Abstract
Abnormal brain oscillatory activity has been found in autism spectrum disorders (ASD) and proposed as a potential biomarker. While several studies have investigated gamma oscillations in ASD, none have examined resting gamma power across multiple brain regions. This study investigated resting gamma power using EEG in 15 boys with ASD and 18 age and intelligence quotient matched typically developing controls. We found a decrease in resting gamma power at right lateral electrodes in ASD. We further explored associations between gamma and ASD severity as measured by the Social Responsiveness Scale (SRS) and found a negative correlation between SRS and gamma power. We believe that our findings give further support of gamma oscillations as a potential biomarker for ASD.
Keywords: Autism, Gamma, EEG, SRS, Resting state, Laterality
Introduction
Autism spectrum disorders (ASD) are characterized by social abnormalities, impaired communication and stereo-typed/repetitive behaviors (American Psychiatric Association 2000). In addition to the triad of characteristic behaviors, many individuals with ASD demonstrate information processing disruptions (Magnee et al. 2011; Marco et al. 2011). Gamma band oscillations (30–200 Hz) are the most commonly studied cortical oscillation in psychiatric disorders, including ASD. Gamma frequency oscillations are thought to represent firing of inhibitory GABAergic interneurons (Gonzalez-Burgos and Lewis 2008; Uhlhaas and Singer 2010). This is consistent with the hypothesis that a loss or reduction of inhibitory interneurons may lead to impaired information processing in ASD, including the processing of social-emotional stimuli (Rubenstein and Merzenich 2003). In fact, pharmacological modulation of parvalbumin containing GABAergic interneurons directly influences cortical rhythms such as gamma (Gonzalez- Burgos and Lewis 2008; Sohal et al. 2009; Gonzalez- Burgos et al. 2010). Most recently, it has been suggested that impaired cortical frequency oscillations may represent an endophenotype that underlies the information processing impairments in ASD (Rojas et al. 2008, 2011; Gandal et al. 2010).
While disrupted oscillations in the gamma frequency band have frequently been found in ASD, the pattern of findings is often complex. Sheikhani and colleagues, for example, reported reduced resting gamma spectra criteria at frontal and right temporal EEG electrodes in children with ASD versus typically developing children (TDC) (Sheikhani et al. 2009). This finding is suggestive of frontal and/or lateral group differences in gamma at rest. In a follow up study, they were able to replicate the right temporal electrode reduction in spectral criteria but also showed an increase in resting gamma coherence in ASD compared to TDC for multiple pairs of electrodes across the brain (Sheikhani et al. 2010).
Inconsistencies in the major findings and methodologies such as reduced resting gamma spectral criteria and increased resting gamma coherence have made the literature unclear regarding gamma trends in ASD. There are likely many reasons for these discrepancies, including the significant heterogeneity with ASD itself, which is clearly not a single disorder, but rather a grouping of diverse etiologies with commonalties in phenotypic expression. There are important methodological differences between studies as well. Germane to this study is the fact that prior studies have reported electrophysiological data without directly comparing multiple brain regions (i.e. scalp topographies) or addressing potential links to the core behavior abnormalities characteristic of ASD. The current study aimed to investigate differences in total resting gamma power across multiple brain sites in children with ASD, relative to well-matched TDC and to associate markers of group differences with individual differences in symptom severity in youth with ASD. Our approach differs from previous studies in that we are examining gamma power across 36 electrodes grouped by brain region to provide a more complete view of the topographic differences in resting physiology between ASD and TDC. In addition, we explored a potential relationship between resting gamma and ASD symptomatology. Based on previous studies, we expected to see a reduction in gamma in ASD over the right hemisphere.
Methods
The study was approved by the Ethics Committee of the RWTH Aachen University Hospital. Informed consent was obtained from all participants and their parents.
Participants
A total of 15 males diagnosed with ASD (14 with Asperger syndrome and 1 with high functioning autism) and 18 typically developing male controls (TDC) were included in the final data analysis. Both groups ranged in age from 9 to 18 years, and all participants were right-handed as assessed by the Edinburgh Inventory (Oldfield 1971). Only participants with a full-scale intelligence quotient (IQ) ≥ 80 (estimated based on a short version of the WISC-III; (Gleissner et al. 2003) were included. The groups did not differ with respect to age, IQ, and handedness (Kohls et al. 2011). Table 1 displays the group demographics.
Table 1.
The participant demographics including age, handedness, intelligence quotient (IQ), Autism Diagnostic Observation Schedule (ADOS-G), Autism Diagnostic Interview (ADI-R), Social Communication Questionnaire (SCQ) and Social Responsiveness Scale (SRS) in 15 participants with autism spectrum disorders (ASD) and 18 typically developing children (TDC)
| ASD mean (SD) | TDC mean (SD) | p values | |
|---|---|---|---|
| Age | 15.1 (2.9) | 14.2 (2.9) | 0.443 |
| Handedness (Edinburgh) | 70.9 (24.2) | 78.3 (17.7) | 0.319 |
| IQ (WISC-III) | 108.6 (16.0) | 110.7 (12.9) | 0.69 |
| ADOS-G_total | 12.0 (3.4) | NA | NA |
| ADI-R_total | 38.4 (8.3) | NA | NA |
| SCQ_total | 23.0 (6.4) | 3.4 (1.9) | <0.01 |
| SRS total | 99.0 (31.1) | 12.2 (7.1) | <0.01 |
Children and adolescents with ASD were recruited from the Departments of Child and Adolescent Psychiatry and Psychotherapy in Aachen. All participants were diagnosed by experienced clinicians according to ICD-10 and DSM- IV criteria. Diagnoses were confirmed using the ADOS-G (Lord et al. 2000) and the Autism Diagnostic Interview-Revised (ADI-R; (Lord et al. 1994)) conducted by trained examiners (Kohls et al. 2011). The final sample of participants with ASD had no history of comorbid psychiatric disorders. At the time of testing, two of the ASD participants were taking atypical neuroleptic medications. Parents also completed the Social Communication Questionnaire (SCQ: Rutter et al. 2003) and the Social Responsiveness Scale (SRS: Constantino et al. 2004). The SRS is a dimensional measure of ASD symptom severity while the SCQ is a screening instrument based on the ADI. The SRS and SCQ were collected from all ASD and TDC subjects.
The TDC group was recruited from local primary or grammar schools and underwent an extensive psychiatric examination conducted by an experienced child psychiatrist using a standardized, semi-structured interview to assess current and past episodes of psychopathology according to DSM-IV criteria (K-SADS-PL) (Kaufman et al. 1997). In addition, parents evaluated the behavior of their children with regard to psychopathology using the Child Behavior Checklist (CBCL 4–18) (Achenbach 1991). Children with a T-score above 63 on the Internalizing, Externalizing, and Total Problems scales of the CBCL were excluded. The SCQ was also administered to the TDC group and no subjects scored above the screening cutoff for ASD. At the time of testing, none of the TDC was taking medication. All participants had normal or corrected-to-normal vision.
Electrophysiological Recording
Participants were seated in a comfortable chair in an electrically shielded, sound-attenuated, dimly lit booth. Participants were asked to relax with their eyes open for 3 min while EEG was recorded. Participants were also videotaped during this period to monitor overt movements, general eye gaze and arousal state. All participants were noted to be awake with their eyes open during the duration of testing. All data were collected at the Cognitive Neurology Section, Institute of Neuroscience and Medicine, Research Centre Jülich, Germany.
During task performance continuous EEG recordings were digitally obtained from 64 Ag/AgCl electrodes using a high-impedance, 64-channel Net Amps 200 system (Electrical Geodesics Inc., Eugene, OR, USA). The electrodes were mounted equidistant in a flexible cap and distributed evenly across the head surface (Easycap GmbH, Herrsching-Breitbrunn, Germany). A vertical electro-oculogram (VEOG) was recorded from above and below the left eye, and a horizontal electro-oculogram (HEOG) was recorded from the outer canthus of each eye. The ground electrode was positioned below the left mastoid, and the nose tip was used as reference. An online band-pass filter was set at 0.1 Hz to 70 Hz, and data were offline low-pass filtered at 55 Hz. Raw data were visually inspected for artifacts, and trials containing overt movement, eye blinks and extreme voltage exceeding 180 μV were rejected using EEGlab software Matlab toolbox (Delorme and Makeig 2004). The sampling rate of the electric signal was 250 Hz. Electrode impedances were kept below 20 kΩ. The raw EEG data were stored on a hard disk and processed offline with EEGlab. As an additional quality control measure, we performed analyses for gross horizontal and vertical eye movements as in Keren et al. (2010). We found no group differences in eye movement (p = 0.674).
Statistics
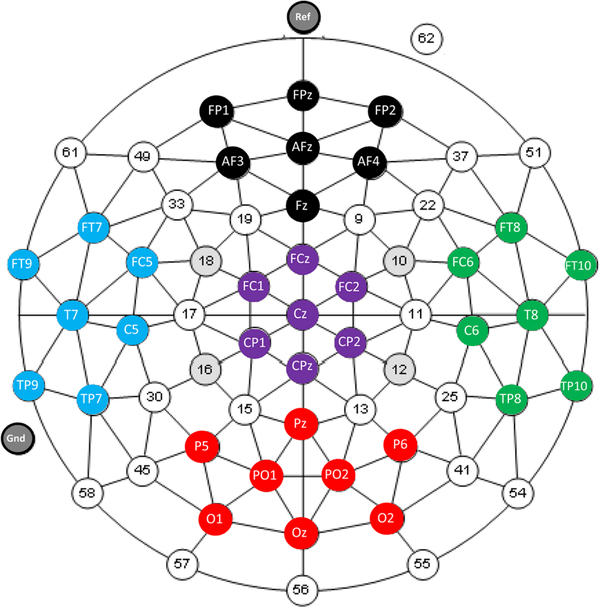
Following filtering and artifact rejection, the 3 min of data were segmented into one second epochs. The total power spectrum per electrode was calculated using the FFT function in EEGlab (window length 256, overlap 128, FFT length 512) for the gamma range of 30–50 Hz and analyzed using SPSS version 19 (IBM). In order to investigate trends in topography without the confounds of multiple comparisons, statistical analyses focused on mean gamma spectral power of 36 electrodes binned across 5 brain regions: frontal (FP1, FPz, FP2, AF3, AFz, AF4, Fz), central (FC1, FCz, FC2, Cz, CP1, CPz, CP2), parietal/ occipital (P5, Pz, P6, PO1, PO2, O1, Oz, O2), left lateral (FT9, FT7, FC5, T7, C5, TP9, TP7) and right lateral (FT10, FT8, FC6, T8, C6, TP10, TP8). Figure 1 shows the electrode groupings. A repeated measures ANOVA was used with diagnostic group (ASD or TDC) as the between subjects factor and topography (frontal, central, parietal/ occipital, left lateral or right lateral) as the repeated measure. Age was used as a co-variate in the ANOVA to account for the large age range and the possibility of age-related changes in EEG. Significant interactions were explored using planned contrasts with and without Bon-ferroni correction. The alpha level was set at 0.05.
Fig. 1.
The electrode groupings of frontal (black: FP1, FPz, FP2, AF3, AFz, AF4, Fz), central (purple: FC1, FCz, FC2, Cz, CP1, CPz, CP2), parietal/occipital (red: P5, Pz, P6, PO1, PO2, O1, Oz, O2), left lateral (blue: FT9, FT7, FC5, T7, C5, TP9, TP7) and right lateral (green: FT10, FT8, FC6, T8, C6, TP10, TP8) clusters
Partial correlations, controlling for age, were calculated to test for association between resting gamma power (at brain regions that showed the greatest group differences) and ASD symptom severity (total SRS score) across participant groups.
Results
Reduced Resting Gamma Power in ASD
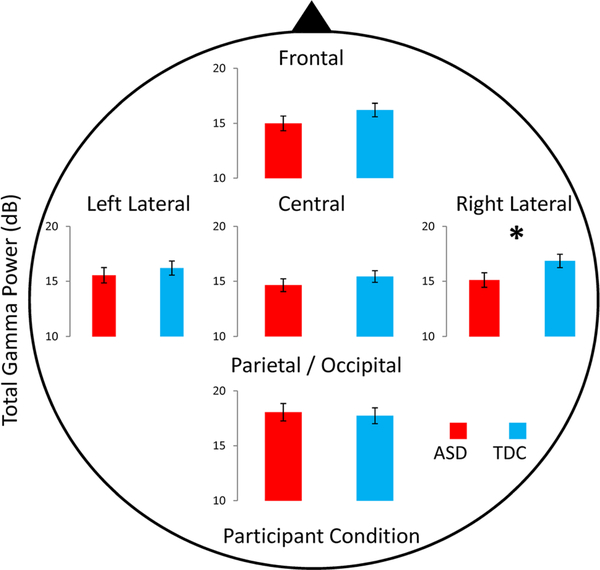
In the gamma frequency band, there were significant main effects of topographic region (p = 0.036), and a topography by group interaction (p = 0.029) (for details see Table 2). Follow-up planned contrasts revealed that at the right lateral electrodes, resting gamma power was significantly reduced in ASD compared to TDC (p = 0.04) which, however, did not survive Bonferroni correction (p = 0.06). Figure 2 demonstrates the mean group differences in gamma power by topographic region. We explored potential reductions in gamma power in the ASD group at other brain regions, but did not find significant group differences (frontal: p = 0.2; central: p = 0.3; parietal/occipital: p = 0.7; left lateral: p = 0.5).
Table 2.
Group (ASD or TDC) and topographic (frontal, central, parietal/occipital, left lateral and right lateral electrodes) statistical differences in the gamma frequency band
| Frequency | Measure | F | p | |
|---|---|---|---|---|
| Gamma (30–50 Hz) | (1) Group | F(1,30) = 0.94 | 0.340 | 0.030 |
| (2) Topography | F(4,120)= 2.66 | 0.036 | 0.081 | |
| 1 × 2 | F(4,120) = 2.79 | 0.029 | 0.085 | |
| Means by group and topography | ASD (std error) | TDC (std error) | ||
| Gamma (30–50 Hz) | Frontal | 14.9 (0.7) | 16.3 (0.6) | |
| Central | 14.6(0.6) | 15.5 (0.5) | ||
| Parietal | 17.9 (0.8) | 17.8 (0.8) | ||
| Left lateral | 15.5(0.7) | 16.2 (0.6) | ||
| Right lateral | 15.0 (0.7) | 16.9 (0.6) | ||
Bold values indicate statistically significant
Means for group by region are also included. Note the significant topography by group interaction. = partial eta squared. Age is a co-variate in this analysis
Fig. 2.
The differences in gamma power between ASD (red bars) and TDC (blue bars) at each electrode group. Note the group difference in resting gamma at the right lateral electrodes. Error bars represent standard error of the mean
Resting Gamma Power and ASD Symptoms
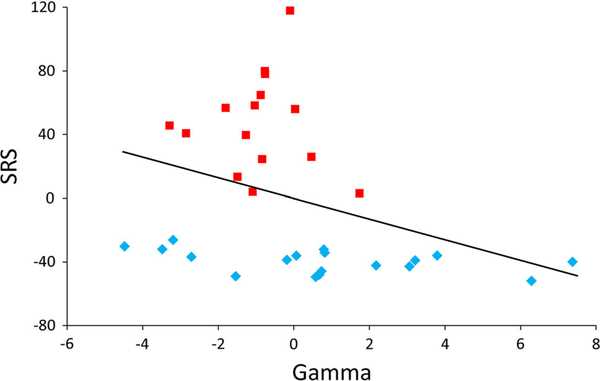
A significant negative correlation was found between gamma power at the right lateral electrode group and the SRS total score across the whole sample, controlling for age (r = −0.35; p = 0.046). This finding is displayed in Fig. 3 using residuals from Age by Gamma and Age by SRS linear regressions to account for the partial correlation statistics.
Fig. 3.
A significant correlation between right lateral resting gamma power and raw SRS total score across groups (red bars represent ASD; blue bars note TDC). Residuals by age are displayed to account for age as a co-variate
Discussion
Our data demonstrate a reduction in resting gamma power at right lateral electrodes in children with ASD compared to TDC. These data suggest a specific reduction of gamma activity in ASD during a no-task, resting study paradigm. Our results are consistent with the right hemisphere (temporal) reduction in gamma spectra criteria in ASD shown in previous reports (Sheikhani et al. 2009, 2010). We also demonstrate a novel association between measures of resting gamma physiology and social reciprocity.
Previous resting state EEG studies have also found abnormal right hemisphere oscillations in ASD. These studies, primarily from Sheikhani and colleagues, have used diverse methods of analysis (gamma spectra criteria versus electrode coherence) to demonstrate group differences in resting gamma between ASD and TDC (Sheikhani et al. 2009, 2010). A recent MEG study reported elevated resting oscillatory activity (eyes closed condition) in ASD across multiple frequency bands including high frequencies such as gamma (20–120 Hz) (Cornew et al. 2012). Interestingly, they showed an association between increased temporal and parietal resting alpha (8–12 Hz) oscillations and ASD symptom severity measured by the SRS. While the findings from Cornew and colleagues are inconsistent with our data, methodological differences including the use of MEG, the wide gamma band range and that the participants were instructed to close their eyes during data recording may account for these discrepancies.
Atypical laterality using EEG has also been studied in healthy adults and typically developing children as well as those with ASD (Muller et al. 1999; Fox et al. 1995; Dawson et al. 1995). In ASD, an investigation by Dawson et al. (1995) demonstrated reduced frontal alpha power and hemispheric asymmetry in affected children during passive visual stimulation (Dawson et al. 1995). However, this study only looked at frontal electrodes and did not examine gamma power. Here, we extend the finding of atypical laterality in ASD focusing on gamma oscillations at right lateral electrodes. Interestingly, Müller and colleagues showed enhanced right hemisphere asymmetry in the gamma band during processing of emotion provoking stimuli (affective images) in typical adults (Muller et al. 1999). Together, these studies suggest the possibility of right hemisphere abnormalities associated with emotional and social information processing which may have specific relevance to the social deficits found in ASD.
The correlation shown between resting gamma power and SRS across diagnostic group suggests a potential association between a physiological measure and a quantitative measure that assesses the severity of social impairment in ASD. This association warrants further investigation with large samples of typically developing individuals or large families affected by ASD in order to help strengthen this finding. Together with our findings, the literature suggests that aberrant gamma oscillations could play an important role in the processing of social and emotional information in children with ASD, warranting further investigation of gamma rhythms, and the disruption thereof in ASD, as a potential biomarker for this neuro-developmental disorder.
Although the present study adds to our understanding of gamma as a potential biomarker for ASD, it could be built upon in a number of important ways. For example, it would be particularly interesting to investigate gamma oscillations with more elaborate source localization techniques, such as low resolution brain electromagnetic tomography (LORETA) or Brain Electrical Source Analysis (BESA), to pinpoint the neural mechanisms and associated neural structures underlying altered gamma functioning in ASD. In addition, an increased sample size may better elucidate differences between diagnostic groups and correlations with psychometric measures of autistic traits. Our ASD group consisted of high-functioning boys with Autistic Disorder and Asperger syndrome. Given the heterogeneity within the autistic spectrum, it is possible that dysfunctions in gamma oscillations manifest differently across the autism spectrum. Our conclusions are thus limited and require replication with larger samples that include children with low-functioning autism versus other clinical comparison groups (e.g., anxiety disorder, ADHD).
Another possible confound of this study is the presence of microsaccade eye movements in EEG. Typically, spike potentials associated with microsaccades occur in the parietal/occipital region (when nose is used as reference site) and can influence stimulus induced gamma activity (Keren et al. 2010). Because we found group differences at rest across right lateral, but not parietal/occipital electrodes, we believe that this phenomenon is a negligible factor to our findings. However, due to methodological constraints, such as a low sampling rate (250 vs. 1,024 Hz used by Keren et al. 2010), we were not able to apply the procedures suggested by Keren and colleagues to detect and reject potential microsaccadic spikes in our data set. Thus, we cannot completely rule out the possibility of microsaccades biasing our gamma findings. Additionally, the examination of gamma band activity in EEG may be confounded by the presence of ocular, forehead and neck muscle activity. While we rejected EEG segments that were contaminated by overt movements, subtle neck and face muscle activity might have contributed to our findings.
In summary, we found reduced resting gamma power in children and adolescents with ASD that is specific to the right lateral hemisphere. We believe that this study provides a foundation for future studies to correlate physiological and behavioral measures of ASD symptoms in order to increase our understanding of the fundamental neural deficits observed in ASD. While changes in gamma have been reported in other neuropsychiatric conditions and thus are not specific to ASD, our findings contribute to the utility of gamma oscillations as a potential biomarker that may cut across diagnostic boundaries (Uhlhaas and Singer 2010). Our findings also highlight the need for those studies that investigate evoked gamma oscillations following sensory stimuli to study group differences in resting state in order to provide a full picture of potential gamma disruptions in ASD.
Acknowledgments
This study was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft/DFG, IRTG 1328). Christina Maxwell is supported by the Institute for Translational Medicine and Therapeutics at the University of Pennsylvania.
Contributor Information
Christina R. Maxwell, Center for Autism Research, Children’s Hospital of Philadelphia, 3535 Market St Suite 860, Philadelphia, PA 19104, USA
Michele E. Villalobos, Center for Autism Research, Children’s Hospital of Philadelphia, 3535 Market St Suite 860, Philadelphia, PA 19104, USA
Robert T. Schultz, Center for Autism Research, Children’s Hospital of Philadelphia, 3535 Market St Suite 860, Philadelphia, PA 19104, USA
Beate Herpertz-Dahlmann, Department of Child and Adolescent Psychiatry and Psychotherapy, RWTH Aachen University, Aachen, Germany; JARA Translational Brain Medicine, Aachen, Germany.
Kerstin Konrad, Department of Child and Adolescent Psychiatry and Psychotherapy, RWTH Aachen University, Aachen, Germany; JARA Translational Brain Medicine, Aachen, Germany; Child Neuropsychology Section, Department of Child and Adolescent Psychiatry and Psychotherapy, RWTH Aachen University, Aachen, Germany; Cognitive Neurology Section, Institute of Neuroscience and Medicine, Research Centre Jülich, Jülich, Germany.
Gregor Kohls, Center for Autism Research, Children’s Hospital of Philadelphia, 3535 Market St Suite 860, Philadelphia, PA 19104, USA.
References
- Achenbach TM (1991). Manual for the child behavior checklist/ 4–18 and 1991 profile. Burlington: VT: University of Vermont. [Google Scholar]
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders—iv-tr (4th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Constantino JN, Gruber CP, Davis S, Hayes S, Passanante N, & Przybeck T (2004). The factor structure of autistic traits. Journal of Child Psychology and Psychiatry, 45, 719–726. [DOI] [PubMed] [Google Scholar]
- Cornew L, Roberts TP, Blaskey L, & Edgar JC (2012). Resting-state oscillatory activity in autism spectrum disorders. Journal of Autism and Developmental Disorders, 42, 1884–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Klinger LG, Panagiotides H, Lewy A, & Castelloe P (1995). Subgroups of autistic children based on social behavior display distinct patterns of brain activity. Journal of Abnormal Child Psychology, 23, 569–583. [DOI] [PubMed] [Google Scholar]
- Delorme A, & Makeig S (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134, 9–21. [DOI] [PubMed] [Google Scholar]
- Fox NA, Rubin KH, Calkins SD, Marshall TR, Coplan RJ, Porges SW, et al. (1995). Frontal activation asymmetry and social competence at four years of age. Child Development, 66, 1770–1784. [PubMed] [Google Scholar]
- Gandal MJ, Edgar JC, Ehrlichman RS, Mehta M, Roberts TP, & Siegel SJ (2010). Validating gamma oscillations and delayed auditory responses as translational biomarkers of autism. Biological Psychiatry, 68, 1100–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleissner U, Von Ondarza G, Freitag H, & Karlmeier A (2003). Auswahl einer HAWIK-III-Kurzform fur Kinder und Jugendliche mit Epilepsie. Zeitschrift fur Neuropsychologie, 14, 3–11. [Google Scholar]
- Gonzalez-Burgos G, Hashimoto T, & Lewis DA (2010). Alterations of cortical GABA neurons and network oscillations in schizophrenia. Current Psychiatry Reports, 12, 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, & Lewis DA (2008). GABA neurons and the mechanisms of network oscillations: Implications for understanding cortical dysfunction in schizophrenia. Schizophrenia Bulletin, 34, 944–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. (1997). Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 36, 980–988. [DOI] [PubMed] [Google Scholar]
- Keren AS, Yuval-Greenberg S, & Deouell LY (2010). Saccadic spike potentials in gamma-band EEG: Characterization, detection and suppression. Neuroimage, 49, 2248–2263. [DOI] [PubMed] [Google Scholar]
- Kohls G, Peltzer J, Schulte-Ruther M, Kamp-Becker I, Rems-chmidt H, Herpertz-Dahlmann B, et al. (2011). Atypical Brain Responses to Reward Cues in Autism as Revealed by Event- Related Potentials. Journal of Autism and Developmental Disorders, 41, 1523–1533. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH Jr, Leventhal BL, Dilavore PC, et al. (2000). The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30, 205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, & Le Couteur A (1994). Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24, 659–685. [DOI] [PubMed] [Google Scholar]
- Magnee MJ, De Gelder B, Van Engeland H, & Kemner C (2011). Multisensory integration and attention in autism spectrum disorder: Evidence from event-related potentials. PLoS ONE, 6, e24196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco EJ, Hinkley LB, Hill SS, & Nagarajan SS (2011). Sensory processing in autism: A review of neurophysiologic findings. Pediatric Research, 69, 48R–54R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MM, Keil A, Gruber T, & Elbert T (1999). Processing of affective pictures modulates right-hemispheric gamma band EEG activity. Clinical Neurophysiology, 110, 1913–1920. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Rojas DC, Maharajh K, Teale P, & Rogers SJ (2008). Reduced neural synchronization of gamma-band MEG oscillations in first-degree relatives of children with autism. BMC Psychiatry, 8, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas DC, Teale PD, Maharajh K, Kronberg E, Youngpeter K, Wilson LB, et al. (2011). Transient and steady-state auditory gamma-band responses in first-degree relatives of people with autism spectrum disorder. Mol Autism, 2, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, & Merzenich MM (2003). Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav, 2, 255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Bailey A, & Lord C (2003). Social Communication Questionnaire Manual for the SCQ. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Sheikhani A, Behnam H, Mohammadi MR, Noroozian M, & Mohammadi M (2010). Detection of abnormalities for diagnosing of children with autism disorders using of quantitative electroencephalography analysis. Journal of Medical Systems, 36, 957–963. [DOI] [PubMed] [Google Scholar]
- Sheikhani A, Behnam H, Noroozian M, Mohammadi MR, & Mohammadi M (2009). Abnormalities of quantitative electroencephalography in children with Asperger disorder in various conditions. Research in Autism Spectrum Disorders, 3, 538–546. [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, & Deisseroth K (2009). Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature, 459, 698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, & Singer W (2010). Abnormal neural oscillations and synchrony in schizophrenia. Nature Reviews Neuroscience, 11, 100–113. [DOI] [PubMed] [Google Scholar]