whole brain tissue segmentation was then performed using FSL’s FAST tool (Zhang et al. 2001), yielding binary masks for white matter, grey matter and CSF. Grey and white matter volumes where combined to compute total brain volume. MR data from thirteen subjects was excluded due to excessive motion. Although little motion was observed in the remaining T1 images, these subject’s structural images were qualitatively scored for motion: none, very mild, mild. Chi square analyses indicted no group differences in motion on the T1 images (χ2 = 1.96, p = 0.58). In addition, visual inspection of each parcellation map showed that the left and right thalamus masks were correctly placed in all individuals.
Results
Relative Alpha Activity
Figure 1 shows VESTAL group-difference relative alpha maps. In both groups, the strongest relative alpha activity was observed in areas bordering the Calcarine Sulcus (TDC shown in red on the left, ASD shown in blue in the center). The statistics map on the right shows that participants with ASD had significantly more relative alpha activity than TDC in regions bordering the Central Sulcus as well as parietal association cortex regions (shown in yellow; p < 0.05, familywise corrected). Calcarine region group differences for absolute alpha power were observed in the left and right hemisphere (see Online Supplement).
Fig. 1.
VESTAL relative alpha power group-difference maps. Best seen in the coronal views (lower row), in both groups the strongest relative alpha activity was observed in primary visual areas (TDC shown in red on the left, ASD shown in blue in the center). No group differences were observed in primary alpha generator areas. Rather, the statistics map (third column) shows greater relative alpha power in ASD than TDC in regions bordering Central Sulcus and parietal-occipital regions (shown in yellow; p < 0.05, familywise corrected). The figure spectrum plot shows the TDC (red) and ASD (blue) relative power spectrum from 1 to 50 Hz for the Central Sulcus and Calcarine region ROIs (alpha range highlighted in gray; for each frequency bin, mean values obtained by averaging across voxels in each ROI for each frequency bin) (Color figure online)
The Fig. 1 spectrum plot shows TDC (red) and ASD (blue) relative power spectrum from 1 to 50 Hz for the group difference Central Sulcus ROI and also the Calcarine region ROI (alpha range highlighted in gray; for each frequency bin, mean values obtained by averaging across voxels in the group difference region of interest for each frequency bin). Although greater relative alpha power in TDC than ASD is suggested in the Calcarine region ROI power spectrum, group differences in this ROI were not observed (p = 0.30).
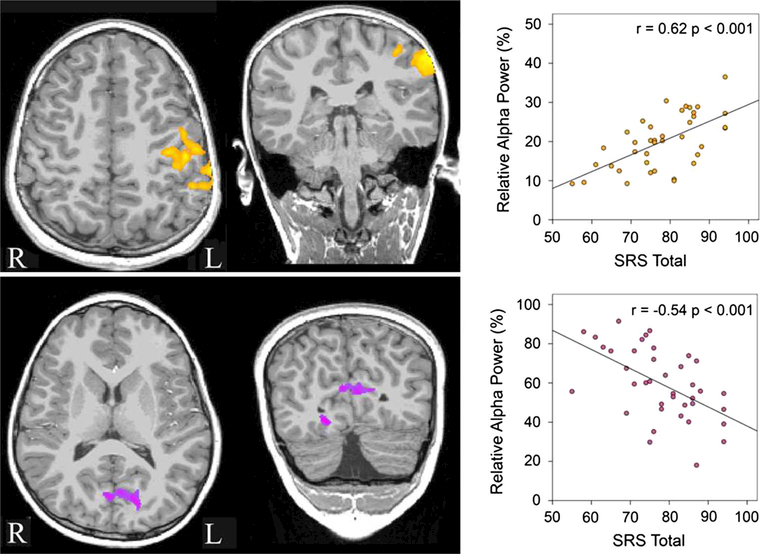
Figure 2 shows associations between relative alpha activity and SRS scores in ASD (p < 0.05, familywise corrected). The Fig. 2 scatterplots show that whereas higher SRS scores were associated with increased left-hemisphere Central Sulcus relative alpha activity (top row—shown in yellow), lower SRS scores were associated with increased Calcarine region relative alpha activity (bottom row—shown in purple). Similar associations were observed in right-hemisphere Central Sulcus regions (not shown), although clusters in this region were not large enough to survive familywise correction.
Fig. 2.
Areas showing associations between relative alpha power and SRS scores in the children with ASD are shown in yellow and purple (p < 0.05, familywise corrected). The scatterplot shows that whereas higher SRS scores were associated with increased left-hemisphere Central Sulcus relative alpha activity (top row—shown in yellow), lower SRS scores were associated with increased Calcarine region relative alpha activity (bottom row—shown in purple) (Color figure online)
Hierarchical regressions of the SRS scores with the alpha activity of the Central Sulcus and Calcarine region ROIs entered in independent steps (first, second, and vice versa) showed that alpha activity in both ROIs predicted unique variance in SRS scores (Central Sulcus + Calcarine region r = 0.72, p < 0.001). SRS Calcarine region associations were not observed for absolute alpha power (see Online Supplement).
Associations Between Age and Alpha Power and Alpha Peak Frequency
The frequency where alpha activity is strongest increases as a function of age, with an alpha peak at ~8 Hz in younger children and an alpha peak between 10 and 12 Hz in older children and adults (Klimesch 1999). To examine this developmental change in ASD, hierarchical regressions with age entered first, group second, and the interaction term last, examined associations between alpha peak frequency and age. Associations between relative alpha power and age were also examined. Regressions were run for two post hoc identified regions-of-interest (ROI): (1) voxels surrounding the Calcarine region (i.e., the region with the greatest concentration of alpha generators), with this region functionally defined as voxels with activity above the 25 percentile of the population (ASD and TD) grand average, and (2) the region where RS relative alpha power group differences were observed (yellow ROI in Fig. 1).
Alpha peak frequency
For the Calcarine region ROI, simple effect analyses of a marginally significant interaction term, F(1,84) = 2.78, p = 0.09, showed a positive relationship between age and relative alpha peak frequency in TDC (r = 0.30, p < 0.05) and a non-significant negative relationship in ASD (r = −0.05, p = 0.74). For the group difference ROI (i.e., near Central Sulcus), although the interaction term was not significant, simple effect analyses again suggested a relationship between age and relative alpha peak frequency in TDC (r = 0.25, p < 0.08) but not ASD (r = 0.03, p = 0.85).
Alpha power
For the Calcarine region ROI, neither the main effects nor interaction term were significant. For the group difference ROI (i.e., near Central Sulcus), the main effect of group (ASD>TDC) was significant, F(1,85) = 20.45, p < 0.001, with simple effect analysis of a significant interaction term, F(1,84) = 3.88, p = 0.05, indicating a negative association (decreased relative alpha activity in older subjects) in TDC (r = −0.36, p < 0.05) but not ASD (r = 0.06, p = 0.70).
Thalamic Volume: Associations with Age and Alpha Power
Hierarchical regressions examined group differences in left and right thalamic volume as well as associations between age and thalamic volume, with age entered first, group second, and the interaction term last. For the left thalamus, only the main effect of age was significant, F(1,73) = 6.8, p < 0.05, indicating that increased thalamic volume was positively associated with age in both groups. For the right thalamus, the main effect of group was marginally significant, F(1,73) = 3.19, p = 0.08, again indicating a positive association between thalamic volume and age. Neither left nor right thalamic volume were associated with SRS scores (r = 0.09, p > 0.05).
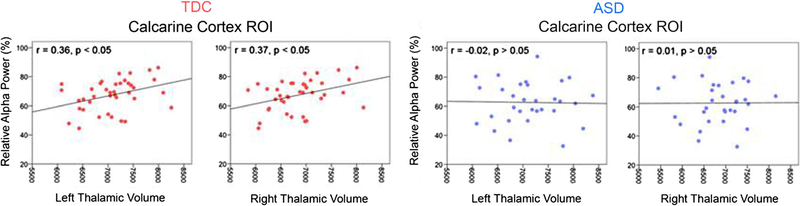
Hierarchical regressions examined associations between left and right thalamic volume and relative alpha power for the two ROIs described in “Associations Between Age and Alpha Power and Alpha Peak Frequency”. For each regression, as is standard, total brain volume was entered in the first block to control for brain size (groups did not differ on total brain volume, p = 0.82, thus allowing the use of brain volume as a covariate), left or right thalamic volume second, group third, and then the thalamic volume × group interaction term last. Examining relative alpha power in the Calcarine region ROI, for both left and right regressions, total brain volume did not explain significant variance in relative alpha power. After removing variance in Calcarine region relative alpha power associated with total brain volume, left and right thalamic volume explained a non-significant 3 % of the variance in relative alpha power (p’s > 0.05). Neither the group nor interaction terms were significant in either model (i.e., left or right thalamus). Although the interaction terms were not significant, Fig. 3 shows significant associations between thalamic volume and relative alpha power in TDC but not ASD. All findings were unchanged when age was added as a covariate (i.e., age and total brain volume in the first block).
Fig. 3.
Scatterplots showing for each group associations between left and right thalamic volume (x axis) and Calcarine region relative alpha power (y axis). Correlation r-values are shown in the top left of each plot (* p < 0.05; ** p < 0.01). Although hierarchical regressions indicated similar associations between thalamic volume and relative alpha power in ASD and TDC (via a non-significant interaction term), the scatterplots show associations between thalamic volume and relative alpha power only in TDC. The lack of a significant interaction term in the regression is likely due to insufficient power
Running analogous regressions for the relative alpha group difference ROI, total brain volume explained no variance in relative alpha power. Neither left nor right thalamic volume were associated with relative alpha power (p’s > 0.05). In the left and right hemisphere group relative alpha differences remained after removing variance in relative alpha power associated with total brain and thalamic volume (p’s < 0.001). The thalamic volume × group interaction terms were not significant. All findings were unchanged when age was added as a covariate (i.e., age and total brain volume in the first block).
Given significant associations between thalamic volume and Calcarine region relative alpha power in TDC, to explore the specificity of the findings, associations with other subcortical structures were examined: left and right caudate, left and right putamen, and left and right hippocampus. Table 2 shows zero-order and partial correlations (i.e., removing variance in each brain structure as well as variance in relative alpha power associated with total brain volume) for each group. Only in the TDC group did relationships remain significant for the left and right thalamus after removing variance in the structural and functional measures associated with total brain volume (shown in the Table 2 TDC partial correlation column). In the ASD group no subcortical brain structure showed significant associations with Calcarine region relative alpha power.
Table 2.
Calcarine region relative alpha and thalamic volume—zero-order and partial correlations
| TDC |
ASD |
|||||
|---|---|---|---|---|---|---|
| Total brain | Relative alpha Zero order | Relative alpha Partial+ | Total brain | Relative alpha Zero order | Relative alpha Partial+ | |
| Calcarine region ROI | ||||||
| Left thalamus | 0.51** | 0.36* | 0.34* | 0.34 | 0.02 | 0.04 |
| Right thalamus | 0.49** | 0.37* | 0.34* | 0.41* | 0.01 | 0.10 |
| Left caudate | 0.56** | 0.23 | 0.04 | 0.19 | 0.05 | 0.10 |
| Right caudate | 0.43** | 0.26 | 0.12 | 0.17 | −0.02 | 0.01 |
| Left putamen | 0.54** | 0.34* | 0.30 | 0.25 | 0.05 | 0.07 |
| Right putamen | 0.53** | 0.30 | 0.26 | 0.15 | −0.18 | −0.14 |
| Left hippocampus | 0.25 | −0.05 | −0.09 | 0.14 | 0.06 | 0.09 |
| Right hippocampus | 0.26 | −0.05 | −0.10 | 0.31 | 0.05 | 0.07 |
p < 0.05;
p < 0.01
Partial correlations removing variance associated with total brain volume from thalamic volume and alpha power (findings remained unchanged computing partial correlations including age)
Discussion
The present study showed abnormalities in ASD in a fundamental RS rhythm. First, increased RS alpha activity in participants with ASD versus TDC was observed in regions surrounding left Central Sulcus and left parietal association cortex (Fig. 1). Alpha activity was of clinical significance, with increased RS Central Sulcus alpha associated with higher SRS scores and increased RS Calcarine region alpha associated with lower SRS scores. Present alpha power findings thus replicate and extend Cornew et al. (2012), using the same RS task but here examining a group distinct from that reported in Cornew et al., using a different MEG system, and applying a more spatially precise source localization method.
Although group differences in alpha power were not observed in the alpha generators near the banks of the Calcarine region (the brain region containing the greatest concentration of alpha generators), the loss of an expected increase in the peak frequency of Calcarine region alpha generators as a function of age in ASD indicated abnormalities in the development of this fundamental RS rhythm. Cortical oscillatory abnormalities (oscillopathies) in ASD have been reported in several studies (Edgar et al. 2013; Rojas et al. 2008; Wilson et al. 2007). It is frequently argued that cortical oscillopathies are due to an imbalance of excitatory (e.g., glutamatergic) and inhibitory (e.g., GABAergic) activity in inhibitory interneuron and pyramidal cell cortical networks (e.g., see Gandal et al. 2010). Evidence in support of this hypothesis includes findings indicating that cortical inhibitory interneurons are abnormal in ASD (Casanova et al. 2002; Levitt et al. 2004). As cortical inhibitory interneurons are thought to play a role in maintaining alpha oscillations (Lorincz et al. 2009), present findings provide support for the above inhibitory-interneuron hypothesis.
Although the source of EEG and MEG alpha recordings are primarily cortical (Huang et al. 2014; Salmelin and Hari 1994), cortical RS alpha rhythms are thought to be highly dependent on thalamic ‘pacemaker’ neurons (e.g., see Anderson and Sears 1964; Jahnsen and Llinas 1984; Steriade and Deschenes 1984). In humans, simultaneous fMRI and EEG studies have observed increased thalamic bold signal during periods of RS alpha (Goldman et al. 2002), and simultaneous PET and EEG studies have observed a strong inverse correlation between alpha power and thalamic metabolism (Danos et al. 2001; Lindgren et al. 1999; Schreckenberger et al. 2004). Consistent with thalamic contributions to RS alpha, present findings showed an association between thalamic volume and alpha power in the TDC group. The failure to observe a similar relationship in ASD suggests that thalamic abnormalities (perhaps via thalamocortical pathways) contribute to RS alpha abnormalities in ASD (see Fig. 3; see also Table 2 showing associations between thalamic volume and alpha power only in controls).
Animal studies have most clearly identified the thalamic mechanisms associated with cortical RS alpha activity and thus inform present human findings. In particular, single unit recordings of lateral geniculate nucleus (LGN) thalamocortical (TC) neurons in awake cats have identified a subset of TC neurons (~20 %) in laminas A and A1 that fire during periods of cortical alpha activity. This firing, a process termed high-threshold (HT) bursting, occurs at relatively depolarized (>−55 mV) membrane potentials (Hughes et al. 2002; Hughes et al. 2004). Synchronization of HT burst-generating TC neurons and the accompanying rhythmic alpha field activities is reliant on thalamic gap junctions (GJ) (Hughes et al. 2002; Hughes et al. 2004). Genes produce GJ channel connexins proteins, and connexins help in the regulation of cell growth and cell-to-cell adhesion (Theis et al. 2004). Observing an increase in Connectin 43 (CX43) in the brains of individuals with ASD, Fatemi et al. (2008) suggested that increased CX43 expression may signify increased glial-neuronal signaling, potentially being responsible for enhanced cell-cell communication. The possibility that thalamic GJs dysfunction in ASD (not examined in Fatemi et al.) accounts for the present findings is thought provoking given the inhibitory interneuron dysfunction ASD hypothesis and given that GJs and inhibitory interneurons perform similar functions.
Although in ASD and TDC cortical RS alpha was predominantly concentrated in areas bordering the Calcarine Sulcus, increased RS alpha activity in ASD was observed only in regions bordering the Central Sulcus. RS 8–12 Hz oscillations over primary motor and somatosensory areas are often spatially defined as Rolandic μ rhythms (Gastaut 1958). Whereas RS alpha activity from generators near the Calcarine Sulcus are modulated by the LGN, μ rhythms appear to be modulated by the ventral posterior nucleus (VPN) (Bouyer et al. 1983; Howe and Sterman 1972). Although it is currently unknown if the mechanisms producing μ rhythms are analogous to those producing posterior alpha rhythms, some form of local interaction between thalamic neurons is thought to be necessary (for a discussion, see Hughes and Crunelli 2005).
The few studies examining thalamocortical communication and pathways from VPN to somatosensory areas do suggest abnormally increased thalamocortical connectivity in ASD. In an fMRI functional connectivity study, Mizuno et al. (2006) observed increased thalamocortical functional connectivity in ASD than TDC in left and right motor and somatosensory areas. Inspection of the Cheon et al. (2011) diffusion tensor imaging data show a marginally significant increase in fractional anisotropy in ASD versus TDC for the left superior thalamic radiations (i.e., fibers traveling from the VPN of the thalamus to primary somatosensory areas, p = 0.06), potentially interpretable as increased connectivity between thalamic VPN and somatosensory areas in ASD. Future work in this area, however, is needed to determine if VPN rather than LGN thalamocortical networks account for the present Central Sulcus alpha power group differences. In future studies, spatial distinction between Calcarine region and Central Sulcus alpha rhythms should also be assessed via functional probes (e.g., Calcarine region alpha modulated by eyes open/closed, and Central Sulcus alpha modulated by fist open/closed).
This is the first study to obtain whole-brain maps of RS alpha activity in children and to report an association between thalamic volume and RS alpha activity. Consistent with MEG investigations of RS alpha activity in adults (Huang et al. 2014; Salmelin and Hari 1994), in both groups, areas showing the strongest concentration of alpha generators bordered the Calcarine Sulcus. As previously noted, although ASD and TDC showed similar amounts of Calcarine region alpha activity, the expected association between alpha peak frequency and age was observed only in the TDC group. In addition, although groups did not differ in thalamic volume, as shown in the Table 2 partial correlations, the relationship between thalamic volume and Calcarine region alpha power appeared to be unique to TDC and, of the subcortical structures examined, also unique to the thalamus.
The present age-frequency findings and the thalamusalpha power findings suggest the loss of age to brain function and brain function to brain structure associations in ASD. An abnormality in the development of the RS alpha circuits in ASD is suggested by other studies. In terms of the frequency of alpha oscillations (and the observed loss of an age and peak alpha frequency relationship in ASD), in vitro cat studies indicate that alpha activity occurs when modulatory corticothalamic feedback in the LGN is mimicked by synaptically or pharmacologically activating the mGluR1a subtype of the metabotropic glutamate receptor located postsynpatically to corticothalamic fibers, with ‘weaker’ mGluR1a activation associated with theta oscillations and ‘stronger’ mGluR1a activation associated with alpha oscillations (e.g., Hughes et al. 2004). Thus, in addition to thalamocortical pathway abnormalities, present findings also suggest the possibility of mGluR1a dysfunction in ASD, with the hypothesis that mGluR1a activity typically changes as a function of age and with mGluR1a age-related changes delayed or absent in ASD.
Studies examining thalamic structure do report age-related delays in ASD, indicating that developmental delays could account for the alpha frequency findings. Nair et al. (2013) observed positive correlations between age and left parietal-occipital thalamocortical fractional anisotropy in typically developing but not ASD children, indicating age-related delays in the development of posterior thalamocortical pathways, and two studies have observed in controls but not ASD a positive association between thalamic volume and total brain volume (Hardan et al. 2006, 2008; Tsatsanis et al. 2003). Although not a focus of this study, it is noteworthy that the Table 2 correlations between total brain volume and subcortical structures appear weaker in ASD than TDC, perhaps suggesting less coordinated development of sub-cortical and cortical brain structures in ASD. The above are all consistent with abnormal brain development and perhaps as a byproduct abnormal subcortical to cortical connections in individuals with ASD accounting for an array of brain imaging findings (e.g., Edgar et al. 2013; Roberts et al. 2013).
Regarding the functional consequences of alpha activity, in the present study an association between increased left-hemisphere alpha activity and higher SRS scores as well as an association between increased Calcarine region alpha activity and lower SRS scores was observed. Alpha has been related to cognitive processes such as memory and attention (for review, see Klimesch 1999), with diminished alpha power commonly interpreted as reflecting cortical activity or task engagement (e.g., Davidson 1988; Markand 1990). The observation of increased RS alpha activity in primary motor and somatosensory as well as parietal multimodal areas—with increased alpha thought to reflect greater inhibition—might impair the ability to identify social cues (related to the sensory aspects of social behavior) or the ability to interpret social cues (the cognitive-behavioral aspect of reciprocal social behavior). The finding that increased Calcarine region alpha activity was associated with lower SRS scores may also reveal in ASD a more widespread RS alpha neural circuit dysfunction that contributes to cognitive control and cognitive flexibility dysfunction.
The consequences of alpha abnormalities in ASD may also be reflected in local and long-range functional connectivity abnormalities in ASD. Several recent studies provide support for this hypothesis. Berman et al. (2014) showed that increased occipital RS alpha was associated with abnormal local RS alpha-to-gamma phase amplitude coupling in ASD (sample distinct from the sample in this study). The Khan et al. (2013) finding of abnormal local fusiform face area alpha-to-gamma phase-amplitude coupling in ASD during a face processing task, as well as observing associations between local and long-range functional connectivity, suggest that alpha abnormalities may contribute to task-related local and long-range connectivity abnormalities in ASD. Studies examining the relationship between RS alpha activity and task-related long-range functional connectivity in ASD are of interest.
Thalamic volume was not associated with SRS scores, indicating that there is variance in cortical alpha power associated with SRS but not thalamic volume (also suggested by the analyses showing that group differences in Central Sulcus alpha power remained after removing variance in alpha power associated with thalamic volume) and thus perhaps indicating that cortical Central Sulcus alpha abnormalities in ASD reflect cortical as well as thalamic or thalamocortical abnormities.
A limitation of the present study is that although the samples were moderate-sized, the samples were not large enough for regression analyses to show group differences in the association between thalamic volume and Calcarine region alpha power. The lack of a thalamic volume x group interaction in the Calcarine region alpha power regressions is likely due to insufficient power (left thalamus interaction p = 0.11; right thalamus interaction p = 0.12).
In sum, present findings identified in ASD a failure to shift Calcarine region peak alpha frequency as a function of age in primary alpha-generating areas, and increased alpha activity in non-primary alpha-generating areas, with abnormally high RS primary motor/somatosensory alpha associated with higher SRS scores. Although groups did not differ in thalamic volume, the relationship between thalamic volume and Calcarine region alpha power appeared to be unique to the TDC group. The present age-frequency findings and the thalamus-alpha power findings suggest the loss of typical age-to-brain-function and brain-function-to-brain-structure relationships in ASD. Given the modulating influence of the thalamus on RS alpha activity, the above findings suggest the possibility that thalamic abnormalities are central to RS alpha abnormalities in ASD.
Acknowledgments
This study was supported in part by NIH Grant R01DC008871 (TR), RC1MH08879 (RTS), a NIH Grant K08 MH085100 (JCE), a NIH Grant R21MH098204 (JCE), a Grant from the Pennsylvania Department of Health (SAP # 4100042728 to RTS), and Award Number P30HD026979 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the NIH. Dr. Roberts gratefully acknowledges the Oberkircher Family for the Oberkircher Family Chair in Pediatric Radiology at CHOP.
Footnotes
Conflict of interest No author declares a conflict of interest.
Electronic supplementary material The online version of this article (doi: 10.1007/s10803-014-2236-1) contains supplementary material, which is available to authorized users.
Contributor Information
J. C. Edgar, Lurie Family Foundations MEG Imaging Center, Department of Radiology, The Children’s Hospital of Philadelphia, 34th and Civic Center Blvd, Wood Building, Suite 2115, Philadelphia, PA 10104, USA
K. Heiken, Lurie Family Foundations MEG Imaging Center, Department of Radiology, The Children’s Hospital of Philadelphia, 34th and Civic Center Blvd, Wood Building, Suite 2115, Philadelphia, PA 10104, USA
V. Chow, Lurie Family Foundations MEG Imaging Center, Department of Radiology, The Children’s Hospital of Philadelphia, 34th and Civic Center Blvd, Wood Building, Suite 2115, Philadelphia, PA 10104, USA
S. Liu, Lurie Family Foundations MEG Imaging Center, Department of Radiology, The Children’s Hospital of Philadelphia, 34th and Civic Center Blvd, Wood Building, Suite 2115, Philadelphia, PA 10104, USA
L. Bloy, Lurie Family Foundations MEG Imaging Center, Department of Radiology, The Children’s Hospital of Philadelphia, 34th and Civic Center Blvd, Wood Building, Suite 2115, Philadelphia, PA 10104, USA
K. M. Cannon, Lurie Family Foundations MEG Imaging Center, Department of Radiology, The Children’s Hospital of Philadelphia, 34th and Civic Center Blvd, Wood Building, Suite 2115, Philadelphia, PA 10104, USA
S. Qasmieh, Lurie Family Foundations MEG Imaging Center, Department of Radiology, The Children’s Hospital of Philadelphia, 34th and Civic Center Blvd, Wood Building, Suite 2115, Philadelphia, PA 10104, USA
T. P. L. Roberts, Lurie Family Foundations MEG Imaging Center, Department of Radiology, The Children’s Hospital of Philadelphia, 34th and Civic Center Blvd, Wood Building, Suite 2115, Philadelphia, PA 10104, USA
Y.-H. Chen, Department of Psychiatry, Center for Psychiatric Research, The University of New Mexico School of Medicine, Albuquerque, NM, USA
J. D. Herrington, Center for Autism Research, Department of Pediatrics, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA
J. Pandey, Center for Autism Research, Department of Pediatrics, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA
S. E. Levy, Center for Autism Research, Department of Pediatrics, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA
R. T. Schultz, Center for Autism Research, Department of Pediatrics, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA
M. Huang, Department of Radiology, San Diego VA Healthcare System, San Diego, CA, USA
References
- Anderson P, & Sears TA (1964). The role of inhibition in the phasing of spontaneous thalamocortical discharge. The Journal of Physiology, 173, 459–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H (1929). Über das elektrenkephalogramm des menschen. Archiv für Psychiatrie und Nervenkrankheiten, 87, 527–570. [Google Scholar]
- Berman J, Liu S, Bloy L, Blaskey L, Roberts TPL, & Edgar JC (2014)Alpha-to-Gamma phase-amplitude coupling methods and application to autism spectrum disorder. Brain Connectivity. doi: 10.1089/brain.2014.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouyer JJ, Tilquin C, & Rougeul A (1983). Thalamic rhythms in cat during quiet wakefulness and immobility. Electroencephalography and Clinical Neurophysiology, 55, 180–187. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, & Roy E (2002). Minicolumnar pathology in autism. Neurology, 58, 428–432. [DOI] [PubMed] [Google Scholar]
- Cheon KA, Kim YS, Oh SH, Park SY, Yoon HW, Herrington J, et al. (2011). Involvement of the anterior thalamic radiation in boys with high functioning autism spectrum disorders: A Diffusion Tensor Imaging study. Brain Research, 1417, 77–86. [DOI] [PubMed] [Google Scholar]
- Cornew L, Roberts TP, Blaskey L, & Edgar JC (2012). Resting-state oscillatory activity in autism spectrum disorders. Journal of Autism and Developmental Disorders, 42, 1884–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danos P, Guich S, Abel L, & Buchsbaum MS (2001). Eeg alpha rhythm and glucose metabolic rate in the thalamus in schizophrenia. Neuropsychobiology, 43, 265–272. [DOI] [PubMed] [Google Scholar]
- Davidson RJ (1988). EEG measures of cerebral asymmetry: conceptual and methodological issues. The International Journal of Neuroscience, 39, 71–89. [DOI] [PubMed] [Google Scholar]
- Edgar JC, Khan SY, Blaskey L, Chow VY, Rey M, Gaetz W, et al. (2013). Neuromagnetic oscillations predict evoked-response latency delays and core language deficits in autism spectrum disorders. Journal of Autism and Developmental Disorders. doi: 10.1007/s10803-013-1904-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Reutiman TJ, & Lee S (2008). Expression of astrocytic markers aquaporin 4 and connexin 43 is altered in brains of subjects with autism. Synapse, 62, 501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal MJ, Edgar JC, Ehrlichman RS, Mehta M, Roberts TP, & Siegel SJ (2010). Validating gamma oscillations and delayed auditory responses as translational biomarkers of autism. Biological Psychiatry, 68, 1100–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastaut H (1958) Some Aspects of the neurophysiological basis of conditioned reflexes and behaviour. In Wolstenholme GEW and O’Connor CM (Eds.), Ciba Foundation Symposium—Neurological Basis of Behaviour John Wiley & Sons, Ltd., Chichester, UK. [Google Scholar]
- Goldman RI, Stern JM, Engel J Jr, & Cohen MS (2002). Simultaneous EEG and fMRI of the alpha rhythm. NeuroReport, 13, 2487–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardan AY, Girgis RR, Adams J, Gilbert AR, Keshavan MS, & Minshew NJ (2006). Abnormal brain size effect on the thalamus in autism. Psychiatry Research, 147, 145–151. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Girgis RR, Adams J, Gilbert AR, Melhem NM, Keshavan MS, et al. (2008). Brief report: abnormal association between the thalamus and brain size in Asperger’s disorder. Journal of Autism and Developmental Disorders, 38, 390–394. [DOI] [PubMed] [Google Scholar]
- Howe RC, & Sterman MB (1972). Cortical-subcortical EEG correlates of suppressed motor behavior during sleep and waking in the cat. Electroencephalography and Clinical Neurophysiology, 32, 681–695. [DOI] [PubMed] [Google Scholar]
- Huang MX, Huang CW, Robb A, Angeles A, Nichols SL, Baker DG, et al. (2014). MEG source imaging method using fast L1 minimum-norm and its applications to signals with brain noise and human resting-state source amplitude images. NeuroImage, 84, 585–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MX, Nichols S, Robb A, Angeles A, Drake A, Holland M, et al. (2012). An automatic MEG low-frequency source imaging approach for detecting injuries in mild and moderate TBI patients with blast and non-blast causes. Neuroimage, 61, 1067–1082. [DOI] [PubMed] [Google Scholar]
- Huang MX, Song T, Hagler DJ Jr, Podgorny I, Jousmaki V, Cui L, et al. (2007). A novel integrated MEG and EEG analysis method for dipolar sources. Neuroimage, 37, 731–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SW, Blethyn KL, Cope DW, & Crunelli V (2002). Properties and origin of spikelets in thalamocortical neurones in vitro. Neuroscience, 110, 395–401. [DOI] [PubMed] [Google Scholar]
- Hughes SW, & Crunelli V (2005). Thalamic mechanisms of EEG alpha rhythms and their pathological implications. The Neuroscientist, 11, 357–372. [DOI] [PubMed] [Google Scholar]
- Hughes SW, Lorincz M, Cope DW, Blethyn KL, Kekesi KA, Parri HR, et al. (2004). Synchronized oscillations at alpha and theta frequencies in the lateral geniculate nucleus. Neuron, 42, 253–268. [DOI] [PubMed] [Google Scholar]
- Jahnsen H, & Llinas R (1984). Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. Journal of Physiology, 349, 205–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Gramfort A, Shetty NR, Kitzbichler MG, Ganesan S, Moran JM, et al. (2013). Local and long-range functional connectivity is reduced in concert in autism spectrum disorders. Proceedings of the National Academy of Science USA, 110(8), 3107–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W (1999). EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Research Reviews, 29, 169–195. [DOI] [PubMed] [Google Scholar]
- Klimesch W (2012). Alpha-band oscillations, attention, and controlled access to stored information. Trends in Cognitive Sciences, 16, 606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt P, Eagleson KL, & Powell EM (2004). Regulation of neocortical interneuron development and the implications for neurodevelopmental disorders. Trends in Neurosciences, 27, 400–406. [DOI] [PubMed] [Google Scholar]
- Lindgren KA, Larson CL, Schaefer SM, Abercrombie HC, Ward RT, Oakes TR, et al. (1999). Thalamic metabolic rate predicts EEG alpha power in healthy control subjects but not in depressed patients. Biological Psychiatry, 45, 943–952. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH Jr, Leventhal BL, DiLavore PC, et al. (2000). The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30, 205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, & Le Couteur A (1994). Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24, 659–685. [DOI] [PubMed] [Google Scholar]
- Lorincz ML, Kekesi KA, Juhasz G, Crunelli V, & Hughes SW (2009). Temporal framing of thalamic relay-mode firing by phasic inhibition during the alpha rhythm. Neuron, 63, 683–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markand ON (1990). Alpha rhythms. Journal of Clinical Neurophysiology, 7, 163–189. [DOI] [PubMed] [Google Scholar]
- Mizuno A, Villalobos ME, Davies MM, Dahl BC, & Muller RA (2006). Partially enhanced thalamocortical functional connectivity in autism. Brain Research, 1104, 160–174. [DOI] [PubMed] [Google Scholar]
- Mosher JC, Leahy RM, & Lewis PS (1999). EEG and MEG: Forward solutions for inverse methods. IEEE Transactions on Bio-medical Engineering, 46, 245–259. [DOI] [PubMed] [Google Scholar]
- Nair A, Treiber JM, Shukla DK, Shih P, & Muller RA (2013). Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain, 136, 1942–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osipova D, Hermes D, & Jensen O (2008). Gamma power is phase-locked to posterior alpha activity. PLoS One, 3, e3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, & Jenkinson M (2011). A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage, 56, 907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G (2006). The cortical activation model (CAM). Progress in Brain Research, 159, 19–27. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancak A Jr, & Neuper C (1996). Event-related synchronization (ERS) in the alpha band-an electrophysiological correlate of cortical idling: a review. international journal of psychophysiology, 24, 39–46. [DOI] [PubMed] [Google Scholar]
- Roberts TP, Lanza MR, Dell J, Qasmieh S, Hines K, Blaskey L, et al. (2013). Maturational differences in thalamocortical white matter microstructure and auditory evoked response latencies in autism spectrum disorders. Brain Research, 1537, 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas DC, Maharajh K, Teale P, & Rogers SJ (2008). Reduced neural synchronization of gamma-band MEG oscillations in first-degree relatives of children with autism. BMC Psychiatry, 8, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmelin R, & Hari R (1994). Characterization of spontaneous MEG rhythms in healthy adults. Electroencephalography and Clinical Neurophysiology, 91, 237–248. [DOI] [PubMed] [Google Scholar]
- Schreckenberger M, Lange-Asschenfeldt C, Lochmann M, Mann K, Siessmeier T, Buchholz HG, et al. (2004). The thalamus as the generator and modulator of EEG alpha rhythm: a combined PET/EEG study with lorazepam challenge in humans. NeuroImage, 22, 637–644. [DOI] [PubMed] [Google Scholar]
- Steriade M, & Deschenes M (1984). The thalamus as a neuronal oscillator. Brain Research, 320, 1–63. [DOI] [PubMed] [Google Scholar]
- Taulu S, & Simola J (2006). Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Physics in Medicine and Biology, 51, 1759–1768. [DOI] [PubMed] [Google Scholar]
- Theis M, Speidel D, & Willecke K (2004). Astrocyte cultures from conditional connexin43-deficient mice. Glia, 46, 130–141. [DOI] [PubMed] [Google Scholar]
- Tsatsanis KD, Rourke BP, Klin A, Volkmar FR, Cicchetti D, & Schultz RT (2003). Reduced thalamic volume in high-functioning individuals with autism. Biological Psychiatry, 53, 121–129. [DOI] [PubMed] [Google Scholar]
- VanRullen R, & Koch C (2003). Is perception discrete or continuous? Trends in Cognitive Sciences, 7, 207–213. [DOI] [PubMed] [Google Scholar]
- Wilson TW, Rojas DC, Reite ML, Teale PD, & Rogers SJ (2007). Children and adolescents with autism exhibit reduced MEG steady-state gamma responses. Biological Psychiatry, 62, 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brady M, & Smith S (2001). Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. iEEE Transactions on Medical imaging, 20, 45–57. [DOI] [PubMed] [Google Scholar]