Abstract
The present study identified the cytotoxic effects of etomidate on the N2a neuroblastoma cell line. Etomidate induced apoptosis in N2a cells in a concentration-dependent manner, which was confirmed by western blotting and flow cytometry. Phase contrast microscopy was used to analyze the effect of etomidate on morphological characteristics. The number of the apoptotic cells was increased and confirmed by DAPI and PI staining, which served as a characteristic hallmark of apoptosis. Additionally, etomidate led to loss of mitochondrial membrane potential and resulted in the generation of reactive oxygen species in N2a cells. The western blot analysis revealed that N2a cells treated with etomidate had a significant modulation of pro-apoptotic proteins, includingpoly ADP-ribose polymerase (PARP), cleaved PARP, caspase-9 and procaspase-3. In conclusion, the present study determined that etomidate induced cytotoxic and apoptotic effects in N2a brain tumor cells in vitro.
Keywords: etomidate, N2a cells, caspase-9, procaspase-3, caspase-9, cytotoxic effect
Introduction
Anesthesia has an important role in invasive medicinal procedures, as it allows for various surgeries to be performed, which may otherwise be impossible to complete due to intolerable pain (1,2). The anesthetic procedures have been further classified based upon the need of medical application, such as general or local anesthesia (3,4). In general anesthesia, the activity of the central nervous system is suppressed, which leads to an overall lack of sensation and unconsciousness, whereas in local anesthesia, the transmission of nerve impulses is blocked between the central nervous system and the part of the body which is targeted (5–9). Among the various anesthetic agents used today to sedate patients, etomidate has been identified as advantageous due to most favorable therapeutic index for single bolus administration (10,11). Etomidate also provides advantages for induction of anesthesia in hemorrhagic shock conditions. It was administered intravenously in order to actasageneral anesthetic without affecting blood pressure and avoid any cardiovascular side effects (12–14). Etomidate has been previously identified to exert protection against cerebral ischemia, cause minimal ventilation suppression and reduce the liberation of histamines (15,16). Previous studies have investigated the cytotoxic effect of etomidate (17–19); however, to the best of our knowledge no previous studies have investigated the underlying mechanism. The present study determined that etomidate induced apoptosis in the N2a neuroblastoma cell line.
Materials and methods
Chemicals and reagents
The reagents and chemicals such as DMEM medium, MTT, dimethyl sulfoxide (DMSO), penicillinG, streptomycin, sodium bicarbonate, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and sodium pyruvate were obtained from Sigma-Aldrich; Merck Millipore, (Darmstadt, Germany). Fetal bovine serum (FBS) was purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and Annex in V-FITC/PI Apoptosis Detection kit (cat. no. 556570) from BD Biosciences (San Jose, CA, USA).
Cell culture
N2a neuroblastoma cell line was obtained from the American Type Culture Collection (Manassas, VA, USA) and strictly grown as per the manufacturer's protocol. Dulbecco's modified Eagle's medium was supplemented with 10% fetal bovine serum (FBS), penicillin G (70 mg/l), streptomycin (100 mg/l) and NaHCO3 (3.7 g/l). Cells were maintained at 37°C in a humidified CO2 incubator with 95% humidity and 5% CO2.
Viability assay
The effect of etomidate on cell viability was investigated using an MTT assay. Briefly, in a 96-well plate, the cells were seeded at a density of 2 ×106 cells/well and allowed to grow for 24 h. After 24 h, the cells were treated with increasing concentrations of etomidate and were maintained for an additional 48 h. The 2.5 mg/ml MTT was added 4 h prior to the termination of experiment. After 4 h, the media was removed and formazan crystals were dissolved by adding 150 µl DMSO per well. The absorbance was quantified using the Synergy MX microplate reader at 570 nm.
Mitochondrial membrane potential
Cells at the density of 2×106 were seeded in 60 mm dish and maintained for 24 h after the 0, 1, 5 and 10 µM etomidate treatment was administered for 48 h. Rhodamine-123 was added to the cells at 37°C for 30 min prior to the termination of the experiment. Finally, the cells were collected in respective tubes, centrifuged for 5 min at 400 × g at 37°C and then washed three times with PBS. In flow tubes, the samples were finally transferred and resuspended in 500 µl PBS. The inverted fluorescent microscope was used to visualize the mitochondrial depolarization level, the mitochondrial depolarization level was quantitatively analyzed by imaging software Image J (version 1.49; National Institutes of Health, Bethesda, MD, USA).
Colony formation assay
In a 6-well plate, N2a cells were seeded at 2.0×106 cells/well cultured for 24 h followed by treatment with 0, 1, 5 and 10 µM etomidate for 48 h at 37°C. Subsequently the cells were trypsinized and replated in a 6-well plate with 500 cells/well. The cells were subsequently cultured for 21 days at 37°C. During termination of the experiment, the cells were washed 3 times with PBS and were subsequently fixed for 10–12 min in 4% paraformaldehyde. Crystal violet (0.06%) was used to stain (30–60 min at 37°C) the live cells and the number of colonies (>50 cells/colony) was counted under an inverted microscope using a camera (Olympus Corporation, Tokyo, Japan).
Reactive oxygen species (ROS) assay
In order to determine the effect of etomidate on the generation of ROS, N2a cells at a cell count of 2.0×106 were seeded in 6-well plates and maintained for 24 h. Subsequently, the cells were treated with 0.5, 1, 5 and 10 µM etomidate for 48 h. Finally, cells were collected in tubes, washed three times with PBS and then resuspended in 500 µl PBS to which 10 µM DCFH-DA was added and cells were incubated for 30 min in dark at 37°C. ROS-induced green fluorescence of DCF-DA was imaged using 488-nm laser excitation. The laser power was set to 1–3%. This power setting allowed complete discrimination of DCF fluorescence and the autofluorescence originating from the oxidized form of mitochondrial flavoproteins. The 515–530 nm emission range was used to monitor an increase in dichlorofluorescein, the oxidized product of DCF-DA.
Detection of apoptosis via Annexin V PI
Cells (1×106) were seeded in 50-ml dishes and incubated for 24 h at 37°C. Subsequently etomidate at different concentration was added directly to the dishes and incubated for additional 24, 48 and 72 h. Cells were collected, washed with PBS and resuspended in PBS. Apoptotic cell death was identified by double supravital staining with recombinant FITC-conjugated Annexin V and PI, using the Annexin V-FITC Apoptosis Detection kit (BD Biosciences) according to the manufacturer's protocol. Flow cytometry analysis was performed immediately following supravital staining. Data acquisition and analysis were performed in a Becton-Dickinson FACS Calibur flow cytometer using Cell Quest software (version 5.1; BD Biosciences).
Western blot analysis
In the 60 mm dishes, the N2a cells (at a density 2×106) were seeded and maintained for 24 h after which they were treated with 0.5, 1, 5 and 10 µM etomidate for 48 h. Cells were trypsinized and lysed using a radioimmunoprecipitation assay buffer (Abcam, Cambridge, MA, USA). Bradford method (Thermo Fisher Scientific, Inc.) was used for protein quantification, after which proteins were separated (10 µg/sample) by 12% SDS-PAGE and were then transferred at 100 V for 2 h onto a PVDF membrane. Blocking of the membrane was performed with 5% skimmed milk for 1 h to avoid non-specific binding of the antibodies. Membranes were incubated with primary antibodies: Caspase-3 (procaspase-3 and active caspase-3; cat. no. 9665; 1:1,000), caspase-9 (procaspase-9 and active caspase-9; cat. no. 9502; 1:1,000), poly ADP-ribose polymerase (PARP; cleaved PARP p89; cat. no. 9542; 1:1,000), β-actin (cat. no. 4967; 1:1,000) all from Cell Signaling Technology, Inc., (Beverly, MA, USA) at 4°C for 12–14 h after which membrane was washed twice with TBST for 5 min each. The secondary antibody, horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG; cat. no. K0211589; 1:3,000; KOMA Biotech, Seoul, South Korea) was added at room temperature for 1 h. The blots were analyzed with enhanced chemiluminescence detection system (GE Healthcare, Chicago, IL, USA) and visualized using a LAS 4000 (GE Healthcare, Chicago, IL, USA) imaging system and images were processed using ImageJ software (version 1.49; National Institutes of Health).
Statistical analysis
All experiments were performed in triplicate and data were presented as the mean ± standard error of the mean. One-way analysis of variance or the independent samples Kruskal-Wallis H test, as appropriate was used to compare differences among normally distributed variables. For statistically significant differences, post hoc pairwise comparisons were performed by using Tukey's honestly significant difference test or Dunn's test. P<0.05 was considered to indicate a statistically significant difference.
Results
Inhibition of cell proliferation by etomidate in N2a cells
N2a cells were first treated with a single concentration of etomidate (20 µM) for 48 h and the cell viability was reduced up to 80% in 48 h (data not shown). For the IC50 value determination, the cells were treated with increasing concentrations of etomidate for 48 h in a 96-well plate. The cell viability was reduced with increased etomidate concentration (0.5, 1, 5 and 10 µM). These findings demonstrated that N2a cells responded to etomidate in a dose-dependent manner. The IC50 was determined as 5 µM (Fig. 1). These findings collectively suggested that etomidate reduced the viability of N2a neuroblastoma cells.
Figure 1.
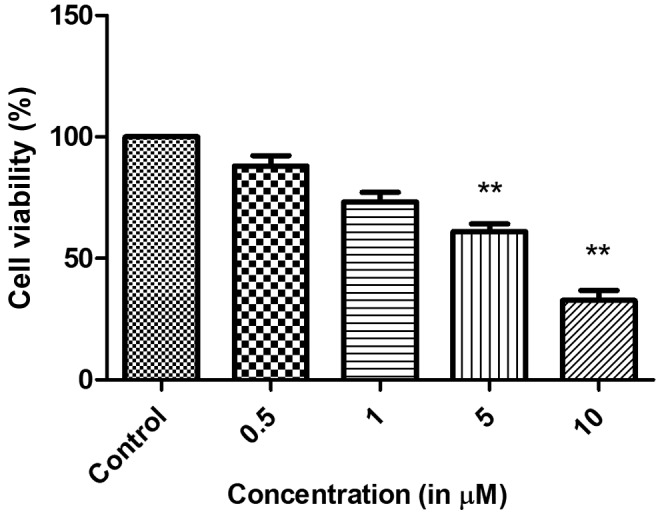
Etomidate inhibits N2a cell proliferation. Data are expressed in mean ± standard error of the mean. **P<0.05 vs. control.
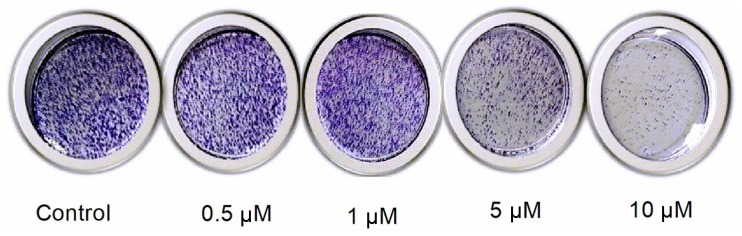
Effect on colony formation
When N2a cells were treated with different concentrations of etomidate, the clonogenic potential of the cells reduced with the increase in etomidate concentration. This led to an inhibition of the colony formation potential of N2a cells in a dose-dependent manner as presented in Fig. 2.
Figure 2.
Effect of different etomidate concentrations on colony formation.
Detection of apoptosis in N2a cells by Annexin V/PI staining
In order to confirm that etomidate induces apoptosis in N2a cells, Annexin V/PI assay was performed. The current study determined that etomidate increased the apoptotic population in a dose-dependent manner as presented in in Fig. 3. These finding sclearly indicated that etomidate induced concentration dependent apoptosis in N2a cells.
Figure 3.
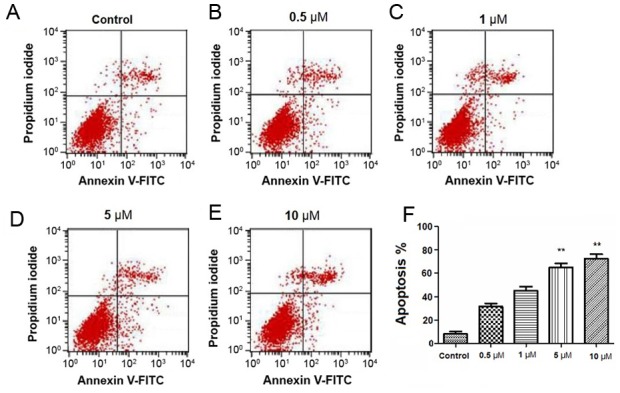
Apoptosis assay was performed with flow cytometry using PI and Annexin V-FITC double staining. Etomidate induced apoptosis in N2a cells in a concentration-dependent manner. N2a cells were treated with four different concentrations of etomidate (A) control, (B) 0.5, (C) 1, (D) 5 and (E) 10 µM. (F) Quanitifaction of % apoptosis. Data are expressed in mean ± standard error of the mean. **P<0.05 vs. control.
Effect of etomidateon generation of reactive oxygen species (ROS) in N2a cells
As a fore mentioned etomidate induced apoptosis in N2a cells; therefore, the current study investigated the effect of etomidate on the generation of reactive oxygen species using DCFH2-DA dye. Cells were treated with different concentrations of etomidate for 48 h and it was evident that etomidate treatment had a considerable impact on generation of reactive oxygen species in N2a cells as presented in Fig. 4. Therefore, these findings clearly indicated that etomidate induced generation of reactive oxygen species that led to apoptosis of N2a cells.
Figure 4.
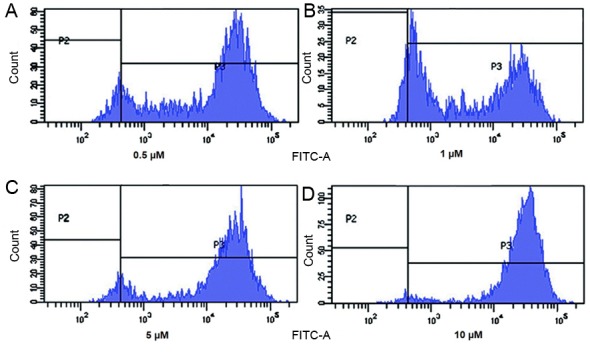
Etomidate treatment at concentration of (A) 0.5, (B) 1, (C) 5 and (D) 10 µM led to generation of reactive oxygen species in N2a cells.
Mitochondrial membrane potential loss is induced by etomidatein N2a cells
The current study investigated whether etomidate treatment of N2a cells had any influence on mitochondrial membrane potential using Rodamine-123 dye. It was evident that etomidate treatment of N2a cells lead to loss of their mitochondrial membrane potential in a dose-dependent manner which led to quenching of the fluorescence when compared with the untreated cells which retained fluorescence. These findings confirmed that etomidate induced apoptosis in N2a cells by reducing the mitochondrial membrane potential of these cells (Fig. 5).
Figure 5.
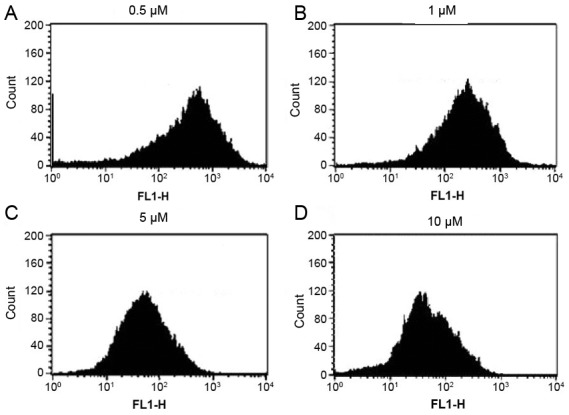
Etomidate treatment of N2a cells at concentration of (A) 0.5, (B), (C) 5 and (D) 10 µM reduced their mitochondrial membrane potential.
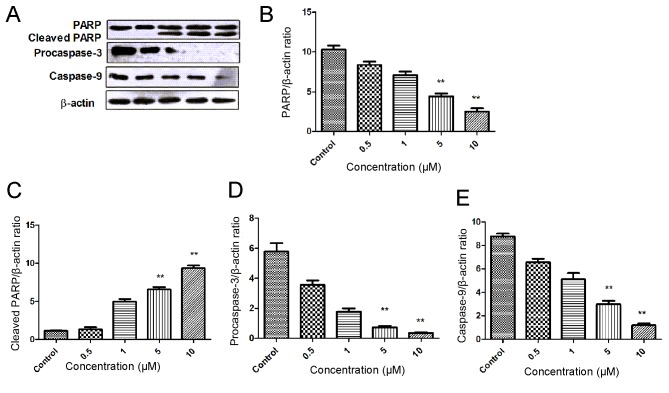
Etomidate induces PARP cleavage in N2a cells
In the present study, the effect of etomidate on the caspase activation and cleaving of PARP was investigated using western blotting. The N2a cells were treated with different concentrations of etomidate for 48 h. An increase in the expression of cleaved PARP was observed in a dose dependent manner. There was also a decrease in the expression level of the initiator caspase (caspase-9), along with a decrease in the expression of procaspase-3 in a dose-dependent manner. These findings suggested that etomidate induces apoptosis in a dose-dependent manner (Fig. 6).
Figure 6.
Western blot analysis of etomidate-treated samples. (A) Reperesentative western blotting image. Quantified expression levels of (B) PARP (C) cleaved PARP, (D) procaspase-3 and (E) caspase-9. Data are presented as the mean ± standard error of the mean. **P<0.05 vs. control. PARP, poly ADP-ribose polymerase.
Discussion
Surgery is one of the most common procedures used to treat patients in various diseases, including cancer (20,21). However, to lessen the pain for the patients, sedatives or anesthetics are administered prior the medical procedures being performed (22,23). Anesthesia is the induction of temporary loss of sensation or awareness (24). It is important as it aids in the execution of surgeries that may be otherwise impossible to be performed due to intolerable pain (24). The type of anesthesia administered to a patient depends upon the type of medical procedure that is to be performed on the patient during toothache, local anesthesia is administered that helps by blocking the transmission of the nerve impulse between the central nervous system and the tooth that is to be treated. During surgeries involving the removal of tumor mass from a patient with cancer, general anesthesia is administered, which leads to total suppression of central nervous system resulting in total lack of sensation and unconsciousness (9,25). Etomidate is a drug that is administered as general anesthetic to patients. Etomidate is widely used due to easy dosing pattern and because it has no effect on the blood pressure of the patient (26,27). Additionally, it also provides myocardial and cerebral ischemia protection, minimal suppression of ventilation and decrease in liberation of histamines (15,28). To the best of our knowledge, whilst the way etomidate functions has been previously established, how etomidate exerts some of its cytotoxic effects remains to be determined. The present study revealed that anesthetic agent etomidate induces apoptosis in the N2a neuroblastoma cell line. The cells responded to etomidate in a concentration-dependent manner with increased cell death. In previous study, etomidate was identified to exert cytotoxic effect on RAW264.7 murine leukemia macrophage cell line (29). It has been determined that etomidate may lead to enhancement of apoptotic cell morphological changes and reduced cell viability in RAW264.7 cells. It also leads to an increase in expression of cytochrome c, apoptosis-inducing factor (AIF), endonuclease G (Endo G), caspase-9, caspase-3 active form and BCL2 associated X (Bax) proteins; however, it inhibited the expression of B cell leukemia/lymphoma 2 extra-large (Bcl-xL), leading to apoptosis (29). This would appear to be the case with etomidate viaa mitochondria-dependent pathway based on the change of the ratio of Bax/Bcl-xL which led to cytochrome c, AIF and Endo G, release from mitochondria (30–32). In present study, western blot analysis of etomidate-treated N2a cells revealed an decrease in the expression of pro-apoptotic proteins, such as initiator caspase-9 and procaspase-3. It was evident that there was an increase in the expression of cleaved PARP in a dose-dependent manner. Additionally, etomidate treatment led to generation of reactive oxygen species in N2a cells. Etomidate treatment also led to loss of mitochondrial membrane potential in N2a cells.
In conclusion, the current study revealed that etomidate is an anesthetic drug that induces apoptosis in the N2a neuroblastoma cell line. However, further studies in vitro and in vivo are required in order to confirm the cytotoxic effects of etomidate.
Acknowledgements
Authors would like to thank the Affiliated Foshan Hospital of Sun Yat-sen University (Guangdong, China) for providing the laboratory facility for the present study.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JZ and ZYY conceived and designed the experiments and wrote the manuscript. HTC, YLF, MX, XQH and CL performed the experiments. BJL, LXF, XQH and LX analyzed the data.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ghoneim MM, Block RI, Haffarnan M, Mathews MJ. Awareness during anesthesia: Risk factors, causes and sequelae: A review of reported cases in the literature. Anesth Analg. 2009;108:527–535. doi: 10.1213/ane.0b013e318193c634. [DOI] [PubMed] [Google Scholar]
- 2.Hsu GL, Hsieh CH, Chen HS, Ling PY, Wen HS, Liu LJ, Chen CW, Chua C. The advancement of pure local anesthesia for penile surgeries: Can an outpatient basis be sustainable? J Androl. 2007;28:200–205. doi: 10.2164/jandrol.106.000679. [DOI] [PubMed] [Google Scholar]
- 3.Lee JS, Hayanga AJ, Kubus JJ, Makepeace H, Hutton M, Campbell DA, Jr, Englesbe MJ. Local anesthesia: A strategy for reducing surgical site infections? World J Surg. 2011;35:2596–2602. doi: 10.1007/s00268-011-1298-x. [DOI] [PubMed] [Google Scholar]
- 4.Breen P, Park KW. General anesthesia versus regional anesthesia. Int Anesthesiol Clin. 2002;40:61–71. doi: 10.1097/00004311-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Craig AD. Interoception: The sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/S0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 6.Bery A, Cardona A, Martinez P, Hartenstein V. Structure of the central nervous system of a juvenile acoel, Symsagittifera roscoffensis. Dev Genes Evol. 2010;220:61–76. doi: 10.1007/s00427-010-0328-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koizumi O. Nerve ring of the hypostome in hydra: Is it an origin of the central nervous system of bilaterian animals? Brain Behav Evol. 2007;69:151–159. doi: 10.1159/000095204. [DOI] [PubMed] [Google Scholar]
- 8.Mace SE. Central nervous system infections as a cause of an altered mental status? What is the pathogen growing in your central nervous system? Emerg Med Clin North Am. 2010;28:535–570. doi: 10.1016/j.emc.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Uhrig L, Dehaene S, Jarraya B. Cerebral mechanisms of general anesthesia. Ann Fr Anesth Reanim. 2014;33:72–82. doi: 10.1016/j.annfar.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Bajwa S, Kulshrestha A. Dexmedetomidine: An adjuvant making large inroads into clinical practice. Ann Med Health Sci Res. 2013;3:475–483. doi: 10.4103/2141-9248.122044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chohan AS. Anesthetic considerations in orthopedic patients with or without trauma. Top Companion Anim Med. 2010;25:107–119. doi: 10.1053/j.tcam.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Sarkiss M. Anesthesia for bronchoscopy and interventional pulmonology: From moderate sedation to jet ventilation. Curr Opin Pulm Med. 2011;17:274–278. doi: 10.1097/MCP.0b013e3283471227. [DOI] [PubMed] [Google Scholar]
- 13.Lü F, Lin J, Benditt DG. Conscious sedation and anesthesia in the cardiac electrophysiology laboratory. J Cardiovasc Electrophysiol. 2013;24:237–245. doi: 10.1111/jce.12001. [DOI] [PubMed] [Google Scholar]
- 14.Hansson L, Zanchetti A, Carruthers SG, Dahlöf B, Elmfeldt D, Julius S, Ménard J, Rahn KH, Wedel H, Westerling S. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: Principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT study Group. Lancet. 1998;351:1755–1762. doi: 10.1016/S0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 15.El-Khatib MF, Bou-Khalil P. Clinical review: Liberation from mechanical ventilation. Crit Care. 2008;12:221. doi: 10.1186/cc6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nedergaard M, Dirnagl U. Role of glial cells in cerebral ischemia. Glia. 2005;50:281–286. doi: 10.1002/glia.20205. [DOI] [PubMed] [Google Scholar]
- 17.Tavare AN, Perry NJ, Benzonana LL, Takata M, Ma D. Cancer recurrence after surgery: Direct and indirect effects of anesthetic agents. Int J Cancer. 2012;130:1237–1250. doi: 10.1002/ijc.26448. [DOI] [PubMed] [Google Scholar]
- 18.Drlica K. Mechanism of fluoroquinolone action. Curr Opin Microbiol. 1999;2:504–508. doi: 10.1016/S1369-5274(99)00008-9. [DOI] [PubMed] [Google Scholar]
- 19.Alonso-Castro AJ, Domínguez F, García-Carrancá A. Rutin exerts antitumor effects on nude mice bearing SW480 tumor. Arch Med Res. 2013;44:346–351. doi: 10.1016/j.arcmed.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Duffy MJ. The war on cancer: Are we winning? Tumor Biol. 2013;34:1275–1284. doi: 10.1007/s13277-013-0759-2. [DOI] [PubMed] [Google Scholar]
- 21.De La Puerta B, Baines S. Surgical diseases of the genital tract in male dogs 1. Scrotum, testes and epididymides. In Pract. 2012;34:58–65. doi: 10.1136/inp.e1102. [DOI] [Google Scholar]
- 22.Hansen TG. Sedative medications outside the operating room and the pharmacology of sedatives. Curr Opin Anaesthesiol. 2015;28:446–452. doi: 10.1097/ACO.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 23.Anderson SL, Duke-Novakovski T, Singh B. The immune response to anesthesia: Part 2 sedatives, opioids, and injectable anesthetic agents. Vet Anaesth Analg. 2014;41:553–566. doi: 10.1111/vaa.12125. [DOI] [PubMed] [Google Scholar]
- 24.Chamisha Y, Shamir MH, Merbl Y, Chai O. Reversible paralysis and loss of deep pain sensation after topical intrathecal morphine administration following durotomy. Vet Surg. 2015;44:41–45. doi: 10.1111/j.1532-950X.2014.12192.x. [DOI] [PubMed] [Google Scholar]
- 25.Dierdorf SF. Awareness during anesthesia. Anesthesiol Clin. 1996;14:369–384. doi: 10.1016/S0889-8537(05)70277-7. [DOI] [Google Scholar]
- 26.de la Grandville B, Arroyo D, Walder B. Etomidate for critically ill patients. Con: Do you really want to weaken the frail? Eur J Anaesthesiol. 2012;29:511–514. doi: 10.1097/EJA.0b013e32835819ca. [DOI] [PubMed] [Google Scholar]
- 27.Janouschek H, Nickl-Jockschat T, Haeck M, Gillmann B, Grözinger M. Comparison of methohexital and etomidate as anesthetic agents for electroconvulsive therapy in affective and psychotic disorders. J Psychiatr Res. 2013;47:686–693. doi: 10.1016/j.jpsychires.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 28.Mendez-Tellez PA, Needham DM. Early physical rehabilitation in the ICU and ventilator liberation. Respir Care. 2012;57:1663–1669. doi: 10.4187/respcare.01931. [DOI] [PubMed] [Google Scholar]
- 29.Wu RS, Wu KC, Yang JS, Chiou SM, Yu CS, Chang SJ, Chueh FS, Chung JG. Etomidate induces cytotoxic effects and gene expression in a murine leukemia macrophage cell line (RAW264.7) Anticancer Res. 2011;31:2203–2208. [PubMed] [Google Scholar]
- 30.Chiang JH, Yang JS, Ma CY, Yang MD, Huang HY, Hsia TC, Kuo HM, Wu PP, Lee TH, Chung JG. Danthron, an anthraquinone derivative, induces DNA damage and caspase cascades-mediated apoptosis in SNU-1 human gastric cancer cells through mitochondrial permeability transition pores and Bax-triggered pathways. Chem Res Toxicol. 2011;24:20–29. doi: 10.1021/tx100248s. [DOI] [PubMed] [Google Scholar]
- 31.Yu FS, Yang JS, Yu CS, Lu CC, Chiang JH, Lin CW, Chung JG. Safrole induces apoptosis in human oral cancer HSC-3 cells. J Dent Res. 2011;90:168–174. doi: 10.1177/0022034510384619. [DOI] [PubMed] [Google Scholar]
- 32.Chen JC, Lu KW, Lee JH, Yeh CC, Chung JG. Gypenosides induced apoptosis in human colon cancer cells through the mitochondria-dependent pathways and activation of caspase-3. Anticancer Res. 2006;26:4313–4326. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.