Abstract
Crohn's disease (CD) is a type of inflammatory bowel disease that cannot be fully cured by medication or surgery. In the present study, the aim was to understand the underlying mechanisms of CD. Two CD microarray datasets were downloaded from The Gene Expression Omnibus database: GSE36807 (13 CD and 7 normal samples) and GSE59071 (8 CD and 11 normal samples). A series of bioinformatics analyses were conducted, including weighted gene co-expression network analysis to identify stable modules, and analysis of differentially expressed genes (DEGs) between CD and normal samples. The common DEGs in the GSE36807 and GSE59071 datasets were screened. Subsequently, overlapping genes in the stable modules and the DEGs were selected to construct a protein-protein interaction (PPI) network using Cytoscape software. Enrichment analysis of genes in the network was performed to explore their biological functions. A total of 10 stable modules and 927 DEGs were identified, of which 234 genes were shared in the stable modules and the DEGs. After removal of 32 uncharacterized genes, 202 genes were selected to build the PPI network. Low density lipoprotein receptor (LDLR), toll-like receptor 2 (TLR2), lipoprotein lipase (LPL), forkhead box protein M1 (FOXM1) and neuropeptide Y (NPY) were revealed as key nodes with high degree. Pathway enrichment analysis demonstrated that LPL was enriched in the peroxisome proliferator-activated receptor (PPAR) signaling pathway. In conclusion, LDLR, TLR2, FOXM1 and NPY, as well as LPL in the PPAR signaling pathway may serve critical roles in the pathogenesis of CD.
Keywords: Crohn's disease, weighted gene co-expression network analysis, meta-analysis, protein-protein interaction network, enrichment analysis
Introduction
Crohn's disease (CD) is a type of inflammatory bowel disease (IBD) that can affect any part of the gastrointestinal tract (1). This disease is caused by immune, bacterial and environmental factors in individuals with genetic susceptibility (2–4). Patients with CD usually suffer from weight loss, abdominal pain, fever and diarrhea (5); other complications associated with CD include arthritis, anemia, inflammation of the eye, fatigue and skin problems (6). CD is a common disease in the developed world, with increasing incidence and prevalence in developing countries (7,8). At present, there are no medical treatments or operative interventions that can fully cure CD, and in some cases surgery may even result in recurrence of the disease (9). Therefore, the underlying mechanisms of CD need to be explored in order to develop novel treatment strategies.
So far, some progress has been made with understanding the pathogenesis of CD. For example, it was demonstrated that mutation of the autophagy-related 16-like 1 gene is associated with CD, and may possibly support the role of autophagy in the development and progression of IBD (10). T helper (Th) 17 cells are a unique Th cell lineage that serve an important role in inflammatory diseases by secreting proinflammatory cytokines, including interleukin 17 (IL-17) and IL-23, and may mediate the Th1/Th17 imbalance seen in CD and ulcerative colitis (11,12). A previous study reported that signal transducer and activator of transcription 3 (STAT3) and Janus kinase 2 (JAK2), which are involved in the STAT-JAK pathway, can increase the risk of CD (13). Nucleotide-binding oligomerization domain-containing 2 (NOD2) is expressed throughout the small intestine, with highest expression in the terminal ileum where there is an abundance of Paneth cells, and is also closely associated with pathogenesis of CD (14,15). Despite these profound findings, pathogenesis of CD has not been fully elucidated.
Bioinformatics analysis is a useful tool for exploring disease pathogenesis and screening novel therapeutic targets (16). Kenny et al performed a genome-wide association study in an Ashkenazi Jewish population with CD and revealed that 16 replicated and novel loci made up 11.2% of the total genetic variations associated with CD risk (17). Fransen et al employed expression quantitative trait loci to select single nucleotide polymorphisms (SNPs) for follow-up and identified two CD-associated SNPs in the ubiquitin-conjugating enzyme E2L 3 and B-cell chronic lymphocytic leukemia/lymphoma 3 genes (18). These findings provide some information on the etiology of CD; however, to the best of our knowledge the mechanisms of CD pathogenesis have not yet been investigated by comprehensive bioinformatics analysis.
In the present study, comprehensive bioinformatics analysis was carried out to understand the pathogenesis of CD and identify novel therapeutic targets. The gene expression profiles of patients with CD were downloaded from a public database, and subsequently a series of bioinformatics analyses were performed, including weighted gene co-expression network analysis (WGCNA), meta-analysis, protein-protein interaction (PPI) network analysis and enrichment analysis for key genes associated with CD. This study contributes towards the further understanding of the mechanisms underlying human CD.
Materials and methods
Data source
The key words ‘Crohn's disease’ and ‘Homo sapiens’ were used to search relevant gene expression profile data on the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/). The inclusion criteria were as follows: i) Gene expression profiles; ii) intestinal tissues from patients with CD (not cells); iii) availability of both CD and normal samples; iv) human samples and v) number of total samples ≥18. Based on these criteria, two microarray datasets were selected: GSE36807 (Platform GPL570; 13 CD and 7 normal samples) (19) and GSE59071 (Platform GPL6244; 8 CD and 11 normal samples) (20).
Data pre-processing
The raw data (CEL files) were downloaded from GEO. Subsequently, the oligo package version 1.41.1 from R (http://www.bioconductor.org/packages/release/bioc/html/oligo.html) (21) was utilized to perform pre-treatments, including conversion of data format, summarization using median-polish, background correction using the MAS method and data normalization by quantile method.
WGCNA analysis
WGCNA is a popular systems biology tool used to construct gene co-expression networks, which can be used to detect disease-associated gene clusters and identify therapeutic targets (22). GSE36807 and GSE59071 were used as the training and validation sets, respectively. Stable gene modules associated with CD were screened using the R WGCNA package version 1.61 (https://cran.r-project.org/web/packages/WGCNA/index.html) (22), with the following parameters; cutHeight=0.95 and ≥30 genes/module; the correlation coefficients (CC) were also calculated.
Meta-analysis
Using the MetaDE.ES function in the R MetaDE package (https://cran.r-project.org/src/contrib/Archive/MetaDE/) (23), differentially expressed genes (DEGs) between CD and normal samples that were common to the two datasets were identified. To ensure homogeneity of genes, tau2=0, Qpval >0.05 and false discovery rate <0.05 were used as the cut-off criteria.
Construction of the PPI network
Genes in the stable modules and DEGs were compared, and overlapping genes were used as candidate genes for construction of the PPI network. An integrated PPI network was generated from the candidate genes by merging numerous PPI databases, including BioGRID version 3.4.153 (http://thebiogrid.org/) (24), Human Protein Reference Database release 9 (http://www.hprd.org/) (25) and STRING version 10.5 (https://string-db.org/) (26). The PPI network was visualized using the Cytoscape software version 3.3.0 (http://www.cytoscape.org/) (27).
Functional and pathway enrichment analyses
The Database for Annotation, Visualization and Integrated Discovery (DAVID) version 6.8 (https://david.ncifcrf.gov/) is an easy-to-use web tool in which genes are annotated and summarized according to shared categorical data (28). Based on DAVID analysis, Gene Ontology (GO) (29) functional enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) (30) pathway enrichment analysis were conducted for the nodes (a node represents a gene) identified in the PPI network, with the criterion of P<0.05.
Results
WGCNA analysis
To ensure the GSE36807 and GSE59071 datasets were comparable, correlation of gene expression and connectivity of common genes was analyzed. The results revealed that the CC for gene expression and connectivity between the two datasets were 0.82 (P<1×10−200; Fig. 1A) and 0.51 (P<1×10−200; Fig. 1B), respectively. Therefore, it was deduced that the GSE36807 and GSE59071 datasets were comparable.
Figure 1.
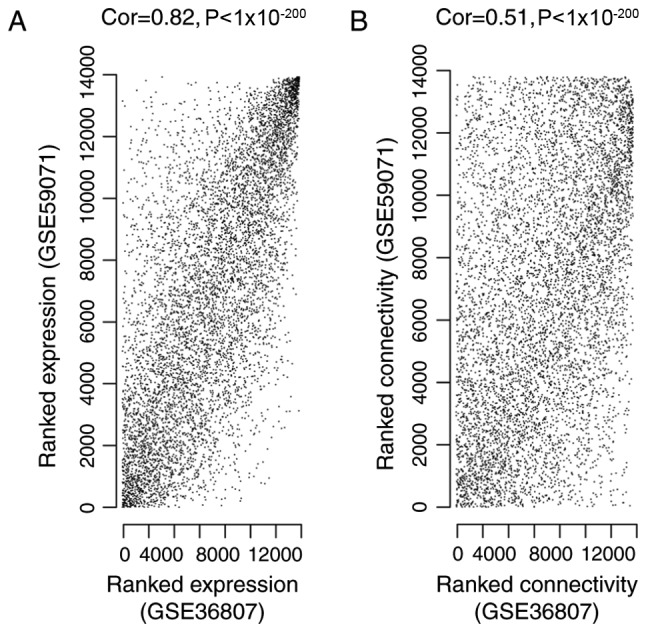
Correlation between (A) gene expression and (B) gene connectivity in the GSE36807 and GSE59071 datasets. Cor, correlation.
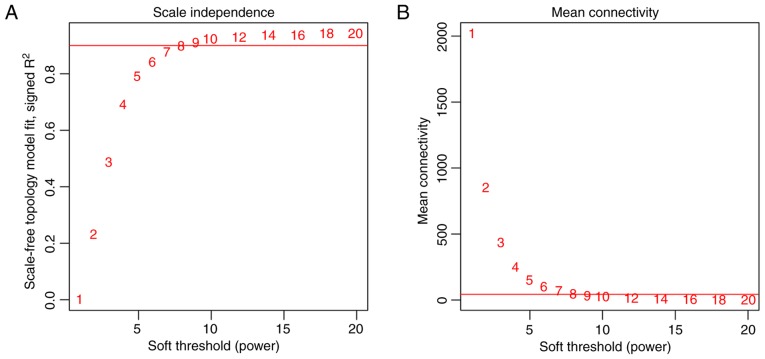
To meet the prerequisite of scale-free network distribution, the value of the adjacency matrix weighting parameter ‘power’ was explored. After setting the ranges of parameters for the network, the scale-free topology matrix was calculated. Using GSE36807 as the essential data, the scale-free topology model fit was calculated and statistics were selected for graphing (Fig. 2A). The greater the value of CC2, the closer the network was to scale-free distribution. Based on this theory, the mean connectivity of genes was calculated using power=8 as this was the minimum power value to achieve R2=0.9. The results demonstrated that the mean connectivity was 16, which conforms to the node connection properties of a scale-free network (Fig. 2B).
Figure 2.
(A) Selection of the weighting parameter ‘power’. The red line indicates when the square of correlation coefficient equals 0.9. (B) Mean connectivity of genes according to ‘power’. The red line indicates a mean connectivity of 16 when the ‘power’ is 8.
Using GSE36807 as the training set, disease-associated modules were screened. Variation in gene expression in the samples was calculated, and the genes with variation coefficients >0.1 were selected. Subsequently, the community dissimilarities among the genes were calculated, and a clustering tree was obtained. A total of 10 modules (D1M1, D1M2, D1M3, D1M4, D1M5, D1M6, D1M7, D1M8, D1M9 and D1M10) were identified using cutHeight=0.95 (Fig. 3A). Similarly, module division was performed for GSE59071 (Fig. 3B) and the modules in this dataset were used to evaluate the stability of those identified in the training set.
Figure 3.
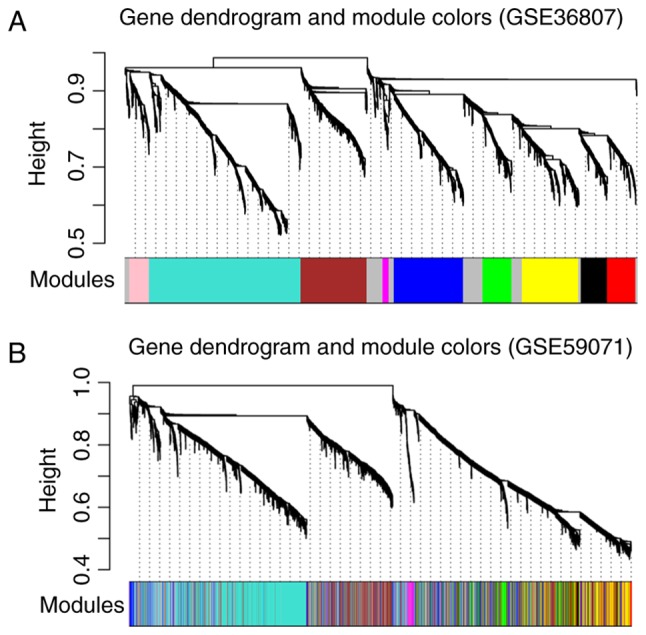
Module partition trees of (A) GSE36807 and (B) GSE59071; each color represents a different module.
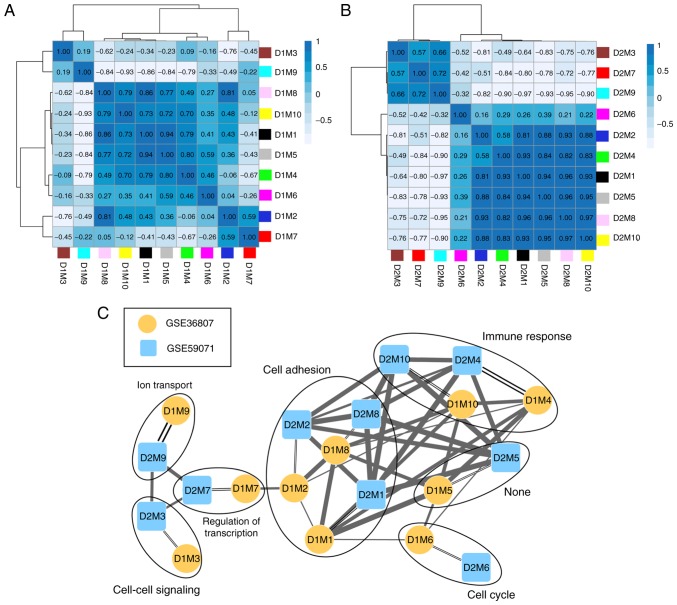
The correlation of gene expression was analyzed for the same color modules in the two datasets, and the results demonstrated that 10 modules were stable (Table I; preservation Z-score >5; P≤0.05). According to the expression similarities of the module genes, the correlation of the modules in GSE36807 (Fig. 4A) and GSE59071 (Fig. 4B) were analyzed (Table I). By combining the correlations among the modules with the GO categories for genes in the stable modules (Table I), a network for the modules was constructed (Fig. 4C). This revealed that genes in three stable modules (M1, M2 and M8) were significantly associated with cell adhesion and genes in two stable modules (M4 and M10) were significantly associated with immune response.
Table I.
| Modules | Module preservation | ||||||
|---|---|---|---|---|---|---|---|
| GSE36807 | GSE59071 | Color | Size | Correlation | P-value | Z-score | Module characterization |
| D1M1 | D2M1 | Black | 163 | 0.40 | 1.2×10−7 | 20.34 | Cell adhesion |
| D1M2 | D2M2 | Blue | 431 | 0.37 | 2.0×10−15 | 10.05 | Cell adhesion |
| D1M3 | D2M3 | Brown | 412 | 0.30 | 5.1×10−10 | 15.88 | Cell-cell signaling |
| D1M4 | D2M4 | Green | 180 | 0.50 | 8.9×10−13 | 16.73 | Immune response |
| D1M5 | D2M5 | Grey | 368 | 0.35 | 4.8×10−12 | 4.12 | – |
| D1M6 | D2M6 | Magenta | 38 | 0.32 | 5.0×10−2 | 11.74 | Cell cycle |
| D1M7 | D2M7 | Pink | 123 | 0.41 | 2.5×10−6 | 5.53 | Regulation of transcription |
| D1M8 | D2M8 | Red | 177 | 0.35 | 1.8×10−6 | 21.44 | Cell adhesion |
| D1M9 | D2M9 | Turquoise | 944 | 0.68 | 4.2×10−129 | 17.93 | Ion transport |
| D1M10 | D2M10 | Yellow | 352 | 0.42 | 1.8×10−16 | 29.51 | Immune response |
Figure 4.
Correlation cluster diagrams for the modules of (A) GSE36807 and (B) GSE59071. (C) Module-module correlation network. Single lines connect modules within the same dataset and double lines connect modules across datasets. The thickness of the links indicates the extent of correlation. D1, dataset 1 GSE36807; D2 dataset 2 GSE59071; M, module.
Meta-analysis
After calculation of the parameter values of each gene, a total of 927 DEGs were screened according to the aforementioned thresholds. The heatmap revealed that changes in expression of the DEGs were similar for GSE36807 and GSE59071 (Fig. 5).
Figure 5.
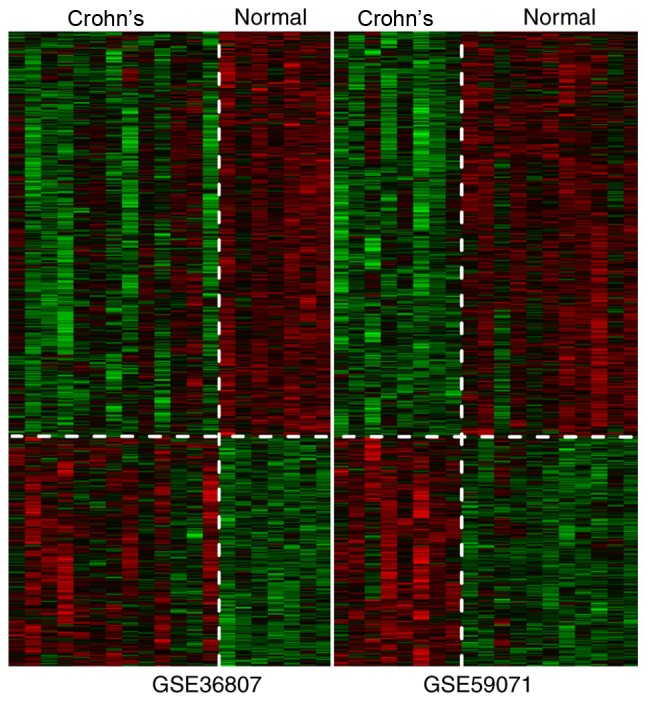
Clustering heatmap of differentially expressed genes.
Construction of the PPI network
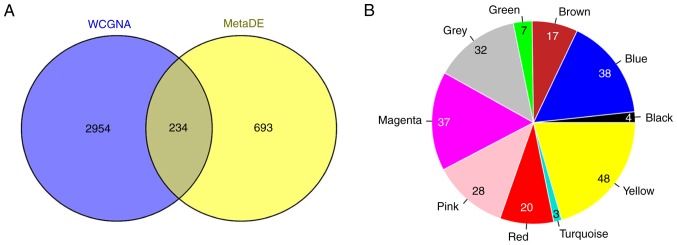
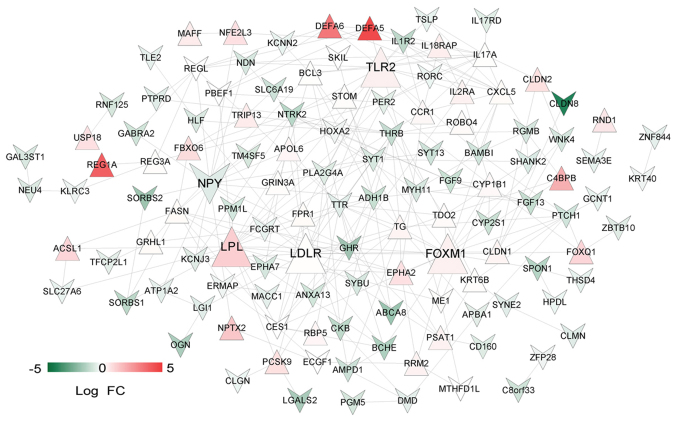
A total of 234 overlapping genes from genes in the stable modules (3,188 genes) and the 927 DEGs were identified and used as candidate genes (Fig. 6A). The number and proportion of these genes in different modules are shown in Fig. 6B. A total of 32 uncharacterized genes in the grey module were removed from subsequent analyses. PPIs were predicted for the remaining 202 genes and a PPI network (123 nodes and 270 edges) was constructed (Fig. 7). Notably, low density lipoprotein receptor (LDLR; node degree=22), toll-like receptor 2 (TLR2; node degree=19), lipoprotein lipase (LPL; node degree=18), forkhead box M1 (FOXM1; node degree=17) and neuropeptide Y (NPY; node degree=16) were the top five nodes with high degrees in the PPI network.
Figure 6.
(A) Venn diagram depicting the overlap between the 3,188 genes in the stable modules and the 927 differentially expressed genes. (B) Pie chart of the number of genes in different modules. WCGNA, weighted gene co-expression network analysis.
Figure 7.
Protein-protein interaction network. Upright red and inverted green triangles represent upregulated and downregulated proteins, respectively. The color intensity indicates gene expression. The enlarged nodes are the top five nodes with highest degrees. FC, fold change.
Functional and pathway enrichment analyses
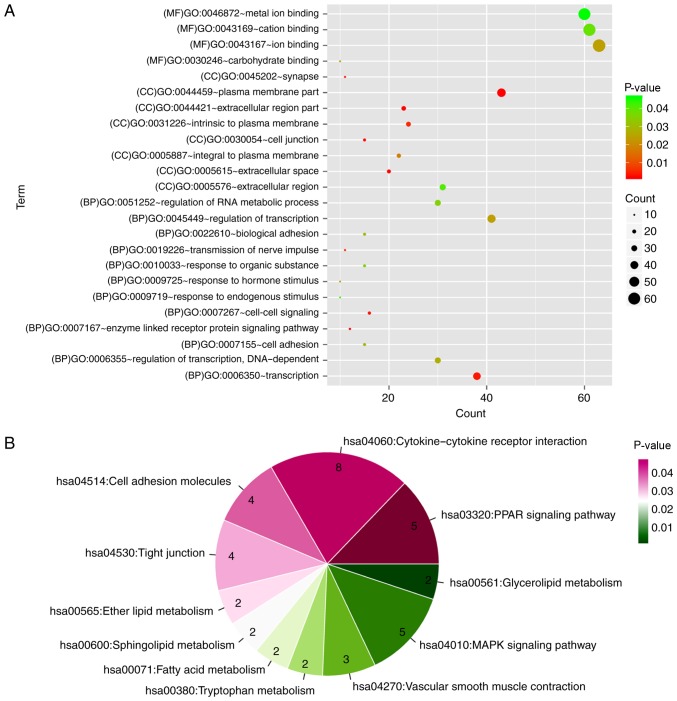
A total of 24 GO categories and 12 KEGG pathways were enriched for the PPI network nodes. Notably, the network nodes were mainly implicated in the enzyme-linked receptor protein signaling pathway (GO category; P=0.00185), cell-cell signaling (GO category; P=0.003505), peroxisome proliferator-activated receptor (PPAR) signaling pathway (KEGG pathway; P=0.001014) and cytokine-cytokine receptor interaction (KEGG pathway; P=0.004187) (Fig. 8).
Figure 8.
(A) Functional categories and (B) pathways enriched for the protein-protein interaction network nodes. BP, biological process; CC, cellular component; GO, Gene Ontology; MAPK, mitogen-activated protein kinase; MF, molecular function; PPAR, peroxisome proliferator-activated receptor.
Discussion
In the present study, a total of 10 stable CD-associated modules were identified using WGCCA; of which three stable modules (M1, M2 and M8) and two stable modules (M4 and M10) were significantly associated with cell adhesion and immune response, respectively. In addition, 927 overlapping DEGs in the GSE36807 and GSE59071 datasets were screened. In the PPI network, five important nodes with relatively high node degrees were identified as LDLR, TLR2, LPL, FOXM1 and NPY.
Previous studies have investigated the relationship between inflammatory cytokines and LDLR (31,32), and demonstrated that the inflammatory cytokines IL-1β and tumor necrosis factor (TNF)-α can regulate LDLR expression leading to accumulation of high concentrations of low-density lipoprotein (33). FOXM1 belongs to the forkhead box (FOX) family of transcription factors, and regulates key genes involved in goblet cell metaplasia and lung inflammation (34,35). FOXM1 is an important mediator of cell proliferation, and its expression is enhanced during gastric carcinogenesis induced by Helicobacter pylori infection (36). Overexpression of triglyceride-rich lipoproteins can induce lipid accumulation and early inflammatory responses, and LPL serves an anti-inflammatory role by hydrolyzing triglyceride-rich lipoproteins (37). PPARs can regulate lipid metabolism-associated genes and control inflammation, and PPARα and -γ activators contribute to macrophage secretion of LPL (38,39). At present, there is no direct evidence for the involvement of LDLR, FOXM1 and LPL in CD development. However, their expression levels may be linked to disease progression. A study demonstrated that polymorphisms of LIGHT (a TNF superfamily member) are associated with CD, and inhibiting LIGHT reduces dyslypidemia in mice lacking LDLR (40). This suggests a possible association between LDLR and CD. In IBD, the susceptibility genes include FYN proto-oncogene and HCK proto-oncogene, which are involved in the phosphoinositide 3-kinase signaling network, which also contains FOXM1 (41). Since CD is a major subtype of IBD, FOXM1 may also be associated with CD. With regards to the LPL gene, decreased expression is observed in CD adipose-derived stem cells (ASCs) independent of clinical stage, compared with in healthy ASCs, indicating that CD modifies the plasticity of mesenteric ASCs (42). In the present study, LPL was upregulated in CD PPI network, indicating a potential role of LPL in the progression of CD; pathway enrichment analysis revealed that LPL was enriched in the PPAR signaling pathway. Interestingly, the alterations in LPL expression levels of ASC cells and CD tissues revealed different trends, suggesting that the creeping fat tissue may be associated with the immunomodulatory properties in patients with CD. Overall, the results demonstrated that increases in LDLR, FOXM1 and LPL may be highly relevant to the development of CD.
TLRs initiate immune responses to microbial infection, with TLR2 recognizing bacterial lipoproteins, zymosan, peptidoglycan and lipoteichoic acids (43,44). TLR2, TLR4, and their transmembrane co-receptor cluster of differentiation 14, are all upregulated in the terminal ileum of patients with IBD, and their dysregulation may serve critical roles in the pathogenesis of IBD (45). NOD2 mediates the anti-inflammatory cytokine bias induced by TLR2 ligands; therefore, defective NOD2 function may promote inflammation and increase the disease risk of CD (46). TLR2 and TLR4 have been reported to be overexpressed in the inflamed colonic mucosa of children with IBD, indicating that innate immunity is associated with the pathogenesis of IBD (47). A previous study discovered that TLR2 and TLR4 are highly expressed on inflammation-associated intestinal macrophages (IMACs), leading to a higher reactivity to lipopolysaccharide and possibly CD (48). In addition, the role of Gp96, an endoplasmic reticulum chaperone for multiple protein substrates, in CD has been investigated. The results demonstrated that the lack of Gp96 in CD IMACs is associated with loss of tolerance against the host gut flora, and that the Gp96 knockdown cell line has decreased TLR2 and TLR4 expression (49). Therefore, it may be hypothesized that TLR2 has an important role in the pathogenesis of CD. Neurogenic inflammation is critical for the development of IBD, and NPY induces oxidative stress and colitis by regulating the expression of neuronal nitric oxide synthase (50). NPY and the NPY receptor Y1 (NPY1R) are associated with intestinal inflammation and suppression of NPY1R signaling may represent a potential therapeutic strategy for colonic inflammation (51,52). NPY peptides have been implicated in various gastrointestinal disorders, including IBD, malabsorption and short gut; and NPY receptor agonists and antagonists can be used for preventing diarrhea and intestinal inflammation (53). Gut neurohormones, including NPY, can affect inflammation by interacting with the immune system, and NPY represents a promising target for treating IBD (54). In the present study, NPY was reported as an important node with a high degree in the CD PPI network; however, the expression levels of NPY were lower in the CD samples of patients compared with in the healthy control, which is inconsistent to the reports (50,52). Considering the important role of NPY in CD and that its expression levels varies from that of previous reports, further validation of NPY mRNA and protein expression in clinical samples is required.
Nevertheless, there are some limitations to the present study. Firstly, the results are solely based on bioinformatics analysis and although various important genes for CD etiology were identified, experimental validation is required. Secondly, only two CD microarray datasets were selected with relatively small sample sizes; therefore, this could affect the robustness of the results. Further experiments will be designed and conducted to validate these findings in follow-up studies.
In conclusion, a total of 10 stable modules and 927 DEGs associated with CD were identified. Notably, LDLR, TLR2, FOXM1, NPY and LPL may be the key genes involved in pathogenesis of the disease. LPL may exert its effects through the PPAR signaling pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
LB performed data analyses and wrote the manuscript. HF made substantial contributions to data analyses. JY conceived and designed the present study. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Baumgart DC, Sandborn WJ. Crohn's disease. Lancet. 2012;380:1590–1605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 2.Cho JH, Brant SR. Recent insights into the genetics of inflammatory bowel disease. Gastroenterology. 2011;140:1704–1712. doi: 10.1053/j.gastro.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dessein R, Chamaillard M, Danese S. Innate immunity in Crohn's disease: The reverse side of the medal. J Clin Gastroenterol. 2008;42(Suppl 3):S144–S147. doi: 10.1097/MCG.0b013e3181662c90. [DOI] [PubMed] [Google Scholar]
- 4.Stefanelli T, Malesci A, Repici A, Vetrano S, Danese S. New insights into inflammatory bowel disease pathophysiology: Paving the way for novel therapeutic targets. Current Drug Targets. 2008;9:413–418. doi: 10.2174/138945008784221170. [DOI] [PubMed] [Google Scholar]
- 5.Hashash JG, Proksell S, Regueiro MD. Crohn's disease with worsening symptoms. Gastroenterology. 2013;145:e5–e6. doi: 10.1053/j.gastro.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 6.Mazor Y, Maza I, Kaufman E, Ben-Horin S, Karban A, Chowers Y, Eliakim R. Prediction of disease complication occurrence in Crohn's disease using phenotype and genotype parameters at diagnosis. J Crohns Colitis. 2011;5:592–597. doi: 10.1016/j.crohns.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Hovde Ø, Moum BR. Epidemiology and clinical course of Crohn's disease: Results from observational studies. World J Gastroenterol. 2012;18:1723–1731. doi: 10.3748/wjg.v18.i15.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burisch J, Munkholm P. Inflammatory bowel disease epidemiology. Curr Opin Gastroenterol. 2013;29:357–362. doi: 10.1097/MOG.0b013e32836229fb. [DOI] [PubMed] [Google Scholar]
- 9.Hut'an M, Hut'an Mm. The role of surgery in Crohn's disease treatment. Rozhl Chir. 2009;88:185–188. (In Slovak) [PubMed] [Google Scholar]
- 10.Prescott NJ, Fisher SA, Franke A, Hampe J, Onnie CM, Soars D, Bagnall R, Mirza MM, Sanderson J, Forbes A, et al. A nonsynonymous SNP in ATG16L1 predisposes to ileal Crohn's disease and is independent of CARD15 and IBD5. Gastroenterology. 2007;132:1665–1671. doi: 10.1053/j.gastro.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi T, Okamoto S, Hisamatsu T, Kamada N, Chinen H, Saito R, Kitazume MT, Nakazawa A, Sugita A, Koganei K, et al. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn's disease. Gut. 2008;57:1682–1689. doi: 10.1136/gut.2007.135053. [DOI] [PubMed] [Google Scholar]
- 12.Siakavellas SI, Bamias G. Role of the IL-23/IL-17 axis in Crohn's disease. Discov Med. 2012;14:253–262. [PubMed] [Google Scholar]
- 13.Ferguson LR, Han DY, Fraser AG, Huebner C, Lam WJ, Morgan AR, Duan H, Karunasinghe N. Genetic factors in chronic inflammation: Single nucleotide polymorphisms in the STAT-JAK pathway, susceptibility to DNA damage and Crohn's disease in a New Zealand population. Mutat Res. 2010;690:108–115. doi: 10.1016/j.mrfmmm.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Nabatov AA. The vesicle-associated function of NOD2 as a link between Crohn's disease and mycobacterial infection. Gut Pathog. 2015;7:1. doi: 10.1186/s13099-015-0049-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balasubramanian I, Gao N. From sensing to shaping microbiota: Insights into the role of NOD2 in intestinal homeostasis and progression of Crohn's Disease. Am J Physiol Gastrointest Liver Physiol. 2017;313:G7–G13. doi: 10.1152/ajpgi.00330.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Szustakowski J, Schinke M. Bioinformatics analysis of microarray data. Methods Mol Biol. 2009;573:259–284. doi: 10.1007/978-1-60761-247-6_15. [DOI] [PubMed] [Google Scholar]
- 17.Kenny EE, Pe'er I, Karban A, Ozelius L, Mitchell AA, Ng SM, Erazo M, Ostrer H, Abraham C, Abreu MT, et al. A genome-wide scan of Ashkenazi jewish Crohn's disease suggests novel susceptibility loci. PLoS Genet. 2012;8:e1002559. doi: 10.1371/journal.pgen.1002559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fransen K, Visschedijk MC, van Sommeren S, Fu JY, Franke L, Festen EA, Stokkers PC, van Bodegraven AA, Crusius JB, Hommes DW, et al. Analysis of SNPs with an effect on gene expression identifies UBE2L3 and BCL3 as potential new risk genes for Crohn's disease. Hum Mol Genet. 2010;19:3482–3488. doi: 10.1093/hmg/ddq264. [DOI] [PubMed] [Google Scholar]
- 19.Montero-Melendez T, Llor X, Garcia-Planella E, Perretti M, Suárez A. Identification of novel predictor classifiers for inflammatory bowel disease by gene expression profiling. PLoS One. 2013;8:e76235. doi: 10.1371/journal.pone.0076235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanhove W, Peeters PM, Staelens D, Schraenen A, Van der Goten J, Cleynen I, De Schepper S, Van Lommel L, Reynaert NL, Schuit F, et al. Strong upregulation of AIM2 and IFI16 inflammasomes in the mucosa of patients with active inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:2673–2682. doi: 10.1097/MIB.0000000000000535. [DOI] [PubMed] [Google Scholar]
- 21.Parrish RS, Spencer HJ., III Effect of normalization on significance testing for oligonucleotide microarrays. J Biopharm Stat. 2004;14:575–589. doi: 10.1081/BIP-200025650. [DOI] [PubMed] [Google Scholar]
- 22.Langfelder P, Horvath S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Kang DD, Shen K, Song C, Lu S, Chang LC, Liao SG, Huo Z, Tang S, Ding Y, et al. An R package suite for microarray meta-analysis in quality control, differentially expressed gene analysis and pathway enrichment detection. Bioinformatics. 2012;28:2534–2536. doi: 10.1093/bioinformatics/bts485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oughtred R, Chatraryamontri A, Breitkreutz BJ, Chang CS, Rust JM, Theesfeld CL, Heinicke S, Breitkreutz A, Chen D, Hirschman J, et al. BioGRID: A resource for studying biological interactions in yeast. Cold Spring Harb Protoc. 2016;2016 doi: 10.1101/pdb.top080754. pdb.top080754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu B, Hu B. HPRD: A high performance RDF database. Int J Parallel Emergent Distributed Systems. 2010;25:123–133. doi: 10.1080/17445760802431839. [DOI] [Google Scholar]
- 26.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–D452. doi: 10.1093/nar/gku1003. (Database Issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohl M, Wiese S, Warscheid B. Cytoscape: Software for visualization and analysis of biological networks. Methods Mol Biol. 2011;696:291–303. doi: 10.1007/978-1-60761-987-1_18. [DOI] [PubMed] [Google Scholar]
- 28.Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4:P3. doi: 10.1186/gb-2003-4-5-p3. [DOI] [PubMed] [Google Scholar]
- 29.Gene Ontology Consortium: Gene ontology consortium: Going forward. Nucleic Acids Res. 2015;43:D1049–D1056. doi: 10.1093/nar/gku1179. (Database Issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2015;44:D457–D462. doi: 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strickland DK, Au DT, Cunfer P, Muratoglu SC. Low-density lipoprotein receptor-related protein-1: Role in the regulation of vascular integrity. Arterioscler Thromb Vasc Biol. 2014;34:487–498. doi: 10.1161/ATVBAHA.113.301924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fantus D, Awan Z, Seidah NG, Genest J. Aortic calcification: Novel insights from familial hypercholesterolemia and potential role for the low-density lipoprotein receptor. Atherosclerosis. 2013;226:9–15. doi: 10.1016/j.atherosclerosis.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 33.Ruan XZ, Varghese Z, Powis SH, Moorhead JF. Dysregulation of LDL receptor under the influence of inflammatory cytokines: A new pathway for foam cell formation. Kidney Int. 2001;60:1716–1725. doi: 10.1046/j.1523-1755.2001.00025.x. [DOI] [PubMed] [Google Scholar]
- 34.Ren X, Shah TA, Ustiyan V, Zhang Y, Shinn J, Chen G, Whitsett JA, Kalin TV, Kalinichenko VV. FOXM1 promotes allergen-induced goblet cell metaplasia and pulmonary inflammation. Mol Cell Biol. 2013;33:371–386. doi: 10.1128/MCB.00934-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim IM, Zhou Y, Ramakrishna S, Hughes DE, Solway J, Costa RH, Kalinichenko VV. Functional characterization of evolutionarily conserved DNA regions in forkhead box f1 gene locus. J Biol Chem. 2005;280:37908–37916. doi: 10.1074/jbc.M506531200. [DOI] [PubMed] [Google Scholar]
- 36.Feng Y, Wang L, Zeng J, Shen L, Liang X, Yu H, Liu S, Liu Z, Sun Y, Li W, et al. FoxM1 is Overexpressed in Helicobacter pylori-induced gastric carcinogenesis and is negatively regulated by miR-370. Mol Cancer Res. 2013;11:834–844. doi: 10.1158/1541-7786.MCR-13-0007. [DOI] [PubMed] [Google Scholar]
- 37.Ziouzenkova O, Perrey S, Asatryan L, Hwang J, MacNaul KL, Moller DE, Rader DJ, Sevanian A, Zechner R, Hoefler G, Plutzky J. Lipolysis of triglyceride-rich lipoproteins generates PPAR ligands: Evidence for an antiinflammatory role for lipoprotein lipase. Proc Natl Acad Sci USA. 2003;100:2730–2735. doi: 10.1073/pnas.0538015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tyagi S, Gupta P, Saini AS, Kaushal C, Sharma S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J Adv Pharm Technol Res. 2011;2:236–240. doi: 10.4103/2231-4040.90879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ooi EM, Watts GF, Sprecher DL, Chan DC, Barrett PH. Mechanism of action of a peroxisome proliferator-activated receptor (PPAR)-delta agonist on lipoprotein metabolism in dyslipidemic subjects with central obesity. J Clin Endocrinol Metab. 2011;96:E1568–E1576. doi: 10.1210/jc.2011-1131. [DOI] [PubMed] [Google Scholar]
- 40.Herro R, Croft M. The control of tissue fibrosis by the inflammatory molecule LIGHT (TNF Superfamily member 14) Pharmacol Res. 2016;104:151–155. doi: 10.1016/j.phrs.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu TC, Naito T, Liu Z, VanDussen KL, Haritunians T, Li D, Endo K, Kawai Y, Nagasaki M, Kinouchi Y, et al. LRRK2 but not ATG16L1 is associated with Paneth cell defect in Japanese Crohn's disease patients. JCI Insight. 2017;2:e91917. doi: 10.1172/jci.insight.91917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serena C, Keiran N, Madeira A, Maymó-Masip E, Ejarque M, Terrón-Puig M, Espin E, Martí M, Borruel N, Guarner F, et al. Crohn's disease disturbs the immune properties of human Adipose-derived stem cells related to inflammasome activation. Stem Cell Reports. 2017;9:1109–1123. doi: 10.1016/j.stemcr.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 44.Yuk JM, Jo EK. Toll-like receptors and innate immunity. J Bacteriol Virol. 2011;41:225–235. doi: 10.4167/jbv.2011.41.4.225. [DOI] [Google Scholar]
- 45.Frolova L, Drastich P, Rossmann P, Klimesova K, Tlaskalova-Hogenova H. Expression of Toll-like receptor 2 (TLR2), TLR4, and CD14 in biopsy samples of patients with inflammatory bowel diseases: Upregulated expression of TLR2 in terminal ileum of patients with ulcerative colitis. J Histochem Cytochem. 2008;56:267–274. doi: 10.1369/jhc.7A7303.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Netea MG, Kullberg BJ, de Jong DJ, Franke B, Sprong T, Naber TH, Drenth JP, Van der Meer JW. NOD2 mediates anti-inflammatory signals induced by TLR2 ligands: Implications for Crohn's disease. Eur J Immunol. 2004;34:2052–2059. doi: 10.1002/eji.200425229. [DOI] [PubMed] [Google Scholar]
- 47.Szebeni B, Mraz M, Veres G, Dezsofi A, Vannay A, Vasarhelyi B, Majorova E, Arato A. Increased expression of toll-like receptor (Tlr) 2 and Tlr4 in the colonic mucosa of children with active inflammatory bowel disease. J Pediat Gastroenterol Nutrition. 2006;42:34–41. doi: 10.1111/j.1365-2249.2007.03531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hausmann M, Kiessling S, Mestermann S, Webb G, Spöttl T, Andus T, Schölmerich J, Herfarth H, Ray K, Falk W, Rogler G. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology. 2002;122:1987–2000. doi: 10.1053/gast.2002.33662. [DOI] [PubMed] [Google Scholar]
- 49.Cosin-Roger J, Spalinger MR, Ruiz PA, Stanzel C, Terhalle A, Wolfram L, Melhem H, Atrott K, Lang S, Frey-Wagner I, et al. Gp96 deficiency affects TLR4 functionality and impairs ERK and p38 phosphorylation. PLoS One. 2018;13:e0193003. doi: 10.1371/journal.pone.0193003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chandrasekharan B, Bala V, Kolachala VL, Vijay-Kumar M, Jones D, Gewirtz AT, Sitaraman SV, Srinivasan S. Targeted deletion of neuropeptide Y (NPY) modulates experimental colitis. PLoS One. 2008;3:e3304. doi: 10.1371/journal.pone.0003304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hassani H, Lucas G, Rozell B, Ernfors P. Attenuation of acute experimental colitis by preventing NPY Y1 receptor signaling. Am J Physiol Gastrointest Liver Physiol. 2005;288:G550–G556. doi: 10.1152/ajpgi.00182.2004. [DOI] [PubMed] [Google Scholar]
- 52.Chandrasekharan B, Nezami BG, Srinivasan S. Emerging neuropeptide targets in inflammation: NPY and VIP. Am J Physiol Gastrointest Liver Physiol. 2013;304:G949–G957. doi: 10.1152/ajpgi.00493.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vona-Davis LC, McFadden DW. NPY family of hormones: Clinical relevance and potential use in gastrointestinal disease. Curr Top Med Chem. 2007;7:1710–1720. doi: 10.2174/156802607782340966. [DOI] [PubMed] [Google Scholar]
- 54.El-Salhy M, Hausken T. The role of the neuropeptide Y (NPY) family in the pathophysiology of inflammatory bowel disease (IBD) Neuropeptides. 2016;55:137–144. doi: 10.1016/j.npep.2015.09.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.